Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that contracts most smooth muscles. Although S1P has been shown to contract bladder smooth muscle, the mechanism(s) by which S1P initiates contraction has not been extensively investigated. The goal of this study was to determine if S1P-induced force generation and myosin light chain (MLC) phosphorylation are dependent on calcium sensitization pathways mediated by protein kinase C (PKC) and Rho kinase (ROCK) and which S1P receptor is important in this response. Bladder smooth muscle strips from rabbit and rat were mounted for isometric force recording and contracted in response to carbachol or S1P in the presence and absence of an inhibitor of PKC (3 μM Bisindolylmaleimide-1) or ROCK (1 μM H-1172). 10 μM S1P produced approximately 40% of the force generated in response to 110 mM KCl in rabbit bladder smooth muscle. S1P, up to 100 μM, did not produce a response in rat bladder smooth muscle, any response evoked was due to solvent (NaOH). S1P-dependent force development was associated with a concomitant increase in Ser19, but not dual Thr18/Ser19 MLC phosphorylation. Inhibition of PKC decreased force development, whereas inhibition of ROCK abolished S1P-induced force. An inhibitor of the S1P2 receptor, JTE-013, relaxed a S1P-induced contraction; whereas, an agonist with low affinity to the S1P2 receptor, dihydro-S1P, did not elicit a contraction. Our results suggest that S1P contracts rabbit, but not rat, bladder smooth muscle via the S1P2 receptor and is dependent on MLC phosphorylation and myofilament calcium sensitization primarily in response to ROCK activation.
Keywords: Rho kinase, Protein kinase C, Calcium sensitization, S1P2 Receptor, Myosin light chain phosphorylation
1. Introduction
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that has been extensively studied for its ability to affect cell growth, motility, and survival. Sphingolipids are components of cell membrane lipid bilayers and numerous agonists regulate their metabolism to generate several signaling molecules, such as ceramide, sphingosine, and S1P. S1P is a component of a signaling pathway which regulates cellular stress responses and apoptosis (Spiegel and Milstein, 2002). More recently it has been shown that S1P contracts vascular (Bischoff et al., 2000), airway (Rosenfeldt et al., 2003), gastrointestinal (Zhou and Murthy, 2004), and bladder smooth muscles (Watterson et al., 2007; Aydin et al., 2010).
Although the role of S1P in several different smooth muscle tissues has been studied, the importance of S1P in bladder smooth muscle contraction has not been rigorously investigated. S1P-induced smooth muscle contraction occurs through extracellular G-protein coupled receptors (GPCRs), although evidence that S1P can also act intracellularly to induce calcium release has been presented (Spiegel and Milstein, 2002). S1P selectively activates a family of GPCRs named lysophospholipid S1P receptors 1-5; formerly part of the endothelial differentiation gene (EDG) receptor family. The coupling of these receptors to various heterotrimeric G-proteins has been studied in smooth muscle, especially in gastric smooth muscle cells (Zhou and Murthy, 2004; Hu et al., 2006). S1P1-3 receptors are widely expressed in many tissues including smooth muscle, and their coupling to G-proteins varies allowing distinct signaling pathways to be activated.
An increase in intracellular calcium concentration is the primary mediator of smooth muscle contraction (Webb, 2003 for review). However, a change in the calcium sensitivity of the contractile apparatus also plays an important role in the regulation of contraction. This process is termed ‘myofilament calcium sensitization’ and is mediated through the regulation of the myosin light chain (MLC) phosphatase by two primary signaling pathways (Somlyo and Somlyo, 2003 for review). Activation of the small GTPase Rho A and Rho kinase (ROCK) result in phosphorylation-dependent inhibition of the MLC phosphatase and a net increase in MLC phosphorylation (Sward et al., 2003). In addition, inhibition of MLC phosphatase by phospho-Thr38-CPI-17 (PKC-potentiated inhibitor 17kDa protein) has been shown to increase force production and MLC phosphorylation (Kitazawa et al., 2003). Phosphorylation of CPI-17 at Thr38 has been shown to be catalyzed by PKC (Eto et al., 1997). Inhibition of MLC phosphatase activity results in greater net levels of MLC phosphorylation and force at any given [Ca2+], thus shifting the force/[Ca2+] relationship to the left demonstrating an increase in myofilament calcium sensitivity.
The objectives of this work were to measure the contractile activity in response to S1P in bladder smooth muscle and determine if force generation and MLC phosphorylation are dependent upon activation of myofilament calcium sensitization pathways mediated by PKC and ROCK. Moreover, using pharmacological techniques we examined the importance of the S1P2 receptor in S1P-induced bladder smooth muscle contraction.
2. Materials and methods
2.1 Materials
All electrophoretic and blotting reagents were obtained from Bio-Rad (Hercules, CA), except for Odyssey blocking buffer obtained from Li-Cor (Lincoln, Nebraska). Carbamoylcholine chloride (Carbachol) was obtained from Sigma-Aldrich (St. Louis, MO). Bis-1 (2-[1-(3-Dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide) and H-1152 [(S)-(+)-2-Methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine)] were obtained from EMD Millipore (Billerica, MA). Sphingosine-1-phosphate (S1P) and dihydro-sphingosine-1-phosphate (dhS1P) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). S1P was dissolved in 0.3 M NaOH before application in the organ baths (Aydin et al., 2010). JTE-013 [1-[1,3-Dimethyl-4-(2-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-4-(3,5-dichloro-4-pyridinyl)-semicarbazide] was obtained from Tocris Bioscience (Minneapolis, MN) and dissolved in ethanol as recommended by the manufacturer. All other reagents were obtained from Fisher Scientific (Pittsburgh, PA) and were of analytical grade or better.
2.2 Animal model and tissue dissection
2.2.1 Rabbit bladder smooth muscle
Male New Zealand White rabbits weighing 2 to 2.5 kg were used in this study. All animal studies and procedures were approved by Drexel University College of Medicine’s Institutional Animal Care and Use Committee (IACUC). Rabbits were euthanized using pentobarbital via the ear vein followed by thoracotomy, their bladders removed, and the mucosal and serosal layers were dissected away in ice cold physiological salt solution (PSS) under a dissecting microscope leaving a primarily smooth muscle layer. PSS contained (in mM) 140 NaCl, 4.7 KCl, 1.2 MgSO4, 1.6 CaCl2, 1.2 Na2HPO4, 2 MOPS (pH 7.4), 5 D-glucose, and 0.02 EDTA. Muscle strips (~1.5 × 6 mm) were cut along the central axis of the bladder from the middle body in the longitudinal orientation as previously described (Su et al., 2003) and stored in PSS at 4°C until further use. Storage in PSS was never longer than 24 hours.
2.2.2 Rat bladder smooth muscle
Male lean Zucker rats were used in this study. All animal studies and procedures were approved by Drexel University College of Medicine’s IACUC. Rats were euthanized using a pre-charged CO2 chamber followed by exsanguination. Bladders were removed and dissected as described in 2.2.1.
2.3 Measurement of Isometric Contraction
Bladder strips were mounted between a Grass FT.03 force transducer and a stationary clip in water-jacketed muscle organ baths containing PSS at 37°C and aerated with 100% O2. A passive force was applied to mounted tissues and they were allowed to stress-relax until they reached an optimal length for active stress development (Lo), as previously described (Su et al., 2003). Bladder strips were then equilibrated for at least 45 min until a stable force recording was obtained.
After the equilibration period, strips were stimulated with 110 mM KCl for 5 min and then relaxed with PSS. This contraction-relaxation cycle was repeated multiple times until peak force did not change more than 10% between trials, normally this required 4 or 5 contraction-relaxation cycles. The initial contraction stretches the series elastic component to allow accurate setting of pre-load in the resting state. Subsequent contractions are required to fully equilibrate the tissues and provide a stable contraction with which to use to normalize the force generated by carbachol or S1P stimulation. The increase in force between the first and last equilibration contraction was 1.12 ± 0.21 grams force or 15.5 ± 2.9% (mean ± S.E.M., P = 0.03). In selected experiments, tissues were contracted at the end of the protocol to ensure that no deterioration of force occurred during the course of the study. 3 μM Bis-1 and 1 μM H-1152 were used to inhibit PKC and ROCK activity respectively during S1P induced contractions. Each inhibitor was added to the organ bath 20 min prior to tissue stimulation. Tissues were contracted for 5 min with S1P or carbachol. Bladder smooth muscle contractions to carbachol were used as positive controls for force generation and MLC phosphorylation.
In selected experiments 10 μM dihydro-sphingosine-1-phosphate (dhS1P) was applied in place of S1P to determine the extracellular receptors responsible for S1P-induced contraction. In other experiments the S1P2 receptor antagonist JTE-013 was applied to the organ baths 10 min before the addition of S1P; force was then measured 10 min after S1P application. Alternatively, JTE-013 was applied after 10 min of S1P stimulation; force was measured 10 min after the application of JTE-013.
2.4 Measurement of myosin light chain (MLC) phosphorylation
2.4.1 Native Gel Electrophoresis
Bladder smooth muscle tissue strips were rapidly frozen in a dry ice/acetone slurry containing 6% trichloroacetic acid (weight per volume) and 10 mM dithiothreitol (DTT). Tissues were extracted for 2 hours at 4°C in a buffer containing (in mM) 6000 Urea, 120 Tris HCl (pH 6.8), 10 DTT, 10 EGTA, 1 Na2EDTA, 5 sodium fluoride, and 2% (v/v) protease inhibitor cocktail (Nauli et al., 2005). The extracts were subjected to non-denaturing PAGE (12% gel) with subsequent transfer to nitrocellulose membranes. After transfer, the membranes were blocked with Odyssey blocking buffer (Li-Cor) for 1 hour and then incubated with 1:10,000 dilution of mouse anti-MLC antibody (Sigma-Aldrich) overnight at 4°C. The membranes were then washed 4 times with a phosphate-buffered saline solution containing 0.1% Tween-20 (PBST) and then incubated at room temperature with a secondary anti-mouse fluorescent antibody (Li-Cor, 1:10,000) for 45 min. After incubation, the membranes were again washed 4 times with 0.1% PBST and then rinsed in deionized water. The membranes were scanned using an Odyssey Imager (Li-Cor) and bands were quantified using the software accompanying the instrument. No loading control was required as phosphorylated MLC was compared to total MLC levels (the sum of the phosphorylated and unphosphorylated bands) as a ratio (phosphorylated MLC: total MLC).
2.4.2 Measurement of dual Thr18/Ser19 MLC phosphorylation
Dual MLC phosphorylation was measured using an antibody recognizing dual phosphorylation at the Ser18 and Thr19 sites of the regulatory MLC (Cell Signaling Technology). For these measurements the tissues were frozen as described in section 2.4.1 but homogenized in a buffer containing 10% glycerol and 1% w/v SDS instead of extraction in urea buffer. Sample extracts were subjected to SDS-PAGE (12% gel) with subsequent transfer to nitrocellulose membranes. Blocking and washing procedures were the same as in section 2.4.1 except that the primary antibodies used were for detection of Ser18/Thr19 p-MLC (Antibody # 3674, Cell Signaling, 1:1,000) and 3-actin (Sigma, 1:250,000). For this procedure the ratio of the intensity of the Ser18/Thr19 band to the 3-actin band was used as the quantitative measurement of MLC phosphorylation.
2.4.3 Measurement of Ser19 MLC Phosphorylation
Tissue homogenization, electrophoresis, transfer, blocking, and washing procedures were the same as described in 2.4.2. The primary antibodies used for detection were rabbit anti-Ser19 phosphorylated MLC (1:1,000 Cell Signaling Technology Inc.) and mouse anti-MLC antibody (1:10,000 Sigma-Aldrich) which were incubated overnight at 4°C. After washes, membranes were incubated at room temperature with secondary anti-mouse and anti-rabbit fluorescent antibodies (1:10,000 Li-Cor) for 45 min. The membranes were scanned using an Odyssey Imager (Li-Cor) and bands were quantified using the software accompanying the instrument. For this procedure the ratio of the intensity of the Ser19 band to the total MLC bands was used as the quantitative measurement of Ser19 MLC phosphorylation level.
2.5 Measurement of S1P2 Receptor Expression
Tissue extracts were run through 10% polyacrylamide gels. Transfer and washing procedures were the same as in section 2.4.1. Membranes were incubated with rabbit anti-EDG5 (1:500 Santa Cruz Biotechnology Inc.) and mouse anti-calponin (1:200,000 Sigma-Aldrich) primary antibodies overnight at 4°C. Secondary antibody and scanning procedures were the same as in section 2.4.2.
2.6 Statistics
Statistical significance between means was determined using the Student’s t-test with or without the Bonferroni correction. When appropriate for multiple comparisons a one-way analysis of variance (ANOVA) test was performed followed by either a Tukey or Bonferroni post hoc test for significance of multiple comparisons. Tukey’s post hoc test was used when comparisons were being made among all of the conditions measured. Selected Bonferroni post hoc tests were used when comparisons were being made among some but not all of the conditions measured. A P value < 0.05 was taken as significant. N values refer to the number of bladder smooth muscle strips/lysates used, with each strip/lysate taken from the bladder of a different animal.
3. Results
3.1 Isometric force production in response to S1P or carbachol
3.1.1 Rabbit bladder smooth muscle
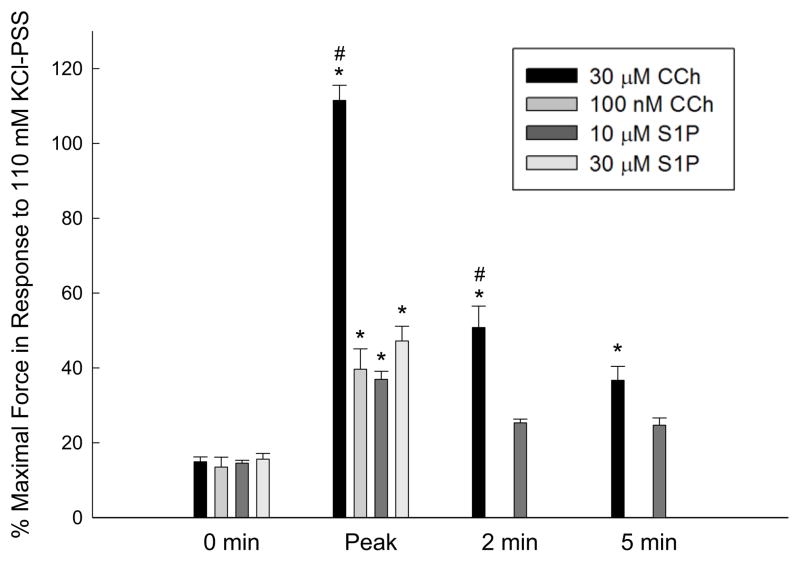
We determined the maximal force in response to the exogenous addition of various concentrations of S1P or carbachol (Fig. 1) in strips of rabbit bladder smooth muscle. Bladder smooth muscle exhibits a phasic-like contraction such that force reaches a peak and then decreases approaching baseline values (time 0 in Fig. 1). Baseline force was defined as the passive force applied to the tissue prior to the addition of stimulus, which for these experiments was ~ 1 gram force to approximate Lo. Both S1P and carbachol produced measurable levels of isometric force. The force generated by 30 μM carbachol was significantly greater than the force produced by 10 μM S1P, 30 μM S1P, and 100 nM carbachol during peak force generation and 2 min after the addition of agonist, but not at 5 min when steady state force in response to 30 μM carbachol only tended to be higher. Force generated by 30 μM carbachol was significantly greater than baseline levels (0 min) at all time points measured (Fig. 1). Peak forces in response to both concentrations of S1P and 100 nM carbachol were similar. As maximal force development was similar in response to either 10 μM or 30 μM S1P, all subsequent studies utilized 10 μM S1P. The mean absolute force in response to 110 mM KCl for these experiments was 7.30 ± 0.14 grams force.
Fig. 1.
Isometric force in response to carbachol (CCh) or S1P. Force was measured prior to contraction (baseline force, 0 min), at the peak of contraction (Peak), 2 min, and 5 min after addition of agonist. Force was expressed as a percentage of the maximal force in response to 110 mM KCl; average force in response to KCl was 7.30 ± 0.14 grams. Statistical comparisons were made by one-way ANOVA followed by a Tukey post-hoc test. Values shown are the means ± S.E.M. of at least 4 experiments. * P < 0.05 compared with values at 0 min for the same agonist and concentration. # P < 0.05 compared with all other measurements at the same time point.
3.1.2 Rat bladder smooth muscle
Rat bladder smooth muscle strips were mounted for isometric force recording and subjected to increasing concentrations of S1P (100 nM to 100 μM) or solvent alone (NaOH). In contrast to rabbit bladder smooth muscle, rat bladder smooth muscle developed similar levels of force in response to 100 μM S1P and vehicle alone, 4.6 μM NaOH final concentration in bath (Fig. 2). The addition of 4.6 μM NaOH increased the pH of the bathing solution from 7.40 to 7.44. Solvent volume could not be further reduced as the stock solution used for the experiments approached the maximal solubility of S1P in 0.3 M NaOH. The solubility of S1P in DMSO was inadequate to provide a suitably greater stock concentration. Therefore, we did not perform any additional studies with rat bladder smooth muscle.
Fig. 2.
Stimulation of rat bladder smooth muscle by S1P. Rat bladder smooth muscle strips were exposed to increasing concentrations of S1P (A) or solvent (B). Tissues responded similarly to either the highest concentration of S1P (100 μM) or solvent alone (4.6 μM NaOH final concentration in bath). Solvent volume could not be reduced further due to the low solubility of S1P.
3.2 Inhibition of PKC and ROCK during S1P-induced isometric force
Isometric force production in response to 10 μM S1P was also measured in the presence or absence of an inhibitor of PKC, Bis-1, or an inhibitor of ROCK, H-1152 (Fig. 3). The mean absolute force in response to 110 mM KCl for these experiments was 7.29 ± 0.20 grams force. This experiment was designed to determine if exogenous S1P activates the myofilament calcium sensitization pathways (ROCK or PKC). The ROCK and PKC signaling pathways both inhibit the MLC phosphatase resulting in greater levels of MLC phosphorylation and force at any given [Ca2+]. 10 μM S1P alone produced a phasic contraction with peak force production ~ 1 min after addition. Inhibition of PKC by the addition of 3 μM Bis-1 significantly decreased force production at 1 and 2 min after S1P addition compared to S1P alone. Force was lower at 5 min in the presence as compared to the absence of Bis-1, but the decrease was not significant. Inhibition of ROCK by the addition of 1 μM H-1152 significantly decreased force production compared to S1P alone at all time points measured after the addition of S1P (1, 2, and 5 min). Consistent with previous results from our laboratory, inhibition of ROCK tended to decrease baseline force (Wang et al., 2009; Wang et al., 2012), although the H-1152-induced decrease in baseline force was not significant in this set of experiments. The decrease in S1P-induced isometric force production by inhibition of ROCK was significantly greater than that produced by inhibition of PKC. It is important to note, that previous studies in our laboratory have shown that 3 μM Bis-1 inhibits PKC-dependent pathways and 1 μM H-1152 inhibits ROCK-dependent pathways in rabbit bladder smooth muscle (Wang et al., 2009; Wang et al, 2012). Preliminary studies from our laboratory demonstrated that these two compounds at these concentrations had no effect on MLC phosphorylation levels in a Triton X-100 skinned preparation of rabbit bladder smooth muscle, suggesting Bis and H-1152 have no non-specific effects on the MLC kinase (unpublished results). The Triton X-100 skinned preparation is devoid of PKC and ROCK thus any inhibitor effect on MLC phosphorylation levels would be on the MLC kinase.
Fig. 3.
The effect of PKC or ROCK inhibition on S1P-induced force in rabbit bladder smooth muscle. Inhibitors were added 20 min prior to stimulation with 10 μM S1P. Force was recorded at the time of inhibitor addition (Inh Addition), at the time of S1P addition (0), and at 1, 2, and 5 min after addition of S1P. Force was expressed as a percentage of the maximal force in response to 110 mM KCl; average force in response to KCl was 7.29 ± 0.20 grams. Values shown are the means ± S.E.M. of 8 – 9 determinations, each from a different bladder. Statistical comparisons were made by one-way ANOVA followed by selected Bonferroni post-hoc tests. * P < 0.05 compared with S1P alone (black circles) at the same time point. # P < 0.05 compared with S1P + Bis (open squares) at the same time point.
3.3 S1P-induced MLC phosphorylation
We measured MLC phosphorylation levels during S1P-induced contraction. MLC phosphorylation is a primary step in the initiation of force production in smooth muscle (Somlyo and Somlyo, 1994). The myofilament calcium sensitization pathways, regulated by PKC and ROCK activity, modulate MLC phosphorylation. Thus the potential role(s) of these kinases on MLC phosphorylation was investigated.
3.3.1 MLC phosphorylation measured by native gel electrophoresis
MLC phosphorylation levels were first measured by the use of native gel electrophoresis (Fig 4). MLC phosphorylation was measured before addition of agonists and at peak force development in response to 100 nM CCh, 300 nM CCh, 30 μM CCh, or 10 μM S1P. Only 300 nM CCh and 30 μM CCh increased MLC phosphorylation significantly above baseline values. Neither 10 μM S1P nor 100 nM CCh significantly increased MLC phosphorylation levels above basal values. There was no increase in MLC phosphorylation due to vehicle alone (either deionized water or 0.3 μM NaOH; data not shown).
Fig. 4.
Native gel electrophoresis for the measurement of S1P-induced MLC phosphorylation in rabbit bladder smooth muscle. MLC phosphorylation levels were measured at peak force in response to carbachol (CCh) or S1P. The top panel shows a representative Western blot of the separation of phosphorylated MLC (‘P’ lower band) from unphosphorylated MLC (‘UnP’ upper band). Both 0.3 μM and 30 μM CCh significantly increased MLC phosphorylation above control levels. Statistical comparisons were made by Student’s t-test with Bonferroni correction. Values shown are the means ± S.E.M. of 4 – 5 experiments. * P < 0.0125 compared with control.
Both 10 μM S1P and 100 nM CCh generated similar and significant levels of peak isometric force production (Fig. 1), but the level of force was less than that generated in response to 30 μM CCh. The absence of an increase in MLC phosphorylation in response to 10 μM S1P or 100 nM CCh could be due to force development independent of MLC phosphorylation or insufficient sensitivity of the detection method. Therefore, we utilized a second method of MLC phosphorylation detection.
3.3.2 MLC phosphorylation measured by SDS-PAGE and Western blot
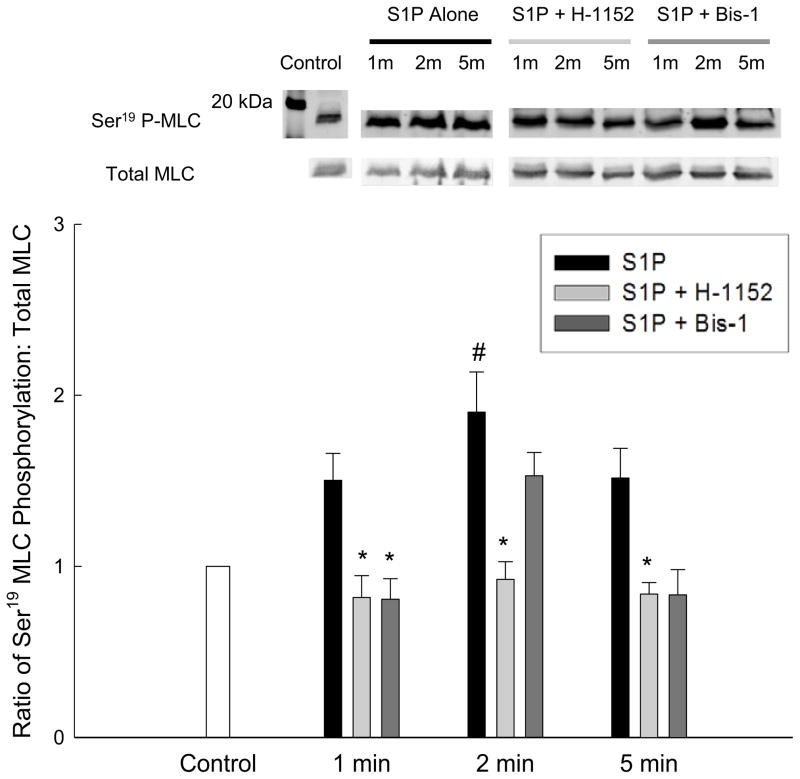
Ser19 MLC phosphorylation was measured by SDS-PAGE and Western blot using an antibody against the Ser19 phosphorylation site (Fig. 5). Ser19 MLC phosphorylation tended to be increased compared to control by 10 μM S1P at all time points measured (1, 2, and 5 min) but was only significantly increased compared to control at 2 min. This suggests that force in response to S1P is accompanied by an increase in MLC phosphorylation levels, but that the increase in phosphorylation was below the detection limits of native gel electrophoresis as we were unable to detect an increase in MLC phosphorylation by the methodology used in 3.3.1.
Fig. 5.
The effect of PKC or ROCK inhibition on S1P-induced Ser19 MLC phosphorylation in rabbit bladder smooth muscle. Ser19 MLC phosphorylation was measured in response to 10 μM S1P in the presence or absence of 3 μM Bis-1 or 1 μM H-1152. The top panel shows representative Western blots whereas the bottom panel shows the averaged results of 3 – 5 blots, depending on the treatment condition. Statistical comparisons were made by one-way ANOVA with selected Bonferroni post-hoc tests. Values shown are the means ± S.E.M. of 3 – 5 experiments. * P < 0.05 compared with S1P alone at the same time point. # P < 0.05 compared with control.
3.3.3 Dual Thr18/Ser19 MLC phosphorylation
MLC has multiple phosphorylation sites (Ikebe and Hartshorne, 1985). Dual phosphorylation of Thr18 and Ser19 has been shown to enhance actomyosin ATPase activity more than when MLC is phosphorylated on Ser19 alone (Ikebe and Hartshorne, 1985). Therefore, to determine if S1P stimulation produces dual MLC phosphorylation, dual Thr18/Ser19 MLC phosphorylation was measured by SDS-PAGE and Western blotting using an antibody against both the Thr18 and Ser19 phosphorylation sites (Fig. 6). The antibody only detects MLC when phosphorylated at both sites (Sutherland and Walsh, 2012). Thr18/Ser19 MLC phosphorylation was significantly increased during peak force production in response to 30 μM carbachol but not to 10 μM S1P. At 5 min after agonist application Thr18/Ser19 MLC phosphorylation was not increased by either carbachol or S1P. This suggests that while carbachol stimulation of bladder smooth muscle results in dual phosphorylation of MLC at Thr18 and Ser19, S1P does not induce dual phosphorylation of MLC.
Fig. 6.
Carbachol or S1P induced dual Thr18/Ser19 MLC phosphorylation in rabbit bladder smooth muscle. Dual Thr18/Ser19 MLC phosphorylation was measured in response to either 30 μM carbachol (black bars) or 10 μM S1P (grey bars). Dual Thr18/Ser19 MLC phosphorylation was quantified by comparison to endogenous 3-actin levels and then normalized to control MLC phosphorylation values from samples not treated with carbachol or S1P. Statistical comparisons were made by Student’s t-test. Values shown are the means ± S.E.M. of 5 experiments. * P < 0.05 compared with control values.
3.3.4 Effect of PKC and ROCK inhibition on MLC phosphorylation
Ser19 MLC phosphorylation was measured in response to 10 μM S1P in the presence or absence of 3 μM Bis-1 or 1 μM H-1152 to determine the effects of inhibition of the myofilament calcium sensitization pathways on MLC phosphorylation (Fig. 5). Inhibition of ROCK with H-1152 significantly reduced Ser19 MLC phosphorylation in response to S1P stimulation at all time points measured (1, 2, and 5 min) as compared to the addition of S1P alone. Inhibition of PKC with Bis-1 decreased MLC phosphorylation levels at 1 and 5 min of stimulation, but the decrease was only statistically significant at 1 min. Levels of MLC phosphorylation were not decreased at 2 min of S1P stimulation in the presence of Bis-1.
3.4 S1P receptor subtype
In addition to measuring isometric force development and MLC phosphorylation levels in response to exogenous S1P, we also investigated the potential involvement of the S1P2 receptor in the S1P-induced contraction. This was performed using a S1P2 receptor antagonist, JTE-013 and a S1P analog (dhS1P), which although it activates all S1P receptors has a 10-fold lower affinity for the S1P2 receptor (Watterson et al., 2007). The rationale being that if the S1P contraction is inhibited by an antagonist of the S1P2 receptor and a S1P analog with a low affinity for the S1P2 receptors does not produce a contraction, then this would suggest that in our studies, the S1P contraction is mediated via the S1P2 receptor. Representative force tracings are shown in Fig 7 which depict the phasic force developed in response to 10 μM S1P alone (Fig. 7A). The addition of the S1P2 receptor antagonist JTE-013 (10 μM) during an S1P-induced contraction reduced force to baseline levels (Fig. 7B). Similar results were obtained with the addition of 10 μM JTE-013 prior to stimulation with S1P. The addition of the S1P analog, dhS1P (10 μM), did not produce a contraction of bladder smooth muscle (Fig. 7C). S1P2 receptor protein expression was probed by Western blot analysis and found to be present in our preparation of rabbit bladder smooth muscle (Fig. 7D). The addition of JTE-013 before or after stimulation of S1P inhibited or relaxed the S1P contraction respectively (Fig. 7E). Moreover, dhS1P did not increase force (Fig. 7E).
Fig. 7.
S1P receptor subtype in rabbit bladder smooth muscle. Panel A shows a representative tracing of force production in response to the addition of 10 μM S1P. Panel B shows a representative tracing of the reduction in S1P-induced force due to blockade of S1P2 receptors by 10 μM JTE-013. Panel C shows a representative tracing of the lack of contraction to an S1P analog, dhS1P (10 μM). Panel D shows a representative Western blot of S1P2 receptor protein in lysates of rabbit bladder smooth muscle. The left image shows the positive control band for S1P2 receptor which appears at approximately 50 kDa. The right image shows that S1P2 receptor is present in the rabbit bladder samples. Panel E shows the quantitative results of several experiments such as those shown in Panels A–C plus experiments performed adding JTE-013 prior to the addition of S1P. Statistical comparisons were made by Student’s t-test. Values shown are the means ± S.E.M. of 3 – 6 experiments. * P < 0.05 compared with S1P-induced contractions in the absence of inhibitors.
4. Discussion
In this study we showed that stimulation of rabbit bladder smooth muscle with S1P increased isometric force and Ser19 MLC phosphorylation, but not dual Thr18/Ser19 MLC phosphorylation. Both S1P-induced force and Ser19 MLC phosphorylation were reduced by inhibition of PKC and ROCK; however, ROCK is the more important kinase for both S1P-induced force and MLC phosphorylation. To the best of our knowledge, this is the first demonstration that S1P-dependent contraction of bladder smooth muscle involves activation of PKC and ROCK mediated myofilament calcium sensitization pathways. Moreover, based on our pharmacological experiments the S1P2 receptor is the primary receptor for S1P-induced force in rabbit bladder smooth muscle. Lastly, in this report, we have shown that in contrast to the S1P-dependent contraction of rabbit bladder smooth muscle, rat bladder smooth muscle does not contract in response to S1P stimulation.
4.1 S1P induced contraction
Rabbit bladder smooth muscle contracted in response to S1P stimulation, but the magnitude of force developed was lower than that in response to carbachol, consistent with previous findings (Watterson et al., 2007). Our results also showed that S1P-induced force and MLC phosphorylation are PKC and ROCK-dependent. In airway smooth muscle S1P-induced MLC phosphorylation was shown to be ROCK-dependent (Kume et al., 2007; Rosenfeldt et al., 2003). Also, S1P dependent contraction of cultured human coronary artery smooth muscle cells was RhoA/ROCK dependent (Ohmori et al., 2003). In rat bladder smooth muscle inhibition of ROCK abolished S1P-induced contraction (Aydin et al., 2010). Interestingly, our rat bladder smooth muscle strips did not respond to S1P and any force developed was due to solvent (NaOH). Whether this is a strain difference in rats is not known, but it is a possibility as we used male lean Zucker rats and Disanto’s group used male Sprague Dawley rats. Nonetheless, these studies all suggest the importance of ROCK in S1P-induced force in rabbit bladder smooth muscle.
During the early phase of S1P-induced contraction (30 s) in gastric smooth muscle cells inhibition of PKC or ROCK had no effect on MLC phosphorylation or contraction although both were calcium and MLC kinase dependent (Zhou and Murthy, 2004). In our preparation, inhibition of either PKC or ROCK reduced both contraction and MLC phosphorylation during peak force production (~ 1.5 min) and sustained contraction (5 min), suggesting that PKC and ROCK are involved in both the peak and sustained phases of S1P-induced contraction in bladder smooth muscle. In contrast, using human airway smooth muscle cells, inhibition of PKC had no effect on any phase of a S1P-induced contraction (Rosenfeldt et al., 2003). It is important to note that different smooth muscles have different time courses of contraction and varying roles of intracellular signaling in the different phases of contraction (Wang et al., 2009). Therefore, our results are consistent with the literature in that S1P-induced smooth muscle contraction and MLC phosphorylation are ROCK-dependent although the precise phase of contraction in which ROCK is important and the relative contribution of ROCK varies among different smooth muscles.
Inhibition of ROCK abolished both force and MLC phosphorylation in response to S1P; whereas inhibition of PKC only partially inhibited either. The complete abolishment of both force and MLC phosphorylation by ROCK inhibition suggests that S1P-induced contraction and MLC phosphorylation may be completely ROCK-dependent in rabbit bladder smooth muscle. This finding also suggests S1P-induced force is predominantly due to inhibition of the MLC phosphatase resulting in a concomitant increase in MLC phosphorylation. Alternatively, S1P-induced increases in MLC phosphorylation may also be the result of direct ROCK-catalyzed MLC phosphorylation, instead of signaling through MYPT or CPI-17 (Amano et al., 1996; Totsukawa et al., 2000). The finding that inhibition of ROCK abolished force while inhibition of PKC only decreased force suggests a relationship between the two enzymes. Several studies have shown cross-talk between the PKC and ROCK myofilament calcium sensitization pathways in smooth muscle contraction (Niiro et al., 2003; Patil et al., 2006; Wang et al., 2009; Wang et al., 2012).
4.2 MLC phosphorylation
We detected MLC phosphorylation using multiple methodologies, due to the possibility that our first method, which combined native PAGE with detection via an anti-MLC antibody, was not sufficiently sensitive to detect changes in S1P-induced MLC phosphorylation. Historically, MLC phosphorylation has been detected by multiple methodologies. However, some methodologies have inherent problems detecting low levels of MLC phosphorylation such as aggregation of MLC by high levels of urea and failure to enter the gel (Takeya et al., 2008). In our second methodology we combined SDS-PAGE, which may increase the solubility and ability of MLC to enter and migrate through the gel, with Western blotting using an anti-phospho-MLC antibody (Fig. 5, 6). Anti-Ser19-phospho MLC antibodies have been shown to generate stronger signals then anti-MLC antibodies (Takeya et al., 2008). While MLC has been shown to be phosphorylated at both Thr18 and Ser19 by MLC kinase (Ikebe et al., 1985), not all agonists result in phosphorylation at both sites (Takeya et al., 2008). Moreover, only phosphorylation at Ser19 is required for actin-activated myosin ATPase activity and muscle contraction (Kamm et al., 1985; Somlyo and Somlyo, 1994). S1P increases Ser19 MLC phosphorylation, but does not increase levels of dual Thr18/Ser19 MLC phosphorylation in rabbit bladder smooth muscle.
4.3 Receptor pharmacology of S1P contraction
The S1P receptor family consists of 5 receptors, although the most prominent and well described are S1P1, 2, and 3 receptors. The S1P1 receptor is coupled to Gi and activation is not believed to result in an increase in cellular calcium (Hu et al., 2006; Rapizzi et al., 2007; Siehler and Guerini, 2006); however, activation of the S1P1 receptor has been shown to increase cellular calcium in endothelial cells (Zhang et al., 2010). The S1P2 receptor is coupled to phospholipase C and increases cellular calcium by IP3-dependent intracellular calcium release as well as opening store-operated calcium influx (Hu et al., 2006; Hopson et al., 2011). Activation of the S1P3 receptor, similar to the S1P2 receptor increases cellular calcium via release from intracellular sources (Rapizzi et al., 2007; Murakami et al., 2010). Our results in rabbit bladder smooth muscle showing a lack of contraction in response to dihydro-S1P, relaxation or inhibition of S1P-induced contraction by a S1P2 receptor antagonist, JTE-013, and the presence of S1P2 receptor protein by Western blotting are consistent with previous observations (Watterson et. al., 2007). Experiments in airway smooth muscle (Rosenfeldt et al., 2003), gastric smooth muscle cells (Zhou and Murthy, 2004), and a vascular preparation (Wamhoff et al., 2008) also suggest the importance of S1P2 receptor in S1P-mediated contraction. The presence of S1P2 receptor protein, reduction of contraction by an S1P2 receptor antagonist, and lack of contraction in response to an S1P receptor agonist which weakly activates S1P2 receptor supports the hypothesis that the S1P2 receptor is the primary receptor in S1P-induced rabbit bladder smooth muscle contraction.
4.4 Myofilament calcium sensitization of S1P-induced bladder smooth muscle contraction
In this study we have shown a decrease in force and MLC phosphorylation in response to inhibition of either PKC or ROCK suggesting activation of both myofilament calcium sensitization pathways by S1P. PKC and ROCK are both known activators of myofilament calcium sensitization, albeit to different degrees depending on the specific smooth muscle and agonist studied. The importance of PKC and ROCK in S1P-induced contraction has been shown in rat bladder smooth muscle (Aydin et al., 2010), gastric smooth muscle cells(Zhou and Murthy, 2004), airway smooth muscle (Kume et al., 2006), and vascular smooth muscle (Hemmings et al., 2006). In contrast, Ratz’s group suggested that S1P does not induce myofilament calcium sensitization based on the finding that the force/calcium ratio in response to S1P stimulation was lower than that measured in response to carbachol; an agonist known to cause myofilament calcium sensitization (Watterson et al., 2007). Our results and the majority of those in the literature are consistent with a primary role of ROCK induced myofilament calcium sensitization and a lesser role for PKC dependent myofilament calcium sensitization in response to S1P stimulation. Moreover, because inhibition of ROCK abolished and inhibition of PKC only depressed S1P-induced contraction, this would be consistent with PKC activation via ROCK as we have previously suggested (Wang et al., 2009; Wang et al., 2012).
5. Conclusion
In summary, we have shown that S1P contracts rabbit bladder smooth muscle via the S1P2 receptor. However, S1P did not elicit a contraction in rat bladder smooth muscle from the lean Zucker strain. S1P-induced contractions are mediated by an increase in Ser19 but not dual Thr18/Ser19 MLC phosphorylation. Inhibition of PKC reduces but does not abolish a S1P-induced contraction, suggesting that S1P binding to the S1P2 receptor activates PKC and increases the myofilament Ca2+ sensitivity, most likely via phosphorylation of CPI-17. Inhibition of ROCK completely abolishes the S1P-induced contraction, suggesting that S1P binding to the S1P2 receptor activates the Rho A/ROCK pathway increasing myofilament Ca2+ sensitivity. Lastly, the Rho A/ROCK pathway is sufficient for contraction in response to S1P.
Acknowledgments
This study was supported, in part, by funds from The National Institute of Diabetes, Digestion, and Kidney Disease grant DK 85734 to RS Moreland. DM Kendig was supported by a predoctoral fellowship from the Drexel University College of Medicine Aging Initiative. The authors would like to thank Sarah E. Sivilich for her technical assistance with the force transduction experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aydin M, Downing K, Villegas G, Zhang X, Chua R, Melman A, Disanto ME. The sphingosine-1-phosphate pathway is upregulated in response to partial urethral obstruction in male rats and activates RhoA/Rho-kinase signalling. BJU Int. 2010;106:562–571. doi: 10.1111/j.1464-410X.2009.09156.x. [DOI] [PubMed] [Google Scholar]
- Bischoff A, Czyborra P, Fetscher C, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br J Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997;410:356–360. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- Hemmings DG, Hudson NK, Halliday D, O’Hara M, Baker PN, Davidge ST, Taggert MJ. Sphingosine-1-phosphate acts via rho-associated kinase and nitric oxide to regulate human placental vascular tone. Biol Reprod. 2006;74:88–94. doi: 10.1095/biolreprod.105.043034. [DOI] [PubMed] [Google Scholar]
- Hopson KP, Truelove J, Chun J, Wang Y, Waeber C. S1P activated store-operated calcium entry via receptor- and non-receptor-mediated pathways in vascular smooth muscle. Am J Physiol: Cell Physiol. 2011;300:C919–C916. doi: 10.1152/ajpcell.00350.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Huang J, Mahavadi S, Li F, Murthy KS. Lentiviral siRNA silencing of sphingosine-1-phosphate receptors S1P1 and S1P2 in smooth muscle. Biochem Biophys Res Commun. 2006;343:1038–1044. doi: 10.1016/j.bbrc.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Bio Chem. 1985;260:10027–10031. [PubMed] [Google Scholar]
- Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Ann Rev Pharm Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–889. doi: 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Takeda N, Oguma T, Ito S, Kondo M, Ito Y, Shimokata K. Sphingosine 1-phosphate causes airway hyper-reactivity by rho-mediated myosin phosphatase inactivation. J Pharm Exp Thera. 2007;320:766–773. doi: 10.1124/jpet.106.110718. [DOI] [PubMed] [Google Scholar]
- Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, Watanabe Y, Mori K, Sato Y, Takahashi A, Mochizuki N, Takahura N. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77:704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Williams JM, Gerthoffer WT, Pearce WJ. Chronic hypoxia modulates relations among calcium, myosin light chain phosphorylation, and force differently in fetal and adult ovine basilar arteries. J Appl Physiol. 2005;99:120–127. doi: 10.1152/japplphysiol.01131.2004. [DOI] [PubMed] [Google Scholar]
- Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin- binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J. 2003;369:117–128. doi: 10.1042/BJ20021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- Patil SB, Bitar KN. RhoA- and PKC-alpha-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol: Gastro Liver Physiol. 2006;290:G83–95. doi: 10.1152/ajpgi.00178.2005. [DOI] [PubMed] [Google Scholar]
- Rapizzi E, Donati C, Cericetti F, Pinton P, Rizzuto R, Bruni P. Sphintosin 1-phosphate receptors modulate intracellular Ca2+ homeostasis. Biochem Biophys Res Commun. 2007;353:268–274. doi: 10.1016/j.bbrc.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- Siehler S, Guerini D. Novel GPCR screening approach: Indirect identification of S1P receptor agonists in antagonist screening using a calcium assay. J Recept Signal Transduct Res. 2006;26:549–575. doi: 10.1080/10799890600932246. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol: Renal Physiol. 2003;284:F644–652. doi: 10.1152/ajprenal.00274.2002. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Walsh MP. Myosin regulatory light chain diphosphorylation slows relaxation of arterial smooth muscle. J Biol Chem. 2012;287:24064–24076. doi: 10.1074/jbc.M112.371609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertension Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- Takeya K, Loutzenhiser K, Shiraishi M, Loutzenhiser R, Walsh MP. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: the first quantification in renal arterioles. Am J Physiol: Renal Physiol. 2008;294:F1487–1492. doi: 10.1152/ajprenal.00060.2008. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff BR, Lynch KR, Macdonald TI, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterio Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Kendig DM, Smolock EM, Moreland RS. Carbachol-induced rabbit bladder smooth muscle contraction: roles of protein kinase C and Rho kinase. Am J Physiol: Renal Physiol. 2009;297:F1534–1542. doi: 10.1152/ajprenal.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Kendig DM, Trappanese DM, Smolock EM, Moreland RS. Phorbol 12,13-dibutyrate-induced, protein kinase C-mediated contraction of rabbit bladder smooth muscle. Front Pharmacol Cardiovasc Smoooth Musc Pharmacol. 2012;2:1–12. doi: 10.3389/fphar.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson KR, Berg KM, Kapitonov D, Payne SG, Miner AS, Bittman R, Milstien S, Ratz PH, Spiegel S. Sphingosine-1-phosphate and the immunosuppressant, FTY720-phosphate, regulate detrusor muscle tone. FASEB J. 2007;21:2818–2828. doi: 10.1096/fj.06-7326com. [DOI] [PubMed] [Google Scholar]
- Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Edu. 2003;27:201–206. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- Zhang G, Xu S. Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules. Am J Physiol: Heart Circ Physiol. 2010;299:H1494–H1504. doi: 10.1152/ajpheart.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol: Cell Physiol. 2004;286:C1130–1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]