Abstract
In this study, we investigated the effect of flow perfusion culture on the mineralization of co-cultures of human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs). Osteogenically precultured hMSCs were seeded onto electrospun scaffolds in monoculture or a 1:1 ratio with HUVECs, cultured for 7 or 14 days in osteogenic medium under static or flow perfusion, and the resulting constructs were analyzed for cellularity, alkaline phosphatase (ALP) activity and calcium content. In flow perfusion, constructs with monocultures of hMSCs demonstrated higher cellularity and calcium content, but lower ALP activity compared to corresponding static controls. ALP activity was enhanced in co-cultures under flow perfusion conditions, compared to hMSCs alone; however unlike the static controls, the calcium content of the co-cultures in flow perfusion was not different from the corresponding hMSC monocultures. The data suggest that co-cultures of hMSCs and HUVECs did not contribute to enhanced mineralization compared to hMSCs alone under the flow perfusion conditions investigated in this study. However, flow perfusion culture resulted in an enhanced spatial distribution of cells and matrix compared to static cultures, which were limited to a thin surface layer.
Keywords: co-culture, hMSCs, HUVECs, flow perfusion bioreactor, bone tissue engineering
Introduction
Bone marrow is populated by a variety of stem and progenitor cells that endow the tissue with a high regenerative capacity.29 One such cell population is mesenchymal stem cells (MSCs), which are capable of differentiating into bone-forming osteoblasts, and reside in the perivascular niche of the bone marrow.8, 26, 29 The localization of MSCs around blood vessels has prompted the investigation of cross-talk between MSCs and vascular cells for a variety of tissue engineering applications. In some cases, the co-culture of MSCs and vascular cells, such as endothelial cells (ECs) has been investigated in an effort to enhance the osteogenic or angiogenic properties of tissue engineered constructs. For example, the addition of ECs to cultures of MSCs has been previously shown to enhance the osteogenic differentiation of the cultures.3, 21, 31–33
While numerous studies have investigated such co-cultures in various culture conditions,21 the majority of research to-date has focused on the co-culture of these cells in static conditions.9 In order to better recapitulate the bone marrow perivascular niche, the dynamic mechanical environment in vivo should also be considered in an in vitro co-culture system.13 Flow perfusion bioreactor systems can be used to apply mechanical stresses to three-dimensional cell cultures.6 Furthermore, relative to static culture, flow perfusion culture has been shown to be capable of improving the infiltration, proliferation, and osteogenic differentiation of MSCs in three-dimensional scaffolds through the enhancement of mass transfer and the application of shear stress to the cells.1, 6, 25, 30 While several studies have investigated the effects of shear stress on the vascularization of EC and MSC co-cultures,18, 24 the effect of flow perfusion culture on the osteogenic differentiation of MSCs in such co-cultures has not been evaluated.
Thus, the goal of this study was to investigate the effects of flow perfusion culture on the mineralization of co-cultures of human MSCs (hMSCs) and human umbilical vein endothelial cells (HUVECs) on three-dimensional porous polymer scaffolds. It was hypothesized that flow perfusion culture would enhance the osteogenenic differentiation of hMSCs and that addition of HUVECs would have a beneficial effect in flow perfusion. In order to evaluate this hypothesis, osteogenically precultured hMSCs were cultured alone or with HUVECs on electrospun microfiber scaffolds in flow perfusion and static conditions and analyzed after 7 and 14 days.
Materials and Methods
Experimental design
Osteogenically precultured hMSCs were seeded onto electrospun poly(ε-caprolactone) (PCL) scaffolds and cultured in osteogenic medium for up to 14 days. In order to investigate the effects of hMSC co-culture with ECs, HUVECs were cultured with hMSCs in a 1:1 ratio on the PCL scaffolds (MH). Additionally, to control for the effects of cell density, low-density (M1) and high-density (M2) hMSC monocultures were investigated. The low-density monocultures (M1) consisted of half the number of hMSCs as the high-density hMSC group (M2) and the same number of hMSCs as the co-culture groups (MH). In order to investigate the effects of flow perfusion on these cell populations, cultures were conducted in static (St) and flow perfusion (Fl) conditions.6 For each time point, two bioreactor units (with 10 constructs in each unit) were employed, and constructs were analyzed for DNA, alkaline phosphatase (ALP) activity, and calcium content (n=10, flow perfusion; n=6, static). Additionally, histological analysis (n=6, flow perfusion; n=2, static) and scanning electron microscopy (SEM) (n=4, flow perfusion; n=2, static) were performed to visualize the presence and distribution of cells and extracellular matrix.
Scaffold fabrication and characterization
Nonwoven PCL microfiber mats with an average fiber diameter of approximately 10 µm (9.4±1.0 µm) were fabricated as previously described,25 using a horizontal electrospinning setup. An 18 wt% PCL solution was prepared by dissolving PCL (inherent viscosity 1.0–1.3 dl/g; Lactel, Pelham, AL) in a solution of 5:1 v/v chloroform:methanol. The PCL solution was pumped at a flow rate of 25 ml/h through a blunt 16 G needle, which continuously received an applied voltage of 25 kV. The needle tip was directed at a copper collecting plate 33 cm away. Fiber diameter and morphology were inspected via SEM. Scaffolds (1.2 to 1.3 mm in thickness) were punched from electrospun mats using a 3 mm biopsy punch and loaded into custom-made polycarbonate holders designed to confine the cell solution during seeding and support the scaffolds during perfusion culture.6 Scaffolds were then sterilized by exposure to ethylene oxide (Anderson Sterilizers, Haw River, NC) for 14 hours and aerated overnight to remove residual gas. Following sterilization, scaffolds were pre-wetted by soaking in a decreasing ethanol gradient (100% to 25%), rinsed three times in phosphate buffered saline (PBS), and soaked in culture medium (α-MEM, 13% fetal bovine serum (FBS) (Atlanta biologicals, Norcross, GA), 1% penicillin/streptomycin/fungizone (Gibco, Grand Island, NY)) for three nights at 37°C prior to cell seeding.
Cell culture and seeding
Frozen bone marrow-derived hMSCs were provided by Dr. Darwin Prockop from the Texas A&M University Health Science Center in Temple, Texas, USA. Per the supplier, cells were confirmed to possess widely accepted CD-markers, including CD90, CD105 and CD73, and to be capable of osteogenic and adipogenic differentiation up to passage 4.8 For this experiment, the hMSCs were thawed and cultured for 2 passages in expansion medium (α-MEM without nucleosides and ribonucleosides (Gibco) with 13% v/v FBS, 100 units/ml penicillin, 100 µg/ml streptomycin (Gibco) and 2–4 mM L-glutamine (Sigma, St. Louis, USA)) and osteogenically precultured for 7 days in osteogenic medium (expansion medium supplemented with 10 nM dexamethasone, 10 mM β-glycerol-phosphate and 0.2 mM ascorbic acid (all from Sigma)).
Frozen primary HUVECs pooled from several donors were purchased from American Tissue Culture Collection, (ATCC, Manassas, VA). According to the supplier’s certification, the cells were von Willebrand factor positive and smooth muscle α-actin negative. HUVECs were thawed and cultured until passage 4 in vascular cell basal medium containing 0.2% bovine brain extract (BBE), 5 ng/ml rhEGF, 10 mM L-glutamine, 0.75 units/ml heparin sulfate, 1 µg/ml hydrocortisone, 50 µg/ml ascorbic acid, 2% v/v FBS (Endothelial Cell Growth Kit-BBE) (all from ATCC) and 10 units/ml penicillin, 10 µg/ml streptomycin and 25 ng/ml amphotericin B (Gibco).
Cells were detached from T-225 Falcon flasks with 0.05% Trypsin-EDTA (for HUVECs) or 0.25% Trypsin-EDTA (for hMSCs) (Sigma), centrifuged at 1200 rpm for 10 min, and resuspended in expansion medium. Cell suspensions in 30 µl of culture medium were then pipetted onto the scaffolds at a density of 35,000 and 70,000 cells per scaffold for monocultures of hMSCs (M1 and M2) and 70,000 total cells at a 1:1 ratio for co-cultures (MH: 35,000 hMSCs and 35,000 HUVECs). After an overnight attachment period, perfusion samples were cultured in a flow perfusion bioreactor with 45 ml of osteogenic medium at a flow rate of 0.03 ml/min through each 3 mm scaffold, as previously described.5, 6 At the same time, static cultures were removed from loading cassettes and transferred to 12 well plates with 3 ml of osteogenic medium in each well. Half of the medium in flow perfusion and static cultures was changed twice a week for up to 14 days. Six constructs from each group were harvested the day after seeding to assess the seeding efficiency. At each time point twenty constructs from two bioreactor units and ten static constructs were harvested, rinsed with PBS, and frozen at −20°C for storage.
Biochemical assays
Prior to biochemical analysis, samples underwent two freeze/thaw cycles in 250 µl of ddH2O, followed by 10 min of sonication.20 The concentration of double stranded DNA (dsDNA) in the supernatant was quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Eugene, OR) according to the manufacturer’s instructions and as previously described.20, 23 Briefly, cell lysate, buffer, and dye were pipetted into an opaque 96 well plate in duplicates, and fluorescence was measured with an excitation wavelength of 485 nm and an emission wavelength of 528 nm (FL x800 Fluorescence Microplate Reader; BioTek Instruments, Winooski, VT). DNA concentrations were determined relative to a lambda DNA standard curve.
ALP activity of different constructs was measured using a previously established colorimetric assay.20 Briefly, samples were combined with buffer and p-nitrophenyl phosphate disodium salt hexahydrate (ALP-substrate) (Sigma, USA) in a clear 96-well plate in duplicates. Plates were incubated for 1 hour at 37°C, and the absorbance of each well was measured at 405 nm (PowerWave 340 Microplate Reader; BioTek Instruments). The ALP activity of samples was determined relative to a p-nitrophenol standard curve and normalized to the total DNA content of each individual sample.
Calcium content of constructs was determined using an established colorimetric assay, as previously described.20 Constructs were placed into 0.2 ml 1 N acetic acid, and left on a shaker table at 200 rpm overnight to dissolve calcium in the constructs. The supernatant and calcium reagent (Arsenazo III, Diagnostic Chemicals, Oxford, CT) were pipetted into a clear 96-well plate, and the absorbance at 650 nm was measured. Samples were run in duplicate and diluted to fit within the range of a standard curve generated using CaCl2.
Histology
Constructs from each group were rinsed with PBS, fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA) for 30 min at room temperature, and then dehydrated for 30 min in 70% ethanol. Samples were then embedded into HistoPrep freezing medium (Fisher Scientific) overnight and frozen. Sections, 7 µm thick, were made using a cryostat (Leica CM 1850 UV; Leica Biosystems Nussloch GmbH, Germany) and mounted onto glass slides. Slides were placed on a 42°C slide warmer for 2–3 days to enhance scaffold adhesion. Sections were stained with Fast Green and von Kossa stains to visualize the distribution of cells and matrix and mineralized matrix, respectively. Images were taken using a ZeissAxio Imager.Z2 microscope equipped with AxioCam MRc5 (Carl Zeiss MicroImaging GmbH, Germany).
Scanning electron microscopy
Constructs from each group were rinsed with PBS, fixed in 2.5% v/v glutaraldehyde in PBS for 30 min at room temperature. They were then dehydrated with a gradient alcohol series and dried under laminar air flow in a culture hood for 1 day. Dried samples were mounted onto aluminum stubs and sputter-coated with 15 nm of gold. Extracellular matrix morphology of constructs was evaluated via SEM (FEI Quanta 400 Environmental, Hillsboro, OR) under high vacuum at a 30 kV voltage.
Statistical analysis
Results were analyzed using one-way ANOVA with Tukey’s post-hoc test using JMP 10 software. Differences were considered significant at p<0.05, and results are presented as mean + standard deviation for n=10 (flow perfusion) and n=6 (static).
Results
Construct cellularity
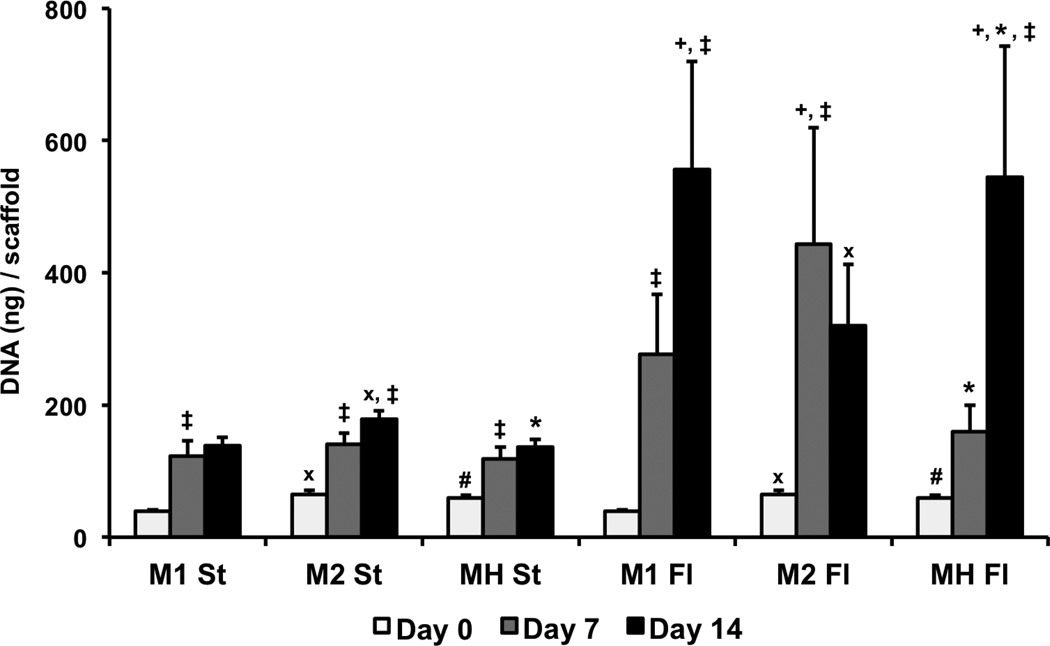
Over the time course of the experiment, all groups showed an increase in DNA content from the day 0 samples, and all cell populations showed an increase in cellularity as a result of flow perfusion culture (Figure 1). As expected, the DNA contents of the high-density monocultures (M2) were greater than the low-density monocultures (M1) immediately after the initial attachment period. However, after 7 days no difference was observed in cellularity between the two seeding densities in either static or flow perfusion. While the DNA content of the high-density static monocultures (M2 St) was greater than the lower density (M1 St) at 14 days, the opposite effect was seen in the flow perfusion cultures (M2 Fl and M1 Fl). The high-density flow perfusion monoculture (M2 Fl) showed an increase in DNA content at day 7 but did not show any significant change in cellularity from day 7 to 14. In contrast, the low-density flow perfusion monoculture (M1 Fl) showed a continuous increase over the course of the study, resulting in a higher cellularity than the high-density monoculture by day 14. The initial cell density in the co-cultures (MH Fl and MH St) was equal to that of the high-density monocultures (M2 Fl and M2 St). However, after 14 days in static culture, the co-cultures (MH St) resulted in lower DNA content than the high-density monocultures (M2 St) and an equivalent amount of DNA as the low-density monocultures (M1 St). Similarly, in flow perfusion, the co-cultures followed the same trend as the low-density monocultures, where after 7 days the co-cultures had lower cellularity than the high-density monocultures but after 14 days exceeded the cellularity of the high-density monocultures.
Figure 1. DNA content of constructs cultured for 14 days in osteogenic medium in static (St) or flow perfusion (Fl) conditions.
Scaffolds were seeded with either 35,000 hMSCs (M1), 70,000 hMSCs (M2) or 35,000 hMSCs and 35,000 HUVECs (MH). Samples were taken after 0, 7 and 14 days of culture. Data are presented as means + standard deviation. At a given time point, statistical differences (p<0.05) between co-cultures (MH) and low-density monocultures (M1) are indicated by #, and differences (p<0.05) between co-cultures (MH) and high-density monocultures (M2) are indicated by *. At a given time point, statistical differences (p<0.05) between high-density monocultures (M2) and low-density monocultures (M1) are indicated by x, and statistical differences (p<0.05) between a flow perfusion group (Fl) and the corresponding static group (St) are indicated by +. Within a group, statistical differences (p<0.05) compared to previous time points are indicated by ‡.
ALP activity
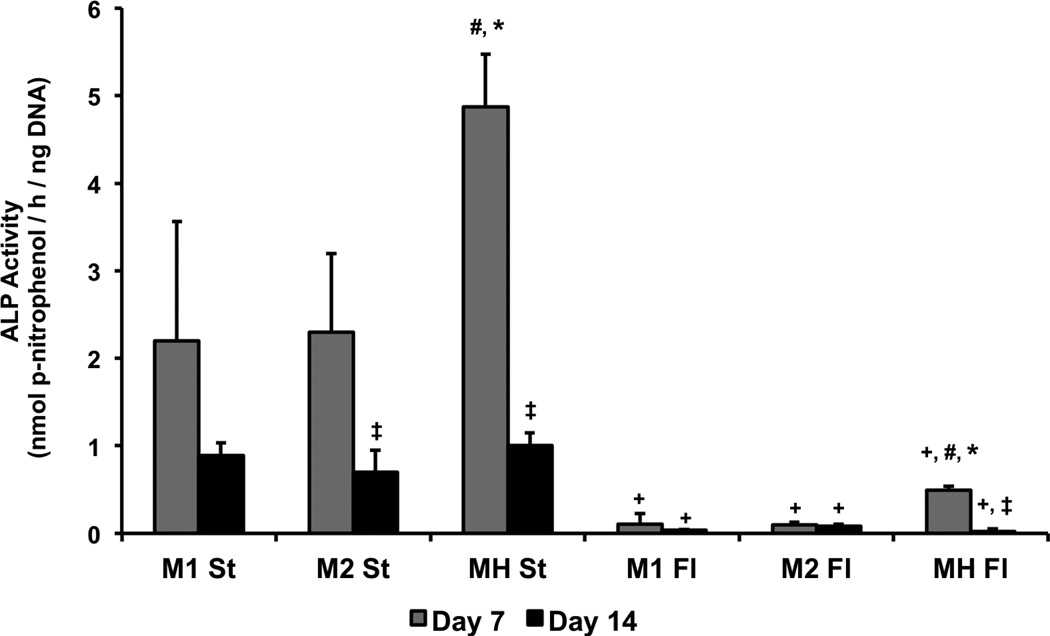
ALP activity was higher in static samples compared to the corresponding perfusion samples at all time points (Figure 2). No difference was observed in ALP activity between the high- and low-densities of hMSCs in either static or flow perfusion. However, a higher level of ALP activity was found after 7 days in the co-cultures in both culture conditions compared to the hMSCs alone. A decrease in ALP activity from 7 to 14 days was observed in the static high-density monoculture (M2 St) and both co-culture (MH St and MH Fl) groups, and by day 14 there was no statistical difference in ALP activity between any group.
Figure 2. ALP activity normalized to DNA in constructs cultured for 14 days in osteogenic medium in static (St) or flow perfusion (Fl) conditions.
Scaffolds were seeded with either 35,000 hMSCs (M1), 70,000 hMSCs (M2) or 35,000 hMSCs and 35,000 HUVECs (MH). Samples were taken after 7 and 14 days of culture. Data are presented as means + standard deviation. At a given time point, statistical differences (p<0.05) between co-cultures (MH) and low-density monocultures (M1) are indicated by #, and differences (p<0.05) between co-cultures (MH) and high-density monocultures (M2) are indicated by *. At a given time point, statistical differences (p<0.05) between high-density monocultures (M2) and low-density monocultures (M1) are indicated by x, and statistical differences (p<0.05) between a flow perfusion group (Fl) and the corresponding static group (St) are indicated by +. Within a group, statistical differences (p<0.05) compared to previous time points are indicated by ‡.
Calcium content
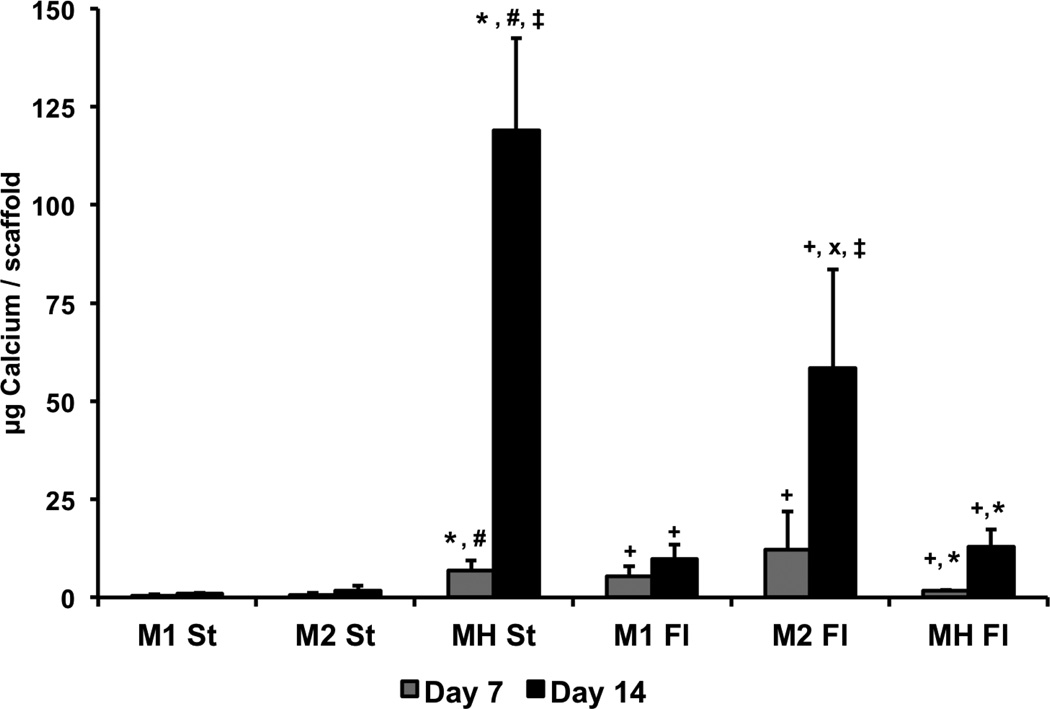
In the monoculture groups, calcium content was higher in flow perfusion than in static at both time points and at both seeding densities (Figure 3). In static, no difference in calcium deposits was observed between the high- and low-densities at either time point, but after 14 days in flow perfusion, the high-density monoculture (M2 Fl) led to greater levels of mineralization compared to the low-density (M1 Fl). Calcium content in the static co-culture group (MH St) was greater than both static monocultures and the flow perfusion co-culture at both time points. In flow perfusion, the co-cultures had no significant effect on the mineralization compared to the low-density monocultures and had lower calcium contents compared to the high-density monocultures.
Figure 3. Calcium content of constructs cultured for 14 days in osteogenic medium in static (St) or flow perfusion (Fl) conditions.
Scaffolds were seeded with either 35,000 hMSCs (M1), 70,000 hMSCs (M2) or 35,000 hMSCs and 35,000 HUVECs (MH). Samples were taken after 7 and 14 days of culture. Data are presented as means + standard deviation. At a given time point, statistical differences (p<0.05) between co-cultures (MH) and low-density monocultures (M1) are indicated by #, and differences (p<0.05) between co-cultures (MH) and high-density monocultures (M2) are indicated by *. At a given time point, statistical differences (p<0.05) between high-density monocultures (M2) and low-density monocultures (M1) are indicated by x, and statistical differences (p<0.05) between a flow perfusion group (Fl) and the corresponding static group (St) are indicated by +. Within a group, statistical differences (p<0.05) compared to previous time points are indicated by ‡.
Histology
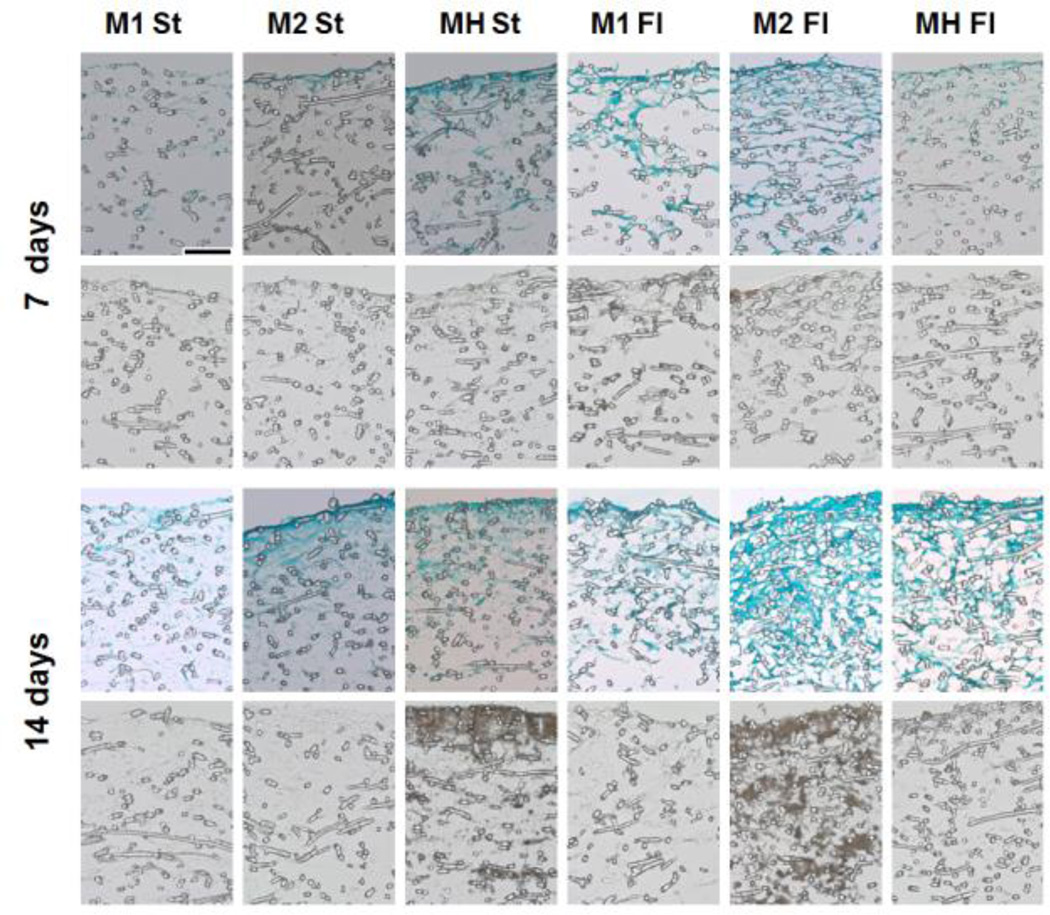
Staining of histological sections was consistent with the results of the biochemical assays (Figure 4). Higher intensity Fast Green staining was observed in flow perfusion samples compared to static samples, indicating higher cellularity and matrix production. Similarly, greater von Kossa staining was observed in the static co-cultures and high-density flow perfusion samples (MH St and M2 Fl). A more homogeneous spatial distribution can be observed in the sections of flow perfusion samples, as staining in static samples was primarily localized to the top region of the construct.
Figure 4. Representative histological sections of constructs cultured for 7 or 14 days in static (St) or flow perfusion (Fl) conditions.
Scaffolds were seeded with either low (M1) or high (M2) densities of hMSCs, or co-cultures of hMSCs and HUVECs (MH). Samples were taken after 7 and 14 days of culture and were stained with Fast Green (green) to visualize the distribution of cells and matrix (first and third row) and von Kossa (brown) to visualize the presence of minerals (second and fourth row). Scale bar represents 100 µm in all images.
Scanning electron microscopy
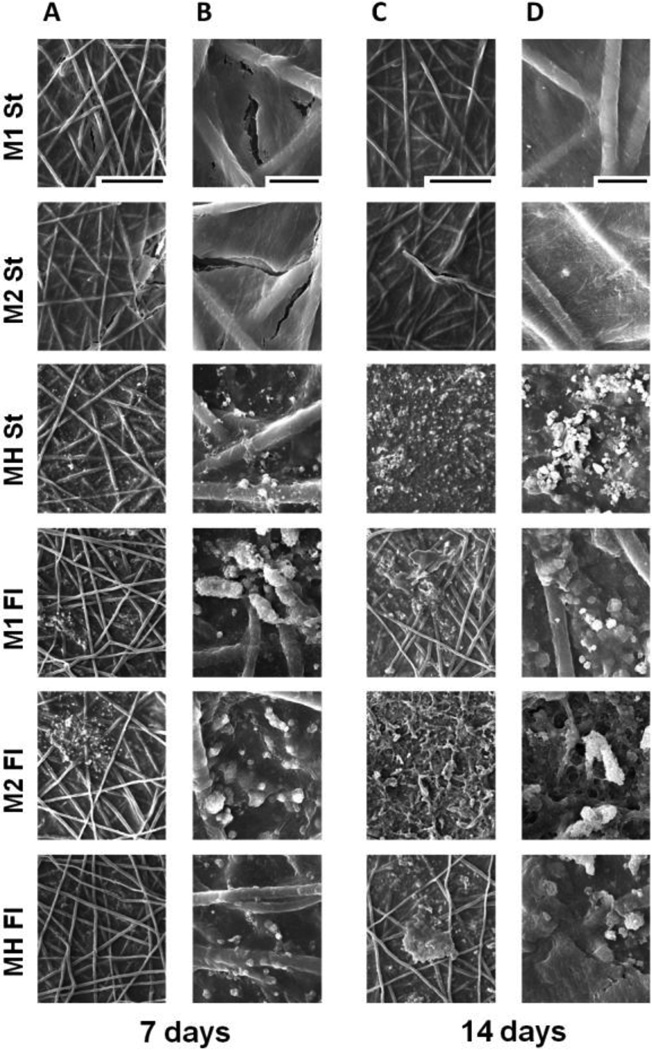
SEM imaging (Figure 5) demonstrated cells and extracellular matrix completely covering the scaffold surfaces as well as cells infiltrating the pores between PCL fibers. SEM imaging confirmed that static co-cultures exhibited the greatest mineralization and that both monocultures in flow perfusion exceeded the mineralization of those in static. Furthermore, high-density flow perfusion cultures displayed the most abundant mineral deposition among all flow perfusion groups.
Figure 5. Scanning electron micrographs of the top surfaces of constructs cultured for 7 or 14 days in static (St) or flow perfusion (Fl) conditions.
Scaffolds were seeded with either low (M1) or high (M2) densities of hMSCs, or co-cultures of hMSCs and HUVECs (MH). Samples were taken after 7 and 14 days of culture. Panels A and C are magnified at 300x, and the scale bar is 200 µm and applies for all images in these panels. Panels B and D are magnified at 1600x, the scale bar is 30 µm and applies for all images in these panels.
Discussion
Previous research has shown that when co-cultured with osteoblasts or MSCs, ECs enhance osteogenic potential as demonstrated by greater ALP activity and mineralization.3, 12, 21, 32 Furthermore, previous studies from our laboratories have demonstrated that co-culturing osteogenically precultured hMSCs with HUVECs in a 1:1 ratio under static conditions led to enhanced osteogenic differentiation compared to cultures of hMSCs alone.9 Both cell populations have previously been shown to be highly responsive to mechanical forces,1, 16, 22 and the effect of mechanical stimulation on the behavior of endothelial cells and vascularization in co-cultures has been investigated.18, 24 However, the effect of mechanical forces on the osteogenic differentiation of hMSC/HUVEC co-cultures has not been evaluated. Thus, the objective of this study was to investigate the effects of flow perfusion culture on co-cultures of hMSCs and HUVECs. Specifically, it was hypothesized that flow perfusion would enhance the osteogenic differentiation of hMSCs in such co-cultures.
In the present study, flow perfusion culture was shown to significantly enhance cell proliferation and cell and matrix distribution through the constructs in all groups. These results are consistent with previous studies,1, 4, 14, 20 which demonstrated enhanced proliferation and distribution of MSCs as a result of enhanced mass transfer,1 as well as through the application of shear stress to the cells.30 Here, it was observed that flow perfusion culture stimulated the proliferation of both high- and low-density hMSC cultures, although the timing of proliferation varied with seeding density. Similarly, previous work investigating the effect of flow perfusion on co-cultures of rat MSCs and aortic ECs found increased proliferation compared to either cell type alone.18 In contrast to the previous study, co-cultures of the present study did not stimulate cell proliferation in flow perfusion conditions, which could be a result of the difference in the culture conditions or cell species used in the two studies.18 Furthermore, while quantifying the total DNA content indicates the proliferation within the construct as a whole, it cannot distinguish between the proliferation rates of the hMSCs or HUVECs in co-culture.
The co-culture ALP activity was enhanced in both static and perfusion culture; however when comparing static to flow perfusion conditions, perfusion culture led to a reduced level of ALP activity at all time points evaluated. The enhanced ALP activity in the co-cultures is consistent with previous studies of co-cultures in static conditions3, 9, 21, 32 and with the enhanced mineralization that was also observed in these cultures. While ALP activity is a transient marker and thus no definitive conclusions can be drawn from this result, the enhanced ALP activity of the perfusion co-cultures at the time points evaluated here, suggests that the addition of HUVECs to hMSC cultures may enhance early stages of osteogenesis in both static and flow perfusion. While reduced ALP activity was observed in flow perfusion compared to static conditions, the cultures were only assayed at 7 and 14 days, and thus different results may have been observed at earlier time points.
Previous research has shown enhanced osteogenic differentiation of rat MSCs in high-density compared to low-density cultures as a result of increased cell-cell communication.15, 17 In the present study, no effect of the seeding density on either ALP activity or calcium content in monocultures was observed in static conditions. While no effect was observed in static cultures for to the specific densities evaluated, increased mineralization (characteristic of late-stage osteogenic differentiation) was observed in the high-density compared to the low-density flow perfusion monocultures after 14 days. The increased levels of calcium could be a result of increased cell number. However after noting the equivalent levels of ALP activity between these two groups, the enhanced late stage ostoegenesis may also be attributed to a higher applied shear stress resulting from a reduced scaffold pore size due to greater quantities of ECM being produced by the high cell density. Previous studies have demonstrated that the quantity of ECM produced by osteogenically differentiating MSCs is sufficient to reduce the average pore size in fibrous scaffolds7 and that a lower scaffold mesh size in flow perfusion can lead to greater mineralization.15 Furthermore, increased shear stress has demonstrated a capacity to enhance mineralization, without significantly affecting ALP activity, which led to the conclusion that variation in shear stress primarily affects the later stages of osteogenic differentiation.1, 30 Additionally, this result is consistent with studies evaluating hMSC seeding density and flow rate in perfusion, in which it was observed that while seeding density affected construct mineralization, seeding density was a less significant factor than flow rate (i.e. shear stress).11
While the mineralization of hMSC monocultures was enhanced in flow perfusion compared to static culture, the opposite outcome was observed in the co-cultures where the calcium content was reduced under flow perfusion conditions. The most likely explanation for this outcome includes the application of suboptimal flow perfusion parameters as well as reduced cell-cell contacts. A flow rate of 0.03 ml/min through each 3 mm scaffold was selected for this study in order to provide an average shear stress approximating the level which was previously shown to be beneficial for osteogenic differentiation of rat MSCs.1, 30 The initial shear stress level was estimated based on a cylindrical pore model previously used to calculate the level of shear stress in scaffolds of this geometry.1, 10, 25 Based on this approximation, the initial shear stress was estimated to be 0.15 dynes/cm2. While this flow rate proved to be beneficial to the osteogenic differentiation of rat MSCs alone, an optimal level of shear stress for the co-culture of human cell populations has not been established. The ideal range of shear stress for the osteogenic co-cultures of these cell types may be a balance between the level that alone is beneficial for osteogenic differentiation and that which promotes the proliferation or specific gene expression of the endothelial cells. For example, studies have demonstrated improved EC proliferation and microvasculature formation in co-cultures with MSCs when exposed to approximately 5 dynes/cm2 in collagen gels.18 ECs are highly sensitive to shear stress, and can exhibit vastly different responses and gene expression patterns depending on the magnitude of shear stress experienced.16, 19, 27 Thus, a different effect may have been observed with different flow rates, a potentially interesting phenomenon for future study.
Reduced cell-cell contacts resulting from increased cellular infiltration into the scaffolds, as seen in Figure 4, is another possible explanation for perfusion-dependent reductions in calcium content in co-cultures. The relationship between seeding density, scaffold pore size, and flow rate for perfusion cultures was emphasized previously2, 11, 28 generating outcomes in agreement with the data presented herein. While soluble signaling is certainly significant in the osteogenesis of co-cultures, bone marrow-derived MSCs are thought to be especially sensitive to cell-cell contacts.3, 12 An enhancement of osteogenic differentiation may result from increased cell density in perfusion co-cultures, which also increased the number of cell-cell contacts. The fact that mineralization was greatest in the high density monoculture group and was virtually equal between the low density monoculture and co-culture groups further supports the conclusion that optimization of the seeding density may enable better osteogenic outcomes in co-cultures under flow perfusion conditions.
Here, a 1:1 ratio of hMSCs to HUVECs was implemented, as previous studies have shown that a 1:1 ratio of hMSCs to HUVECs led to the highest levels of mineralization and ALP activity compared to the other ratios evaluated (80:20 to 98:2), and that the cell ratio had a significant effect on the osteogenic outcome.21 In the present perfusion cultures, the change in cell ratio in the co-culture over the duration of the experiment is unknown. However, the highdensity monoculture exhibited the greatest calcium mineralization, indicating that enhanced osteogenesis may be achieved by increasing the proportion of hMSCs relative to HUVECs in the perfusion co-cultures above a 1:1 ratio.
Conclusions
Flow perfusion culture was found to enhance the mineralization of hMSC cultures at both high- and low- densities. While hMSC density had no effect on mineralization in static culture, increased hMSC density led to enhanced mineralization in perfusion cultures, possibly due to the higher effective shear stress experienced by cells in these constructs. While co-cultures of hMSCs and HUVECs had enhanced mineralization compared to monocultures in static conditions, the same effect was not observed in perfusion cultures. Co-cultures of hMSCs and HUVECs did not contribute to enhanced mineralization compared to hMSCs alone under the flow perfusion conditions investigated in this study. However, flow perfusion culture did result in an enhanced spatial distribution of cells and matrix compared to static cultures.
Acknowledgements
This work was supported by grants from the National Space Biomedical Research Institute Postdoctoral Fellowship Program through NCC 9–58 (JGG) and the National Institutes of Health (R01 AR057083) (AGM).
References
- 1.Bancroft GN, Sikavitsas VI, Van Den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3d perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerre L, Bunger CE, Kassem M, Mygind T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials. 2008;29(17):2616–2627. doi: 10.1016/j.biomaterials.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Bulnheim U, Muller P, Neumann HG, Peters K, Unger RE, Kirkpatrick CJ, Rychly J. Endothelial cells stimulate osteogenic differentiation of mesenchymal stem cells on calcium phosphate scaffolds. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1590. [DOI] [PubMed] [Google Scholar]
- 4.Cartmell SH, Porter BD, Garcia AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9(6):1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 5.Dahlin RL, Meretoja VV, Ni M, Kasper FK, Mikos AG. Hypoxia and flow perfusion modulate proliferation and gene expression of articular chondrocytes on porous scaffolds. AIChE Journal. 2012 [Google Scholar]
- 6.Dahlin RL, Meretoja VV, Ni M, Kasper FK, Mikos AG. Design of a high-throughput flow perfusion bioreactor system for tissue engineering. Tissue Eng Part C Methods. 2012 doi: 10.1089/ten.tec.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3d osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103(8):2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Gershovich JG, Dahlin RL, Kasper FK, Mikos AG. Enhanced osteogenesis in co-cultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2013.0256. Submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22(11):1279–1288. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 11.Grayson WL, Bhumiratana S, Cannizzaro C, Chao PHG, Lennon DP, Caplan AI, Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14(11):1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grellier M, Bordenave L, Amedee J. Cell-to-cell communication between osteogenic and endothelial lineages: Implications for tissue engineering. Trends in Biotechnol. 2009;27(10):562–571. doi: 10.1016/j.tibtech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Gurkan UA, Akkus O. The mechanical environment of bone marrow: A review. Ann Biomed Eng. 2008;36(12):1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 14.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res Part A. 2005;72A(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 15.Holtorf HL, Datta N, Jansen JA, Mikos AG. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bioreactor. J Biomed Mater Res Part A. 2005;74A(2):171–180. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 16.Inoguchi H, Tanaka T, Maehara Y, Matsuda T. The effect of gradually graded shear stress on the morphological integrity of a huvec-seeded compliant small-diameter vascular graft. Biomaterials. 2007;28(3):486–495. doi: 10.1016/j.biomaterials.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ, Niklason LE. A novel flow bioreactor for in vitro microvascularization. Tissue Eng Part C Methods. 2010;16(5):1191–1200. doi: 10.1089/ten.tec.2009.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38(10):1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Liao J, Guo X, Nelson D, Kasper FK, Mikos AG. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta Biomater. 2010;6(7):2386–2393. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma JL, Van Den Beucken JJJP, Yang F, Both SK, Cui FZ, Pan JL, Jansen JA. Coculture of osteoblasts and endothelial cells: Optimization of culture medium and cell ratio. Tissue Eng Part C Methods. 2011;17(3):349–357. doi: 10.1089/ten.TEC.2010.0215. [DOI] [PubMed] [Google Scholar]
- 22.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 23.Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33(27):6362–6369. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishi M, Matsumoto R, Dong J, Uemura T. Engineered bone tissue associated with vascularization utilizing a rotating wall vessel bioreactor. J Biomed Mater Res Part A. 2013;101A(2):421–427. doi: 10.1002/jbm.a.34340. [DOI] [PubMed] [Google Scholar]
- 25.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 26.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 27.Rotenberg MY, Ruvinov E, Armoza A, Cohen S. A multi-shear perfusion bioreactor for investigating shear stress effects in endothelial cell constructs. Lab Chip. 2012;12(15):2696–2703. doi: 10.1039/c2lc40144d. [DOI] [PubMed] [Google Scholar]
- 28.Sellgren KL, Ma T. Perfusion conditioning of hydroxyapatite-chitosan-gelatin scaffolds for bone tissue regeneration from human mesenchymal stem cells. J Tissue Eng Regen M. 2012;6(1):49–59. doi: 10.1002/term.396. [DOI] [PubMed] [Google Scholar]
- 29.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 30.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3d perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usami K, Mizuno H, Okada K, Narita Y, Aoki M, Kondo T, Mizuno D, Mase J, Nishiguchi H, Kagami H, Ueda M. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res Part A. 2009;90(3):730–741. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
- 32.Wang DS, Miura M, Demura H, Sato K. Anabolic effects of 1,25-dihydroxyvitamin d3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology. 1997;138(7):2953–2962. doi: 10.1210/endo.138.7.5275. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Vandevord PJ, Mao L, Matthew HW, Wooley PH, Yang SY. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30(4):508–517. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]