INTRODUCTION
The purpose of a drug delivery system (DDS) [1, 2] is to devise a method that enables delivery of a therapeutic agent that may have sub-optimal physicochemical properties in biological tissue, thus enhancing efficacy and safety by controlling the rate, time, and release of the agent [3]. Targeted DDS (TDDS) includes the administration of the therapeutic substance, the release of the active ingredients from the DDS, and the subsequent transport of the active ingredients across the biological membranes to the specific site of action, often using what is so called “smart drug carriers” or “smart nanoparticles (NP)”. Smart NP can improve drug delivery mechanisms based on their particular formulations [4, 5]. NP used in such biomedical applications include lipid-based NP (liposomes) [6, 7], polymeric micelles [8, 9], block ionomer complexes [10, 11], water-soluble synthetic polymers (dendrimers) [12, 13], inorganic [14] and polymeric NP [15], nanorods [16, 17], quantum dots [18, 19], carbon nanotubes [20, 21], silica-based NP [22], metal and semiconductor NP, upconversion NP [23, 24], self-illuminating NP [25, 26] and polymer-drug conjugates [27]. However the main limitations of employing NP for drug delivery are the following: 1) the relatively small amount of drug that can be linked to each NP; 2) the possibility of drug deactivation once it is chemically bound to the NP; 3) the possibility of immediate uncontrollable passive release (burst effect); and 4) NP agglomeration producing quick elimination from bloodstream by macrophages before reaching the target cells [28]. Most of these factors in turn require injection of unnecessarily high concentrations of NP, with potential systemic toxic effects. In order to overcome some of these hurdles, physical energy methods have been explored to enhance not only NP but also drug and gene delivery.
The enhancement of effective drug delivery to the target achieved by the addition of physical energy to the traditional TDDS systems has been widely explored in recent years [29–36]. These techniques have the potential to be used in different biological and medical applications as an advanced TDDS that can minimize the current limitations and unwanted effects. Biophysical energy such as electric fields, magnetic fields, ultrasound and mechanical forces, light and temperature gradients can act as enhancers for drug delivery. Technique-specific terms have been associated with each form of physical energy such as electroporation [37, 38] for electric current, iontophoresis for electric potential, magnetoporation [39, 40] for magnetic field, sonoporation [41, 42], mechanoporation [43] and phonophoresis [44, 45] for ultrasound, optoporation [46, 47] for pulsed light and thermoporation [48, 49] for temperature, respectively (Figure 1). Table 1 summarizes all of the different types of physical energy used in this review for porating the cells. The advantages and disadvantages of the techniques are analyzed in the table and the corresponding gene transfection names are also included. Synergistic combinations of two or more physical energies such as magnetic-electroporation [50] are also of special interest and have been explored as a potential system for effective drug delivery. The formation of temporary pores in the cell membrane achieved through the application of various physical energies is the common phenomenon involved in such systems. Once the pores have been formed, drug-carrying NP or smart drug carriers (SDC) [51] can easily penetrate through these pores in the membrane and enter the cells where the drug/gene is further released and activated. Thereby, some of the above-mentioned constraints inherently limiting the efficacy of NP can be overcome by use of physical energies.
Figure 1. Different physical energy modules used for drug delivery.
Drug delivery enhancers such as electroporation, magnetoporation, thermoporation, sonoporation and optoporation are illustrated.
Table 1.
| Poration | Electroporation | Magnetoporation | Thermoporation | Sonoporation | Optoporation |
|---|---|---|---|---|---|
| Attributes | |||||
| Physical energy | Electric Field | Magnetic Field | Temperature | Ultrasound | Light |
| Limitations |
|
|
Low Penetration depth.(since only applied topically so far) |
|
|
| Disadvantages |
|
|
|||
| Advantages |
|
|
Smart NP along with the application of physical energy comprise a new arena of novel nanotechnology that enables electrical engineers, physicists, chemists, biologists and pharmaceutical engineers to explore and develop interdiciplinary research. For instance, physicists and engineers can come up with new devices by understanding the concept of “poration” using different modalities. Although, there are several factors that will affect the efficiency of drug delivery while using such physical energies, the key is the possible optimization techniques for individual “poration” methods. The aim of this review is to cover comprehensive up-to-date information about the usage of various such types of physical energy applications called “poration”
Though many different types of physical energy have been used for poration and some for concentration, activation is the final step that completes TDDS after the drug has reached its target. Photodynamic therapy (PDT) [52, 53], is an emerging therapeutic modality. It is a two-component system that uses light to activate the photosensitizer (PS) drug after delivery by physical enhancement has been applied and reaches its target. Special emphasis will be given in the review to applications in the PDT arena (when relevant) partly due to the experience of the authors’ laboratory in PDT; nevertheless other types of drugs, DNA vectors and transfection procedures will also be covered in this review. In a similar manner, photothermal therapy [54] is also a light-mediated activation process, and will be covered as well. Cancer therapy will be emphasized due to the prevailing demand in this area of biomedicine. The efficacy of each physical energy system and the limitations and possible optimizations techniques will be given special importance in the review. Theranostics [55, 56], an emerging multifunctional modality, will be discussed in context with the various techniques of biophysical action.
Photodynamic Therapy
PDT involves the use of photosensitizers (PS) in combination with visible light of the correct wavelength to excite the PS. In the presence of molecular ground (triplet) state oxygen (3O2), the excited state PS transfers energy or electrons to produce reactive oxygen species (ROS) that are able to kill cells. PDT has mostly been developed as a cancer therapy [57], but recently has been proposed as an antimicrobial therapy.
The PS molecule has a stable electronic configuration in the singlet state with the most energetic electrons in the highest occupied molecular orbital (HOMO) [58]. Following absorption of a photon of light of the specific wavelength according to its absorption spectrum (Figure 2 shows a Jablonski diagram illustrating the process), an electron in the HOMO is excited to the lowest unoccupied molecular orbital (LUMO), causing the PS to reach the unstable and short-lived excited singlet state. In this state, several processes may rapidly occur such as fluorescence and internal conversion to heat, but the most critical of these to PDT, is the reversal of the spin of the excited electron (known as intersystem crossing) to the triplet state of the PS. This excited triplet state is less energetic than the excited singlet state, but has a considerably longer lifetime, as the excited electron, now with a spin parallel to its former paired electron, may not immediately fall back down (as it would then have identical quantum numbers to that of its paired electron, thus violating the Pauli Exclusion Principle). This much longer lifetime (many μs as compared to a few ns) means the triplet PS can survive long enough to carry out chemical reactions which would not have been possible with the excited singlet PS. Dyes without a significant triplet yield may be highly absorbent or fluorescent but are not good PS.
Figure 2. Jablonski Diagram.
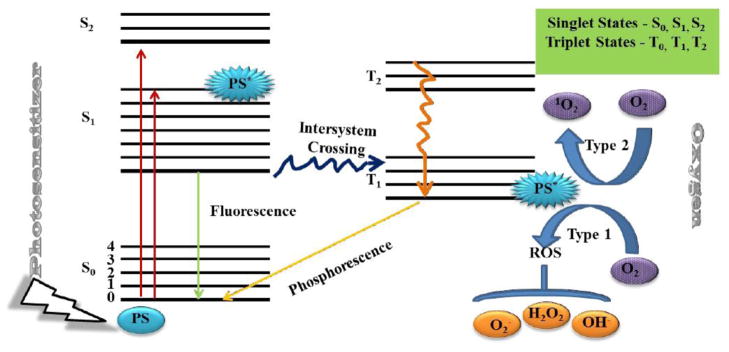
In the presence of molecular ground (triplet) state oxygen (3O2), the excited state PS transfers energy or electrons to produce reactive oxygen species (ROS).
The photochemical reactions of the triplet state can be divided into two different pathways, either the Type I mechanism involving e− or hydrogen atom transfer from one molecule to another, or the Type II mechanisms involving energy transfer to molecular oxygen. It should be noted that both these mechanisms can occur at the same time but the relative proportions may depend on the PS structure and also on the microenvironment.
The type I pathway can involve an electron-transfer reaction from the PS to O2 in the triplet state which results in the formation of toxic oxygen species such as O2•− that can further transfer to form ROS, such as H2O2 and •OH, which are formed by the Fenton reaction in the presence of divalent metal ions such as Fe2+ [59] (Eq. 7–9). Another possible mechanism has been proposed that may operate in cases where the triplet state PS is a good e− donor. Here H2O2 (formed from O2•−) can undergo a one e− reduction to form •OH + −OH (the redox potential is only +0.32V, see Table 1).
The two most prevalent damaging ROS (•OH and 1O2) are able to react with many biomolecules in cells.. The exact targets and reaction mechanisms involved depend on the following considerations. Firstly the localization of the PS generation is critical because most of the ROS are highly reactive and cannot travel far from their site of production before disappearing (these distances are the order of nm to μm). Secondly the relative abundance of the target biomolecule is important. Thirdly we have the question of whether Type 1 or Type 2 mechanisms produce the ROS in question.
PS are usually organic delocalized aromatic molecules consisting of a central chromophore with auxiliary branches (auxochromes) that are responsible for further electron delocalization of the PS, thus altering the absorption spectra of the PS. Due to extensive electron delocalization, PS tend to be deeply colored. This means that the energy required to excite the e− in the HOMO to the LUMO is low compared with less delocalized molecules and therefore, the absorption bands are in the longer wavelength (red) spectral region and are large, reflecting the high probability of excitation.
Magnetic Drug and Gene Targeting: Magnetoporation
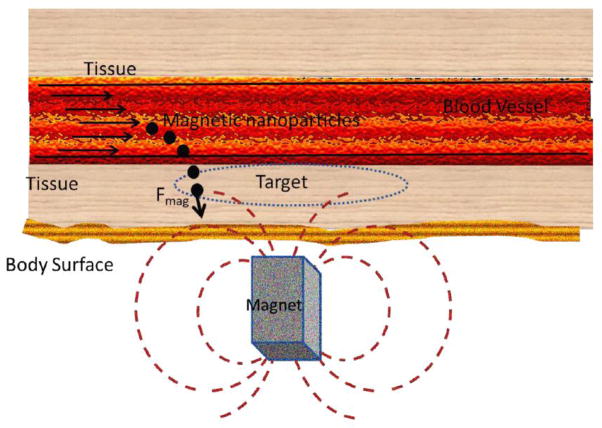
Magnetic fields have promising applications in drug delivery due to their relatively non-invasive nature compared to their counterpart of electrical fields. Magnetic energy is an important technique for delivering drugs to specific locations within the body [60]; this phenomenon of application of magnetic field for drug delivery has been called magnetic drug targeting (MDT) [61, 62]. In principle, MDT consists of the following steps (Figure 3) [63]: 1) coupling the drug to the magnetic NP (MNP); 2) applying an external magnetic field to attract the MNP to the desired location such as a tumor; and 3) the release of the drugs from the NP under the influence of external alternating magnetic field once it reaches the target. Furthermore applied magnetic fields can also be used to release drugs from nanocarriers such as liposomes or nanoemulsions. These nanoparticles can contain superparamagnetic iron oxide nanoparticles (SPION) that heat up to temperatures of the order of 45C under the influence of alternating magnetic fields [64].
Figure 3. Schematic representation of a magnetic nanoparticle-based drug delivery system.
Steps for magnetic nanoparticle-based drug delivery are; coupling the drug to the magnetic NP (MNP), applying an external magnetic field to attract the MNP to the desired location such as a tumor, and the release of the drugs from the NP under the influence of external alternating magnetic field once it reaches the target.
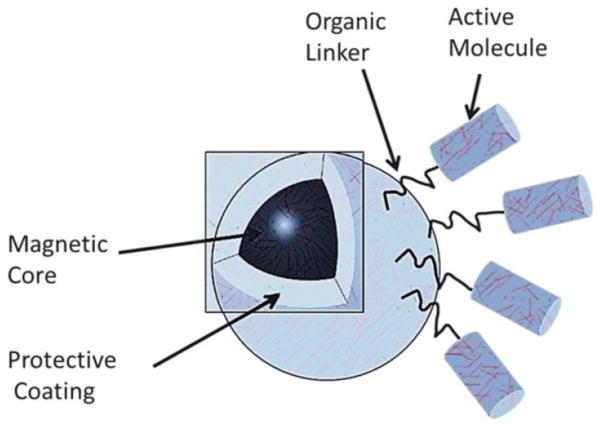
MNP have been tested pre-clinically or clinically as targeted drug and gene delivery agents and also to enhance diagnostic imaging such as magnetic resonance imaging (MRI) [4]. The primary attractive features of MNP for drug delivery include their simplicity of synthesis, ability to be controlled remotely via magnetic fields, the fact that magnetic fields easily penetrate through biological tissue and their biocompatibility and low toxicity [65]. One of the unique properties of MNP, exclusively available on the nanoscale level, is the switchable magnetic properties of superparamagnetic NP, which may pave the way for applications in the biomedical field which include advanced medical imaging and cell tracking. The primary step in MDT is that therapeutic agents are attached to or encapsulated within these MNP which are exclusively used as magnetic delivery vehicles. These MNP can be constructed from magnetic cores with a polymer or metal coating that can be functionalized (Figure 4). The polymer (that can be modified) acts as the most important material for modification of MNP for drug delivery. Sometimes the units may consist of porous polymers that contain MNP precipitated within the pores. Through functionalization of the polymer or metal coating it is possible to attach, for example, cytotoxic drugs for chemotherapy or DNA for gene delivery [66]. Different kinds of functionalization have been described in detail by Chomoucka et al. [67]. Once functionalized, the MNP/therapeutic agent complexes are injected into the bloodstream, often using a catheter to position the injection site near to the target. When solid (rare-earth) external magnets, typically with high-field and high-gradient, are focused over the target site, the magnetic fields drive the MNP to be accumulated at the target site. This technique is advantageous over simple direct injection of the drug into the diseased area as the natural route of drug absorption into the tissue is via the capillaries rather than a direct bolus of drug into the tissue that may not be distributed into all the cells. Thus, trajectories of such MNP are almost fully controlled by the externally applied magnetic field though other smaller forces may also coexist. While this may be effective for targets close to the body surface, due to the fact that the magnetic field strength attenuates rapidly as a function of distance, deeper areas within the body become more difficult to be targeted [66]. Sometimes complicated trajectories are possible in the presence of an applied field; drugs attached to MNP can be slowed down by drag or even captured by other cells that are non-targeted. The drag force experienced by the MNP is mostly due to the blood flow inside the blood vessels. In order to effectively overcome the drag, the magnetic force due to the external field must be greater than the drag force [68].
Figure 4. A typical magnetic nanoparticle.
Magnetic nanoparticle consists of a magnetic core, a protective coating, an organic linker and an active molecule
Where, η is the viscosity of blood, rh is the hydrodynamic radius of the particle and v is the velocity of the particles. It is important to note that the hydrodynamic radius of the particles could be much larger than the magnetic radius of the particles, this is especially the case if the particles are coated with polymers and proteins (typical values: rm = 5nm, rh > 20 nm) [4].
In general, the force experienced by the material (cells/physical body) when placed in an external magnetic field is given by [68]:
Where, V is the volume of the material, μ0 = 4π 10−7 N/ A2 is the magnetic permeability of vacuum, M is the magnetization (magnetic dipole moment per unit volume) and H is the applied (auxiliary) magnetic field. The magnetization of the cells/NP can be taken approximately proportional to the applied magnetic field up to a certain value called the saturation magnetization Msat. Beyond this level all the constituent dipoles are aligned with the field and no further increase in magnetization is possible. It is critical that these conditions have to be clearly taken into account during drug delivery; be it the external magnetic field or the particle that carries the magnetic field, effective delivery may be successfully accomplished only when a comprehensive understanding of the behavior of fields is considered.
Recently multi wall carbon nanotubes (MWCNT) have been used as magnetically driven nanotools for effective cell transfection without noticeable cell damage [69]. The paramagnetic MWCNT interact with cells when exposed to magnetic fields, driving cell migration toward the magnetic source. Decreased viability of the tumor cells caused by cell membrane damage accompanied by cell component leakage paves way for the possibility of an image-guided in situ ablation of tumors. An increased uptake of cytotoxic agents and magneto-cell lysis producing mechanical tumor ablation is speculated. For instance, the effect of TiO2 and Fe-doped TiO2 MNP on the activity of HL60 leukemic cells was studied by observing the influence on the activity of cells exposed to light compared to un-exposure [70]. It was observed that the PS (TiO2) completely eliminated tumor cells using illumination under an applied magnetic field..
When using magnetic fields for gene transfection the phenomenon is called magnetofection. For magnetofection to take place in vitro, a high-field, high-gradient magnetic force is focused onto a multi-well culture plate in which the cells are growing [66]. Next, the gene/DNA is attached to magnetic NP and the external magnetic field is turned on; the external field apart from controlling trajectory of the MNP also enhances sedimentation rates, particle internalization and gene expression. Although the mechanism of increased internalization is not completely understood it is hypothesized that the oscillating fields introduce extra energy to the system which produces a non-linear motion of the particles as they move along the field gradient that may aid cell and tissue penetration. For in vitro controlled transfection, three important points of magnetofection needs to be considered, they are: 1) very low vector dose; 2) reduced incubation time for achieving high magnetofection/transduction efficiency; and 3) the possibility of gene delivery to otherwise non-permissive cells (cells that do not easily internalize DNA material). It is presently uncertain if this magnetofection technique could be usefully applied in vivo.
Non-viral gene vectors are considered safer than viruses, but owing to their inherent low efficiencies, limitations apply to their utility in gene therapy. Therefore, gene vectors with superparamagnetic NP in conjunction with TDDS using magnetic fields have been explored [71]. The use of super paramagnetic iron oxide nanoparticles (SPION) under oscillating magnetic fields (OMF) have been shown to improve diffusive transport of drugs across biological barriers by increasing the local temperature by inductive heating [72]. SPION often tend to remain in circulation unaffected by the liver or the immune system. The two functionalities associated with SPION for drug delivery are: 1) increased delivery into cells and tissues due to heating and motion induced in the SPION by the oscillating field that affect the potential for MNP to be used as targeted drug delivery agents under low magnetic field strengths; and 2) activation for achieving triggered release of drug attached to the surfaces of the particles using OMF with minimal damage caused due to heating of the surrounding tissues. In essence, such biocompatible magnetic field conditions avoid significant disruption that can be caused by the use of synthetic biopolymers-based NP for drug or gene delivery [65]. Combined thermosensitive and magnetic NP have been utilized in controlled TDDS with added advantages [73]. In superparamagnetism thermal fluctuations take place such that they can spontaneously demagnetize a NP that had previously been magnetically saturated leaving these particles with no hysteresis [67].
Multi-functional SPION consisting of tetra-ethyl-ortho-silicate (TEOS) encapsulating monodisperse Fe3O4 NP (which serve as a core) and further grafted by poly (N-isopropylacrylamide) (PNIPAM) are of special significance. These SPION could be introduced to the desired location by external magnetic field by controlling the magnetite core [73]. There is tremendous potential to further explore MNP as facilitators of drug delivery provided all factors that affect its efficiency are controlled.
Parameters that affect Magnetic Drug Delivery efficiency
In MDT the magnetic field can be modulated externally in order to control the drug-release with the characteristics of driven magnetic accuracy, targeting, and high drug-carrying capacity cells. This property of MDT can be used to lower toxic effects and to enhance the therapeutic effect. The efficiency of the MDT for targeted drug delivery depends on at least a dozen of different factors [4] that includes: 1) the size of the MNP; 2) magnetic property of the MNP; 3) the biocompatibility of MNP; 4) physicochemical properties of the drug-loaded in the MNP; 5) applied magnetic field strength and geometry of the system; 6) penetration depth of the target tissue; 7) resistivity caused by blood flow; 8) vascular supply; 9) NP aggregation due to large surface/volume ratio; 10) the short half-life of the particles in blood circulation; 11) the low efficiency of the intracellular uptake of NP; and 12) nonspecific targeting. Although small MNP have much better drug delivery properties than larger ones due to their ability to pass through biological barriers, their small size may make them insufficiently responsive to the applied magnetic field.
Limitations and possible solutions
There are several limitations that hinder full clinical therapeutic usage of MDT. The limitations include inappropriate gradient modulations by applied magnetic fields which causes non-specific targeting [4], the potential for embolization (blocking blood flow) when the particles accumulate within the bloodstream, and the development of cytotoxicity as an unwelcome side-effect with the increased concentration of MNP in the liver. However, some of these problems may be potentially turned to advantage and could increase targeting, for example, in the case of tumors in the liver the blood supply to the tumor mass gets blocked [63]. Some of the other problems of efficient drug delivery can be avoided if SPION are used as MNP as described earlier, however, further investigations need to be carried out for a comprehensive understanding of the field.
Electric Field Drug and Gene Delivery: Electroporation and Iontophoresis
Depending on the size and the shape of the cell, when a pulse of intense external electric field is applied, polarization [74, 75] takes place; the anode facing side becomes hyperpolarized and the cathode facing side becomes depolarized [76]. This leads to a phenomenon called “cell poration” or “cell fusion” that involves the fundamental behavior of cell membranes defined by the term “electroporation” (EP). EP can introduce dipolar molecules into the cell by varying membrane permeability of the cell or tissue [75]. EP is inexpensive and simple to perform in the laboratory, and therefore has been widely used in biomedical research. Since traditional methods of increasing cell uptake can involve biological and chemical side effects due to over dosage, EP can be used as enhancer for TTDS with higher spatial resolution. Though the fundamental principle by which the electric field induces cell poration or cell fusion is not yet thoroughly understood, it is widely assumed that electrical breakdown of the cell membrane could be a key explanation of the phenomenon. Assuming a spherical cell the cell membrane potential is given by
Where θ is the angle between the electric field and the normal vector of the cell membrane, r is the radius of the cell when placed in an external electric field of strength E, Vmax is the potential maximum near the cell poles where θ = 0° and 180°. For very large E, Vmax reaches a critical value V (on the order of 1 V), the field within the cell membrane is high enough to cause an electrical breakdown of the lipid bilayer [77]. Membrane pores could be created as a result of such breakdown [76] as shown in Figure 5. Apart from the primary electrical breakdown of the membrane a second breakdown mechanism of the cell membrane is possible caused by a phenomena called “electro-mechanical coupling effect” [78].
Figure 5. Electroporation phenomenon.
Electric field disrupts cell membrane allowing drug/genes poration. The status of drug/genes before pulse, during the electric field (E-field) and after pulse is shown.
EP has a wide range of applications, including drug delivery [79–83] and gene transfection (electrofection) [84–87]. EP has been incorporated for drug and gene delivery in various areas of the human body that include lung [88–90], skin [91–93], cornea [94, 95], spinal cord [96, 97], heart [98, 99], brain [100–102] and dental tissues [103, 104]. Owing to its high gene transfection efficiency, safety, low toxicity and reproducibility, EP also has the potential for introducing genes into mammalian cells [105]. DNA, (pSV2-CAT gene) when introduced into a mammalian cell in the presence of electric field in vivo the transfection efficiency was found to multiply by 100–1000 times compared to the one without field [106].
Similarly, experiments have demonstrated that when EP was introduced in PDT malignant cells were eradicated in a much shorter time compared to PDT without field. To demonstrate such capabilities of EP, in a study, a Chinese hamster fibroblast cell line (DC-3F) was targeted using two different PS; chlorin(e6) (Ce6) and aluminum phthalocyanine tetrasulfonate (ALPeS4)] with different dose levels followed by EP with 8 electric pulses at 1200 V/cm intensity, 0.1 ms duration and 1 Hz frequency. After 20 min of incubation the cells were irradiated using a visible light source (660 nm). The malignant cells were found to be dead in a significantly shorter time span proving enhanced efficacy contributed by EP [107]. Another group applied EP on human histiocytic lymphoma U937 and Saccharomyces cerevisiae cells incorporating some of the reliable PDT agents, such as, thiopyronine, protoporphyrin, zinc phthalocyanine, copper phthalocyanine sulfonate, adriamycin and daunomysin and achieved remarkable results from their experiments. The dye diffused rapidly into the porated cells and the membranes resealed over a period of 6–10 min. Faster destruction of all resealed cells compared to control cells (without EP treatment) has been observed in these experiments demonstrating the advantages of the applied electric field.
Field strength is an important parameter in EP that needs to be controlled for better efficacy of drug/gene delivery. Recently high-intensity direct current (DC) electric field has been applied for electroporation in cancer therapy. In an experiment, macromolecular chromophore dextrans (cibacron-dextran) acting as PS were transported into cancer cells(U-937 cells) by electroporation of their membrane using short pulses in between two electrodes dipped in culture medium. After electroporation by electric pulses the penetration of cibacron blue dextran and the resealing of membrane pores, during 20 min in the dark, took place and the following irradiation by 66 J/cm2 light produced a distinct PDT effect on cancer cells [108]. However, in the control measurements, PDT alone using cibacron-dextran produced negligible effect. Likewise, Xu et al [109] observed that a combination of EP and TiO2-conjugated NP with monoclonal antibody showed significant photokilling after photoexcitation. While EP accelerated the delivery speed of the TiO2 NP to cancer cells, use of monoclonal antibody increased the photokilling selectivity of TiO2 NP to cancer cells with less damage to healthy cells.
There are several ongoing clinical trials that are associated with EP-mediated gene delivery [110]. However, efficient use of EP for drug/gene delivery is still in a preliminary stage due to possible side-effects of electric fields and currents, and further research is underway.
Parameters that affect electric field drug/gene delivery efficiency
Gene/drug delivery efficacy during EP relies on cell membrane permeability and electrophoresis [111]. Formation of pores such that membrane reseals when field is turned off is a prerequisite for drug/gene entry. The size of pores and number of pores created are important parameters to be considered for drug/gene delivery efficacy. The pore size is directly proportional to the applied electrophoretic force. The pore size is also affected by the presence of anionic lipids during EP [112]. Peng et al investigated the number of pores created and the dependence on the interaction of the DNA plasmid fragments with the electroporated membranes [110]. It was found that a relatively low number of electropores was necessary to avoid toxicity and that polymeric forms of the DNA vectors enhanced the transgene expression to give efficient delivery. Electrofection efficiency depends on a number of optimizing parameters including: 1) electric pulse parameters; 2) electrode design; 3) DNA buffer composition; and, 4) pretreatments of the target tissue. The overall goal is to produce the optimum number of temporary pores to allow DNA penetration into the cell without allowing the pores to remain open long enough to adversely affect critical cellular physiology leading to cell death.
Limitations and possible solutions
Strong electrical fields can produce permanent cell permeabilization and thereby consequent loss of cell homeostasis eventually lead to cell death, a process known as irreversible EP [113]. Intense electric field impulses produce a mechanical shock (similar to forced oscillation using ultrasound) disrupting the structure of the membrane locally. For the thickness of the membrane (6 × 10−7 cm) when the membrane potential is on the order of 1 V, the electric field within the cell membrane will exceed 10(6) V/cm and the following two processes can take place [113]: 1) compression of the lipid bilayer normal to the surface leading to electrical breakdown of the membrane; 2) the charged groups of the membrane are forced to move in the direction of the field leading to mechanical stress in the membrane eventually disrupting it. In order to avoid breakdown it would be more effective to apply an oscillating electric field to induce poration than to apply a DC field. EP has been widely applied for gene delivery in experimental animals to improve cancer treatment, vaccination and wound healing [114–116] and has been tested in a clinical trial of interleukin-12 plasmid delivery for melanoma [117].
Iontophoresis
Iontophoresis which also uses an electric field is an emerging modality of drug delivery through the skin by using a potential producing only a gentle electrical current [118, 119]. Unlike formation of pores in EP, in iontophoresis the ionized form of the drug penetrates through sweat ducts, sebaceous glands, hair follicles and imperfection sites in the skin layer. The iontophoresis unit carries an anode drug reservoir that holds and releases the drug and a cathode that collects the opposite ions when a potential is applied across the two polarities as shown in Figure 6 [120, 121]. When a potential difference is applied the cationic molecules that are placed under the cathode are repelled due to the same charge and are transported through the dermal layers into deeper structures underlying the skin and absorbed into the bloodstream. Similarly, anions originating from tissues below the surface of the skin are extracted into the counter electrode which is the “anode”. Iontophoresis has been proposed for numerous uses, including the delivery of local anesthetics before skin procedures in children thus avoiding injections, local drug delivery for agents such as non-steroidal anti-inflammatory drugs (NSAID) or corticosteroids for musculoskeletal inflammatory disorders [122]. Singh et al. [123] investigated modulated iontophoresis that involves microneedles in transdermal permeation of ropinirole hydrochloride in Parkinson’s disease therapy and succeeded in delivering therapeutic amounts over a suitable patch area and time.
Figure 6. Iontophoresis method.
The iontophoresis unit carries an anode drug reservoir that holds and releases the drug and a cathode that collects the opposite ions when a potential is applied across the two polarities
In PDT for skin cancer (actinic keratosis (AK)) [124], when traditional method is used, the PS 5-aminolevulinic acid (ALA) is absorbed percutaneously through passive diffusion which requires 4–6 h waiting time before performing the procedure. In order to find an alternative method to accelerate the PS delivery, ALA was iontophoresed using direct-current pulsed iontophoresis. Protoporphyrin IX (PpIX) production following iontophoresis were comparable to the conventional occlusive dressing technique (ODT) and biopsies from the treated lesion demonstrated disappearance of AK.
Parameters affecting the efficiency of Iontophoresis
Several parameters affect the transdermal absorption of drugs through iontophoresis including: 1) concentration of drug; 2) polarity and ionizable property of drugs; 3) pH of donor solution; 4) co-ions availability; 5) ionic strength of solution; and 6) electrode polarity.
Light Induced Gene and Drug Delivery: Optoporation
Owing to the ready availability of light sources and the good biocompatibility of light in biological systems, light has been evaluated for its use as an effective drug delivery enhancer. When light is used as an enhancer in drug/gene delivery then the phenomenon is called as “optoporation”. From fundamental experimental research to clinical practice, successful optoporation techniques have been investigated. Since light spans a wide electromagnetic spectrum (Figure 7) and the fact that some of the wavelengths can penetrate deep into the body, optoporation takes advantage of this special property. Optoporation has the potential to facilitate the permeation of topically applied drugs into deep tissue layers, mainly by means of light or lasers (light amplification by stimulated emission of radiation). Particularly in the skin, ablative fractional resurfacing procedures using CO2 (10,600 nm) and erbium-doped: yttrium-aluminum-garnet (Er: YAG – 2,940 nm) lasers have gained popularity due to their capability to disrupt the major cutaneous barrier - the stratum corneum - by creating vertical micro channels (Figure 8) which serve as pathways for the deep penetration of drug into skin layers [63].
Figure 7. Light penetration as a function of wavelength.
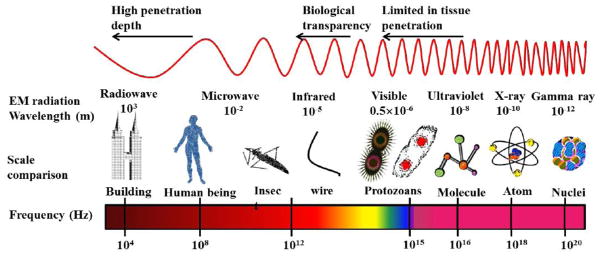
There exists a ‘window of transparency’ for human tissues in the far-red spectrum light is still limited in its penetration depth, or how far in can penetrate into the human body.
Figure 8. Optoporation.

Schematic representation of micro-channels created by ablative fractional lasers in skin.
The light beam disrupts the major skin barrier, the stratum corneum (SC), and reaches deeper layers (dermis).
The ablative fractional laser technique (Fraxel) was found to be an effective clinical procedure to enhance targeted drug delivery. Using this technique Togsverd-Bo et al. [125] conducted a randomized clinical trial comparing the fractional laser-assisted PDT with ordinary PDT for actinic keratosis (AK) in a field-cancerized face and scalp using CO2 Fraxel laser on fifteen patients. Patients had two symmetrical sides treated one with ablative fractional laser prior to the PDT and the other with PDT only. When the drug methyl aminolevulinate (MAL), a precursor of PpIX, was topically occluded followed by PDT illumination with red light, a better therapeutic effect on the Fraxel-treated site compared with the non-treated site was observed. During such ablative techniques it is necessary to understand that the depth of drug entry into the skin depends on the energy of the light transmitted and also the pulse duration. In order to understand depth as a function of the energy of the light a study was conducted on ablative fraction laser technique [126]. In this study MAL was applied on Yorkshire swine normal skin treated with CO2 laser with pulse durations of 3 ms and delivering energy of 37, 190, and 380 mJ per laser channel. This procedure created three different hole-depths, which reached down to 2,100 μm into deep dermis/subcutaneous layer. It was found that different intradermal laser channel depths enhance the drug permeation throughout the skin layers but it did not affect the PpIX accumulation.
CO2 fractional lasers are now popularly used for such studies owing to their inherent capabilities. In order to achieve similar results, recently YAG laser was also suggested as an alternative method. For instance, using the Er: YAG (2,940 nm) laser in an experimental ex vivo study, Forster et al.[127] observed the penetration enhancement of topical 5-aminolevulinic acid (ALA) 20% - a precursor PpIX, on a porcine skin model. It was observed that the PpIX fluorescence was observed in deeper skin layers compared with non-ablated skin. Lee et al. compared the ability of Er:YAG and CO2 lasers and microdermabrasion in terms of enhancement and control of in vitro skin permeation and deposition of vitamin C [128]. The flux and skin deposition of vitamin C across microdermabrasion-treated skin was found to be almost 20-fold higher than that across intact skin. Among the modalities tested Er:YAG laser demonstrated the greatest enhancement of skin permeation of vitamin C. It was further emphasized that the laser fluence and spot size played important roles in controlling drug absorption.
There are also other kinds of lasers used for optoporation. Using an argon ion laser (488 nm) and phenol red as a light absorbing dye the plasma membrane of Chinese hamster ovary cells was made permeable when the beam was focused [129]. With irradiation with laser, gene transfection has also been studied. Optical transfection is sometimes called “optofection”. Optofection effects were studied using a green fluorescent protein (GFP) encoding plasmid [129]. The optofection rates after laser irradiation were around 30% for younger cells and less than 10% for aged cells.
Limitations of Light
Although optoporation has shown promising results, some reports raise concerns about being used with PDT owing to the phototoxic effects of PDT, such as inflammatory response and exacerbation of pain [130–132] attributed to the use of high PS concentration and high light fluences [133–135]. Another remarkable side effect is prolonged skin pigmentation. A recent study on ex vivo full-thickness porcine skin model has reported damage to the epidermis by thermal effects and other toxic outcomes at higher fluence rates which can lead to ultrastructural changes in epidermis [136]. However, these drawbacks can be avoided by decreasing the amount of light energy delivered to the tissue. There have been a few clinical studies with very small sample size conducted in order to understand such effects. However, controlled clinical studies that include histopathological outcomes need to be conducted in order to establish safe and effective parameters required for this therapy.
Nanobiophotonics is also an emerging field that uses light for molecular diagnostics that involves specific sequence characterization of nucleic acids for gene delivery systems of relevance for therapeutic strategies [137]. Though light can enhance the drug delivery into deeper layers of the tissue by opening microchannels that allows the molecule uptake, further randomized clinical trials are required to ensure standards in optoporation efficacy.
Temperature Induced Drug and Gene Delivery: Thermoporation and Photothermal therapy
Thermoporation (TP) also alternatively called ‘microporation’ makes use of heat to produce tiny openings in the stratum corneum [138]. Heat is generated by an array of metallic filaments held temporarily against the skin. An activation electric pulse induces heating of the filaments. A vaccine or drug is applied over the created pores via an adhesive patch for treatment. In a study Ivanov et al. studied the structural changes in erythrocyte membrane during thermoporation involved in thermohaemolysis [138]. When intense electric fields (1.5kVcm−1 for 5s) were applied on cell suspension medium, heat variations were observed. However, corresponding classical thermal treatments at equivalent temperatures had no effect on the viability of cells [139]. It is commonly assumed that electrical conductivity variations of the intra- and extra-cellular matrix caused by ions and solute transfer across the membrane were shown to be involved in the observed heating. Though thermoporation is a less common technique that is used for poration and concentration of drug or gene, the most popular technique that combines both heat and light for activation is called “photothermal therapy” is rapidly growing recently.
Photothermal Therapy for activation
Photothermal therapy (PTT) is a model with some similarities to PDT. Unlike PDT, where the excited PS releases singlet oxygen or free radicals, PTT takes advantage of the excited PS releasing vibrational energy in the form of heat. PTT uses near infrared radiation (NIR) and many different NP as agents to induce killing of the unwanted cells or tissues. When lasers are used as the activation source several factors need to be considered that for efficacy: 1) tissue absorption coefficient, 2) thermal relaxation time; 3) the sensitization of the tissue; 4) time duration of the laser exposure; and 5) tissue thickness [140]. Photothermal sensitizers such as metallo derivatives of porphyrins and porphyrinoid compounds, azo dyes and triphenylmethane derivatives are used in this case for PTT [140]. Photothermal damage of tissues may be induced by pulsed irradiation of either endogenous chromophores added or dyes. Dyes have the advantage of significant absorbance at wavelengths longer than 600 nm that can penetrate deep into biological tissues. Spatial confinement of the photothermal process depends on the absorption coefficient of the photoexcited chromophore and its thermal relaxation time. In addition, the use of lasers in PTT has shown promising results for cancer therapy, since the start of the decade. In the study conducted by Chen et al., murine mammary tumors were treated using 808nm laser with indocyanine as the PTT agent [141]. Tumor regression was clearly observed when the treatment involved 3–5min time duration with 5–10W power; however the efficacy of the PTT against cancer was of concern. In a clinical study, feasibility and safety of image guided targeted photothermal therapy for localized prostate cancer was tested. PTT was employed to low risk prostrate patients using optical fibres and treated with minimal adverse effects. Successful results from such in vitro and in vivo studies for cancer application through various agents have helped in moving forward with further exploration towards translational studies [142]. PTT has proved effective against breast tumors [143], Lewis lung carcinoma in mice [144], localized prostate cancer [142], murine glioma model [145] and Ehrlich carcinoma tumors [146] and gene therapy. For instance, Au NP covalently linked to a viral vector was used in gene cancer therapy [147]. When gene transfection takes place using thermal vibrations the phenomenon is known as “thermofection”. PTT killing can be broadly classified according to photosensitizer-induced [148] and hyperthermia-induced [149] based on the type of molecule is encapsulated by the NP.
Advancements in synthesis of NP provide a wide choice of materials for encapsulating PTT drugs which include but not limited to gold nanoshell, gold nanocages, gold nano particles, carbon nanotubes, graphene, copper sulfide (CuS) and Single Wall Carbon Nanotube (SWCNT). The unique optical properties of such NP makes them strong optical absorbers suitable for PTT [150]; The NIR spectral range of 750–1300 nm falls in the optical therapeutic window which allows maximum penetration depth into the biological tissue. Among the NP, gold nanorods are widely preferred for PTT due to their antenna-like structure that has two absorption bands due to the surface plasmon resonance oscillation. These physical properties improve the absorption of the light used to excite the nanorods. Gold- and CNT- conjugates show high selectivity towards cancer cells with destructive effects of laser irradiation. One of the upcoming gold nanostructures is the hollow gold nano cages for anticancer applications. They are usually of 30–50 nm in size and highly tunable to the NIR region. Likewise polyethylene glycol (PEGylated) nanogels (Figure 9) containing gold nanoparticels are of great interest [151]
Figure 9. Photothermal therapy.
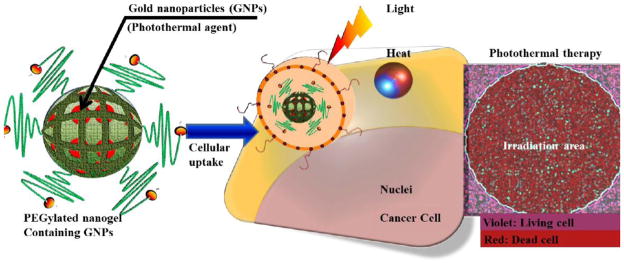
PEGylated nanogels containing gold nanoparticels are uptaken by the cells. Irradiation (both heat and light) kills the cancer cells.
Another carbon nanomaterial known for NIR absorption is single wall carbon nanotubes SWCNT with low cytotoxicity. For instance, Cobalt Molybdenum Catalyst (CoMoCAT) SWCNT [152] with a narrow absorption peak at 980 nm, were conjugated with folate receptor for tumor targeting. Laser irradiation (fluence 300 J/cm2) was directed onto the tumor site 8 days after the injection of folate-SWCNT directly into the center of the tumor (EMT6 cells) in a Balb/c female mouse. The experiment was reported to produce significant tumor destruction.
Though both in vivo and in vitro experimental trials show promising potential for PTT, further research is necessary to improve the efficacy of PTT
Limitations of Thermoporation and Photothermal therapy
Excess heat can produce burns (thermal or photothermal damage) on normal cells [153]. Only applied topically and deeper penetration into tissue is a challenge with this technique. Since the heat filaments are connected electrically some of the limitations of using electric field also apply here.
Acoustic-Mediated Drug Delivery: Sonoporation (Mechanoporation), Phonophoresis, Sonodynamic therapy
Acoustic targeted drug delivery (ATDD) has been explored as a promising method to enhance the delivery of therapeutic agents to the target tissues. The underlying mechanism for ATDD is the use of ultrasound energy mediated transport of molecules into the target tissues. Ultrasound is defined as the transmission of sound pressure waves with a frequency greater than 20,000 Hz, the upper limit of human hearing. The technique of using ultrasound for drug/gene delivery is called “sonoporation”. Ultrasound waves can be refracted or reflected similar to light waves. However, in ultrasound waves the actual movement of molecules with compression or expansion of medium takes place. Thus, ultrasound waves being very physical in nature they can dynamically interact with biomolecules or cells. Another unique property of ultrasound waves that there is relatively little absorption of these waves by body fluids and tissues. Therefore, ultrasound can be used for non-invasive, painless transmission of energy at specific body locations, which is the foundation for ATDD. Thus, ultrasound enhances drug delivery by various mechanisms including induction of tissue hyperthermia [154, 155] and the recently discovered remarkable ability of these waves to produce cavitation activity [156–159]. The exposure of a medium to ultrasound causes formation and/or activity of gas-filled microbubbles called cavitation bubbles [160]. The use of ultrasound as an enhancer is referred to as phonophoresis or sonophoresis [44, 161]. “Sonoporation” can be defined as a “physical” method, in which an ultrasound is applied, in the presence of microbubbles that acts as cavitation nuclei, to porate the plasma membrane in order to allow direct transfer of molecules into the cytoplasm [162] of the cell. Sonoporation, involves ultrasound contrast agents (UCA) [42], to permit the transfer of drug and genes into cells. However, it is a transient and reversible phenomenon that ensures relatively good cell viability. A major challenge to the application of sonoporation in case of gene delivery is to optimize the ultrasound parameters both to obtain high gene transfection and to maintain good cell viability [163]. Another requirement is to avoid premature release of the cargo after ultrasound activation (dose-dumping) to produce predictable controlled release kinetics.
Cavitation bubbles expand and shrink in response to low-and high-pressure portion of the ultrasound wave. If the resultant oscillation in the bubble size is stable (repeatable over many cycles), it is called ‘stable’ or ‘non-inertial’ cavitation. A circulating fluid flow (microstreaming) [164, 165] is created by such oscillations around the bubble with velocities and shear rates proportional to the oscillation amplitude. The associated shearing forces generated at high amplitudes have the capacity to shear open synthetic vesicles such as liposomes [164]. Therefore, ultrasound-mediated delivery of drugs and genes is a complex mechanism with interplay among target tissue, therapeutic agent, nature of ultrasound energy, and characteristics of microbubbles.
While using ultrasound, the microbubble formation reduces the peak negative pressure required for enhanced drug delivery by acting as nuclei for cavitation, thereby decrease the threshold of ultrasound energy required for this phenomenon. The transport of drug is significantly enhanced over diffusion alone by the presence of these oscillating bubbles. Stable oscillating bubbles create convection by evolution of circulating eddies around the oscillating bubble (microstreaming) and produce a force acting on other suspended particles in the vicinity of an oscillating bubble (acoustic pressure). The particle is pushed towards the bubble if it is denser than the surrounding liquid and vice versa [166]. Most of the drug carriers, such as micelles and liposomes, being denser than water are convected towards the bubble, and thus augment the dispersive transport of the drug carrier. The dispersive transport of the drug is further increased if the vesicle is drawn into the microstreaming field around the bubble and can be ruptured by high shear rate, thus releasing the drug. Another key mechanism contributing to ultrasound enhanced drug or gene delivery is related to the stress inflicted by cavitation events on tissue and cells. The ultrasound-induced microbubble cavitation induces pore formation in the cell membranes, aiding the drug/gene deposition. In a recent study Deng et al. [167] demonstrated ultrasound-induced increased transmembrane current as a direct result of membrane resistance due to pore formation. Microbubbles have another unique characteristic of increased adherence to the damaged vascular endothelium. This characteristic enable selectively concentrated delivery of drug or genes bound to albumin-coated microbubbles at the vascular injury site [168].
One of the most promising clinical applications of sonoporation technologies is efficient delivery of genetic material, such as plasmids and antisense oligonucleotides, for gene therapy. The process of gene transfection using ultrasound is known as “sonofection”. The delivery of genetic material to the target tissue is tricky because it has to be tissue specific and at same time it has to be protected from degradation by DNase. Since the ultrasound beam can be focused on a particular tissue, ultrasound-mediated gene delivery holds tremendous potential. The first use of surface ultrasound in targeted DNA delivery was done in 1996, using intravenously delivered microbubbles carrying antisense oligonucleotide [169]. Since then, many studies, both in vitro and in vivo, have described the efficient use of ultrasound-mediated microbubble destruction by cavitation and subsequent cargo release for gene delivery [156, 170–177].
Another potential application of this technology is ultrasound-assisted delivery of large protein molecules, such as regulatory hormones. The delivery of large protein molecules is very complex compared to small molecular weight drugs because proteins are poorly diffusible through solids and gels. Therefore, the lipid bilayer of cell membrane prevents the transport of protein. Current research is primarily focused on transdermal delivery of insulin for diabetes and some efforts for birth control hormones. Recent studies have reported increased permeability of the skin by ultrasound with collapse cavitation as a causative mechanism [178–180]. The ultrasound-induced cavitation events cause opening of reversible channels in the lipid bilayer of stratum corneum and thus easing the transport of proteins into the skin [181, 182]. The ultrasound-induced delivery has been used for other protein, such as thrombolytic enzymes [183–185] and ultrasonic nebulization by the pulmonary route [186–188]. Use of ultrasound for the potentiation of chemotherapeutic agent delivery has been recently explored. Studies have suggested that ultrasound has synergistic effects with drugs [159, 189, 190] due to increased delivery of the drugs to the tissues. Further advancement has been made in ultrasound-induced chemotherapeutic agent delivery by developing molecular vehicles that can deliver the drug locally using ultrasound stimulus at tumor site and thus reducing systemic side effects. Two such carrier molecules for delivery of chemotherapeutic agents are liposomes [191–193] and micelles [194, 195].
Use of ultrasound-assisted targeted drug delivery technology is ever-increasing in clinical medicine and research due to its use in delivery of various therapeutic agents including genetic materials, chemotherapy drugs, and proteins. In future, this technique is believed to further advance with improved efficacy and reduced side effects.
Sonodynamic Therapy
Sonodynamic Therapy (SDT) uses ultrasound and PDT agents in synchronization for activation. SDT is a non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer for cancer therapy [196]. For instance, PPIX produced from 5-aminolevulinic acid (5-ALA)) is reported to be useful with low-intensity but focused ultrasound in deep-seated glioma models [197]. Also, 5-ALA-induced PPIX fluorescence has been used in malignant glioma in order to render more complete resection in surgical operations [198]. Further, various novel PS have been used for SDT such as a homogeneous complex of oligomers of hematoporphyrin, called photofrin II, a gallium porphyrin complex, ATX-70, a hydrophilic chlorin derivative, A7X-S10, and a novel porphyrin derivative devoid of photosensitivity and DCPH-P-Na (I) [199]. The mechanisms of action of SDT are still under investigation but are believed to be based on either the action of PS nanoaggregates as nuclei for formation of cavitation microbubbles that mechanically disrupt the cells, or on the emission of small “flashes” of light caused by collapse of cavitation microbubbles that are absorbed by the PS producing singlet oxygen that kills the cells as in PDT.
The applications and properties of various resonances from magnetic, fluorescence, nuclear and surface plasmon plays a significant role in the enhancement and activation of SDT. [200]. Recently, a triple-functional core-shell structured upconversion luminescent nanoparticles, NaGdF(4):Yb,Er@CaF(2)@SiO(2)-PS (hematoporphyrin and silicon phthalocyanine dihydroxide) which possess a multifunctionality in the fields of PDT, MRI and fluorescence/luminescence imaging was developed to investigated the effect of PDT in vitro in HeLa cells using a fluorescent probe. It has low dark toxicity and could be a promising modality for PDT [201]. Also, another study showed caspase-3 activation in single cells with FRET that induces apoptosis through PDT [202] and complexes like Fe3O4/SiO2 core/shell NP functionalized by phosphorescent iridium have been designed and synthesized that makes it well-suited for phosphorescent labeling and simultaneous singlet oxygen generation to induce apoptosis through PDT [203]. Thus, the effective use of various resonating techniques could provide a promising methodology for enhancing SDT.
SDT opens a new arena of medical-activated sensitizer therapy [204]. SDT makes use of sonosensitizers that are similar to photosensitizers but sensitive to ultrasound instead of light. Recently, Dai et al. applied the use of sonosensitizers in combination with liposomes to target chemotherapy drugs directly to tumor cells. SDT with low-intensity ultrasound combined with a sonosensitizer proved to be a promising approach to cancer therapy [205]. The effects of combined sonodynamic and photodynamic therapies with photolon on a glioma C6 tumor model were studied and found that the maximal tumor necrosis area that underwent sonication followed by PDT at a dose of 100 J/cm² was 100% in rats [206].
Limitations
Being a very active process, the consequences of ultrasound-mediated targeted drug delivery could be detrimental for the tissues [160, 166, 207]. The ultrasonic cavitation bubble formation, a requisite for helping the transport of drugs into the cells and promoting the physical or chemical release of drugs from the carrier, is clearly not a healthy environment for the cells. A certain intensity of cavitational activity is essential for effective delivery of the drug. Thus, intensity of this cavitational activity should be at a level sufficient enough to permeabilize the cells without destroying them or a level that creates adequate microstreaming to break open drug carriers, such as liposomes, without damaging the host cells. However, these shear forces and liquid micro-jets could potentially damage the cell membrane. The essential cellular biochemical processes could be inhibited by free radical formation. On the other hand, microbubbles with stable cavitation can potentially be beneficial for the cells by increasing the convection of nutrients and oxygen [208]. Therefore, there is an urgent need to further understand ultrasound-microbubbles interaction and cavitation physics. Another pressing need is to develop better protected genetic material that is not degraded by the action of enzymes or shear forces before it gets delivered to the target cells. To bring ultrasound-assisted transdermal protein delivery to the clinic, further studies are required to conclusively understand the effects of ultrasound on protein conformation and reveal the underlying mechanism of enhanced transdermal protein delivery.
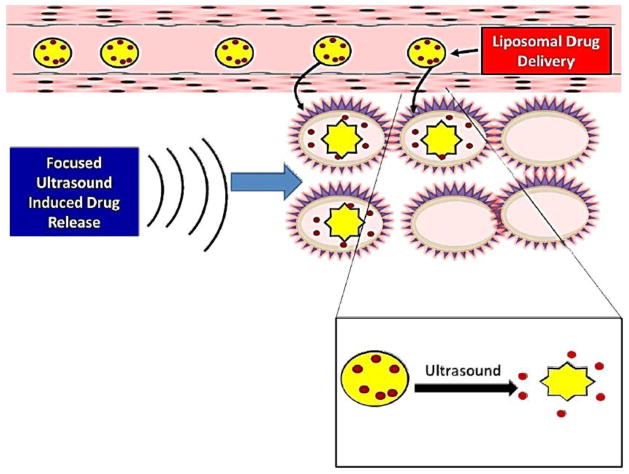
Moreover acoustic cavitation increases cell permeability and facilitates molecular internalization; however, it may also damage or even kill the cells if the pores induced by cavitation are too large such that the cell membrane cannot reseal quickly [162]. The problem is magnified for the effective delivery of large molecules, such as plasmid DNA, which requires that sufficiently large pores are created on the cell surface, but which risks that the disrupted cell membrane will lose vital cytoplasmic compounds, resulting in cell death. Moreover, it appears that the cells return almost immediately to an impermeable state after ultrasound exposure (Figure 10), suggesting that sonoporation is a transient phenomenon, with pore opening lasting on the order of milliseconds to seconds.
Figure 10. Sonoporation.
Liposome bound drug is delivered to desired site. Ultrasound-sensitive liposomes burst open upon exposure to ultrasound leading to effective local delivery of the drug.
Theranostics
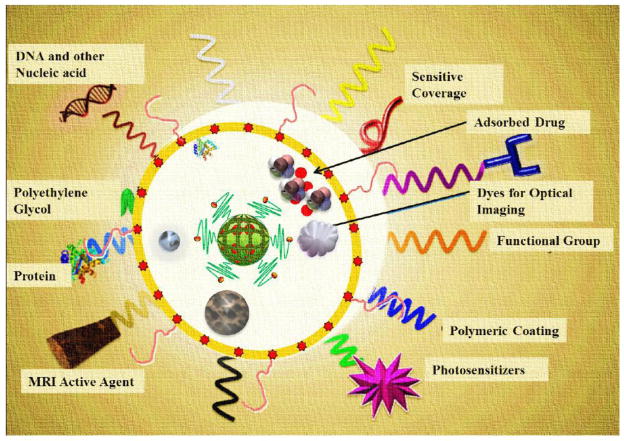
Drug standardization protocols and the prevailing concept of universal or generalized medicine have caused a lot of problems in the past for the drug companies as well as consumers Research and common-sense ideas raise a lot of questions on the credibility of the current medical system as a whole. Questions like “why use common medicines for everyone when individual genotypes are different?” are raised. Scientists and physicians are increasingly orienting their research towards the use of personalized medicine. Theranostics aims to develop such an idea of personalized medicine intended for a specific individual’s illness by understanding the cellular phenotype(s) and heterogeneity of the target [209, 210] in the individual’s body and designing an individualized treatment. Coined by Funkhouser [211], theranostics describes a multimodal functionalized system that functions at the molecular level [212] as shown in Figure 11. Any of the physical methods described above, can be used for porating and concentrating the drugs in therapeutic strategies such as drug, gene, nucleic acid delivery and chemotherapy and activated by hyperthermia/photothermal ablation or PDT which are combined with one or more imaging functionalities for both in vitro and in vivo studies.
Figure 11. Nanoplatforms with theranostic functionalities.
Fe2O3, magnetic NP, photosensitizer, appropriate polymeric coating, surface-charge tuning groups, optical imaging dyes, targeting agents, adsorbed/bonded drugs, sensitive coverage, DNA and other nucleic acids, polyethylene glycol, proteins and MRI active agents.
For instance, recently, PDT has been used as a theranostics modality for various cancers and diseases [213]. With the use of highly tumor-specific albumin NP as efficient therapeutic agents and photodynamic imaging (PDI) reagents in cancer treatment, hydrophobic photosensitizer chlorin(e6) (Ce6) was first chemically conjugated to human serum albumin (HSA)[214]. Next, upon illumination with the appropriate wavelength of light the conjugate produced singlet oxygen and damaged target tumor cells in a cell culture system.
The progress of novel theranostic approaches based on carbon nanostructure is attractive in the field of nanomedicine. The inherent optical properties such as fluorescence make carbon nanostructures useful contrast agents in optical imaging and sensing [215, 216]. Functionalization of SWCNT with different moieties enables targeting and imaging therapy. One of the most interesting examples of such multimodal SWCNT constructs is carrying a fluorescein probe together with the antifungal drug amphotericin B or fluorescein and the antitumor agent methotrexate [217]. Fullerenes have shown potential applications in several different cancer therapeutic approaches such as PDT, photothermal treatment, radiotherapy and chemotherapy which can also serve as novel contrast agents in MRI [218]. The authors performed extensive research on PDT and emphasized on the application of fullerenes in various studies [219–221]. Similarly, graphene monolayers have also aroused widespread interest in the applications of biomedicine [222]. Unique physical and surface features of carbon-based nanomaterials make them a key player in the field of nanomedicine. Besides all its wide application in theranostics, still more research needs to be carried out on these materials to explore the interaction of such multifunctional and combinational therapy in both in vitro and in vivo applications.
Conclusions
The use of physical energy methods for enhancing drug and gene delivery is a good example of the type of multi-disciplinary collaborations that have become increasingly common in the modern era of personalized medicine and nanomedicine. Many of the interactions between physical energy and cells or tissues take place on the nano-scale, and need to be studied and optimized using nanotechnology techniques. A common unifying motif in many (if not most) of these applications is the creation of temporary pores or channels in the plasma membranes of cells or in the stratum corneum of skin. The desired therapeutic molecules (drugs or DNA) pass through these pores or channels, sometimes driven by gradients produced by the applied energy differential. These pores and channels should be self-sealing, in other words they should stay open long enough to allow the therapeutic cargo to pass through, but not stay open long enough to cause cell death or tissue injury. Many of the nanocarriers used for drug and gene delivery are considerably more efficient if they can be disrupted at their desired site of action thus releasing their drug cargo in a predictable controlled manner, rather than relying on the natural release mechanisms that may increase non-targeted drug distribution. Since many physical energy modalities can be spatially focused onto the tumor or other target tissue, they have been extensively used for drug release or drug activation. Magnetic and thermal energy can be further used to concentrate systemically administered drugs or drug carriers in the desired anatomical area by external application of the energy. Potential clinical applications of these technologies will however be complicated by their multidisciplinary nature. One other limitation is that unless and until location of the affected region is known with a high spatial resolution the use of physical energy may not be as applicable. By definition these technologies will be drug-device combinations which historically have been much harder to progress to regulatory approval than drugs or devices alone. With the introduction of nanocarriers into the picture, the path to regulatory approval becomes even more complicated. Nevertheless the relentless growth in biomedical research based on nanomedicine will undoubtedly continue and physical energy modalities are expected to be increasingly involved in these efforts.
Acknowledgments
This work was supported by the US NIH (R01AI050875 to MRH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomed - Nanotechnol. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Poste G, Kirsh R. Site-Specific (Targeted) Drug Delivery in Cancer Therapy. Nat Biotech. 1983;1:869–878. [Google Scholar]
- 3.Jain KK. Drug Delivery Systems. In: Walker JM, editor. Method Mol Biol. Humana Press; Totowa, NJ: 2008. pp. 1–251. [Google Scholar]
- 4.Hofmann-Amtenbrink M, Rechenberg Bv, Hofmann H. Supermagnetic Nanoparticles for Biomedical Application. Transworld Research Network; Trivandrum, Kerala, India: 2009. [Google Scholar]
- 5.Gupta A, Avci P, Sadasivam M, Chandran R, Parizotto N, Vecchio D, de Melo WCMA, Dai T, Chiang LY, Hamblin MR. Shining light on nanotechnology to help repair and regeneration. Biotechnol Adv. doi: 10.1016/j.biotechadv.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X, Chen YJ, Peng DY, Li QL, Wang XS, Wang DL, Chen WD. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloid Surfaces B. 2013;102:391–397. doi: 10.1016/j.colsurfb.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv. 2006;3:139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 9.Kwon GS, Okano T. Polymeric micelles as new drug carriers. Adv Drug Deliv Rev. 1996;21:107–116. [Google Scholar]
- 10.Kim JO, Nukolova NV, Oberoi HS, Kabanov AV, Bronich TK. Block Ionomer Complex Micelles with Cross-Linked Cores for Drug Delivery, Polymer science. Series A. Chemistry, physics. 2009;51:708–718. doi: 10.1134/S0965545X09060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pothayee N, Pothayee N, Jain N, Hu N, Balasubramaniam S, Johnson LM, Davis RM, Sriranganathan N, Riffle JS. Magnetic block ionomer complexes for potential dual imaging and therapeutic agents. Chem Mater. 2012;24:2056–2063. [Google Scholar]
- 12.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliver Rev. 2005;57:2203–2214. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Sun M, Fan A, Wang Z, Zhao Y. Dendrimer-mediated drug delivery to the skin. Soft Matter. 2012;8:4301–4305. [Google Scholar]
- 14.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornara A, Recalenda A, Qin J, Sugunan A, Ye F, Laurent S, Muller RN, Zou J, Usama AR, Toprak MS, Muhammed M. Polymeric/Inorganic multifunctional nanoparticles for simultaneous drug delivery and visualization. MRS Online Proceedings Library. 2010;1257:0. [Google Scholar]
- 16.Zhang C, Li C, Huang S, Hou Z, Cheng Z, Yang P, Peng C, Lin J. Self-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug delivery. Biomaterials. 2010;31:3374–3383. doi: 10.1016/j.biomaterials.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Qi L, Gao X. Emerging application of quantum dots for drug delivery and therapy. Expert Opin Drug Deliv. 2008;5:263–267. doi: 10.1517/17425247.5.3.263. [DOI] [PubMed] [Google Scholar]
- 19.Ozkan M. Quantum dots and other nanoparticles: what can they offer to drug discovery? Drug Discov Today. 2004;9:1065–1071. doi: 10.1016/S1359-6446(04)03291-X. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilder TA, Hill JM. Carbon nanotubes as drug delivery nanocapsules. Curr Appl Phys. 2008;8:258–261. [Google Scholar]
- 22.Slowing II, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. 2007;17:1225–1236. [Google Scholar]
- 23.Wang C, Cheng L, Liu Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials. 2011;32:1110–1120. doi: 10.1016/j.biomaterials.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–1637. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Yu WW, Chang E, Drezek R, Colvin VL. Water-soluble quantum dots for biomedical applications. Biochem Biophys Res Co. 2006;348:781–786. doi: 10.1016/j.bbrc.2006.07.160. [DOI] [PubMed] [Google Scholar]
- 26.De M, Ghosh PS, Rotello VM. Applications of Nanoparticles in Biology. Adv Mater. 2008;20:4225–4241. [Google Scholar]
- 27.Duncan R. Drug-polymer conjugates: potential for improved chemotherapy. Anti-Cancer Drug. 1992;3:175–210. doi: 10.1097/00001813-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Giani G, Fedi S, Barbucci R. Hybrid Magnetic Hydrogel: A potential system for controlled trug delivery by means of alternating magnetic fields. Polymers. 2012;4:1157–1169. [Google Scholar]
- 29.Asmatulu R, Fakhari A, Wamocha HL, Chu HY, Chen YY, Eltabey MM, Hamdeh HH, Ho JC. Drug-carrying magnetic nanocomposite particles for potential drug delivery systems. Journal of Nanotechnology. 2009;2009:1–6. [Google Scholar]
- 30.Kassan DG, Lynch AM, Stiller MJ. Physical enhancement of dermatologic drug delivery: iontophoresis and phonophoresis. J Am Acad Dermatol. 1996;34:657–666. doi: 10.1016/s0190-9622(96)80069-7. [DOI] [PubMed] [Google Scholar]
- 31.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 32.Golzio M, Rols MP, Teissie J. In vitro and in vivo electric field-mediated permeabilization, gene transfer, and expression. Methods. 2004;33:126–135. doi: 10.1016/j.ymeth.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, Wu TC, Pai SI. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–5459. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Kyrtatos PG, Lehtolainen P, Junemann-Ramirez M, Garcia-Prieto A, Price AN, Martin JF, Gadian DG, Pankhurst QA, Lythgoe MF. Magnetic tagging increases delivery of circulating progenitors in vascular injury. JACC Cardiovasc Interv. 2009;2:794–802. doi: 10.1016/j.jcin.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 37.Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO journal. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostrow A. U.S. Patents, editor. Transdermal magnetic delivery sytem and method. US, United States; 2002. pp. 1–11. [Google Scholar]
- 40.Liu D, Wang L, Wang Z, Cuschieri A. Magnetoporation and magnetolysis of cancer cells via carbon nanotubes induced by rotating magnetic fields. Nano Lett. 2012;12:5117–5121. doi: 10.1021/nl301928z. [DOI] [PubMed] [Google Scholar]
- 41.Pan H, Zhou Y, Sieling F, Shi J, Cui J, Deng C. Sonoporation of cells for drug and gene delivery, in: Engineering in medicine and biology society, 2004. IEMBS ‘04. 26th Annual International Conference of the IEEE; 2004. pp. 3531–3534. [DOI] [PubMed] [Google Scholar]
- 42.Karshafian R, Bevan PD, Williams R, Samac S, Burns PN. Sonoporation by Ultrasound-Activated Microbubble Contrast Agents: Effect of Acoustic Exposure Parameters on Cell Membrane Permeability and Cell Viability. Ultrasound Med Biol. 2009;35:847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Yanyan Z, Ballas CB, Rao MP. Towards ultrahigh throughput microinjection: MEMS-based massively-parallelized mechanoporation. Engineering in Medicine and Biology Society (EMBC); 2012 Annual International Conference of the IEEE; 2012. pp. 594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yueh-Ling H. Effects of ultrasound and diclofenac phonophoresis on inflammatory pain relief: Suppression of inducible nitric oxide synthase in arthritic rats. Phys Ther. 2006;86:39–49. doi: 10.1093/ptj/86.1.39. [DOI] [PubMed] [Google Scholar]
- 45.Tyle P, Agrawala P. Drug Delivery by Phonophoresis. Pharm Res. 1989;6:355–361. doi: 10.1023/a:1015967012253. [DOI] [PubMed] [Google Scholar]
- 46.Soman P, Zhang W, Umeda A, Zhang ZJ, Chen S. Femtosecond laser-assisted optoporation for drug and gene delivery into single mammalian cells. J Biomed Nanotechnol. 2011;7:1–8. doi: 10.1166/jbn.2011.1295. [DOI] [PubMed] [Google Scholar]
- 47.Gu L, Mohanty SK. Targeted microinjection into cells and retina using optoporation. J Biomed Opt. 2011;16:128003–128003. doi: 10.1117/1.3662887. [DOI] [PubMed] [Google Scholar]
- 48.Popescu D, Stelian I, Popescu AG, Neacsu N, Flonta ML. The effect of lipid bilayer hydration on transbilayer pores appearance. Romanian J Biophys. 2006;16:39–56. [Google Scholar]
- 49.Dumitru Popescu SI, Popescu Aurel, Movileanu Liviu. Planar Lipid Bilayers (BLMs) and thier applications. In: Tien HT, Ottava-Leitmannova A, editors. Elastic properties of bilayer lipid Membranes and Pore Formation. Elsevier Scince B.V; Netherlands: 2003. p. 174. [Google Scholar]
- 50.Kardos TJ. U.S. Patents, editor. Method of Contactless Magnetic Electroporation. CA, US: 2010. pp. 1–9. [Google Scholar]
- 51.Nishiyama N, Bae Y, Miyata K, Fukushima S, Kataoka K. Smart polymeric micelles for gene and drug delivery. Drug Discov Today: Technologies. 2005;2:21–26. doi: 10.1016/j.ddtec.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamblin MR, Luke Newman E. New trends in photobiology: On the mechanism of the tumour-localising effect in photodynamic therapy. J Photoche Photobio B. 1994;23:3–8. doi: 10.1016/s1011-1344(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, Jain P, El-Sayed I, El-Sayed M. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 55.Kelkar SS, Reineke TM. Theranostics: Combining imaging and therapy. Bioconjug Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Liang XJ. Nano-carbons as theranostics. Theranostics. 2012;2:235–237. doi: 10.7150/thno.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: An update. CA: Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.St Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2 doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glaeser J, Nuss AM, Berghoff BA, Klug G. Singlet oxygen stress in microorganisms. Adv Microb Physiol. 2011;58:141–173. doi: 10.1016/B978-0-12-381043-4.00004-0. [DOI] [PubMed] [Google Scholar]
- 60.Silva AKA, Silva ELd, Oliveira EE, Egito EST, Carrico AS. Polymeric superparamagnetic carriers as potential drug delivery systems. PublICa. 2005;1:8–15. [Google Scholar]
- 61.Lübbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95:200–206. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- 62.Alexiou C, Jurgons R, Schmid RJ, Bergemann C, Henke J, Erhard W, Huenges E, Parak F. Magnetic Drug Targeting—Biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J Drug Targ. 2003;11:139–149. doi: 10.1080/1061186031000150791. [DOI] [PubMed] [Google Scholar]
- 63.Dobson J. Magnetic nanoparticcle for drug delivery. Drug Develop Res. 2006;67:1–2. [Google Scholar]
- 64.Upadhyay D, Scalia S, Vogel R, Wheate N, Salama R, Young P, Traini D, Chrzanowski W. Magnetised thermo responsive lipid vehicles for targeted and controlled lung drug delivery. Pharm Res. 2012;29:2456–2467. doi: 10.1007/s11095-012-0774-9. [DOI] [PubMed] [Google Scholar]
- 65.McGill SL, Cuylear CL, Adolphi NL, Osinski M, Smyth HDC. Magnetically responsive nanoparticles for drug delivery applications using low magnetic field strengths, nanobioscience. IEEE Transactions. 2009;8:33–42. doi: 10.1109/TNB.2009.2017292. [DOI] [PubMed] [Google Scholar]
- 66.McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomed. 2008;3:169–180. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010;62:144–149. doi: 10.1016/j.phrs.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Haverkort JW, Kenjeres S. Optimizing drug delivery using non-uniform magnetic fields: a numerical study. In: Sloten JV, Verdonck P, Nyssen M, Haueisen J, editors. ECIFMBE 2008. Springer-Verlag Berlin Heidelberg; Heidelberg: 2008. pp. 2623–2627. [Google Scholar]
- 69.Graham AP, Duesberg GS, Hoenlein W, Kreupl F, Liebau M, Martin R, Rajasekharan B, Pamler W, Seidel R, Steinhoegl W, Unger E. How do carbon nanotubes fit into the semiconductor roadmap? Appl Phys A. 2005;80:1141–1151. [Google Scholar]
- 70.Xiong YY. Study on Photodynamic Therapy for HL60 cells inactivation by visible light-induced Fe-Doped nano-TiO2 under magnetic field. Adv Mat Res. 2010;136 [Google Scholar]
- 71.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 72.Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005;293:483–496. [Google Scholar]
- 73.Lien YH, Wu TM. The application of thermosensitive magnetic nanoparticles in drug delivery. Adv Mat Res. 2008;47–50:528–531. [Google Scholar]
- 74.Vicente-Manzanares M, Sánchez-Madrid F. Cell polarization: A comparative cell biology and immunological view. Dev Immunol. 2000;7:51–65. doi: 10.1155/2000/70801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huttenlocher A. Cell polarization mechanisms duringdirected cell migration. Nat Cell Biol. 2005;7:336, 337. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- 76.Chang DC. Cell poration and cell fusion using an oscillating electric field. Biophys J. 1989;56:641–652. doi: 10.1016/S0006-3495(89)82711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chernomordik LV, Sukharev SI, Popov SV, Pastushenko VF, Sokirko AV, Abidor IG, Chizmadzhev YA. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochimica et Biophysica Acta. 1987;902:360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- 78.Grodzinsky AJ. Electromechanical and physicochemical properties of connective tissue. Crit Rev Biomed Eng. 1983;9:133–199. [PubMed] [Google Scholar]
- 79.Murthy SN, Zhao YL, Sen A, Hui SW. Cyclodextrin enhanced transdermal delivery of piroxicam and carboxyfluorescein by electroporation. J Control Release. 2004;99:393–402. doi: 10.1016/j.jconrel.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 80.Huang JF, Sung KC, Hu OY, Wang JJ, Lin YH, Fang JY. The effects of electrically assisted methods on transdermal delivery of nalbuphine benzoate and sebacoyl dinalbuphine ester from solutions and hydrogels. Int J Pharm. 2005;297:162–171. doi: 10.1016/j.ijpharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24:1389–1395. doi: 10.1007/s11095-007-9308-2. [DOI] [PubMed] [Google Scholar]
- 82.Wong TW, Zhao YL, Sen A, Hui SW. Pilot study of topical delivery of methotrexate by electroporation. Br J Dermatol. 2005;152:524–530. doi: 10.1111/j.1365-2133.2005.06455.x. [DOI] [PubMed] [Google Scholar]
- 83.Tokudome Y, Sugibayashi K. Mechanism of the synergic effects of calcium chloride and electroporation on the in vitro enhanced skin permeation of drugs. J Control Release. 2004;95:267–274. doi: 10.1016/j.jconrel.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Nishikawa M, Yamauchi M, Morimoto K, Ishid E, Takakura Y, Hashida M. Hepatocyte-targeted in vivo gene expression by intravenous injection of plasmid DNA complexed with synthetic multi-functional gene delivery system. Gene Ther. 2000;7:548–555. doi: 10.1038/sj.gt.3301140. [DOI] [PubMed] [Google Scholar]
- 85.Heller LC, Jaroszeski MJ, Coppola D, McCray AN, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007;14:275–280. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Preat V. Drug and gene delivery using electrotransfer. Ann Pharm Fr. 2001;59:239–244. [PubMed] [Google Scholar]
- 87.Chesnoy S, Huang L. Enhanced cutaneous gene delivery following intradermal injection of naked DNA in a high ionic strength solution. Mol Ther. 2002;5:57–62. doi: 10.1006/mthe.2001.0511. [DOI] [PubMed] [Google Scholar]
- 88.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou R, Norton JE, Dean DA. Electroporation-mediated gene delivery to the lungs. Methods Mol Biol. 2008;423:233–247. doi: 10.1007/978-1-59745-194-9_17. [DOI] [PubMed] [Google Scholar]
- 90.Pringle IA, McLachlan G, Collie DD, Sumner-Jones SG, Lawton AE, Tennant P, Baker A, Gordon C, Blundell R, Varathalingam A, Davies LA, Schmid RA, Cheng SH, Porteous DJ, Gill DR, Hyde SC. Electroporation enhances reporter gene expression following delivery of naked plasmid DNA to the lung. J Gene Med. 2007;9:369–380. doi: 10.1002/jgm.1026. [DOI] [PubMed] [Google Scholar]
- 91.Vanbever R, LeBoulenge E, Preat V. Transdermal delivery of fentanyl by electroporation. I. Influence of electrical factors. Pharm Res. 1996;13:559–565. doi: 10.1023/a:1016093920875. [DOI] [PubMed] [Google Scholar]
- 92.Denet AR, Preat V. Transdermal delivery of timolol by electroporation through human skin. J Control Release. 2003;88:253–262. doi: 10.1016/s0168-3659(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 93.Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci U S A. 1993;90:10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou R, Dean DA. Gene transfer of interleukin 10 to the murine cornea using electroporation. Exp Biol Med (Maywood) 2007;232:362–369. [PMC free article] [PubMed] [Google Scholar]
- 95.Blair-Parks K, Weston BC, Dean DA. High-level gene transfer to the cornea using electroporation. J Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Echeverri K, Tanaka EM. Electroporation as a tool to study in vivo spinal cord regeneration. Dev Dyn. 2003;226:418–425. doi: 10.1002/dvdy.10238. [DOI] [PubMed] [Google Scholar]
- 97.Lin CR, Tai MH, Cheng JT, Chou AK, Wang JJ, Tan PH, Marsala M, Yang LC. Electroporation for direct spinal gene transfer in rats. Neurosci Lett. 2002;317:1–4. doi: 10.1016/s0304-3940(01)02354-0. [DOI] [PubMed] [Google Scholar]
- 98.Hargrave B, Downey H, Strange R, Jr, Murray L, Cinnamond C, Lundberg C, Israel A, Chen YJ, Marshall W, Jr, Heller R. Electroporation-mediated gene transfer directly to the swine heart. Gene Ther. 2013;20:151–157. doi: 10.1038/gt.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ayuni EL, Gazdhar A, Giraud MN, Kadner A, Gugger M, Cecchini M, Caus T, Carrel TP, Schmid RA, Tevaearai HT. In vivo electroporation mediated gene delivery to the beating heart. PLoS One. 2010;5:e14467. doi: 10.1371/journal.pone.0014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellis TL, Garcia PA, Rossmeisl JH, Jr, Henao-Guerrero N, Robertson J, Davalos RV. Nonthermal irreversible electroporation for intracranial surgical applications. Laboratory investigation. J Neurosurg. 2011;114:681–688. doi: 10.3171/2010.5.JNS091448. [DOI] [PubMed] [Google Scholar]
- 101.Garcia PA, Rossmeisl JH, Jr, Neal RE, 2nd, Ellis TL, Olson JD, Henao-Guerrero N, Robertson J, Davalos RV. Intracranial nonthermal irreversible electroporation: in vivo analysis. J Membr Biol. 2010;236:127–136. doi: 10.1007/s00232-010-9284-z. [DOI] [PubMed] [Google Scholar]
- 102.Neal RE, 2nd, Rossmeisl JH, Jr, Garcia PA, Lanz OI, Henao-Guerrero N, Davalos RV. Successful treatment of a large soft tissue sarcoma with irreversible electroporation. J Clin Oncol. 2011;29:e372–377. doi: 10.1200/JCO.2010.33.0902. [DOI] [PubMed] [Google Scholar]
- 103.Yao S, Gutierrez DL, Ring S, Liu D, Wise GE. Electroporation to deliver plasmid DNA into rat dental tissues. J Gene Med. 2010;12:981–989. doi: 10.1002/jgm.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao S, Rana S, Liu D, Wise GE. Electroporation optimization to deliver plasmid DNA into dental follicle cells. Biotechnol J. 2009;4:1488–1496. doi: 10.1002/biot.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng LH, Tsang SY, Tabata Y, Gao JQ. Genetically-manipulated adult stem cells as therapeutic agents and gene delivery vehicle for wound repair and regeneration. J Control Release. 2012;157:321–330. doi: 10.1016/j.jconrel.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 107.Zhou A, Liu M, Baciu C, Glück B, Berg H. Membrane electroporation increases photodynamic effects. J Electroanal Chem. 2000;486:220–224. [Google Scholar]
- 108.Lambreva M, Glück B, Radeva M, Berg H. Electroporation of cell membranes supporting penetration of photodynamic active macromolecular chromophore dextrans. Bioelectrochemistry. 2004;62:95–98. doi: 10.1016/j.bioelechem.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 109.Xu YS Juan, Huang Junjie, Chen Chunmei, Liu Guoyuan, Jiang Yan, Zhao Yaomin, Jiang Zhiyu. Photokilling cancer cells using highly cell-specific antibody TiO2 bioconjugates and electroporation. Bioelectrochemistry. 2007;71:217–222. doi: 10.1016/j.bioelechem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Peng LH, Tsang SY, Tabata Y, Gao JQ. Genetically-manipulated adult stem cells as therapeutic agents and gene delivery vehicle for wound repair and regeneration. J Control Release. 2012;157:321–330. doi: 10.1016/j.jconrel.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 111.Boccaccini AR, Ma R, Li Y, Zhitomirsky I. Electrophoretic deposition of biomaterials. J R Soc Interface. 2010;7 doi: 10.1098/rsif.2010.0156.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sen A, Zhao YL, Hui SW. Saturated anionic phospholipids enhance transdermal transport by electroporation. Biophys J. 2002;83:2064–2073. doi: 10.1016/S0006-3495(02)73967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rubinsky B, Onik G, Mikus P. Irreversible Electroporation: A new ablation modality Clinical implications. Technol Cancer Res T. 2007;6:1–12. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 114.Peng J, Zhao Y, Mai J, Guo W, Xu Y. Short noncoding DNA fragment improve efficiencies of in vivo electroporation-mediated gene transfer. J Gene Med. 2012;14:563–569. doi: 10.1002/jgm.2667. [DOI] [PubMed] [Google Scholar]
- 115.Shoji M, Katayama K, Tachibana M, Tomita K, Sakurai F, Kawabata K, Mizuguchi H. Intramuscular DNA immunization with in vivo electroporation induces antigen-specific cellular and humoral immune responses in both systemic and gut-mucosal compartments. Vaccine. 2012;30:7278–7285. doi: 10.1016/j.vaccine.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 116.Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-β1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db Mice. J Invest Dermatol. 2004;123:791–798. doi: 10.1111/j.0022-202X.2004.23309.x. [DOI] [PubMed] [Google Scholar]
- 117.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther. 2013;10:312–317. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 118.Mannem V, Nanjarapalle C, Stagni G. Iontophoresis of amoxicillin and cefuroxime: rapid therapeutic concentrations in skin. Drug Dev Ind Pharm. 0:1–5. doi: 10.3109/03639045.2012.760579. [DOI] [PubMed] [Google Scholar]
- 119.Malinovskaja K, Laaksonen T, Kontturi K, Hirvonen J. Ion-exchange and iontophoresis-controlled delivery of apomorphine. Eur J Pharm and Biopharm. doi: 10.1016/j.ejpb.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 120.Minkowitz HS. Fentanyl iontophoretic transdermal system: a review. Techniques in Regional Anesthesia and Pain Management. 2007;11:3–8. [Google Scholar]
- 121.Silber S, Richartz BM. Evidence-based application of cardiac magnetic resonance and computed tomography for primary diagnosis of stable coronary artery disease with special attention to disease management program and the German National Medical Care Guidelines. Herz. 2007;32:139–158. doi: 10.1007/s00059-007-2973-4. [DOI] [PubMed] [Google Scholar]
- 122.Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis - An approach for controlled drug delivery: A review. Curr Drug Deliv. 2007;4:1–10. doi: 10.2174/1567201810704010001. [DOI] [PubMed] [Google Scholar]
- 123.Singh ND, Banga AK. Controlled delivery of ropinirole hydrochloride through skin using modulated iontophoresis and microneedles. J Drug Target. 2013 doi: 10.3109/1061186X.2012.757768. [DOI] [PubMed] [Google Scholar]
- 124.Mizutani K, Watanabe D, Akita Y, Akimoto M, Tamada Y, Matsumoto Y. Photodynamic therapy using direct-current pulsed iontophoresis for 5-aminolevulinic acid application, Photodermatol. Photoimmunol Photomed. 2009;25:280–282. doi: 10.1111/j.1600-0781.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 125.Togsverd-Bo K, Haak CS, Thaysen-Petersen D, Wulf HC, Anderson RR, Hædesdal M. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262–1269. doi: 10.1111/j.1365-2133.2012.10893.x. [DOI] [PubMed] [Google Scholar]
- 126.Haak CS, Farinelli WA, Tam J, Doukas AG, Anderson RR, Hædersdal M. Fractional laser-assisted delivery of methyl aminolevulinate: Impact of laser channel depth and incubation time. Lasers Surg Med. 2012;44:787–795. doi: 10.1002/lsm.22102. [DOI] [PubMed] [Google Scholar]
- 127.Forster B, Klein A, Szeimies RM, Maisch T. Penetration enhancement of two topical 5-aminolaevulinic acid formulations for photodynamic therapy by erbium:YAG laser ablation of the stratum corneum: continuous versus fractional ablation. Exp Dermatol. 2010;19:806–812. doi: 10.1111/j.1600-0625.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 128.Lee WR, Shen SC, Wang KH, Hu CH, Fang JY. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J Invest Dermatol. 2003;121:1118–1125. doi: 10.1046/j.1523-1747.2003.12537.x. [DOI] [PubMed] [Google Scholar]
- 129.Schneckenburger H, Hendinger A, Sailer R, Strauss WSL, Schmitt M. Laser-assisted optoporation of single cells. J Biomed Opt. 2002;7:410–416. doi: 10.1117/1.1485758. [DOI] [PubMed] [Google Scholar]
- 130.Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- 131.Fijan S, Honigsmann H, Ortel B. Photodynamic therapy of epithelial skin tumours using delta-aminolaevulinic acid and desferrioxamine. Br J Dermatol. 1995;133:282–288. doi: 10.1111/j.1365-2133.1995.tb02630.x. [DOI] [PubMed] [Google Scholar]
- 132.Grapengiesser S, Ericson M, Gudmundsson F, Larko O, Rosen A, Wennberg AM. Pain caused by photodynamic therapy of skin cancer. Clin Exp Dermatol. 2002;27:493–497. doi: 10.1046/j.1365-2230.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- 133.Ji Y, Powers SK, Brown JT, Walstad D, Maliner L. Toxicity of photodynamic therapy with photofrin in the normal rat brain. Lasers Surg Med. 1994;14:219–228. doi: 10.1002/lsm.1900140304. [DOI] [PubMed] [Google Scholar]
- 134.Chen Q, Chopp M, Madigan L, Dereski MO, Hetzel FW. Damage threshold of normal rat brain in photodynamic therapy. Photochem Photobiol. 1996;64:163–167. doi: 10.1111/j.1751-1097.1996.tb02437.x. [DOI] [PubMed] [Google Scholar]
- 135.Chen Q, Wilson BC, Dereski MO, Patterson MS, Chopp M, Hetzel FW. Effects of light beam size on fluence distribution and depth of necrosis in superficially applied photodynamic therapy of normal rat brain. Photochem Photobiol. 1992;56:379–384. doi: 10.1111/j.1751-1097.1992.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 136.Forster B, Klein A, Szeimies RM, Maisch T. Penetration enhancement of two topical 5-aminolaevulinic acid formulations for photodynamic therapy by erbium:YAG laser ablation of the stratum corneum: continuous versus fractional ablation. Exp Dermatol. 2010;19:806–812. doi: 10.1111/j.1600-0625.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 137.Conde J, Rosa J, Lima JC, Baptista PV. Nanophotonics for molecular diagnostics and therapy applications. Int J Photoenergy. 2012;2012 [Google Scholar]
- 138.Ivanov IT, Todorova R, Zlatanov I. Spectrofluorometric and microcalorimetric study of the thermal poration relevant to the mechanism of thermohaemolysis. Int J Hypertherm. 1999;15:29–43. doi: 10.1080/026567399285837. [DOI] [PubMed] [Google Scholar]
- 139.Stéphane G, Eric F, Jean-Baptiste B, Patrick G. Yeast cell inactivation related to local heating induced by low-intensity electric fields with long-duration pulses. Int J Food Microbiol. 2007;113:180–188. doi: 10.1016/j.ijfoodmicro.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 140.Jori G, Spikes JD. Photothermal sensitizers: possible use in tumor therapy. J Photochem Photobiol B. 1990;6:93–101. doi: 10.1016/1011-1344(90)85078-b. [DOI] [PubMed] [Google Scholar]
- 141.Chen WR, Adams RL, Higgins AK, Bartels KE, Nordquist RE. Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: an in vivo efficacy study. Cancer Lett. 1996;98:169–173. [PubMed] [Google Scholar]
- 142.Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, Wilson BC, Fenster A, Trachtenberg J. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 143.Gu Y, Chen WR, Xia M, Jeong SW, Liu H. Effect of photothermal therapy on breast tumor vascular contents: noninvasive monitoring by near-infrared spectroscopy. Photochem Photobiol. 2005;81:1002–1009. doi: 10.1562/2004-09-05-RA-305. [DOI] [PubMed] [Google Scholar]
- 144.Liu H, Chen D, Tang F, Du G, Li L, Meng X, Liang W, Zhang Y, Teng X, Li Y. Photothermal therapy of Lewis lung carcinoma in mice using gold nanoshells on carboxylated polystyrene spheres. Nanotechnology. 2008;19:455101. doi: 10.1088/0957-4484/19/45/455101. [DOI] [PubMed] [Google Scholar]
- 145.Day ES, Thompson PA, Zhang L, Lewinski NA, Ahmed N, Drezek RA, Blaney SM, West JL. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2011;104:55–63. doi: 10.1007/s11060-010-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khlebtsov BN, Panfilova EV, Terentyuk GS, Maksimova IL, Ivanov AV, Khlebtsov NG. Plasmonic nanopowders for photothermal therapy of tumors. Langmuir. 2012;28:8994–9002. doi: 10.1021/la300022k. [DOI] [PubMed] [Google Scholar]
- 147.Everts M, Saini V, Leddon JL, Kok RJ, Stoff-Khalili M, Preuss MA, Millican CL, Perkins G, Brown JM, Bagaria H, Nikles DE, Johnson DT, Zharov VP, Curiel DT. Covalently linked Au nanoparticles to a viral vector: potential for combined photothermal and gene cancer therapy. Nano Lett. 2006;6:587–591. doi: 10.1021/nl0500555. [DOI] [PubMed] [Google Scholar]
- 148.Jang B, Choi Y. Photosensitizer-conjugated gold nanorods for enzyme-activatable fluorescence imaging and photodynamic therapy. Theranostics. 2012;2:190–197. doi: 10.7150/thno.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choi WI, Kim JY, Kang C, Byeon CC, Kim YH, Tae G. Tumor regression in vivo by photothermal therapy based on gold-nanorod-loaded, functional nanocarriers. ACS Nano. 2011;5:1995–2003. doi: 10.1021/nn103047r. [DOI] [PubMed] [Google Scholar]
- 150.Choi WI, Sahu A, Kim YH, Tae G. Photothermal cancer therapy and imaging based on gold nanorods. Ann Biomed Eng. 2012;40:534–546. doi: 10.1007/s10439-011-0388-0. [DOI] [PubMed] [Google Scholar]
- 151.Oishi M, Tamura A, Nakamura T, Nagasaki Y. A Smart Nanoprobe Based On Fluorescence-Quenching PEGylated Nanogels Containing Gold Nanoparticles for Monitoring the Response to Cancer Therapy. Adv Func Mater. 2009;19:827–834. [Google Scholar]
- 152.Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt. 2009;14:021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhen Fan MS, Singh AK, Senapati D, Khan SA, Ray PC. Multifuntional plasmonic shell magnetic core nanoparticles for targated diagnostics, isolation and photothermal destruction of tumor cells. ACS Nano. 2012;6:1065–1073. doi: 10.1021/nn2045246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gasselhuber A, Dreher MR, Partanen A, Yarmolenko PS, Woods D, Wood BJ, Haemmerich D. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: computational modelling and preliminary in vivovalidation. Int J Hyperthermia. 2012;28:337–348. doi: 10.3109/02656736.2012.677930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rapoport N. Ultrasound-mediated micellar drug delivery. Int J Hyperthermia. 2012;28:374–385. doi: 10.3109/02656736.2012.665567. [DOI] [PubMed] [Google Scholar]
- 156.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 157.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 158.Paliwal S, Mitragotri S. Ultrasound-induced cavitation: applications in drug and gene delivery. Expert Opin Drug Deliv. 2006;3:713–726. doi: 10.1517/17425247.3.6.713. [DOI] [PubMed] [Google Scholar]
- 159.Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- 160.Barnett SB, ter Haar GR, Ziskin MC, Nyborg WL, Maeda K, Bang J. Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol. 1994;20:205–218. doi: 10.1016/0301-5629(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 161.Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Journal of the A Physical Ther Assoc. 2013;75:539–553. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 162.Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J Control Release. 2005;104:213–222. doi: 10.1016/j.jconrel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 163.Karshafian R, Bevan PD, Williams R, Samac S, Burns PN. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol. 2009;35:847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 164.Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423:153–156. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- 165.Nyborg WL. Ultrasonic microstreaming and related phenomena. Br J Cancer Suppl. 1982;5:156–160. [PMC free article] [PubMed] [Google Scholar]
- 166.Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med Biol. 2001;27:301–333. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 167.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30:519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 168.Porter TR, Hiser WL, Kricsfeld D, Deligonul U, Xie F, Iversen P, Radio S. Inhibition of carotid artery neointimal formation with intravenous microbubbles. Ultrasound Med Biol. 2001;27:259–265. doi: 10.1016/s0301-5629(00)00338-0. [DOI] [PubMed] [Google Scholar]
- 169.Porter TR, Iversen PL, Li S, Xie F. Interaction of diagnostic ultrasound with synthetic oligonucleotide-labeled perfluorocarbon-exposed sonicated dextrose albumin microbubbles. J Ultrasound Med. 1996;15:577–584. doi: 10.7863/jum.1996.15.8.577. [DOI] [PubMed] [Google Scholar]
- 170.Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953–959. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 171.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 172.Chen S, Shohet RV, Bekeredjian R, Frenkel P, Grayburn PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol. 2003;42:301–308. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 173.Teupe C, Richter S, Fisslthaler B, Randriamboavonjy V, Ihling C, Fleming I, Busse R, Zeiher AM, Dimmeler S. Vascular gene transfer of phosphomimetic endothelial nitric oxide synthase (S1177D) using ultrasound-enhanced destruction of plasmid-loaded microbubbles improves vasoreactivity. Circulation. 2002;105:1104–1109. doi: 10.1161/hc0902.104720. [DOI] [PubMed] [Google Scholar]
- 174.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 175.Manome Y, Nakamura M, Ohno T, Furuhata H. Ultrasound facilitates transduction of naked plasmid DNA into colon carcinoma cells in vitro and in vivo. Hum Gene Ther. 2000;11:1521–1528. doi: 10.1089/10430340050083252. [DOI] [PubMed] [Google Scholar]
- 176.Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, Mourad PD. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol. 2002;28:389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 177.Cho CW, Liu Y, Cobb WN, Henthorn TK, Lillehei K, Christians U, Ng KY. Ultrasound-induced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharm Res. 2002;19:1123–1129. doi: 10.1023/a:1019837923906. [DOI] [PubMed] [Google Scholar]
- 178.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 179.Mitragotri S, Kost J. Low-frequency sonophoresis: a noninvasive method of drug delivery and diagnostics. Biotechnol Prog. 2000;16:488–492. doi: 10.1021/bp000024+. [DOI] [PubMed] [Google Scholar]
- 180.Tezel A, Sens A, Tuchscherer J, Mitragotri S. Frequency dependence of sonophoresis. Pharm Res. 2001;18:1694–1700. doi: 10.1023/a:1013366328457. [DOI] [PubMed] [Google Scholar]
- 181.Mitragotri S, Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 182.Mitragotri S, Edwards DA, Blankschtein D, Langer R. A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J Pharm Sci. 1995;84:697–706. doi: 10.1002/jps.2600840607. [DOI] [PubMed] [Google Scholar]
- 183.Francis CW, Onundarson PT, Carstensen EL, Blinc A, Meltzer RS, Schwarz K, Marder VJ. Enhancement of fibrinolysis in vitro by ultrasound. J Clin Invest. 1992;90:2063–2068. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suchkova V, Carstensen EL, Francis CW. Ultrasound enhancement of fibrinolysis at frequencies of 27 to 100 kHz. Ultrasound Med Biol. 2002;28:377–382. doi: 10.1016/s0301-5629(01)00522-1. [DOI] [PubMed] [Google Scholar]
- 185.Lauer CG, Burge R, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–1264. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- 186.Kosugi T, Saitoh S, Tamaki N, Hanashiro K, Nakahodo K, Nakamura M. Inhalation of platelet-activating factor increases respiratory resistance in rats: determination by means of an astograph under nonanesthetized conditions. Laryngoscope. 1993;103:428–430. doi: 10.1002/lary.5541030411. [DOI] [PubMed] [Google Scholar]
- 187.Flament MP, Leterme P, Gayot A. Study of the technological parameters of ultrasonic nebulization. Drug Dev Ind Pharm. 2001;27:643–649. doi: 10.1081/ddc-100107320. [DOI] [PubMed] [Google Scholar]
- 188.Flament MP, Leterme P, Gayot A. Influence of the technological parameters of ultrasonic nebulisation on the nebulisation quality of alpha1 protease inhibitor (alpha1PI) Int J Pharm. 1999;189:197–204. doi: 10.1016/s0378-5173(99)00248-3. [DOI] [PubMed] [Google Scholar]
- 189.Singh A, Singh SP, Bamezai R. Modulatory influence of arecoline on the phytic acid-altered hepatic biotransformation system enzymes, sulfhydryl content and lipid peroxidation in a murine system. Cancer Lett. 1997;117:1–6. doi: 10.1016/s0304-3835(97)04733-2. [DOI] [PubMed] [Google Scholar]
- 190.Yu T, Hu K, Bai J, Wang Z. Reversal of adriamycin resistance in ovarian carcinoma cell line by combination of verapamil and low-level ultrasound. Ultrason Sonochem. 2003;10:37–40. doi: 10.1016/s1350-4177(02)00106-2. [DOI] [PubMed] [Google Scholar]
- 191.Bednarski MD, Lee JW, Callstrom MR, Li KC. In vivo target-specific delivery of macromolecular agents with MR-guided focused ultrasound. Radiology. 1997;204:263–268. doi: 10.1148/radiology.204.1.9205257. [DOI] [PubMed] [Google Scholar]
- 192.Vyas SP, Singh R, Asati RK. Liposomally encapsulated diclofenac for sonophoresis induced systemic delivery. J Microencapsul. 1995;12:149–154. doi: 10.3109/02652049509015285. [DOI] [PubMed] [Google Scholar]
- 193.Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33:886–892. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 194.Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically activated chemotherapeutic drug delivery in a rat model. Cancer Res. 2002;62:7280–7283. [PubMed] [Google Scholar]
- 195.Rapoport NY, Christensen DA, Fain HD, Barrows L, Gao Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 196.Shibaguchi H, Tsuru H, Kuroki M. Sonodynamic cancer therapy: a non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011;31:2425–2429. [PubMed] [Google Scholar]
- 197.Ohmura T, Fukushima T, Shibaguchi H, Yoshizawa S, Inoue T, Kuroki M, Sasaki K, Umemura S. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011;31:2527–2533. [PubMed] [Google Scholar]
- 198.Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly. 2008;138:180–185. doi: 10.4414/smw.2008.12077. [DOI] [PubMed] [Google Scholar]
- 199.Kuroki M, Hachimine K, Abe H, Shibaguchi H, Maekawa S, Yanagisawa J, Kinugasa T, Tanaka T, Yamashita Y. Sonodynamic therapy of cancer using novel sonosensitizers. Anticancer Res. 2007;27:3673–3677. [PubMed] [Google Scholar]
- 200.Li L, Zhao JF, Won N, Jin H, Kim S, Chen JY. Quantum Dot - Aluminum phthalocyanine Conjugates perform photodynamic reactions to kill cancer cells via fluorescence resonance energy transfer (FRET) Nanoscale Res Lett. 2012;7:386. doi: 10.1186/1556-276X-7-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qiao XF, Zhou JC, Xiao JW, Wang YF, Sun LD, Yan CH. Triple-functional core-shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitro. Nanoscale. 2012;4:4611–4623. doi: 10.1039/c2nr30938f. [DOI] [PubMed] [Google Scholar]
- 202.Wu Y, Xing D, Luo S, Tang Y, Chen Q. Detection of caspase-3 activation in single cells by fluorescence resonance energy transfer during photodynamic therapy induced apoptosis. Cancer Lett. 2006;235:239–247. doi: 10.1016/j.canlet.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 203.Lai CW, Wang YH, Lai CH, Yang MJ, Chen CY, Chou PT, Chan CS, Chi Y, Chen YC, Hsiao JK. Iridium-Complex-Functionalized Fe3O4/SiO2 Core/Shell Nanoparticles: A Facile Three-in-One System in Magnetic Resonance Imaging, Luminescence Imaging, and Photodynamic Therapy. Small. 2008;4:218–224. doi: 10.1002/smll.200700283. [DOI] [PubMed] [Google Scholar]
- 204.Kenyon JN, Fuller RJ, Lewis TJ. Activated Cancer Therapy Using Light and Ultrasound - A case series of sonodynamic photodynamic therapy in 115 patients over a 4 year period. Curr Drug Ther. 2012 [Google Scholar]
- 205.Dai ZJ, Li S, Gao J, Xu XN, Lu WF, Lin S, Wang XJ. Sonodynamic therapy (SDT): A novel treatment of cancer based on sonosensitizer liposome as a new drug carrier. Med Hypotheses. 2013 doi: 10.1016/j.mehy.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 206.Tserkovsky DA, Alexandrova EN, Chalau VN, Istomin YP. Effects of combined sonodynamic and photodynamic therapies with photolon on a glioma C6 tumor model. Exp Oncol. 2012;34:332–335. [PubMed] [Google Scholar]
- 207.Barnett SB, Rott HD, ter Haar GR, Ziskin MC, Maeda K. The sensitivity of biological tissue to ultrasound. Ultrasound Med Biol. 1997;23:805–812. doi: 10.1016/s0301-5629(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 208.Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98:290–293. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- 209.McCarthy JR. The future of theranostic nanoagents. Nanomedicine (Lond) 2009;4:693–695. doi: 10.2217/nnm.09.58. [DOI] [PubMed] [Google Scholar]
- 210.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine (Lond) 2008;3:137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 211.Funkhouser J. Reintroducing pharma: Theranostic revolution. Curr Drug Discovery. 2002 [Google Scholar]
- 212.Lee DY, Li KC. Molecular theranostics: a primer for the imaging professional. AJR Am J Roentgenol. 2011;197:318–324. doi: 10.2214/AJR.11.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vatansever F, Chandran R, Sadasivam M, Chiang LY, Hamblin MR. Multi-Functionality in Theranostic Nanoparticles: is more Always Better? Nanomed Nanotech. 2012;3:1–5. doi: 10.4172/2157-7439.1000e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jeong H, Huh M, Lee SJ, Koo H, Kwon IC, Sy SYJ, Kim K. Photosensitizer-conjugated human serum albumin nanoparticles for effective photodynamic Therapy. Theranostics. 2011;1:230–239. doi: 10.7150/thno/v01p0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol. 2009;4:773–780. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang X, Wang C, Cheng L, Lee ST, Liu Z. Noble metal coated single-walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapy. J Am Chem Soc. 2012;134:7414–7422. doi: 10.1021/ja300140c. [DOI] [PubMed] [Google Scholar]
- 217.Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008;41:60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 218.Chen Z, Ma L, Liu Y, Chen C. Applications of functionalized fullerenes in tumor theranostics. Theranostics. 2012;2:238–250. doi: 10.7150/thno.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sharma SK, Chiang LY, Hamblin MR. Photodynamic therapy with fullerenes in vivo: reality or a dream? Nanomedicine (Lond) 2011;6:1813–1825. doi: 10.2217/nnm.11.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang M, Huang L, Sharma SK, Jeon S, Thota S, Sperandio FF, Nayka S, Chang J, Hamblin MR, Chiang LY. Synthesis and photodynamic effect of new highly photostable decacationically armed[60] and [70]fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J Med Chem. 2012;55:4274–4285. doi: 10.1021/jm3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Thota S, Wang M, Jeon S, Maragani S, Hamblin MR, Chiang LY. Synthesis and characterization of positively charged pentacationic [60]fullerene monoadducts for antimicrobial photodynamic inactivation. Molecules. 2012;17:5225–5243. doi: 10.3390/molecules17055225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shen H, Zhang L, Liu M, Zhang Z. Biomedical applications of graphene. Theranostics. 2012;2:283–294. doi: 10.7150/thno.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Joseph RDPKT. U.S. Patent, editor. Method of Contactells Magnetic Electrporation. MagneGene, Inc; US: 2010. [Google Scholar]
- 224.Rao R, Nanda S. Sonophoresis: recent advancements and future trends. J Pharm Pharmacol. 2009;61:689–705. doi: 10.1211/jpp.61.06.0001. [DOI] [PubMed] [Google Scholar]
- 225.Oni G, Brown SA, Kenkel JM. Can fractional lasers enhance transdermal absorption of topical lidocaine in an in vivo animal model? Lasers Surg and Med. 2012;44:168–174. doi: 10.1002/lsm.21130. [DOI] [PubMed] [Google Scholar]
- 226.Rubinsky B. Irreversible Electroporation in Medicine. Technol Cancer Res Treat. 2007;6:255–259. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 227.Yi J, Barrow AJ, Yu N, O’Neill BE. Efficient electroporation of liposomes doped with pore stabilizing nisin. J Liposome Res. 0:1–6. doi: 10.3109/08982104.2013.788024. [DOI] [PubMed] [Google Scholar]
- 228.Chen X, Lv H, Ye M, Wang S, Ni E, Zeng F, Cao C, Luo F, Yan J. Novel superparamagnetic iron oxide nanoparticles for tumor embolization application: Preparation, characterization and double targeting. Int J Pharm. 2012;426:248–255. doi: 10.1016/j.ijpharm.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 229.Ge G, Wu H, Xiong F, Zhang Y, Guo Z, Bian Z, Xu J, Gu C, Gu N, Chen X, Yang D. The cytotoxicity evaluation of magnetic iron oxide nanoparticles on human aortic endothelial cells. Nanoscale Res Lett. 2013;8:215. doi: 10.1186/1556-276X-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ivanov IT, Todorova R, Zlatanov I. Spectrofluorometric and microcalorimetric study of the thermal poration relevant to the mechanism of thermohaemolysis. Int J Hyperthermia. 1999;15:29–43. doi: 10.1080/026567399285837. [DOI] [PubMed] [Google Scholar]
- 231.Park D, Ryu H, Kim HS, Kim Y-s, Choi K-S, Park H, Seo J. Sonophoresis Using Ultrasound Contrast Agents for Transdermal Drug Delivery: An In Vivo Experimental Study. Ultrasound Med Biol. 2012;38:642–650. doi: 10.1016/j.ultrasmedbio.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 232.Pahade VMJA, Kadam VJ. Sonophoresis: An Overview. Int J Pharm Sci Revi Res. 2010;3:24–32. [Google Scholar]
- 233.Bloom JABBS, Geronemus RG. Ablative Fractional Resurfacing in Topical Drug Delivery: An Update and Outlook. Dermatol Surg. 2013 doi: 10.1111/dsu.12111. [DOI] [PubMed] [Google Scholar]
- 234.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 235.Kardos DPRTJ. Contactless magneto-permeabilization for intracellular plasmid DNA delivery in-vivo. Hum Vaccin Immunothe. 2012;8:1707–1713. doi: 10.4161/hv.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rekha Rao SN. Sonophoresis: recent advancements and future trends. J Pharm Pharmacol. 2009;61:689–705. doi: 10.1211/jpp.61.06.0001. [DOI] [PubMed] [Google Scholar]
- 237.Soughayer TK Joseph S, Jacobson Stephen C, Ramsey J Michael, Tromberg Bruce J, Allbritton Nancy L. Characterization of Cellular Optoporation with Distance. Anal Chem. 2000;72:1342–1347. doi: 10.1021/ac990982u. [DOI] [PubMed] [Google Scholar]
- 238.Baumgart J, Humbert L, Boulais É, Lachaine R, Lebrun JJ, Meunier M. Off-resonance plasmonic enhanced femtosecond laser optoporation and transfection of cancer cells. Biomaterials. 2012;33:2345–2350. doi: 10.1016/j.biomaterials.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 239.Stevenson JFG-MDJ, Campbell P, Dholakia K. Single cell optical transfection. J R Soc Interface. 2010;7:863–871. doi: 10.1098/rsif.2009.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]