Abstract
Purpose
To determine the prevalence of thyroid eye disease among dysthyroid Korean patients and to analyze the relationship between demographic data, lifestyle risk factors, and status of thyroid disease and thyroid eye disease.
Methods
All dysthyroid patients who visited endocrinology clinics in 24 general hospitals in Korea during a chosen one-week period were enrolled in this cross-sectional study. Data were collected during an interviewer-administered questionnaire and chart review. Demographic data, lifestyle risk factors, and status of thyroid disease variables were analyzed as risk factors using multivariable regression models to identify independent associations with thyroid eye disease.
Results
A total of 1,632 dysthyroid patients were included (1,301 females [79.7%] and 331 males [20.3%]). Two hundred eighty-three of these patients (17.3%) had thyroid eye disease. Multiple logistic regression analyses revealed that female gender, young age, Graves' disease, dermopathy, anti-thyroid medication treatment, and radioiodine treatment were independent risk factors for thyroid eye disease.
Conclusions
The lower prevalence of thyroid eye disease in dysthyroid Korean patients and the influence of gender on risk factors in this study are novel findings compared to studies performed involving Europeans. Although the risk factors for thyroid eye disease are understood in part, a more in-depth comparative study of gender and ethnic groups is needed to fully understand the biological significance of the demographic factors.
Keywords: Epidemiology, Graves ophthalmopathy, Korea, Prevalence, Risk factors
Thyroid dysfunction, consisting of heterogeneous autoimmune disorders affecting systemic organs, is a relatively common malady in endocrinology clinics. Thyroid eye disease may be the first noticeable warning sign prompting dysthyroid patients to seek medical advice at the onset of the disease or may complicate the disease progression [1,2].
Thyroid eye disease is characterized by various clinical features [3]. Inflammatory reactions to the orbital and periocular tissues cause discomfort and pain and also result in eyelid malposition and exophthalmos, distorting self-image and provoking psychological or social problems. Extraocular muscle changes lead to more serious complications, such as strabismus or visual disturbances. The activity and severity of thyroid eye disease is diverse as well [4]. The subclinical form of thyroid eye disease can be detected with imaging evidence of ultrasonographic [5] or computed tomographic [6] findings or other diagnostic methods, such as measurement of intraocular pressure [7].
A study based on data from published articles indicated that the mean weighted prevalence rate of hyperthyroidism was 1,151 per 100,000 people [8]. According to community-based studies, the prevalence of thyroid dysfunction was reported to be 8.5% to 13.6% in an elderly age group [9,10].
Few data are available on the incidence or prevalence of thyroid eye disease in patients with thyroid dysfunction. It has been suggested that up to 50% of patients with thyroid dysfunction have some form of clinical thyroid eye disease, and 90% have evidence of eye disease on imaging studies, primarily consisting of mild and subclinical forms of disease [11,12]. Using diagnostic criteria of clinical signs, Bartley et al. [13] evaluated the incidence of thyroid eye disease in Olmsted County, Minnesota and showed that new onset thyroid eye disease occurred in 18.8 per 100,000 people (16.0 females and 2.9 males) per year [14]. Manji et al. [15] reported that moderate-to-severe thyroid eye disease (NOSPECS score ≥2) occurred in 51.5% of female and 52.7% of male patients in a cohort of Caucasians with Graves' disease and Hashimoto's thyroiditis.
Asians are known to have different patterns of morbidity compared to non-Asians, and this also applies to thyroid disorders [16]. Asian patients have a higher prevalence of thyroid eye disease [11] but present with a less severe disease pattern [11,17,18]. The clinical factors related to the development of thyroid eye disease have been reported to include age [15,19], gender [15,19], smoking [15,20-23], family history [15,24], and pregnancy [25,26]. The diversity of genetic predisposition, lifestyle, and environmental factors between ethnic groups may influence differences in disease patterns between Asian and non-Asian people. For instance, the smoking rate of Koreans has been reported to be as high as 60.1% [27] to 74.8% [28] in males and as low as 2.9% [28] in females, which is one of the distinctive findings characterizing the lifestyle of Koreans [29]. The disease pattern of Koreans is expected to be different from Westerners; however, a detailed investigation has not yet been performed.
The aim of the present study was to determine the prevalence of thyroid eye disease among Korean patients with thyroid dysfunction and the risk factors associated with thyroid eye disease. To the best of our knowledge, this is the first multi-center study in Korea and the first study to report the prevalence of thyroid eye disease among dysthyroid patients in Asia.
Materials and Methods
A cross-sectional study of thyroid eye disease was conducted on patients with thyroid dysfunction visiting the endocrinology clinic at 24 general hospitals in Korea. The study was performed during a one-week period from 21 to 28 November, 2005.
All patients with thyroid dysfunction presenting to the endocrinology clinic during the study period were identified and enumerated. The initial diagnosis was categorized as Graves' disease and hypothyroidism. Patients with malignant disease of the thyroid gland and thyroid nodules were excluded. All patients >15 years of age were informed of the study and invited to participate in the interview, which was conducted by an ophthalmologist. All study participants were Korean. All study procedures adhered to the tenets of the Declaration of Helsinki.
Self-reported demographic data, past medical history, family history, and life-style data were collected during the interviewer-administered questionnaire. An ophthalmologist in each institution interviewed the patients and noted the presence of symptoms relevant to thyroid eye disease using the VISA classification including vision, inflammatory, strabismus, and appearance categories [30]. For the patients with eye symptoms, the ophthalmologist interviewer performed an ophthalmic examination. Patients with at least one symptom and one sign in any category in the VISA classification were considered to have thyroid eye disease. The clinical data pertaining to the present illness were retrieved by chart review.
Categorical variables were compared using the chi-square test, a t-test was used for continuous variables, and continuous variables for multiple groups were compared using analysis of variance (ANOVA). Multivariable logistic regression analyses were carried out to evaluate the independent relationship of significant risk factors for thyroid eye disease. The SAS ver. 8 software package (SAS Inc., Cary, NC, USA) was used for statistical analyses. Odds for thyroid eye disease were presented with 95% confidence internals (CIs). A p-value of <0.05 was considered significant.
Results
Of the 2,605 patients who were included as eligible for the study, 2,044 (78.5%) agreed to and completed the interviewer-administered questionnaire. Among the total patients, 1,397 (68.3%) patients were diagnosed with Graves' disease, 642 (31.4%) patients were treated for hypothyroidism, and 5 (0.2%) had incomplete information. Of the total 2,044 patients, 31 who had more than one visit, 4 younger than 15 years of age, and 377 who provided incomplete data for multivariate analysis were excluded. Thus, 1,632 patients served as the basis for the analysis.
Of the 1,632 dysthyroid patients, 1,301 (79.7%) were females and 331 (20.3%) were males (female-to-male [3.9 : 1]). The mean age and standard deviation of females and males was 45.0 ± 14.2 and 45.4 ± 13.3 years, respectively; there was no significant difference in age between females and males.
Thyroid eye disease presented in 283 (17.34%; 95% CI, 15.50 to 19.18) of the 1,632 patients examined (225 females and 58 males, female-to-male [3.9 : 1]) (Table 1). There was no difference in the prevalence of thyroid eye disease between genders (females, 225 / 1,301 [17.3%]; males, 58 / 331 [17.5%]; p = 0.6847). In the age distribution in male patients, there was a higher prevalence of men in their 30s and 50s; however, there was no significant difference in the prevalence between age groups (p = 0.13). For female patients, there was a statistically significant difference in the prevalence between age groups (p < 0.001), with a higher prevalence in the younger age group (Table 1).
Table 1.
Prevalence and age distribution of thyroid eye disease in Korean dysthyroid patients
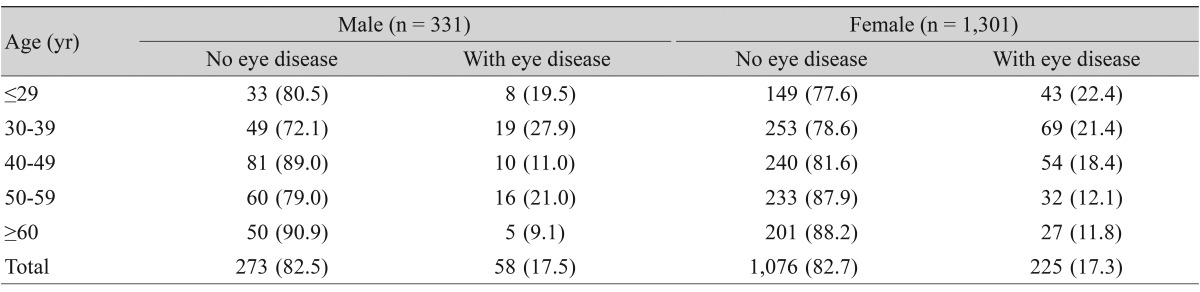
Values are presented as number (%).
Since the gender distribution in the dysthyroid patients showed a predominance of females and the pattern of age distribution in the thyroid eye disease group was dissimilar between genders, gender-specific factors which might contribute to thyroid eye disease were studied. Various factors, including demographics, family history, medical history, and lifestyle, were analyzed in each gender (Table 2).
Table 2.
Comparison of characteristics between patients with and without thyroid eye disease according to gender
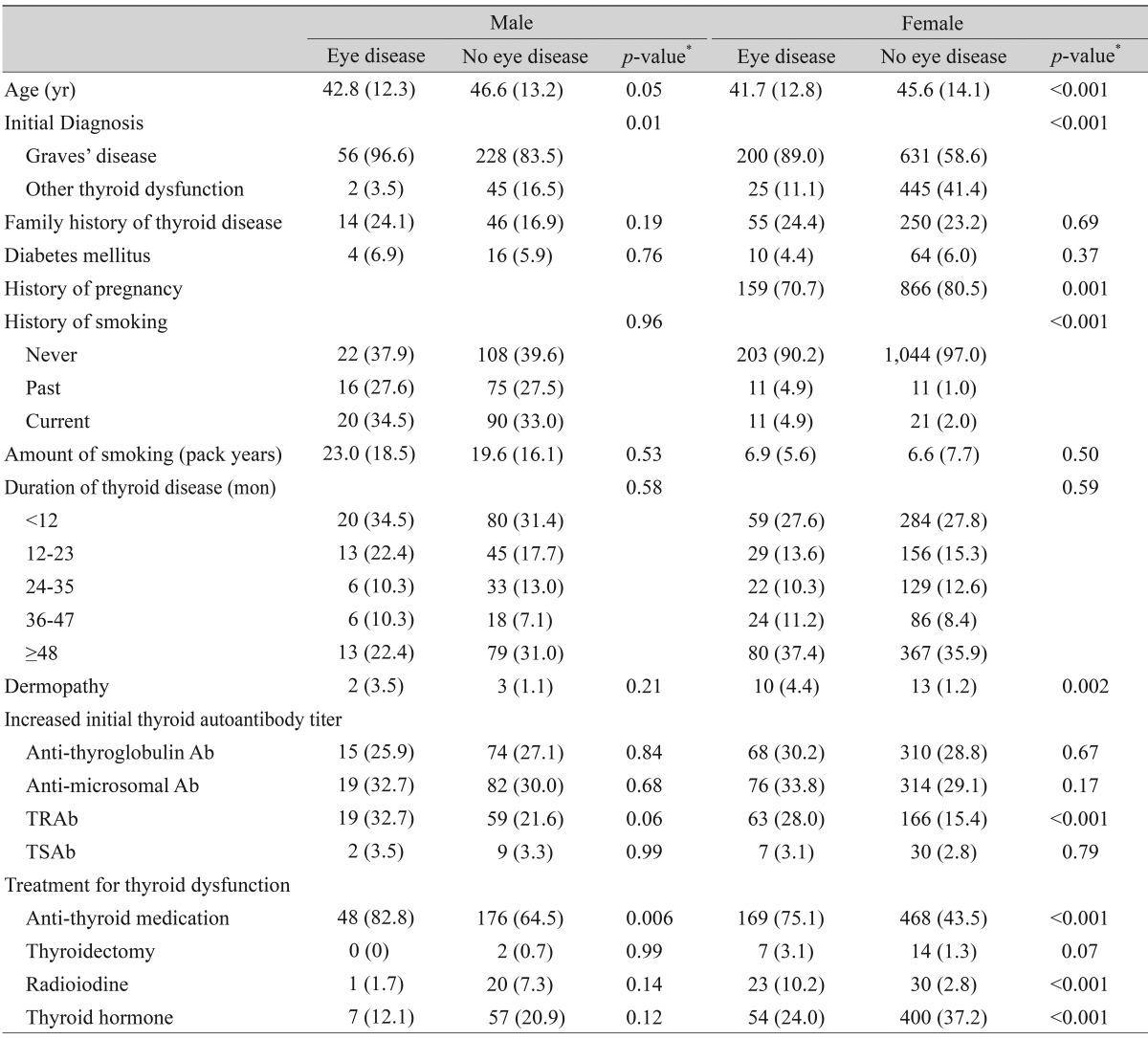
Values are presented as mean (SD) or number (%).
TRAb = thyroid stimulating hormone-receptor antibodies; TSAb = thyroid stimulating antibodies.
*p-values were obtained by t-test or chi-square test.
Age and Graves' disease in both genders, history of smoking in females, dermopathy in females, initially high anti-thyroid stimulating hormone receptor antibody titer in females, anti-thyroid medication in both genders, and radioiodine treatment in females were significantly more prevalent in the thyroid eye disease group than the no eye disease group. On the contrary, history of pregnancy and thyroid hormone therapy in females were more prevalent in the no eye disease group than in the thyroid eye disease group. With respect to lifestyle factors, smoking was shown to have an association with thyroid eye disease in females (p < 0.001) but not in males (p = 0.96).
Multivariable logistic regression analyses for thyroid eye disease revealed that female gender, Graves' disease, dermopathy, anti-thyroid medication, and radioiodine treatment were strongly associated with thyroid eye disease. The risk of thyroid eye disease was shown to be lower with age (Table 3).
Table 3.
Multivariable-adjusted associations* between thyroid eye disease and selected variables in Korean dysthyroid patients (n = 1,632)
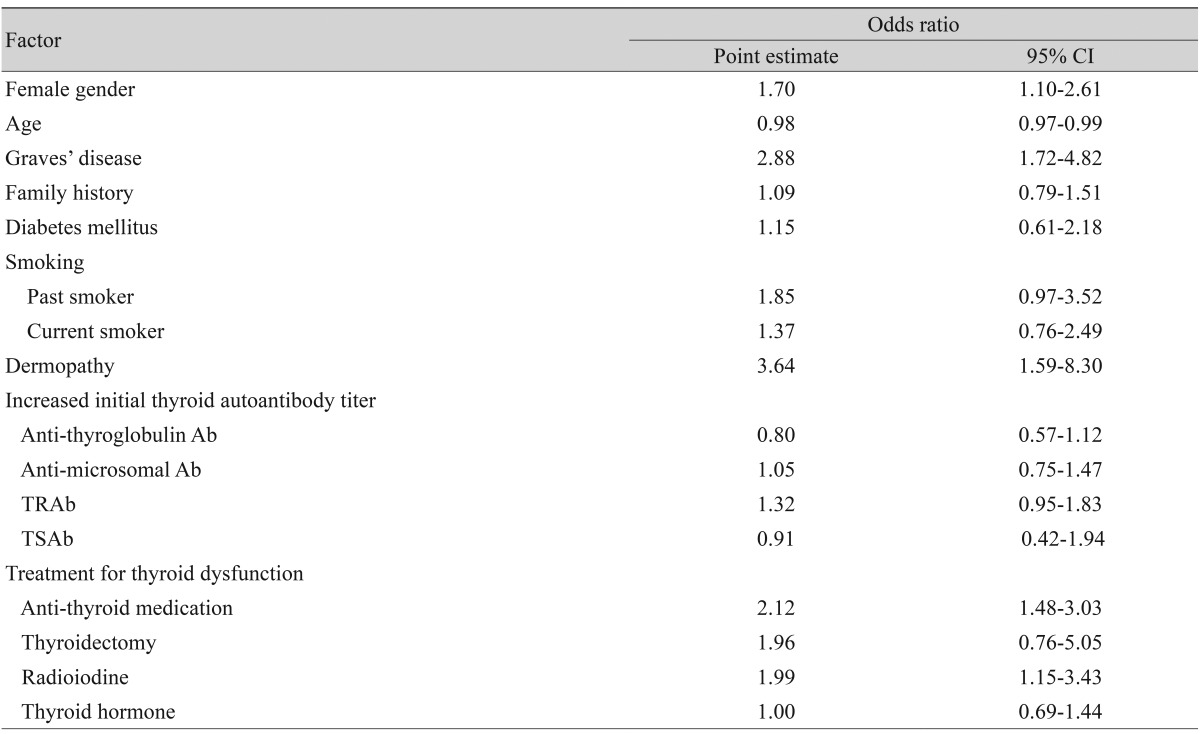
CI = confidence interval; TRAb = thyroid stimulating hormone-receptor antibodies; TSAb = thyroid stimulating antibodies.
*Estimated by multiple logistic regression analysis with an adjustment for other factors presented in the table.
Discussion
Thyroid eye disease was present in 17.3% of dysthyroid patients in this study. Comparing this result directly to other studies may be flawed because the inclusion criteria and study settings are not equivalent. In British studies, the prevalence of thyroid eye disease (NOSPECS score ≥2) was reported to be 51.7% in a Graves' disease cohort of 2,405 patients [15], 40.3% among a Graves' disease cohort of 536 patients [31], and 42% of 116 patients with newly-diagnosed Graves' disease [18]. The prevalence of thyroid eye disease in Graves' disease in the current study was 21.3% (237 / 1,115) if the NOSPECS score ≥2 criteria was applied, which is much lower than in the British studies.
There has been controversy in the reported prevalence of thyroid eye disease in Asian patients [11,18]. Some studies have suggested ethnic differences in the prevalence to be related to different smoking rates [12,18]. Multifactorial etiologies may affect the difference in thyroid eye disease between diverse ethnic groups.
The prevalence of eye disease was 22.3% (139 / 623) in the <40-years-age group and 14.3% (144 / 1,009) in the >40-years-age group in this study (Table 1). Compared to the results reported by Allahabadia et al. [31], the prevalence pattern in each age group was reversed; the prevalence in the former group was 32.7% and the prevalence in the latter group was 54.5%. In another inter-racial comparison, an age of onset of Graves' disease >42 years had an effect on the development of Graves' orbitopathy in Polish patients. In contrast, an age of onset of Graves' disease patients <32 years had a significant effect on the development of Graves' orbitopathy in Japanese patients [32]. Thyroid eye disease is more prevalent in younger people compared to older people amongst Asians, and eye disease is more prevalent in older people than younger people amongst Caucasians. Younger patients with thyroid eye disease may experience greater psychosocial distress from facial disfigurement than older patients [33], which might therefore be a more significant problem for Asian patients.
The risk factors related to thyroid eye disease showed a dissimilar pattern between genders in the current study. Females had more prominent risk factors than did male patients. Although the reason for this gender dissimilarity might be partially related to the small number of male patients, gender characteristics may affect the factors associated with the occurrence of thyroid eye disease in patients with thyroid dysfunction. In a study of the influence of gender on Graves' disease, psychological stress and smoking were positively associated and drinking was negatively associated with Graves' disease in females, whereas no such associations were observed in males [23]. As gender differences influence the immune response to lifestyle factors in patients with Graves' disease, gender characteristics may also affect risk factors in thyroid eye disease.
There are few data available on the effect of pregnancy on thyroid eye disease. Pregnancy has been regarded as a risk factor for Graves' disease [25,26] and may change the course of the disease due to increased thyroid activity in the first trimester, with alteration of the immune system in the second trimester and postpartum [26]. A clinically significant number of women develop Graves' disease after childbirth compared to nulliparas [25]. Graves' disease patients in a postpartum group were shown to have a greater likelihood of positive family history, less eye disease, and a lower relapse rate than the others. The disease is considered as a milder form in such cases due to the transient nature of postpartum maternal immune system changes [25]. Moreover, in the current study, younger patients with higher rates of eye disease might have influenced the higher rate of eye disease in the non-pregnant patient group.
Smoking has been regarded as a very significant risk factor for thyroid eye disease [15,20-23]. A two-fold increased risk of thyroid eye disease was evident in current smokers, and an increased risk was also present in ex-smokers compared with non-smokers [15]. The smoking factor provided an interesting result in this study. The association of smoking with eye disease in male patients was not significant; the smoking rate was 62.1% in the eye disease group and 60.4% in the group without eye disease (Table 2). Female patients had a lower smoking rate, 9.2% in the eye disease group and 3.0% in the group without eye disease, and showed a significant difference of smoking rates between the two groups (Table 2). In a Japanese study, smoking was independently associated with risk for Graves' disease in women but not in men [23]. Gender may have influenced the effect of smoking on the development of thyroid eye disease in dysthyroid patients in the current study group.
In a study of Polish and Japanese Graves' disease patients on factors contributing to thyroid eye disease, smoking was a risk factor in Polish people but not in Japanese people [32]. In another comparative study on the prevalence of thyroid eye disease between European and Asian populations, the prevalence was 42% in Europeans compared to 7.7% in Asians, and the overall risk for Europeans for developing thyroid eye disease was 6.4 times higher than for Asians [18]. In this group, the smoking rate was 61.2% in Europeans and 23% in Asians. The higher prevalence in Europeans can be partly explained by ethnic differences, as well as the higher smoking rate in Europeans. The smoking factor is known to be a risk factor in Europeans; however, the role of smoking in the Asian population is complex and warrants further studies.
Dermopathy showed a strong association with eye disease in this study and is also known to be related to the severity of thyroid eye disease [34]. This might reflect a common pathogenesis of the two diseases. Patients with Graves' dermopathy need more thorough follow-up and more aggressive therapy for eye disease.
With regard to the treatment modalities for thyroid disease, anti-thyroid medication and radioiodine therapy were associated with the occurrence of eye disease. Although anti-thyroid medication was used mostly for Graves' disease patients with higher eye disease rates, the medication also affected the occurrence of eye disease in the multivariate analysis. Further studies are needed to clarify the role of anti-thyroid medication in the occurrence of thyroid eye disease.
This study has several limitations; all of the patients were interviewed by ophthalmologists, but not all of them underwent ophthalmologic examinations in a mass screening setting.
This study on the prevalence and risk factors of thyroid eye disease among dysthyroid Korean patients revealed novel findings associated with ethnic differences. The prevalence of thyroid eye disease in dysthyroid Korean patients was lower than in Europeans. Younger female patients were more prevalent in Koreans in contrast to Europeans. The risk factors in thyroid eye disease showed dissimilar patterns between genders. To understand the biological significance of ethnic factors, further studies on differences in thyroid eye disease between ethnic groups are needed.
Acknowledgements
The authors wish to thank Yun-Mi Song, MD for her assistance with the statistical analyses performed for this study. We thank all of the participants in this project: Sang In Khwarg, MD (Seoul National University Hospital), Yong Jae Lee, MD (Seoul Asan Medical Center), Sung Bok Lee, MD (Chungnam National University Hospital), Kyung-Chul Yoon, MD (Chonnam National University Hospital), Keun-Hae Kim, MD (Daegu Catholic University Hospital), Jae Wook Yang, MD (Inje University Pusan Baik Hospital), Yoon-Duck Kim, MD (Samsung Medical Center), Joo Wan Park, MD (The Catholic University of Korea, St. Mary's Hospital), Tae Soo Lee, MD (Korea University Guro Hospital), Sang Yeul Lee, MD (Yonsei University Severance Hospital), Min Ahn, MD (Chonbuk National University Hospital), Kyung In Woo, MD (Kangbuk Samsung Hospital), Sang Duck Kim, MD (Wonkwang University Hospital), Helen Lew, MD (CHA University Bundang CHA General Hospital), Sun Joo Lee, MD (Hallym University Hospital), Joo Heon Roh, MD (Kosin University Gospel Hospital), Nam Joo Kim, MD (Seoul National University Bundang Hospital), Ho Kyung Chung, MD (Boramae Hospital), Hee Bae Ahn, MD (Donga University Hospital), Yoon Jung Lee, MD (Hanyang University Kuri Hospital), Suk-Woo Yang, MD (The Catholic University of Korea, Kangnam St. Mary's Hospital), Byoung Jin Kim, MD (Hallym University Kangdong Hospital), Se Hyun Baek, MD (Korea University Ansan Hospital), and Sung Joo Kim, MD (Kim's Eye Hospital).
Footnotes
This was presented at 2nd International Orbital Society Symposium, October 26, 2008, New York, NY, USA.
No potential conflict of interest relevant to this article was reported.
References
- 1.Bartley GB, Fatourechi V, Kadrmas EF, et al. Chronology of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:426–434. doi: 10.1016/s0002-9394(14)70439-8. [DOI] [PubMed] [Google Scholar]
- 2.Wiersinga WM, Smit T, van der Gaag R, Koornneef L. Temporal relationship between onset of Graves' ophthalmopathy and onset of thyroidal Graves' disease. J Endocrinol Invest. 1988;11:615–619. doi: 10.1007/BF03350193. [DOI] [PubMed] [Google Scholar]
- 3.Werner SC. Classification of the eye changes of Grave's disease. J Clin Endocrinol Metab. 1969;29:982–984. doi: 10.1210/jcem-29-7-982. [DOI] [PubMed] [Google Scholar]
- 4.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner SC, Coleman DJ, Franzen LA. Ultrasonographic evidence of a consistent orbital involvement in Graves's disease. N Engl J Med. 1974;290:1447–1450. doi: 10.1056/NEJM197406272902602. [DOI] [PubMed] [Google Scholar]
- 6.Forbes G, Gorman CA, Brennan MD, et al. Ophthalmopathy of Graves' disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986;7:651–656. [PMC free article] [PubMed] [Google Scholar]
- 7.Gamblin GT, Harper DG, Galentine P, et al. Prevalence of increased intraocular pressure in Graves' disease: evidence of frequent subclinical ophthalmopathy. N Engl J Med. 1983;308:420–424. doi: 10.1056/NEJM198302243080803. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 9.Diez JJ, Molina I, Ibars MT. Prevalence of thyroid dysfunction in adults over age 60 years from an urban community. Exp Clin Endocrinol Diabetes. 2003;111:480–485. doi: 10.1055/s-2003-44707. [DOI] [PubMed] [Google Scholar]
- 10.Empson M, Flood V, Ma G, et al. Prevalence of thyroid disease in an older Australian population. Intern Med J. 2007;37:448–455. doi: 10.1111/j.1445-5994.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 11.Rootman J. Diseases of the orbit: a multidisciplinary approach. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 169–177. [Google Scholar]
- 12.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002;12:855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 13.Bartley GB, Fatourechi V, Kadrmas EF, et al. The incidence of Graves' ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol. 1995;120:511–517. doi: 10.1016/s0002-9394(14)72666-2. [DOI] [PubMed] [Google Scholar]
- 14.Bartley GB, Gorman CA. Diagnostic criteria for Graves' ophthalmopathy. Am J Ophthalmol. 1995;119:792–795. doi: 10.1016/s0002-9394(14)72787-4. [DOI] [PubMed] [Google Scholar]
- 15.Manji N, Carr-Smith JD, Boelaert K, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873–4880. doi: 10.1210/jc.2006-1402. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson LJ, Taylor JB. Patterns of Asian and non-Asian morbidity in hospitals. Br Med J (Clin Res Ed) 1983;286:949–951. doi: 10.1136/bmj.286.6369.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai CC, Kau HC, Kao SC, Hsu WM. Exophthalmos of patients with Graves' disease in Chinese of Taiwan. Eye (Lond) 2006;20:569–573. doi: 10.1038/sj.eye.6701925. [DOI] [PubMed] [Google Scholar]
- 18.Tellez M, Cooper J, Edmonds C. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf) 1992;36:291–294. doi: 10.1111/j.1365-2265.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 19.Perros P, Crombie AL, Matthews JN, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf) 1993;38:367–372. doi: 10.1111/j.1365-2265.1993.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 20.Bartalena L, Marcocci C, Tanda ML, et al. Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med. 1998;129:632–635. doi: 10.7326/0003-4819-129-8-199810150-00010. [DOI] [PubMed] [Google Scholar]
- 21.Eckstein A, Quadbeck B, Mueller G, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87:773–776. doi: 10.1136/bjo.87.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann K. Risk of smoking in thyroid-associated orbitopathy. Exp Clin Endocrinol Diabetes. 1999;107(Suppl 5):S164–S167. doi: 10.1055/s-0029-1212176. [DOI] [PubMed] [Google Scholar]
- 23.Yoshiuchi K, Kumano H, Nomura S, et al. Stressful life events and smoking were associated with Graves' disease in women, but not in men. Psychosom Med. 1998;60:182–185. doi: 10.1097/00006842-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851–1856. doi: 10.1002/art.10370. [DOI] [PubMed] [Google Scholar]
- 25.Benhaim Rochester D, Davies TF. Increased risk of Graves' disease after pregnancy. Thyroid. 2005;15:1287–1290. doi: 10.1089/thy.2005.15.1287. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus JH. Epidemiology and prevention of thyroid disease in pregnancy. Thyroid. 2002;12:861–865. doi: 10.1089/105072502761016485. [DOI] [PubMed] [Google Scholar]
- 27.Cho HJ, Song YM, Smith GD, Ebrahim S. Trends in socio-economic differentials in cigarette smoking behaviour between 1990 and 1998: a large prospective study in Korean men. Public Health. 2004;118:553–558. doi: 10.1016/j.puhe.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Chung MH, Chung KK, Chung CS, Raymond JS. Health-related behaviors in Korea: smoking, drinking, and perinatal care. Asia Pac J Public Health. 1992-1993;6:10–15. doi: 10.1177/101053959200600105. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Prevalence of cigarette use among 14 racial/ethnic populations: United States, 1999-2001. MMWR Morb Mortal Wkly Rep. 2004;53:49–52. [PubMed] [Google Scholar]
- 30.Dolman PJ, Rootman J. VISA Classification for Graves orbitopathy. Ophthal Plast Reconstr Surg. 2006;22:319–324. doi: 10.1097/01.iop.0000235499.34867.85. [DOI] [PubMed] [Google Scholar]
- 31.Allahabadia A, Daykin J, Holder RL, et al. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1038–1042. doi: 10.1210/jcem.85.3.6430. [DOI] [PubMed] [Google Scholar]
- 32.Bednarczuk T, Hiromatsu Y, Fukutani T, et al. Association of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) gene polymorphism and non-genetic factors with Graves' ophthalmopathy in European and Japanese populations. Eur J Endocrinol. 2003;148:13–18. doi: 10.1530/eje.0.1480013. [DOI] [PubMed] [Google Scholar]
- 33.Kahaly GJ, Petrak F, Hardt J, et al. Psychosocial morbidity of Graves' orbitopathy. Clin Endocrinol (Oxf) 2005;63:395–402. doi: 10.1111/j.1365-2265.2005.02352.x. [DOI] [PubMed] [Google Scholar]
- 34.Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves' dermopathy and acropachy are markers of severe Graves' ophthalmopathy. Thyroid. 2003;13:1141–1144. doi: 10.1089/10507250360731541. [DOI] [PubMed] [Google Scholar]


