Abstract
Low-dose tolerance therapy with nucleosomal histone peptide epitopes blocks lupus disease in mouse models, but effect in humans is unknown. Herein, we found that CD4+CD25highFoxP3+ or CD4+CD45RA+FoxP3low T-cells, and CD8+CD25+FoxP3+ T-cells were all induced durably in PBMCs from inactive lupus patients and healthy subjects by the histone peptide/s themselves, but in active lupus, dexamethasone or hydroxychloroquine unmasked Treg-induction by the peptides. The peptide-induced Treg depended on TGFβ/ALK-5/ pSmad 2/3 signaling, and they expressed TGF-β precursor LAP. Lupus patients’ sera did not inhibit Treg induction. The peptide epitope-induced T cells markedly suppressed type I IFN related gene expression in lupus PBMC. Finally, the peptide epitopes suppressed pathogenic autoantibody production by PBMC from active lupus patients to baseline levels by additional mechanisms besides Treg induction, and as potently as anti-IL6 antibody. Thus, low-dose histone peptide epitopes block pathogenic autoimmune response in human lupus by multiple mechanisms to restore a stable immunoregulatory state.
Keywords: Systemic Lupus Erythematosus; Human, T cells; Peptide epitopes; Tolerance; Autoimmunity
1. INTRODUCTION
1.1
Remission maintenance therapy for SLE that specifically targets pathogenic autoimmune cells could prevent recurrence and halt smoldering inflammatory damage. In SLE, cognate interactions between autoimmune T helper (Th) cells and B cells against epitopes from apoptotic nuclear autoantigens lead to production of pathogenic IgG autoantibodies [1-7]. The IgG autoantibodies form inflammatory immune complexes (IC) containing apoptotic cell derived DNA/RNA, and along with CD40L signals from hyperactive lupus T cells, stimulate lupus APC to produce IL-6, IFNα, and other amplifiers of the pathogenic response [8-15].
1.2
Five critical autoepitopes in apoptotic cell derived nucleosomes that are recognized by autoimmune T and B cells of patients and various mouse strains with SLE are in histone (H) regions, H1′22-42, H382-105, H3115-135, H416-39 and H471-94 [2, 16-19], and these epitopes are promiscuously bound by all major MHC molecules. The peptides delay lupus progression and even restore normal life span, reducing proteinuria in mice with established renal disease upon administration in soluble form (tolerization) at high doses intravenously [20]. The peptides are also therapeutically effective when administered intranasaly, or in low doses subcutaneously [21-24]. In such lupus-prone mice, tolerance therapy with nanoMolar doses of histone peptide epitope/s, which contain both MHC class II and class I binding motifs, induces expansion of potent, autoantigen-specific CD8+, and CD4+CD25+ regulatory T cell (iTreg) cells which suppress via TGFβ the responses of lupus T cells to nuclear autoantigens, and reduce autoantibody production by inhibiting the T cell help; leading to normal survival span. The stable, autoantigen-specific Treg generated in vivo by the peptide therapy can also block accelarated disease upon adoptive transfer into lupus mice [22]. The therapy especially reduces inflammatory cell reaction in the kidney [22, 23]; a major complication of human lupus [25, 26]. Only 1 μg (0.34 nM) of the histone peptide epitope/s is effective in low-dose tolerance therapy of mice with lupus, which would be equivalent to 0.2 to 2 mg range in lupus patients. Moreover, similar to the potent CD8 iTreg generated by histone peptide therapy above, or by other autoantigens in mouse models [27-34], we found that in humans, autologous hematopoietic stem cell transplantation (HSCT) for severe lupus also generates identical FoxP3+, LAPhigh CD103high CD8+TGFβ-producing regulatory T cells (CD8 iTreg), which repairs immunoregulatory deficiency in lupus to maintain patients in true immunological remission [19].
1.3
Because effect of the nucleosomal peptide epitopes in humans is unknown, we studied herein if the histone peptide epitopes have any immunoregulatory effects on lupus patients’ autoimmune cells in vitro.
2. MATERIALS AND METHODS
2.1. Subjects
We enrolled 30 lupus patients (10 active and 20 in remission, 22-63 years) who fulfilled the American College of Rheumatology revised criteria for SLE [35], and 15 healthy subjects (23-57 years). Disease activity was scored by the Systemic Lupus Activity Measure index (SLAM) [36]. Patients with SLAM score < 7 were considered inactive (remission), and those with SLAM ≥ 7 were considered active. Clinical demographic profile is shown in Table I. The study was approved by Institutional Review Board of Northwestern University.
Table 1.
Patient demographic: clinical and treatment status of SLE patients in the study1
| Patient Code | Age/Sex/Race | SLAM Score | Current Treatment |
|---|---|---|---|
| 1 | 51/F/C | 4 | HCQ, SSZ |
| 2 | 60/F/AA | 8 | none |
| 3 | 27/F/AA | 7 | MMF |
| 4 | 40/F/H | 1 | HCQ, Vit D |
| 5 | 22/F/AA | 9 | MMF, HCQ, Pred |
| 6 | 41/F/AA | 5 | HCQ |
| 7 | 41/F/C | 0 | AZT, HCQ |
| 8 | 24/F/W | 15 | MMF |
| 9 | 63/F/C | 5 | none |
| 10 | 50/F/C | 5 | HCQ |
| 11 | 25/F/AA | 2 | none |
| 12 | 32/F/C | 1 | AZT, HCQ |
| 13 | 29/M/C | 5 | MMF, HCQ, Pred |
| 14 | 50/F/C | 2 | HCQ |
| 15 | 63/F/C | 3 | HCQ |
| 16 | 34/F/C | 2 | HCQ |
| 17 | 43/F/AA | 1 | HCQ, Pred |
| 18 | 28/M/H | 17 | MMF, HCQ, Pred |
| 19 | 31/F/W | 16 | AZT, HCQ, Pred |
| 20 | 40/F/C | 5 | HCQ |
| 21 | 43/F/AA | 9 | HCQ, Pred |
| 22 | 24/F/H | 11 | MMF, Pred |
| 23 | 41/F/C | 7 | AZT, HCQ, Pred |
| 24 | 49/F/C | 1 | HCQ |
| 25 | 32/F/C | 2 | HCQ |
| 26 | 63/M/C | 5 | HCQ |
| 27 | 44/M/C | 16 | HCQ, Pred |
| 28 | 41/F/C | 4 | HCQ |
| 29 | 26/F/A | 6 | MTX, HCQ, Pred |
| 30 | 40/F/W | 12 | Pred, HCQ, MMF |
Abbreviations: SLE, Systemic Lupus Erythematosus; SLAM, Systemic Lupus Activity Measure; AA, African American; H, Hispanic; C, Caucasion; F, Female; M, Male; HCQ, Hydroxycholoquine (Plaquenil); Pred., Prednisone or Steroids; SSZ, Sulfasalazine; MMF, Mycophenolate Mofetil(CellCept); Vit D, Vitamin D; AZT, Azathioprine; MTX, Methotrexate.
2.2. Cytokines and reagents
IL-2 was purchased from R&D Systems (Minneapolis, MN), TLR9 ligand CPG-containing oligonucleotide (ODN) 2216 was from InvivoGen (San Diego, CA), anti-IL6 (BD Pharmingen, San Jose, CA), SB-431542 (Glaxo Smith Kline, King of Prussia, PA), Dexamethasone (DEX), Hydroxychloroquine (HCQ), Retinoic acid (RA), 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), Rapamycin (RAPA), Apigenin (APG), and Trichostatin A (TSA) were all from Sigma (St. Louis, MO).
2.3. Peptides
Peptides were synthesized by F-moc chemistry and their purity checked by amino acid analysis by manufacturer (New England Peptide, Gardner, MA).
2.4. Cell culture
PBMC from subjects were isolated with Ficoll-Paque Plus gradient. To test induction of Treg cells, PBMC were cultured with low doses of histone peptide autoepitopes (4 μM of each peptide): H122-42, H382-105, H3115-135, H416-39, and H471-94, as well as a control peptide called “control peptide A” [30] for 7-11 days, along with 50 U/ml of hIL-2 in RPMI 1640 (w/L-glutamine, Gibco, Foster City, CA) supplemented with 10% heat-inactivated FCS, or in some experiment with 10% patient's autologous serum, and 1 mM Sodium Pyruvate, 1 X Nonessential Amino Acids, 1 mM HEPES, 2 mM L-glutamine, 5×10−5 β-Mercaptoethanol, 100U/ml Penicillin/streptomycin. Then percent of stable CD4 and CD8 FoxP3+ Treg cells were analyzed by Flow Cytometry. The positive responders in each experiment were identified when the % of FoxP3 positive cells in their peptide-treated cultures was ≥ 2 SD as compared to that in corresponding PBS-treated culture. Expression of the precursor of active TGFβ, Latency Associated Peptide (LAP), and CD103 and CD39 in cultured T cells were also measured. In some experiments, to explore the effects of anti-inflammatory drugs or antibodies on FoxP3 expression, 1 × 10−5 M of Dexamethasone (DEX), 25 μM of hydroxychloroquine (HCQ), 10 nM or 100 nM of Retinoic acid (RA), 5 ng/ml of TGF-β, 5 μg/ml of anti-IL6 neutralizing antibody, 100 ng/ml of Rapamycin (RAPA), 20 ng/ml of 1,25-ddihydroxyvitamin D3 (1,25(OH)2D3), 12.5 μM of Apigenin (APG), or 0.25 μM or 0.1 μM of Trichostatin A (TSA) was also added to PBMCs cultured with the histone peptides or PBS control. To study the importance of TGFβ/ALK-5/Smad2/3 signaling pathway in Treg cells, 5 μM of ALK5 inhibitor (SD-431542) was added to PBMCs cultures with peptide or PBS control.
To analyze the suppressive effect of single peptides or peptide cocktail on pathogenic autoantibody production by active lupus patients, their PBMC (5 × 105 cells) in 200 μl /microwell were cultured in the presence of different low-dose peptide epitopes or peptide cocktails, or PBS in complete RPMI with 10 % FCS and 50 U/ml IL-2 for 13 days. To exclude autoantibody production by B cells that were already preactivated in vivo, 120 μl of medium from each culture was replaced with fresh medium and corresponding peptides on day 5 without disturbing the cells at bottom of the microwells. On day 13, production of IgG autoantibodies to double-stranded DNA (dsDNA), single-stranded DNA (ssDNA), and nucleosomes (histone/DNA complex) was quantitated in the culture supernatants. In subsequent text and Figure legends, PBS means adding PBS instead of peptide in cell culture. The identity of peptide epitopes are abbreviated showing the first residue only: H1(22), H3(82), H3(115), H4(16) and H4(71) means low doses of indicated peptide added to the 13-day cultures. Peptide cocktail-1 (C1) means a mixture of H1(22), H3(115), H4(16) at 1.5 μM of each; peptide cocktail-2 (C2) means mixture of H1(22), H3(115), H4(16) 4 μM of each; peptide cocktail-3 (C3) means the mix of H1(22), H3(82), H4(71) 1.5 μM each; peptide cocktail-4 (C4) means a mix of the five peptides at1.0 μM of each. C1, C2, C3 or C4 was added in 13-day cultures as with the single epitopes. In some experiments, the low doses of anti-IL6 antibody (600 ng/ml) was used to detect it's suppressive function.
To measure the suppressive effect of peptide cocktail on type I IFN genes expression,PBMC from inactive SLE patients who were taking very low doses of steroids were cultured for five days in the presence or absence of peptide cocktail-2 and then stimulated with the TLR9 ligand, CPG-containing oligonucleotide (ODN) 2216, for 4 hours before the RNA were taken for qPCR. In some experiments, T and B cells were depleted with positive selection beads from PBMC before cell culture (STEMCELL Technologies Inc. Canada).
2.5. ELISA
IgG class autoantibodies to dsDNA, ssDNA and nucleosomes were measured as described [8]. Standard curves were obtained by serial dilutions of one active lupus patient's plasma with high IgG autoantibody titer. The addition of a mixture (cocktail) of the histone peptide epitopes to the lupus plasma did not affect the standard curves. Autoantibody levels are expressed as the absorbance value at 405 nm. The absorbance values of 1:1000 dilution of the reference patient's plasma was considered to be equivalent to 1 U/ml of anti-dsDNA, -ssDNA or anti-nucleosome autoantibodies for calculating the % inhibition by each peptide or cocktail [8].
2.6. Flow cytometry
Antibodies to stain following markers were used in different combinations: CD4-PerCP, CD4-PE-Cy7, CD8-APC, CD8-PerCP-Cy5.5, CD25-FITC, CD25-APC, CD45RA-V450, LAP-PE, CD103-PE, CD39-FITC, CD1c-FITC, CD80-Alexa Fluor700, HLA-DR-APC (BD Biosciences), FoxP3-PE and Isotype control (eBioscience, San Diego, CA), Rabbit IgG or pSmad 2,3/goat anti rabbit–FITC (Cell Signaling, Danvers, MA). For intracellular staining, cells were treated with Fix/Perm Buffer set (eBioscience) and stained with anti-FoxP3-PE or phospho-Smad 2(Ser465/467)/Smad3 (Ser423/435) and then goat anti-rabbit-FITC, and corresponding isotype controls. Data collected on a LSR II flow cytometer (BD Biosciences) were analyzed with FlowJo (Tree Star, Ashland, OR).
2.7. Real-time quantitative PCR
Total RNA was extracted using an RNeasy Mini kit (Qiagen, Valencia, CA) with DNase treatment, and 1.25 μg of total RNA was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Quantitative PCR was performed using TaqMan Gene Expression Master Mix and a 7300 Fast Real-time PCR System (Applied Biosystems). Expression of the tested genes was normalized relative to the levels of GAPDH. The relative expression levels were calculated using the 2−ΔΔCt threshold cycle method.
2.8. Statistical analysis
Data analyses were performed using Prism 4.0 software (GraphPad software). Comparisons were performed by Student t tests. Results are expressed as mean ± SEM; p values < 0.05 were considered significant.
3. RESULTS
3.1. Low doses of histone peptide epitopes by themselves durably induce FoxP3+Treg in vitro
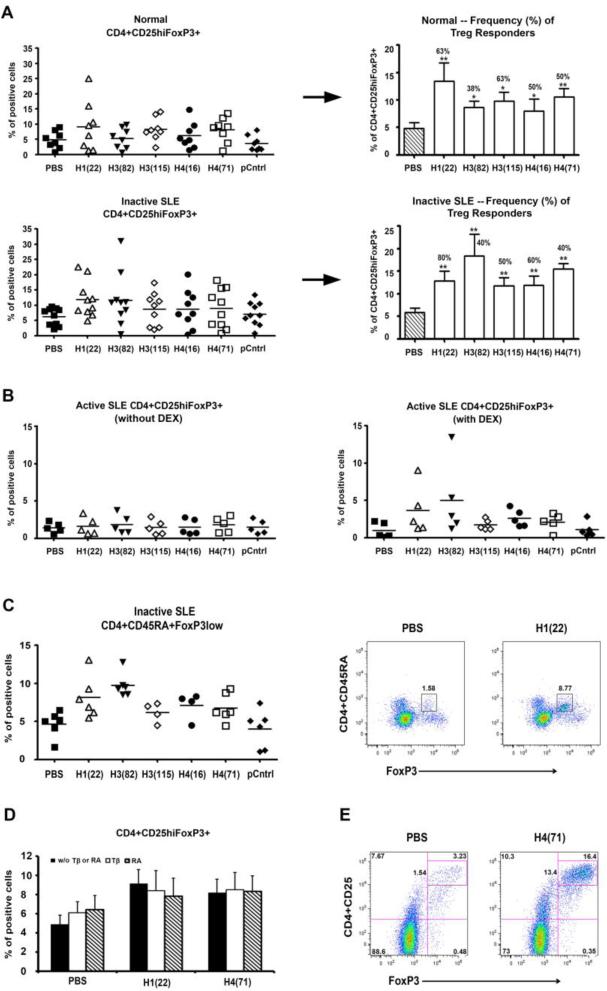
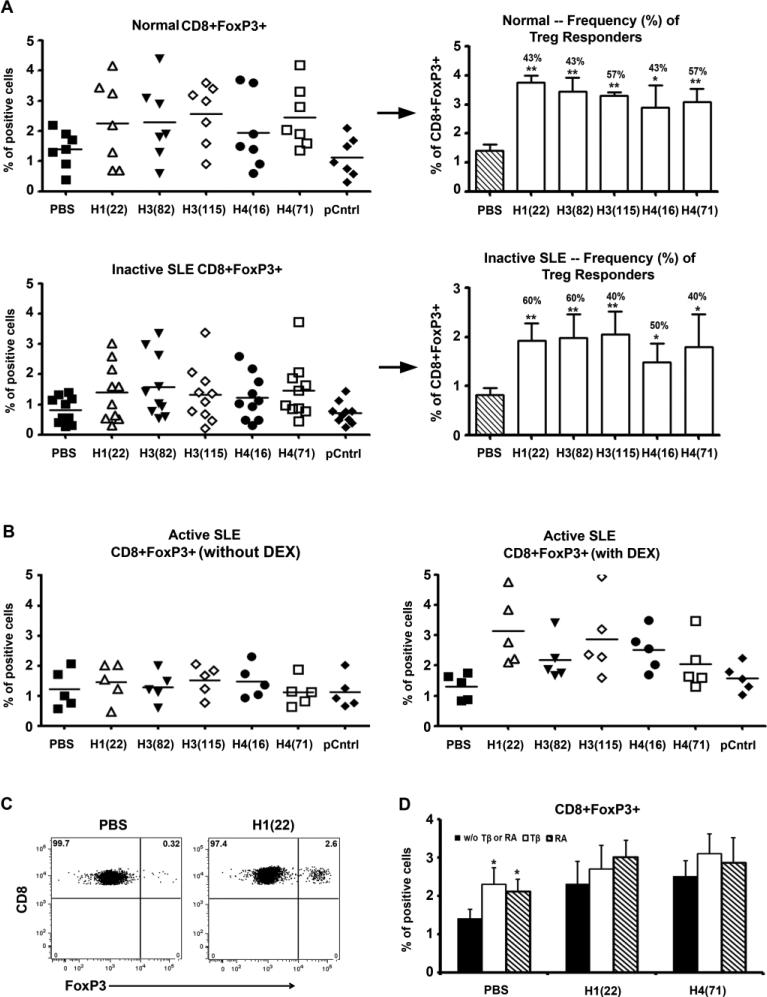
In lupus-prone mice, the histone peptide epitope/s induce autoantigen-specific CD4+CD25+ and CD8+ Treg cells in vivo, blocking lupus disease [22, 23]. To detect whether the histone peptides can induce such Treg cells in humans, fresh PBMCs from active and inactive lupus patients and healthy subjects were cultured with low doses of the peptides (4 μM of each peptide): H122-42, H382-105, H3115-135, H416-32, H471-94, control peptide A or PBS for 3, 5, 7, 9 or 11 days, all with 50 U/ml IL2; the cells were cultured at 2.5 × 106/ml with 10% of FBS complete RPMI and then analyzed by flow cytometry. CD4+CD25highFoxP3+ and the CD8+FoxP3+ cells began to increase after culture for 3 days, and up to 11 days tested. At day 7, the percentage of CD4+CD25highFoxP3+ cells (Figure 1), CD8+FoxP3+ cells (Figure 2) were significantly increased in PBMCs when cells were cultured with low-dose histone peptide, compared with control peptide A or PBS (P <0.01). As shown in the left panels of Figures 1A and 2A, for induction of FoxP3+ Tregs, one peptide epitope may induce positive response in an individual patient but may be negative in another patient. Therefore, we summarized the Treg responder frequency as % of positive responders in right panels of Figures 1A and 2A. A patient was considered to be a positive responder to a peptide if % of FoxP3 Tregs increased above 2 SD over its PBMC cultured without peptide (PBS). The peptide H122-42 induced the highest frequency (up to 80%) of FoxP3+Treg response in PMBCs from inactive lupus patients, followed by H382-105 and H416-39.
Figure 1.
Durable induction of CD4+CD25hiFoxP3+ and CD4+CD45RA+FoxP3low Treg cells in vitro by low-dose histone peptides. (A) Fresh PBMCs from healthy subjects and inactive SLE patients were cultured with histone peptide epitopes, control peptide or PBS, all in the presence of 50 U/ml IL2 for 7 days, and then stained for CD4, CD25, and FoxP3. Peptide epitope names are abbreviated in X-axis (see Methods). Y-axes show % of FoxP3 positive cells among viable T cells gated for being CD4+CD25hi; and each symbol represents data from one individual. pCntrl = control peptide A; horizontal lines indicate the mean. Right panels (next to →): The Y-axis here is same as in the left panels, designating the % of CD4+CD25hi cells that were FoxP3 positive. Except for PBS, the other bars represent mean ± SD of the individual data points shown in the left panels, but for only the positive Treg responders to each peptide. These positive responders in each experiment were identified when the % of FoxP3 positive cells in their peptide-treated cultures was ≥ 2 SD as compared to that in corresponding PBS-treated culture. The numbers above the bars represent the frequency (%) of positive Treg responders for each peptide. P values of FoxP3+ cells over control are indicated, ** p < 0.01, * p < 0.05. n= 5 to 10. (B) Fresh PBMCs from active SLE patients were cultured with histone peptide epitopes and IL2, in the presence or absence of Dexamethasone (DEX) for 7 days, and then stained for CD4, CD25, and FoxP3. (C) Fresh PBMCs of inactive SLE patients were cultured with histone peptides for 7 days and stained for CD4, CD45RA, and FoxP3. The right 2 panels showed the Representative Dot Plots of CD4+CD45RA+FoxP3+ cells induced by peptide H122-42. (D) Shows the effects of TGF-β (Tβ) or Retinoic Acid (RA) on induction of CD4+CD25hiFoxP3+ Treg cells by two of the peptide epitopes, n = 3. (E) Representative Dot Plots show Treg induction in PBMC after culturing with the peptide epitope H122-42 or H471-94 for 7 days: the two panels show gated CD4+T cells in PBMC for CD25+FoxP3+ cells.
Figure 2.
Durable induction of CD8+FoxP3+ cells in vitro by low-dose histone peptides. (A) Fresh PBMCs samples derived from healthy subjects and inactive SLE patients were cultured as described in Figure 1A and stained for CD8 and FoxP3. Y-axes show % of FoxP3 positive cells among viable T cells gated for being CD8+. pCntrl = control peptide A; horizontal lines indicate the mean. Similar to right panels in Figure 1A and as explained in the corresponding legend to Fig. 1A, the right panels here (next to →) show the degree of CD8+FoxP3+ responses (bars = mean ± SD), except for the bar for PBS, and the frequency (% numbers above bars) of positive CD8 Treg responders for each peptide. ** p < 0.01, * p < 0.05. n= 5 to 10. (B) Fresh PBMCs from active SLE patients were cultured with histone peptide epitopes and IL2, in the presence or absence of Dexamethasone (DEX) for 7 days, and then stained for CD8 and FoxP3. (C) Representative Dot Plots show induction of CD8+FoxP3+ T cells in PBMC after culturing with the histone peptide epitope H122-42 for 7 days. (D) Shows the effects of TGF-β (Tβ) or Retinoic Acid (RA) on induction of CD8+FoxP3+ Treg cells by two of the peptide epitopes, n = 3, * p < 0.05.
In humans, unlike mice, FoxP3 can be induced in naïve CD4+FoxP3– T cells by activation, without suppressive activity. Only CD4+CD25hiFoxP3+ T cells show Treg function in humans. Moreover, FoxP3+ T cells are heterogeneous in humans; in fresh PBMCs, CD45RA+FoxP3low CD4 T cells are naïve Tregs (nTregs) and CD45RA–FoxP3hi CD4 T cells are considered effector Tregs (eTregs); and similar to CD25hiCD4+ cells, both of them are potently suppressive in vitro. Effector Tregs are reported to be poorly proliferative, when stimulated with antigen, whereas human nTregs can easily expand upon stimulation, and acquire eTreg phenotype as they upregulate FoxP3, CD25, CD45RO (CD45RA–) when stimulated with antigen [37]. We found that peptide epitopes significantly increased CD4+CD45RA+FoxP3low, but did not increase CD4+CD45RA–FoxP3hi cells (Figure 1C), indicating that most of the Treg were newly induced by the peptide epitopes.
3.2. Effect of exogenous agents on Treg induction by the peptide epitopes
We tested whether patient's serum, which contains Type I IFN-inducing inflammatory immune complexes [12, 13], would block the FoxP3+Treg inducing effect. We supplemented the culture medium with 10% autologous serum of each patient instead of 10% FBS and compared with FBS supplemented medium, and found no significant differences, indicating that patient's serum could not block peptide-induction of FoxP3+Tregs (data not shown).
We tested whether conventional lupus drugs, such as Dexamethasone (DEX) or Hydroxychloroquine (HCQ) could enhance the FoxP3+Treg inducing effect of peptide epitopes. PBMCs from lupus patients were cultured with low-dose peptide in the presence or absence of DEX (1× 10−5 M) or HCQ (25 μM) for 7 days. We had found that unlike inactive patients, in PBMC from active lupus patients, low-dose histone peptides have very weak FoxP3+Tregs inducing effect (Figures 1B and 2B, left panels), however, when cultured with DEX, FoxP3+Tregs increased significantly in the latter (Figures 1B and 2B, right panels). HCQ also had enhancing effect on peptides inducing Tregs as DEX did. Compared to control cultures with HCQ and control peptide or PBS, HCQ plus the different peptide epitopes increased CD4+CD25hiFoxP3+ Tregs by 2.2 to 5.5 fold and CD8+FoxP3+ Tregs by 1.9 to 4.9 fold in active SLE PBMCs. Certain agents are known to enhance the effect of anti-CD3 and -CD28 induced Tregs in healthy subjects, such as the major vitamin A metabolite all-trans Retinoic acid (RA), TGFβ, anti-IL6 antibody, the potent immunosuppressive drug Rapamycin (RAPA), the histone deacetylase (HDAC) inhibitor Trichostatin A (TSA), 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), which can inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Therefore, we also explored the effects of these drugs or antibodies on peptide inducing FoxP3+Tregs in lupus patients. Our results indicated that RA, TGFβ, 1,25(OH)2D3, APG, and TSA did not show any enhancing effect in the induction of FoxP3+Tregs above and beyond that induced by low-dose histone peptide/s themselves (Figures 1D and 2D, and data not shown). RA or TGFβ could increase the baseline for % of CD4 and CD8 Tregs without added peptides (PBS only), but could not significantly increase % Tregs induced by the peptide epitopes any further (Figure 1D & 2D). Similarly, Rapamycin (RAPA) could increase the background of FoxP3 expression in T cells (with PBS only), but could not enhance histone peptide induced FoxP3+ expression in T cells. Anti-IL6 neutralizing antibody also could not enhance histone peptide induced FoxP3+T cell expression any further, although it did by itself markedly increase FoxP3 and CD25 expression in CD4 and CD8 T cells in the 7-day PBMC cultures from normal and inactive lupus subjects (data not shown)
3.3. Low doses of histone peptides induced LAP expression on CD4 and CD8 T cells in vitro
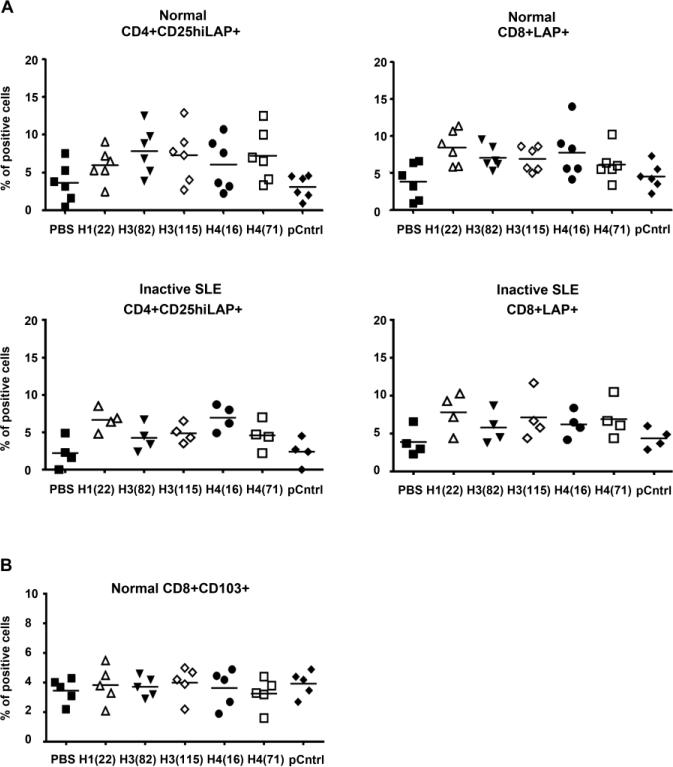
In lupus-prone mice, the histone peptides induce Tregs that mediate disease suppression by TGFβ [22, 23], and such remission inducing Treg can express latency-associated peptide (LAP) on their cell surface and other TGFβ dependent markers, such as CD103 and CD39 [19] Therefore, we added the histone peptides into the PBMCs from healthy subjects and patients with inactive SLE, and cultured the cells for one day or three days before staining for T cell expression of LAP, CD103 and CD39. After one day of culture both CD4+CD25+ T cells and CD8 + T cells expressed markedly higher levels of TGFβ-LAP when cultured with histone peptides as compared to PBS (Figure 3A), but CD103 (Figure 3B) and CD39 expression did not increase significantly (data not shown).
Figure 3.
Low-dose histone peptides induce LAP expression on CD4 and CD8 T cells in vitro. (A) Fresh PBMCs samples from healthy subjects (upper panels) and inactive SLE patients (lower panels) were cultured with peptide epitopes for 1 day before staining CD4, CD8, CD25 and LAP. Y-axes show % of LAP+ cells among viable T cells gated for being CD4+CD25hi or CD8+, n = 4 and 6, the horizontal line = mean. P values for each histone peptide over controls (PBS or pCntrl) were < 0.05 to <0.01; and PBS vs. pCntrl P > 0.05. (B) Fresh PBMCs samples from healthy subjects were cultured with peptide epitopes for 3 day before staining for CD8 and CD103.
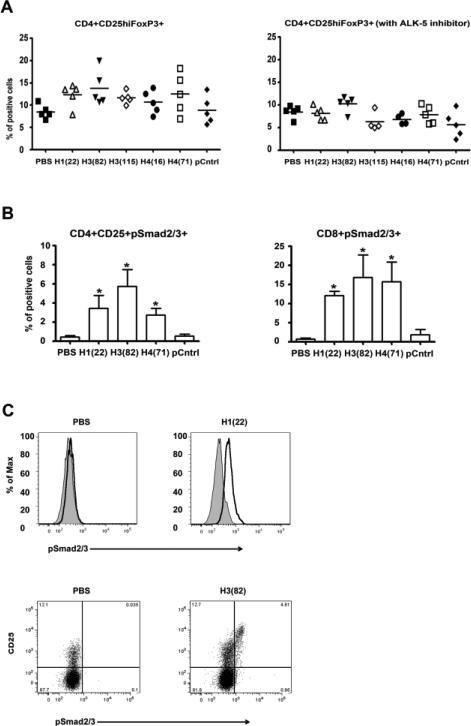
3.4. Low doses of peptide epitopes induced Treg cells through TGFβ/ALK-5 /pSmad 2 and 3 signaling pathway
Because the histone peptides induced high level LAP expression on CD4CD25 and CD8 T cells, we tested whether the epitopes induced Tregs through TGFβ/ALK-5/pSmad 2 and 3 signaling pathway. TGF-β is secreted in a latent form, which first needs to be activated by proteases or thrombospondin before it can bind to its specific type I and type II serine/threonine kinase receptors. Most cell types contain a TGFβ type I receptor active receptor-like kinase 5 (ALK5), which propagates the signal to the nucleus through phosphorylation of Smad2 and Smad3 proteins. Therefore, we assessed the effect of blocking TGFβ/ALK5/pSmad2 and 3 signaling by an ALK5 inhibitor (SD-431542). The PBMCs from active or inactive SLE patients were cultured with or without low-dose histone peptides in the presence or absence of ALK5 inhibitor SD-431542 (5 μM) for 7 days, and then CD4+CD25hiFOXP3 expression was analyzed. The ALK5 inhibitor markedly diminished the inducing effect of low-dose histone peptides for CD4+CD25hiFOXP3+ cells (Figure 4A). We also measured phosphorylated Smad2 and Smad3 protein expression on CD4+CD25+ cells and CD8+ T cells after SLE PBMC were cultured with low-dose histone peptides or the control peptide A for 7 days. We found that pSmad 2/3 expression in T cells was induced by the histone peptide epitopes, but PBS or control peptide A did not show such an effect (Figure 4B). Moreover, Dot Plots showed that the pSmad 2/3 positive cells were within the CD4+CD25hi subpopulation (Figure 4C), suggesting that these cells belong to the peptide-induced Treg population.
Figure 4.
Low-dose peptide epitopes induce Treg cells through TGF/β-ALK-5/ pSmad 2 and 3 signaling pathway. Active or inactive SLE PBMCs were cultured with peptide epitopes in the presence of IL2 or IL2 plus ALK-5 inhibitor SB-431542 for 7 days, followed by staining for CD4, CD25 and FoxP3. We also measured pSmad2/3 expression among viable CD4 or CD8 T cells under those conditions. (A) Y-axes show % of FoxP3 positive cells among viable T cells gated for being CD4+CD25+, n= 5, the horizontal line = mean. The P values for each peptide by ALK-5 inhibitor compared to no inhibitor were < 0.05 to < 0.01. (B) Y-axes show % of pSmad2/3 positive cells among viable T cells gated for being CD4+CD25hi, or CD8+. Results from five experiments; and the bars = mean ± SD, * p < 0.01. (C) Upper 2 panels show representative histogram of pSmad2/3 positive cells (solid line) among CD8+ cells (filled = isotype control). Lower 2 panels show representative Dot Plots of pSmad2/3 positive cells among CD4+CD25+ T cells.
3.5. Low dose of peptide epitopes individually or in a mixture (cocktail) inhibit the production of the pathogenic variety of autoantibodies by active lupus patient's PBMCs
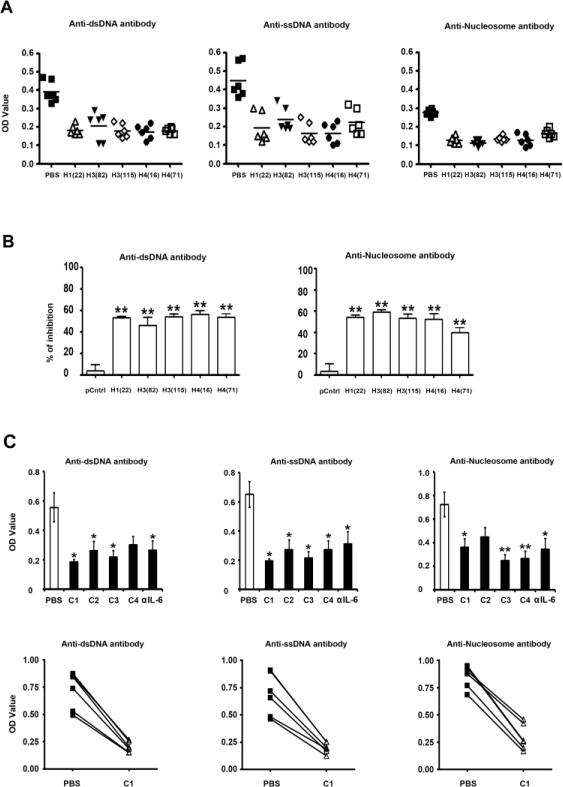
Production of pathogenic autoantibodies, such as IgG autoantibodies to dsDNA, ssDNA and nucleosomes by B cells in vitro, requires T cell help and is detectable only in PBMCs from patients with active lupus [8]. Herein, we confirmed that the pathogenic IgG autoantibodies were detectable in PBMC cultures from patients with active lupus, but not inactive lupus. We then measured the suppressive function of low-dose single histone peptide on the production of pathogenic IgG autoantibodies by PBMCs from active lupus patients. Individual peptide showed significant suppressive effects on autoantibody production in PBMCs from active lupus patients (Figure 5A). The five peptide epitopes tested individually suppressed (mean ± SEM of percent inhibition) IgG anti-dsDNA by 32% to 70%, IgG anti-ssDNA production by 41% to 74%, and IgG antinucleosome autoantibodies by 30% to 67% while the control peptide A had much less suppressive effect (Figure 5B). The single peptides (low-dose) were present in PBMCs cultures throughout the 13-d culture period (see Methods); and drugs received by the patients when the blood was obtained are shown in Table I.
Figure 5.
Low-dose peptide epitopes individually and as peptide cocktails mediate potent suppression of pathogenic autoantibody production by PBMC from active lupus patients. (A) The suppressive effect of single peptide epitope on the production of pathogenic autoantibodies. Y-axes show the absorbance at 405 nm. The horizontal line indicates the mean. (B) The data in “A” is shown as % of inhibition by each peptide as compared to control peptide A (pCntrl). (C) The upper panels show the suppressive effect of cocktails of peptide epitopes or a low dose of anti-IL6 antibody (600 ng/ml) on the production of pathogenic autoantibodies by PBMC in the culture. Peptide Cocktails: C1 is H1(22)+H3(115)+H4(16) at1.5 μM/peptide. C2 is H1(22)+H3(115)+H4(16), 4 μM/peptide. C3 is H1(22)+ H3(82)+H4(71), 1.5 μM/peptide. C4 is H1(22)+H3(82)+H3(115)+H4(16)+H4(71), 1.0 μM/peptide. The bars = mean ± SEM, The lower panels show the examples of inhibition of autoantibodies by cocktail-1(C1) in three independent experiments; Y-axes show the absorbance value at 405 nm. *P < 0.05, **P < 0.01.
However, not every peptide showed the suppressive function on every patient at all times; H122-42 and H3115-135, were the most consistent inhibitors. Therefore, we measured the suppressive function of mixtures or cocktail of the different peptide epitopes on autoantibody production and found that cocktails showed very uniform and potent suppressive function, especially peptide cocktail-1: (H1(22) + H3(115) + H4(16) at 1.5 μM/peptide, and peptide cocktail-3: (H1(22) + H3(82 ) + H4(71) at 1.5 μM/peptide. The potency of the peptide cocktails was as good as anti-IL6 neutralizing antibody in suppressing autoantibody production (Figure 5C).
3.6. Nucleosomal histone peptide cocktails suppress the expression of type I IFN related genes in lupus PBMC
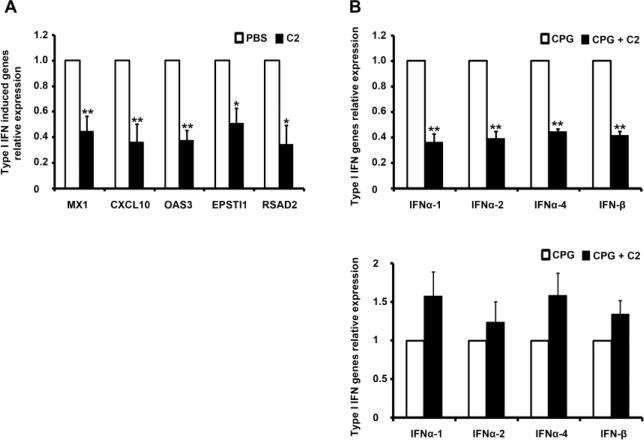
Type I IFNs are produced at a high level, predominantly by pDC stimulated by anti-nuclear autoantibodies complexed with nuclear antigens in lupus [10-15], and in mouse models of lupus low-dose therapy by the histone peptide epitopes target pDC to make them tolerogenic [23]. For these reasons, we first tested whether the histone peptide epitopes individually or peptide cocktails cultured with CD14+ monocytes from normal human peripheral blood under myeloid DC maturation culture conditions (GMCSF and IL4) could inhibit or delay maturation of myeloid DC. We measured DC surface markers of maturation by flow cytometry (such as CD1c, CD80 and HLA-DA) and gene expression for ITGB6, ITGB8, IL-10, TAB-1, BAFF, THBS1, and IL6 by real-time PCR at different culture time points (24 and 48 hours). The results did not show any significant change (data not shown). Then we explored whether the low doses of histone peptides individually or peptide cocktails inhibited type I IFN induced genes expression in PBMCs from lupus patients. At the same time we measured autoantibody levels in supernatants of peptide treated PBMCs from active lupus patients described above, we also harvested the cells and measured expression of type I IFN stimulated genes (ISG ) from the cultured cells. In total, we measured 12 ISG genes reported to be upregulated in lupus patients [10, 11, 15], and the results showed that peptide cocktails (especially peptide Cocktail-2 consisting of H1(22) + H3(115 ) + H4(16), at 4 μM/peptide) could significantly reduce type I IFN induced genes expression, particularly CXCL10, MX1, OAS3, EPSTI1 and RASAD2 (Figure 6A), however, other inflammation or immune regulation related genes expression, including ISG15, IFI44, LY6E, BAFF, HERC5, SOCS1 and SOCS3, did not show significant change (data not shown). Finally, the peptide Cocktail-2 also markedly reduced expression of type I IFN genes themselves in TLR9 stimulated PBMCs from inactive lupus patients which were cultured for 5 days with TLR9-ligand CPG-containing oligonucleotide (ODN) 2216, and the suppressed type I IFN genes included INFα-1, INFα-2, INFα-4 and INF-β (Figure 6B, upper panel), that are the major members of this family [38]. However, when the T and B cells were depleted from the whole PBMC before starting the 5-day cultures, the suppressive effect of peptide cocktail-2 on type I IFN genes expression disappeared completely (Figure 6B lower panel). Because the peptide-induced Treg cells appear in significant numbers only after 5 days of culture, they could not be depleted at the onset of the cultures.
Figure 6.
Histone peptide cocktails suppress expression of type I IFN genes and IFN-induced genes in lupus PBMC. (A) PBMC from active SLE patients were treated with or without peptide cocktail-2 (C2) for 13 days before mRNA of type I IFN induced genes were quantified by qPCR. The bars represent the mean ± SEM from five individual experiments. (B) The upper panel shows results with whole PBMC from inactive SLE patients who were taking very low doses of steroids. The PBMC were cultured for five days in the presence or absence of peptide cocktail-2 and then stimulated with TLR9 ligand CPG-containing oligonucleotide (ODN) 2216 for 4 hours before type I IFN genes mRNA level were quantified by qPCR. The lower panel shows the qPCR results of same experiments as done in upper panel but T cells were depleted from the PBMC before cell culture. The results show mean ± SEM, from four individual experiments. * p < 0.01, * * p < 0.001.
4. DISCUSSION
4.1
Each of the histone peptide epitopes efficiently induced expansion of both CD4+ and CD8+ Treg in vitro in lupus patients’ PBMC, similar to what they do in vivo in lupus-prone mice [22, 23]. Treg induction by the peptides was evident in PBMC of normal subjects and inactive lupus patients, but in active patients, dexamethasone or hydroxychloroquine treatment of the cultures brought out the Treg inducing ability. Thus, the peptide epitopes could be most beneficial in maintaining patients in remission by inducing Treg after inflammation is suppressed. The peptides induced Treg even in the presence of patients’ serum, and without assistance from exogenously added TGFβ, retinoic acid or other Treg enhancing agents. Peptide-induced Treg cells with FoxP3+ phenotype steadily increased in the cultures from day 3 onwards for up to 11 days tested. Because FoxP3 expression can occur transiently on human T cell activation, it was reassuring that FoxP3+ T cells were maintained throughout the test period of 11 days. Moreover, confirming that the Treg induced were real, TGF-β precursor LAP was also induced in both Treg populations by the epitopes. Furthermore, the tolerogenic signal generated by the epitopes in the iTreg cells required TGFβ signaling, as evidenced by phosphorylation of Smad-2 and 3. Blocking TGF-β signal by an ALK 5 inhibitor further confirmed that endogenous TGFβ signal was involved in generation and maintenance of the iTreg cells. In animal models these epitopes could induce a tolerogenic program by decreasing IL-6 and increasing TGF-β production by pDCs, which in turn induce Smad-3 phosphorylation (TGF-β signal) in target autoimmune T cells [23, 39]. However, in the case of human lupus Treg cells induced here, Smad-2/3 phosphorylation was evident even after 7 days in the T cells, when most APCs could be dead in the culture, indicating that a stabilized program of intrinsic and ongoing TGFβ signaling possibly by autocrine and paracrine TGFβ produced by the iTreg cells. It was necessary for us to add IL-2 once to all the cultures including controls, at the beginning of the 7-11 day cultures for survival of the T cells. IL-2 could have contributed to the peptide induced Treg generation as in other systems [40, 41]. However, IL-2 was also added to the cultures with control peptide or PBS, which did not show any Treg inducing effect. IL-2 production is impaired in lupus [42, 43], and low dose IL-2 administration may facilitate CD4+CD25+ Treg homeostasis in chronic GVHD [44]. However, the histone peptide epitopes administered by themselves, without exogenous IL-2, can induce expansion of potent Treg in vivo, blocking lupus disease in three different mouse models of spontaneous SLE, namely, SNF1, B/WF1 and MRL-lpr [21-24, 34].
In other approaches, a synthetic peptide (pConsensus) related to VH region of anti-DNA antibodies, or a human Ig VH4-family CDR1 peptide could suppress lupus in mice and induce iTreg cells [27-29, 45]. Induction of CD4+25+ Treg in vitro has also been demonstrated with other peptide antigens in low doses [46, 47]. Importantly, in vivo, antigen specific T cell receptor stimulation is required to further sustain functionally stable Treg [48]. Therefore, even adoptively transferred Treg cells generated first in vitro may require periodic administration of peptide epitope vaccine to maintain and further generate new antigen specific Treg recruits by tolerance spreading [20, 22, 23, 31].
The CD4+CD25+ Treg generated by the histone peptide epitopes mediate their suppression by TGFβ [22, 23], resembling the Treg induced by mucosal tolerance [49], and as in lupus-prone mice, these iTreg will be beneficial in lupus patients who suffer a deficiency in CD4+CD25+ Treg [19, 50].
On the other hand, very little is known about the CD8+ iTreg generated by the peptides in humans, although studies in animal models, indicate their key role in preventing, and suppressing established disease [19, 22, 28, 30, 31, 51]. The peptide cocktail vaccine provide an opportunity to study the unusual iTreg cells in human lupus. Remarkably, HSCT induced remission in refractory lupus patients is dependent on the same type of potent CD8 Tregs generated by nucleosomal peptide therapy in lupus-prone mice [19], which act by producing TGFβ, and are distinct from other CD8 Treg cells that act by contact cytotoxicity [52-54]. Overall, the Treg induced by the histone peptide epitopes are both autoantigen specific and polyclonal Treg [19, 22, 23], probably because of the promiscuous nature of lupus autoantigenic epitopes [55], and also tolerance spreading by tolerogenic APC and Treg cells themselves that secrete TGFβ [19, 22, 23]. However, suppression by the Treg is preferentially directed against lupus autoimmune response overall, as immune response to foreign antigens is not compromised [22, 23]. Herein, in humans, the peptide epitopes significantly increased CD4+CD45RA+FoxP3low, but did not increase CD4+CD45RA–FoxP3hi cells (Figure 1C), indicating that most of the Treg were newly induced by the peptide epitopes.
4.2
Each of the histone peptide epitopes also inhibited pathogenic IgG autoantibody production in vitro by the PBMC of active lupus patients, without any addition of steroids or exogenous TGFβ. These patients’ cells were producing relatively high levels of autantibodies to the nuclear antigens to begin with, so that marked inhibition by the peptides (up to 74%) in vitro could be evident. Cocktails of the epitopes in different combinations were even more efficient in uniformly suppressing pathogenic autoantibody production; especially the cocktail C1 inhibited up to 90%. The inhibition of autoantibody production by the epitope cocktails was even superior to anti-IL6 antibody. This potency of the peptide epitopes is very encouraging, because anti-IL6 therapy, although targeting a critical pathogenic cytokines in lupus has serious side effects [56]. The iTreg generated by the peptide epitopes could have suppressed autoimmune Th cells and APC [23, 29, 57]. However, induction of conventional Treg by the peptides in active lupus PBMC was relatively less efficient in contrast to that in inactive lupus patients or healthy subjects, yet surprisingly the epitopes caused a marked inhibition of autoantibody production in active lupus patients’ PBMC. These results indicate that lupus B cells that also recognize the epitopes could have been directly tolerized by the peptides [18, 58], in addition to their direct tolerogenic effect on autoantigen presenting pDC, inducing them to produce endogenous TGFβ [23]. Inhibition of autoantibody production by the peptide epitopes was real and not due to binding up of the autoantibodies in culture supernatants by the peptides via nuclear antigens in the autoantibody combining sites, because addition of the peptides to serial dilutions of active lupus patient's plasma did not make a difference in IgG anti-nuclear autoantibody titer measurements.
4.3
The peptides also suppressed IFNα gene expression by lupus PBMC, which was dependent on T cells. This result was probably through generation of iTreg by the epitopes, which then suppressed IFN producing APC by TGFβ. In addition, the peptide epitopes could have a direct tolerogenic effect on pDC in the early stages of the cultures to facilitate the induction of iTreg, as found in mouse models [23].
4.4. Concluding Remarks
Developing tolerance therapy for SLE that specifically targets pathogenic autoimmune cells by utilizing the major nucleosome-derived peptide epitopes would have following advantages: a) the peptides are unaltered, naturally occurring, ubiquitous peptide ligands (UPL), expressed in the thymus and bone marrow during ontogeny of the immune system, and therefore, unlike artificially altered peptide ligands or APLs [59], they are not associated with anaphylactic/allergic reactions [20, 22, 23, 60]; b) they are effective at very low doses, and by subcutaneous or intranasal administration in animal models of lupus; c) they generate long-lasting, antigen-specific iTreg that suppress disease pathology; d) they induce cross-reactive, “tolerance spreading” or linked tolerance to other pathogenic T and B cell autoepitopes of lupus, but not to exogenous antigens; e) they are recognized by autoimmune T and B cells of all lupus patients tested irrespective of their HLA type [2, 17-20, 55]. The histone peptide epitope therapy might be most suitable for restoring immunoregulation and maintaining lupus patients in remission after inflammation is suppressed by more toxic or global immunosuppressive agents. Importantly, the peptide epitopes are effective even when the autoimmune disease is already established [20, 22, 23].
Highlights.
Immunoregulatory effects of nucleosomal histone peptide epitopes in humans revealed
Histone peptide epitopes induce Treg cells in healthy humans and lupus patients
Peptide epitope-induced human Treg are TGFβ–dependent and express TGFβ-LAP
Peptides inhibit autoantibody production in human lupus by additional mechanisms
Peptide-induced Treg suppress type I interferon gene expression in human lupus
ACKNOWLEDGMENTS
Supported by funding from Alliance for Lupus Research (TIL grant #187305 to S.K.D.), and National Institutes of Health (NIAID grant, R01AI41985 to S.K.D, and NIAMS P60 AR30692 to R. R-G).
Abbreviations
- SLE
systemic lupus erythematosus
- iTreg
Induced Regulatory T cells
- H
Histone
- HSCT
hematopoietic stem cell transplantation
- LAP
latency associated peptide
- DEX
Dexamethasone
- HCQ
Hydroxychloroquine
- RA
Retinoic acid
- (1,25(OH)2D3)
1,25-dihydroxyvitamin D3
- RAPA
Rapamycin
- APG
Apigenin
- TSA
Trichostatin A
- ODN
oligonucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
This work did not receive any support from commercial sources. The histone peptide epitope sequences have been published by us repeatedly [2, 16-18], and they are available in databases open to the public, such as in:
Many other investigators have published their work independently in their own experimental systems using the peptide epitopes we have identified [21, 24, 34]. The peptide epitope sequences have been patented, but have not been licensed to any commercial entities. The NIH and Northwestern University have the main rights to the patented discoveries. We have published the sequences of the peptides for open access to everyone.
REFERENCES
- 1.Adams S, Leblanc P, Datta SK. Junctional region sequences of T-cell receptor b chain genes expressed by pathogenic anti-DNA autoantibody-inducing T helper cells from lupus mice: Possible selection by cationic autoantigens. Proc. Natl. Acad. Sci. USA. 1991;88:11271–11275. doi: 10.1073/pnas.88.24.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta SK. Major peptide autoepitopes for nucleosome-centered T and B cell interaction in human and murine lupus. Ann. NY. Acad. Sci. 2003;987:79–90. doi: 10.1111/j.1749-6632.2003.tb06035.x. [DOI] [PubMed] [Google Scholar]
- 3.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: A major immunogen for the pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, Lee KD, Gavalchin J, Kaplan MJ. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J. Immunol. 2006;176:2095–3104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 6.van der Vlag J, Berden JH. Lupus nephritis: role of antinucleosome autoantibodies. Semin Nephrol. 2011;31:376–389. doi: 10.1016/j.semnephrol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Stummvoll GH, Fritsch RD, Meyer B, Hoefler E, Aringer M, Smolen JS, Steiner G. Characterisation of cellular and humoral autoimmune responses to histone H1 and core histones in human systemic lupus erythaematosus. Ann Rheum Dis. 2009;68:110–116. doi: 10.1136/ard.2007.082032. [DOI] [PubMed] [Google Scholar]
- 8.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Invest. 1996;97:2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J. Clin. Invest. 1996;98:826–837. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, Stichweh D, Blankenship D, Li L, Munagala I, Bennett L, Allantaz F, Mejias A, Ardura M, Kaizer E, Monnet L, Allman W, Randall H, Johnson D, Lanier A, Punaro M, Wittkowski KM, White P, Fay J, Klintmalm G, Ramilo O, Palucka AK, Banchereau J, Pascual V. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 13.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 14.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikpour M, Dempsey AA, Urowitz MB, Gladman DD, Barnes DA. Association of a gene expression profile from whole blood with disease activity in systemic lupus erythaematosus. Ann Rheum Dis. 2008;67:1069–1075. doi: 10.1136/ard.2007.074765. [DOI] [PubMed] [Google Scholar]
- 16.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J. Exp. Med. 1996;183:2459–2469. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Kaliyaperumal A, Boumpas DT, Datta SK. Major peptide autoepitopes for nucleosome-specific T cells of human lupus. J. Clin. Invest. 1999;104:345–355. doi: 10.1172/JCI6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaliyaperumal A, Michaels MA, Datta SK. Naturally processed chromatin peptides reveal a major autoepitope that primes pathogenic T and B cells of lupus. J. Immunol. 2002;168:2530–2537. doi: 10.4049/jimmunol.168.5.2530. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-b producing CD8+ Treg cells are associated with immunological remission of lupus. J. Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaliyaperumal A, Michaels MA, Datta SK. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: Tolerance spreading impairs pathogenic function of autoimmune T and B cells. J. Immunol. 1999;162:5775–5783. [PubMed] [Google Scholar]
- 21.Wu HY, Ward FJ, Staines NA. Histone Peptide-induced nasal tolerance: suppression of murine lupus. J. Immunol. 2002;169:1126–1134. doi: 10.4049/jimmunol.169.2.1126. [DOI] [PubMed] [Google Scholar]
- 22.Kang H-K, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J. Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 23.Kang H-K, Liu M, Datta SK. Low-Dose Peptide Tolerance Therapy of Lupus Generates Plasmacytoid Dendritic Cells That Cause Expansion of Autoantigen-Specific Regulatory T Cells and Contraction of Inflammatory Th17 Cells (cover page Figure) J Immunol. 2007;178:7849–7858. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 24.Suen J-L, Chuang Y-H, Tsai B-Y, Yau PM, Chiang B-L. Treatment of murine lupus using nucleosomal T cell epitopes identified by bone marrow-derived dendritic cells. Arthritis Rheum. 2004;50:3250–3259. doi: 10.1002/art.20520. [DOI] [PubMed] [Google Scholar]
- 25.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winchester R, Wiesendanger M, Zhang HZ, Steshenko V, Peterson K, Geraldino-Pardilla L, Ruiz-Vazquez E, D'Agati V. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell beta-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 2012;64:1589–1600. doi: 10.1002/art.33488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh RR, Ebling FM, Albuquerque DA, Saxena V, Kumar V, Giannini EH, Marion TN, Finkelman FD, Hahn BH. Induction of autoantibody production is limited in nonautoimmune mice. J. Immunol. 2002;169:587–594. doi: 10.4049/jimmunol.169.1.587. [DOI] [PubMed] [Google Scholar]
- 28.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB × NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J. Immunol. 2008;180:2069–2080. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 29.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black × New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J. Immunol. 2004;173:3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 30.Hahn BH, Anderson M, Le E, La Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2488–2497. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- 31.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr., Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 32.Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ, Williams CB, Drobyski WR. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012;189:464–474. doi: 10.4049/jimmunol.1200886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skaggs BJ, Singh RP, Hahn BH. Induction of immune tolerance by activation of CD8+ T suppressor/regulatory cells in lupus-prone mice. Hum Immunol. 2008;69:790–796. doi: 10.1016/j.humimm.2008.08.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapira E, Proscura E, Brodsky B, Wormser U. Novel peptides as potential treatment of systemic lupus erythematosus. Lupus. 2011;20:463–472. doi: 10.1177/0961203310389484. [DOI] [PubMed] [Google Scholar]
- 35.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 36.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis. Rheum. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 37.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang HK, Chiang MY, Liu M, Ecklund D, Datta SK. The Histone Peptide H4(71-94) Alone Is More Effective than a Cocktail of Peptide Epitopes in Controlling Lupus: Immunoregulatory Mechanisms. J Clin Immunol. 2011 doi: 10.1007/s10875-010-9504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtsuka K, Gray JD, Quismorio FP, Jr., Lee W, Horwitz DA. Cytokine-mediated down-regulation of B cell activity in SLE: effects of interleukin-2 and transforming growth factor-beta. Lupus. 1999;8:95–102. doi: 10.1191/096120399678847498. [DOI] [PubMed] [Google Scholar]
- 41.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 42.Crispin JC, Tsokos GC. Transcriptional regulation of IL-2 in health and autoimmunity. Autoimmun Rev. 2009;8:190–195. doi: 10.1016/j.autrev.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwitz DA. The clinical significance of decreased T cell interleukin-2 production in systemic lupus erythematosus: connecting historical dots. Arthritis Rheum. 2010;62:2185–2187. doi: 10.1002/art.27538. [DOI] [PubMed] [Google Scholar]
- 44.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, Armand P, Blazar BR, Antin JH, Soiffer RJ, Ritz J. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5:179ra143. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8810–8815. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long SA, Rieck M, Tatum M, Bollyky PL, Wu RP, Muller I, Ho JC, Shilling HG, Buckner JH. Low-dose antigen promotes induction of FOXP3 in human CD4+ T cells. J Immunol. 2011;187:3511–3520. doi: 10.4049/jimmunol.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and foxp3 expression are independent and complementary events required for treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J. Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 51.Horwitz DA. Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther. 2008;10:227. doi: 10.1186/ar2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlad G, Chang CC, Colovai AI, Berloco P, Cortesini R, Suciu-Foca N. Immunoglobulin-like transcript 3: A crucial regulator of dendritic cell function. Hum Immunol. 2009;70:340–344. doi: 10.1016/j.humimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 53.McPhee CG, Sproule TJ, Shin DM, Bubier JA, Schott WH, Steinbuck MP, Avenesyan L, Morse HC, 3rd, Roopenian DC. MHC class I family proteins retard systemic lupus erythematosus autoimmunity and B cell lymphomagenesis. J Immunol. 2011;187:4695–4704. doi: 10.4049/jimmunol.1101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–789. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y, Kaliyaperumal A, Lu L, Southwood S, Sette A, Michaels MA, Datta SK. Promiscuous presentation and recognition of nucleosomal autoepitopes in lupus: Role of autoimmune T cell receptor alpha chain. J. Exp. Med. 1998;187:367–378. doi: 10.1084/jem.187.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, Lipsky PE. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 58.Wiesendanger M, Stanevsky A, Kovsky S, Diamond B. Novel therapeutics for systemic lupus erythematosus. Curr. Opin. Rheumatol. 2006;18:227–235. doi: 10.1097/01.bor.0000218941.04613.85. [DOI] [PubMed] [Google Scholar]
- 59.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 60.Michaels MA, Kang HK, Kaliyaperumal A, Satyaraj E, Shi Y, Datta SK. A defect in deletion of nucleosome-specific autoimmune T cells in lupus-prone thymus: role of thymic dendritic cells. J. Immunol. 2005;175:5857–5865. doi: 10.4049/jimmunol.175.9.5857. [DOI] [PubMed] [Google Scholar]