Abstract
The regulation of blastocyst implantation in the uterus is orchestrated by the ovarian hormones estrogen and progesterone. These hormones act via their nuclear receptors to direct the transcriptional activity of the endometrial compartments and create a defined period in which the uterus is permissive to embryo implantation termed the “window of receptivity”. Additional members of the nuclear receptor family have also been described to have a potential role in endometrial function. Much of what we know about the function of these nuclear receptors during implantation we have learned from the use of mouse models. Transgenic murine models with targeted gene ablation have allowed us to identify a complex network of paracrine signaling between the endometrial epithelium and stroma. While some of the critical molecules have been identified, the mechanism underlying the intricate communication between endometrial compartments during the implantation window has not been fully elucidated. Defining this mechanism will help identify markers of a receptive uterine environment, ultimately providing a useful tool to help improve the fertility outlook for reproductively challenged couples. The aim of this review is to outline our current understanding of how nuclear receptors and their effector molecules regulate blastocyst implantation in the endometrium.
1. Introduction: The Nuclear Hormone Receptor Gene Superfamily
The nuclear receptor family is composed of a large number of intracellular proteins with unique roles in transcriptional regulation and very specific patterns of expression. Sequencing of the human genome has identified 48 nuclear receptors (NRs) and allowed for the phylogenetic classification into six evolutionary groups. NRs have modular domains for transactivation, ligand-binding and DNA-binding [1]. Sequence variation at the DNA binding domain allows NRs to bind discrete DNA sequences in the regulatory regions of genes known as hormone response elements (HRE). Along with DNA sequence binding similarity, members of each group also share dimerization abilities. Endogenous molecules such as steroid hormones, thyroid hormones, oxysterols and fat-soluble vitamins have been identified to regulate the activity of more than half of all NRs. Members with no known ligand are called orphan receptors. Naturally occurring and synthetic compounds have been progressively identified to bind some of these orphan receptors, resulting in their newer classification as “adopted receptors”. Various ligands have been identified to stabilize different combinations of NR dimer pairs, promote specific post-translational modifications, regulate DNA binding and cellular localization or promote NR degradation. Ligand bound or unbound NRs can engage in transcriptional crosstalk with pioneer transcription factors, co-regulators, chromatin remodeling enzymes and transcriptional machinery. These interactions result in the activation or repression of gene transcription and are highly context specific. Ultimately, NRs mediate cellular responses to hormones and ligands by regulating various cellular processes including cell survival, proliferation and differentiation [1]. Several of these members have been described to have pivotal roles in the regulation of endometrial function and dysfunction. For this reason, NRs have been extensively studied and targeted for the pharmacological manipulation of various cellular processes in the treatment gynecological malignancies and infertility.
This review will discuss the role of NRs in the regulation of the “window of receptivity”, a short and tightly regulated period in the female reproductive cycle in which the endometrium becomes receptive to the implanting blastocyst. It is only during the window of receptivity that conditions are permissive of blastocyst implantation for the initiation of pregnancy [2]. A number of studies in humans and non-human primates have looked at some of the morphological and transcriptional changes occurring during the window of receptivity. However, most of what we know about uterine biology and implantation comes from murine studies and transgenic models [3]. Several protocols have been developed to successfully mimic events of early pregnancy in the mouse, providing investigators with useful strategies to interrogate implantation mechanisms [4]. The NRs show very dynamic and compartment specific expressions through the window of receptivity in the murine endometrium (Figure 1.) The review will begin by providing a brief comparative description of the female reproductive cycle in women and mice, highlighting the role of the ovarian steroid hormone estrogen and progesterone. These hormones play a central role in the regulation of implantation via their cognate nuclear receptors to govern the proliferation and differentiation of the endometrial compartments through an intricate mechanism of paracrine crosstalk [5]. Finally, there will be a brief description of other members of the nuclear receptor family known to be expressed in the endometrium. However, characterization of these NRs during implantation may not be fully developed in many cases due to lack of appropriate model systems. Final remarks will highlight important advances and future directions in the molecular understanding of NR biology during implantation.
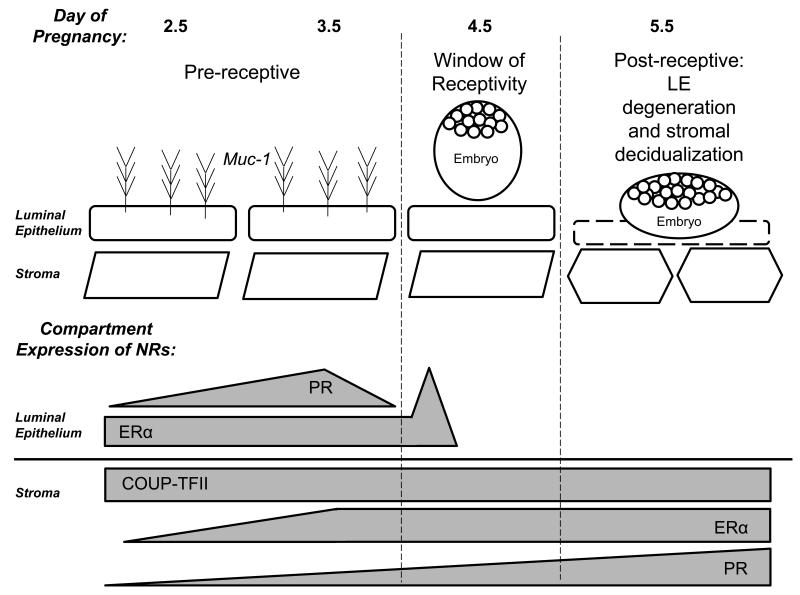
Figure 1. Peri-implantation Expression of the Nuclear Receptors.
The implantation period in the mouse can be divided into pre-receptive, receptive and post-receptive. The nuclear receptors ERα, PR and COUP-TFII have distinct spatiotemporal expression patterns during implantation.
2. The Window of Receptivity
Uterine receptivity to the implanting blastocyst is regulated by the ovarian steroid hormones estrogen and progesterone [6]. In humans, a rise in estrogen levels occurs during the ovulatory/follicular phase of the menstrual cycle. Estrogen stimulates proliferation and growth of the epithelial and stromal compartments of the endometrium. Differentiation of the ovarian granulosa cells into the corpus luteum results in the production of progesterone and the transition into the secretory phase. At this time, proliferation in the epithelial compartment is ceased and the stromal compartment undergoes a progesterone dependent process of differentiation termed decidualization. In species where the blastocyst invades through the epithelial layer into the underlying stroma, decidualization is absolutely required for implantation of the blastocyst and pregnancy progression. Decidualization is a highly complex differentiation process involving an extensive reprogramming of families of genes that result in changes in stromal cell morphology, steroid responsiveness, secretory profile, resistance to oxidative stress and extra cellular matrix remodeling [7,8]. In humans, the decidual reaction occurs recurrently as part of the menstrual cycle and is a blastocyst-independent process. Decidualization initiates near the spiral arteries and spreads throughout the endometrium. The uterus becomes receptive 7-10 days after ovulation during the mid-secretory phase. During the second half of the secretory phase, the endometrium becomes refractory and non-receptive, ultimately concluding in menses in the absence of pregnancy. This menstrual cycle is repeated every 28-30 days [9].
In contrast, mice have shorter and often irregular cycles. The estrous cycle in mice last 4-5 days and can be divided into 4 phases: proestrous, estrous, metestrous and diestrous. In proestrous, the ovary accumulates a mature batch of follicles ready for ovulation during the subsequent estrous phase. Female mice are most receptive to mating during the estrous phase. The morning after mating, termed day 0.5 of pregnancy, a preovulatory surge of estrogen acts upon the uterine epithelium to stimulate proliferation. Estrogen subsequently decreases by day 1.5. At day 2.5, the formation of corpora lutea results in the production of progesterone and the proliferation of the endometrial stroma. Copulation is able to extend this metestrous stage for up to 10 days in a state called pseudopregnancy, but in the absence of pregnancy the uterus enters the diestrous phase. On day 3.5 of pregnancy there is a nidatory estrogen spike that is absolutely critical for inducing uterine receptivity [10,11]. The window of receptivity opens on day 4.0, allowing for implantation to occur. Unlike humans, decidualization of the murine stroma requires mechanical stimulation by the blastocyst at the site of attachment. The decidual reaction subsequently spreads throughout the implantation chamber [12]. In both species, sustained progesterone signaling is critical for the maintenance of pregnancy.
3. Estrogen Receptor
The steroid hormone estrogen acts via the nuclear receptors estrogen receptor α (NR3A1) and estrogen receptor β (NR3A2). The estrogen receptor- α (ERα) and β (ERβ) isoforms arise from two distinct genes. ERα is predominantly expressed in the mammary glands, pituitary, hypothalamus, ovarian theca cells and uterus. In contrast, ERβ is primarily expressed in the ovarian granulosa cells, lung and prostate [13]. In both human and macaque endometrium, ERα and ERβ are expressed in the nuclei of the glandular epithelium and the stroma. Expression of ERα is high in the glands and stroma during the estrogen dominated proliferative phase but declines during the secretory phase [14-16]. Interestingly, the functionalis and basalis layers of the endometrium display subtle differences in the expression of ERα and ERβ during artificial cycles in the macaque [15]. ERα expression is spatially and temporally regulated during implantation in the mouse. Its expression can be observed strongly in the glandular epithelium and more moderately in the luminal epithelium and subepithelial stroma at day 3.5 of pregnancy. At day 4.5, strong expression persists in the glands while most luminal and subepithelial expression is detected in the mesometrial pole of the implantation chamber. Decidual cells surrounding the blastocyst lack ERα expression while the undifferentiated stromal cells maintain strong expression [17].
Uterine responsiveness to estradiol has been attributed primarily to the action of ERα, due to the lack of uterine stimulation and mitotic growth response to estrogen observed in the ERα null mice (ERαKO) [18,19]. The role of ERβ remains controversial. While one study reported that ablation of ERβ in mice (ERβKO) did not result in a significant difference in induction of estrogen responsive genes compared to wild-type mice [19], a separate study reported a significant difference in estrogen and progesterone responses in the ERβKO when an exaggerated estrogen responsiveness and lack of differentiation of the epithelium following acute estrogen treatment was observed [20]. The ERαKO mouse has a hypoplastic uterus and is infertile due to an implantation failure [21]. In contrast, the ERβKO mouse maintains normal implantation, further suggesting estrogen signals primarily via the α isoform [22]. Interestingly, the ratio of ERα to ERβ is altered in ectopic lesions of women with endometriosis, a disease characterized by growth endometrial tissue outside the uterine cavity causing inflammation and chronic pain. Lesions have been shown to be dependent on estrogen and resistant to progesterone signaling [23]. Together with the observation of exaggerated estrogen responsiveness in the ERβKO, this evidence indicates a distinct role of the ER isoforms in the regulation of uterine function and homeostasis.
Estrogen regulation of gene transcription is mediated by ERα interaction with chromatin near the promoter regions of estrogen responsive genes. The ERα cistrome was determined in the murine uterus using chromatin immunoprecipitation (ChIP) followed by whole genome sequencing (ChIP-seq). ChIP-seq identified 5,184 binding sites after vehicle treatment and 17,240 binding sites after treatment with estradiol for one hour. Motif analysis of these binding sites revealed the highest enrichment was for estrogen response element (ERE) motifs, but also reported were significant enrichments in the motifs of other members of the NR family including: peroxisome proliferator associated receptor γ, the thyroid, vitamin D, androgen and glucocorticoid receptors, COUP-TFII, DAX1, retinoid-related orphan receptor-α and estrogen-related receptor. Additionally, there were significant increases in many other non-NR transcription factors, most notably the homeobox (Hox) motifs. ERα binding was overlapped with transcriptional datasets for genes increased or decreased by estrogen relative to vehicle after 2, 6 or 24 hours. This overlap revealed that genes that were up regulated after 2 or 6 hours of estrogen treatment exhibited enriched binding proximal to the transcriptional start site (<10kb) while, in contrast, ER was found to be typically bound at more distal regions surrounding genes that were down regulated. Among the estrogen up-regulated genes were Igf1 and Cdk1, important regulators of early and late estrogen mitogenic events, respectively [24].
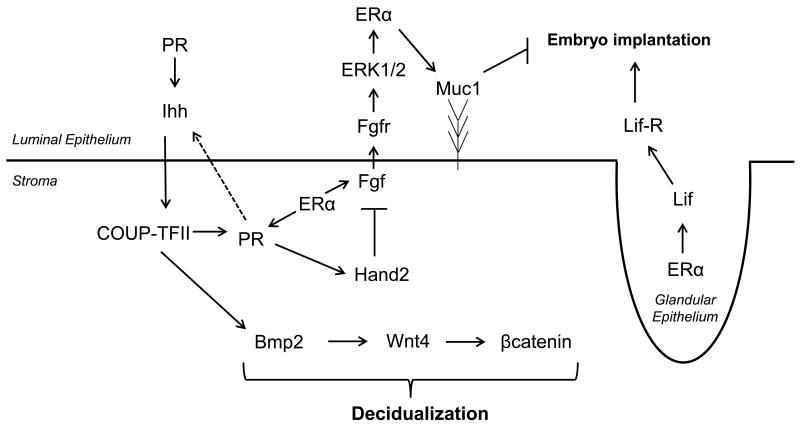
In the mouse, the nidatory estrogen surge in the morning of the fourth day of natural pregnancy coincides with the up-regulated expression of the IL-6 family member leukemia inhibitory factor (Lif) [25]. Estrogen directly mediates Lif signaling via binding of ERα to both the Lif and Lifr genes and regulated their expression in the estrogen treated murine uterus [24]. Lif is a secreted glycoprotein that binds to LIF receptor (LIFR) and gp130 and activates the downstream signal transducer STAT [26]. Both of these receptors are expressed by the blastocyst as well as the uterine luminal epithelium, identifying a potential mechanism of autocrine/paracrine signaling during implantation. LIF expression is seen in the glandular epithelium at day 3.5 and subsequently in the stroma at the site of blastocyst attachment [27]. It was shown that in mice lacking Lif (Lif−/−), the uterus did not respond to the presence of an embryo and was unable to produce a decidual response by artificial stimulation. Interestingly, intraperitoneal injection of LIF was able to rescue implantation and was sufficient to substitute for the nidatory estrogen surge [28]. Altogether this evidence suggests that the nidatory estrogen surge, mediated by ERα, is only required to induce Lif and that the glandular secretion of LIF is only required to initiate the window of receptivity but not subsequently required for the maintenance of pregnancy [28]. LIF expression is also high in humans around the time of implantation [6]. Clinical data has shown that endometria of women with unexplained infertility and multiple implantation failures often display significantly lower levels of Lif during the midsecretory phase of their menstrual cycle when compared to healthy fertile controls [29].
3.1 Estrogen Regulation of Proliferation
The molecular pathways regulated by estrogen have been extensively evaluated in the human and murine endometrium and have identified the conserved role of estrogen in the regulation of proliferation [9]. In humans, the regeneration of the endometrial layers after every menstrual cycle initiates with the proliferation of the basal compartments before an increase in circulating estrogen [30]. The proliferative activity dramatically increases with increasing levels of estrogen and peaks between days 8 and 10 of the menstrual cycle [31]. The epithelial and stromal compartments of the endometrium are highly sensitive to estrogen stimulation and their response has been shown to occur in a biphasic mode during which distinct sets of genes are regulated during early and late events. These responses have been extensively characterized at the physiological, morphological and molecular level. In the mouse early uterine responses to estrogen include transcription of early cell cycle genes, hyperemia, infiltration of immune cells and water imbibition into the uterine tissue [32,33]. Later responses include the further infiltration of immune cells, increased uterine weight and the induction of late cell cycle genes resulting in robust DNA synthesis and mitosis of epithelial cells [33].
Epithelial proliferation in the ovariectomized murine endometrium can be observed by a wave of DNA synthesis starting 6 hours after exposure to estrogen. The wave peaks approximately 12-15 hours with the progression through the G2 and M phases of the cell cycle [34,35]. Estrogen stimulates the activation of the insulin-like growth factor 1 receptor (IGF1R) which leads to the activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway to execute an inhibitory phosphorylation of the glycogen synthase kinase (GSK-3β) [36]. Inactivation of GSK-3β limits the inhibitory phosphorylation of cyclin D1 and allows for its nuclear translocation. Estrogen also induces cyclin E- and cyclin A-cdk2 kinases which promote the hyperphosphorylation of Rb and p107 proteins. Hyperphosphorylated Rb and p107 proteins are unable to inhibit E2F-mediated transcriptional transactivation. The result is the activation of the DNA replication machinery and entry into S-phase of the cell cycle ensuring robust proliferation of the uterine epithelium [37]. The inhibition of proliferation is subsequently necessary for successful implantation [38]. The proposed mechanisms for this inhibition are regulated by progesterone and will be discussed ahead in this review.
3.2 Compartment Specific Roles of ER
ERα expression is under a very strict hormonal and temporal control, indicative of spatiotemporal roles during implantation [17]. The most substantial evidence for the compartment specific roles for ERα was the observation that the uterine epithelium in neonatal mice displayed a mitogenic response to estrogen stimulation despite lack of epithelial ERα expression. Because the mesenchymal/stromal cells of neonatal uteri express ERα, it was hypothesized that the epithelial mitogenic response to estrogen was mediated by stromal ERα paracrine action [39-41]. In order to better elucidate the compartment specific roles of ERα, studies used tissue recombinants of epithelial and stromal cells isolated from wild-type and ERα knock-out uteri were conducted. Tissue recombinants were grafted under the kidney capsule of intact nude mice and estrogen dependent induction of proliferation was measured by [3H] thymidine incorporation into DNA. Wild-type reconstitutions and those with wild-type stroma both exhibited robust levels of epithelial [3H] thymidine labeling. In contrast, tissue grafts containing wild-type epithelium and ERα knock-out stroma were unable to stimulate epithelial proliferation in response to estrogen [42]. This evidence indicated that stromal ERα was both necessary and sufficient to induce estrogen-dependent epithelial proliferation.
The epithelial-specific role for ERα was evaluated in vivo using an epithelial specific ERα knock-out mouse model. This mouse model selectively ablates the Esr1 gene by utilizing the Esr1-loxP and the epithelial specific Wnt7aCre expressing system (UTEpiαERKO). Selective ablation of ERα in the uterine epithelium results in female infertility due to an impaired estrogen response. Interestingly, estrogen stimulated DNA synthesis and expression of mitogenic mediators of epithelial proliferation in wild-type and UTEpiαERKO mice. However, expression of the luminal epithelial estrogen target lactoferrin (Ltf) and a subset of estrogen targets were compromised in the UTEpiαERKO mice. The expression of the apoptotic inhibitor Birc1a was significantly lower after uterine growth was induced by estrogen treatment for 3 days. Consequently, UTEpiαERKO displayed a significant increase in apoptosis. This evidence suggests the role of epithelial ERα is to prevent epithelial apoptosis and ensuring a full epithelial response, while stromal ERα is responsible for estrogen-driven epithelial proliferation [43]. These observations are consistent with the tissue reconstitution studies and collectively illustrate the differential roles for ERα during implantation.
4. Progesterone Receptor
The hormone progesterone acts via the progesterone receptor (NR3C3). Progesterone receptor (PR) exists as two isoforms, PR-A and PR-B, arising from alternative promoter usage in the same gene. PR isoforms are each composed of multiple domains including the activation domain (AF-1) in the N-terminus, the DNA binding domain and the ligand binding domain which contains a second activation domain (AF-2). The PR-B isoform has an additional 164 amino acids in the N-terminus which contains an additional activation domain (AF-3) [44]. There has been evidence of a third truncated isoform, PR-C. This isoform lacks the N-terminus region containing the DNA binding domain, thereby hindering binding of PR-C to DNA. Because PR-C retains the ability to bind hormone, it has been implicated in the functional withdrawal of progesterone in the regulation of parturition [45]. The total levels of PR and the ratio of PR-A/PR-B vary across reproductive tissues. Since PR-A lacks the amino terminal transactivation, it may serve to dampen PR-B driven transcriptional activation when these isoforms are co-expressed [46]. PR-A and PR-B can form heterodimers that result in a dominant repression of PR-B activity [47]. The differential transactivation properties of each isoforms and stoichiometry collectively result in a complex mechanism of progesterone responsiveness in specific tissues and cellular contexts.
Molecular profiling of the human endometrium throughout the menstrual cycle indicates expression of PR is highest in the estrogen-driven proliferative phase and decreases in the progesterone-dominated secretory phase [16]. These observations are consistent with the evidence that PR expression is induced by estrogen action on an ERE in the promoter region of PR while progesterone inhibits its own expression by action of PR/ER regulation of the same ERE [48]. Expression of both PR isoforms is observed in the murine uterus, and ablation of both isoforms (PRKO) results in multiple reproductive abnormalities, including a hyperplastic response to estrogen and progesterone and an implantation defect [49]. However, specific ablation of PR-A (PRAKO) and PR-B (PRBKO) individually has shed light on the role of these isoforms in the mouse uterus. Ablation of PR-B resulted in no discernible uterine phenotype and display normal fertility. PRBKO mice display reduced pregnancy-associated mammary gland morphogenesis, indicating PR-B is a major regulator of mammary gland maturation during pregnancy [50]. Ablation of PR-A phenocopies the PRKO mouse phenotype and indicates that the PR-A isoform is the predominant functional isoform in the mouse uterus [51]. Contrary to what is observed in wild type mice where progesterone antagonizes estrogen-induced epithelial proliferation, progesterone treatment induced proliferation of the uterine epithelium in the PRAKO mice and this proliferation was shown to be dependent on PR-B [51]. This PR-B-dependent gain of function in proliferative activity indicates an important role for PR-A in the regulation of the potentially adverse effects of PR-B-driven proliferation as well as inhibition of estrogen-driven hyperplasia. Collectively, these observations identify functional differences between these two isoforms in response to progesterone in the regulation of epithelial proliferation during early pregnancy.
4.1 Progesterone Receptor Targets
One of the earlier murine studies identified progesterone targets by comparing the uterine gene expression of wild-type and PRKO mice after acute and chronic treatment with progesterone [52]. However, in this study the most robust PR-dependent gene expression change occurred after acute progesterone treatment with the up-regulation of 55 genes. Among these was Indian Hedgehog (Ihh), a morphogen expressed in the luminal epithelium shown to be absolutely required for implantation and decidual response [53]. Chronic progesterone treatment resulted in the robust down-regulation of 102 genes including many of the transcription factors, transport proteins, signal transduction, cell growth, and enzyme genes that had been induced by acute progesterone treatment. The progesterone-PR axis was shown to regulate retinoic acid metabolism, known to play a role in female fertility and the Klks gene family associated with regulation of the inflammatory process, vasodilatation and vascular permeability [54-57]. The P4-PR axis was also shown to regulate other important transcription factors and co-regulators including Gata2 and Cited1 [52].
PR regulation of gene expression occurs by direct binding of the receptor in the regulatory promoter regions of targets genes [58]. In order to identify direct genomic targets of PR in the murine endometrium the genome-wide PR cistrome was determined using ChIP-seq. In the absence of ligand, ChIP-seq identified 6,367 PR-binding sites. The number of binding sites increased nearly 3-fold (18,432) after acute progesterone treatment. Over 73% of these intervals contain progesterone response elements (PRE) or half-PREs. Intervals were enriched in the promoter regions proximal to the transcriptional start sites of genes. PR binding datasets were overlapped with microarray gene expression comparing genes significantly induced by acute progesterone treatment. This analysis confirmed PR binding on both up-regulated (Gata2, Egfr, Ihh, Fkbp5, Cyp26a1) and down-regulated (Pgr, Wnt7a, Lifr) progesterone target genes [59]. Most notably, the ChIP-seq and microarray datasets overlap identified a novel role for progesterone in the regulation of uterine circadian rhythm discussed in greater detail further ahead in this review.
4.2 Progesterone Inhibition of Estrogen Signaling
The use of progesterone supplementation has been an invaluable resource to Assisted Reproductive Technology (ART) efforts [60,61]. However, little is known about the action of progesterone during the implantation window in humans because the numerous molecular profiling efforts have been limited by the technologies available at the time or imprecise methods for endometrial cycle dating [62-66]. Nevertheless, these studies have consistently identified a conserved role of progesterone in the inhibition of estrogen-driven epithelial proliferation. The antiproliferative action of progesterone in the endometrium has also been the focus of extensive research for its potential therapeutic role regulating progression of estrogen-dependent pathologies such as endometrial cancer and endometriosis [67]. Contrasting mechanisms have been proposed for the progesterone-mediated inhibition of estrogen induced epithelial cell proliferation. One line of evidence supports a paracrine mechanism while the alternative proposes a direct role of PR in the uterine epithelial cells.
The paracrine mechanism for the inhibition of epithelial proliferation was strongly supported when the basic helix-loop-helix transcription factor Hand2 was shown to play an essential role in the regulation of growth factor signaling in a progesterone-dependent manner. Hand2 was identified as a significantly regulated gene in the stroma of mice treated with the PR antagonist RU486 [68]. Conditional ablation of Hand2 using the PRcre/+ Hand2f/f (Hand2d/d) bigenic mouse model results in infertility due to an implantation defect. Progesterone inhibition of epithelial proliferation and down regulation of estrogen driven genes including Mucin 1 (Muc1) and Lactoferrin (Ltf) was altered in the Hand2d/d mice. Progesterone responsive genes, including Ihh, Alox15 and Irg1 were not affected. Interestingly, several members of the fibroblast growth factor family (FGFs) and HB-EGF showed a significantly high level of expression in Hand2d/d mice. Fgfs act on Fgf receptors (Fgfr) to activate downstream extracellular signal regulated kinase (ERK) 1 and 2 or the PI3K/AKT pathways. Interestingly, the Fgfr’s are expressed on day 1 and 4 of pregnancy in the epithelium of mice. In the uterus of Hand2d/d, aberrant activation of Fgfr was observed on day 4 of pregnancy, suggesting a mechanism for stromal Hand2 mediated regulation of ERK1/2 and PI3K/AKT signaling in the epithelium [69]. Collectively these results identified a complex mechanism of stromal-epithelial crosstalk regulates proliferation during implantation.
The direct action of progesterone on epithelial cell proliferation is based on the observation that progesterone has been shown to completely inhibit estrogen-induced DNA synthesis and cell proliferation [70,71]. It was found that progesterone inhibits the estrogen regulation of cyclin D1 nuclear translocation resulting in the hypophosphorylation of Rb and p107 proteins and a block in G1-S phase progression [72]. PR also regulates the members of the MCM family of proteins to inhibit epithelial proliferation [37,73]. Substantial evidence implicates the Kruppel-like factor family in this mechanism. The Kruppel-like factor family is composed of zinc finger transcription factors involved in many cellular processes including proliferation, differentiation, apoptosis and development [74]. KLF4 and KLF15 play a significant role in the regulation of epithelial proliferation. KLF4 is up regulated only in the epithelium of ovariectomized mice after treatment in estradiol and inhibited when mice were pretreated with progesterone. In contrast, expression of KLF15 is dramatically increased after estrogen and progesterone treatment in the epithelium and moderately in the stroma. The temporal expression of these KLFs was associated with steroid control of epithelial proliferation via transcriptional regulation of Mcm2, a positive regulator of proliferation required for DNA replication licensing. Estradiol treatment stimulated ERα, KLF4 and RNAPol II binding at transcriptional regulatory sites of the Mcm2 and induced Mcm2 expression. Treatment with estradiol and progesterone ablated this binding but favored binding of KLF15. KLF15-mediated estrogen and progesterone inhibition of Mcm2 promoter activity, Mcm2 expression and proliferation was confirmed in the Ishikawa epithelial cancer cell line, providing a mechanism for KLF regulation of epithelial proliferation. [75]
The above observations suggest that there are direct actions of progesterone and PR, as well as paracrine mechanisms of action. The above conflicting conclusions likely arise from the use of different hormone treatment in these murine models. These strategies could be mimicking events that occur synchronously and synergistically across compartments during different stages of early pregnancy. A more refined hypothesis would be that both of these mechanisms could be occurring at specific times during early pregnancy and disruption of either of these results in the disruption of implantation. This holistic hypothesis highlights the complexity of progesterone signaling and underscores the need to develop new strategies to address remaining questions. Therefore, understanding the compartmental specific expression and action of PR in the uterus is critical.
4.3 Compartment Specific Roles of PR
Expression of PR is observed in the luminal epithelium and subepithelial stroma at day 3.5 of pregnancy. By day 4.5, PR expression in the luminal epithelium ceases and is restricted to the subepithelial stroma at the site of implantation and is undetected in the glandular and luminal epithelium. Expression becomes strong and persistent in the stromal compartment throughout the process of decidualization [17]. In order to begin elucidating the compartment specific roles of PR, studies used tissue recombinants of epithelial and stromal cells isolated from wild-type and PRKO uteri. Tissue recombinants were grafted under the kidney capsule of intact nude mice. Subsequently, mice were ovariectomized and treated with estradiol alone or in combination with progesterone. All tissue grafts combinations (wild-type epithelium with wild-type stroma, PRKO epithelium with wild-type stroma, wild-type epithelium and PRKO stroma) displayed comparable levels of epithelial [3H] thymidine labeling in response to estradiol. Tissue recombinants containing wild-type stroma, independent of the expression of PR in the epithelial tissue, displayed a significant inhibition of estradiol-induced epithelial proliferation with progesterone co-treatment. Tissue grafts with PRKO stroma with wild-type or PRKO epithelium, were not able to suppress epithelial proliferation in response to progesterone treatment. This indicated that expression of PR in the stroma was both necessary and sufficient to inhibit the estrogen-induced epithelial proliferation by progesterone. These results provided evidence for the existence of paracrine signals from the stroma to the epithelium to mediate progesterone signaling.
In order to understand progesterone signaling in compartment specific manner in vivo under the physiological context of implantation the epithelial PR was ablated using a bigenic mouse model expressing Cre recombinase under the epithelial specific promoter of Wnt7a and containing PR allele flanked by loxP sites. This mouse model selectively ablates the Pgr gene by utilizing the Pgr-loxP and the epithelial specific Wnt7aCre/+ expressing system (Wnt7aCre PRf/−). As a consequence of disrupted progesterone signaling, the epithelial PR knock-out mice were infertile due to a defect in blastocyst attachment and an absent decidual response. Expression of the epithelial progesterone targets, Ihh and Areg where significantly down regulated when PR was ablated in the luminal epithelium [76]. In the absence of epithelial PR, the progesterone-dependent down-regulation of estrogen targets cyclin D1 and MCM3 was not observed. In accordance with the defect in down regulation of estrogen proliferation targets, it was observed that ablation of epithelial PR results in retained proliferation of the luminal epithelium at days 2.5 and 3.5 of natural pregnancy. These results are in disagreement with the findings determined by tissue reconstitution studies and demonstrated an essential role of the epithelial PR in the progesterone inhibition of estrogen-driven epithelial proliferation [76]. Notably, it was shown that epithelial PR regulated epithelial expression of Ihh after acute progesterone stimulation. However, the natural pregnancy data indicated only a trend supportive of the hypothesis that epithelial PR was necessary and sufficient for this induction. The inconsistent observations between tissue reconstitution studies and the Wnt7aCre PRf/− mouse model underscore the urgent need to investigate role of the stromal PR in the regulation of pre-implantation events in vivo. As mentioned above, the differences in these observations may be due to hormonal treatment and the time in which the proliferation is evaluated. In the pre-implantation uterus PR is expressed in the epithelial compartment and its actions to regulate gene expression and cell proliferation are direct. During the window of receptivity, PR expression ceases in the epithelium and increases in the stromal compartment. At this time the paracrine mechanism of regulation may dominate.
5. Androgen Receptor
The androgen receptor (NR3C4) has been extensively studied for its role in male reproductive tissues. However, the androgen receptor (AR) is also expressed in female reproductive tissues including the ovary and the uterus [77]. AR is expressed in both stromal and epithelial compartments throughout the menstrual cycle, with expression being highest in the stromal compartment during the proliferative phase and lowest in the secretory phase [78]. Expression of AR is elevated in the endometrium of women with polycystic ovarian syndrome (PCOS) and premature ovarian failure (POF). [77,79,80] Although expression of AR decreases in the decidualizing stroma during the secretory phase, androgen signaling seems to play a critical role in the regulation of a distinct subset of genes during in vitro decidualization of primary human endometrial stromal cells (HESC). Addition of dihydrotestosterone (DHT) enhances the progestin and cAMP-dependent activation of the promoter for the decidual marker prolactin (PRL) and enhances the expression of PRL in a dose dependent manner. Gene expression analysis in decidualizing HESC identified genes involved in cytoskeletal organization, cell motility and cell cycle regulation to be deregulated after AR depletion. Additionally, stromal cells failed to adequately form differentiation-dependent stress fibers [81]. It has been suggested that AR has antiproliferative effects that overlap or may even complement those of progesterone in estrogen-induced replication [82]. Knockdown of AR significantly promotes cell proliferation and partially reverses the hyperphosphorylation of RB associated with HESC differentiation [83]. Additionally, androgens have been shown to inhibit cell growth and DNA synthesis in HESC and cancer cell lines [84,85]. However, it is unknown if this mechanism plays a role in the regulation of the window of receptivity. The AR knockout (ARKO) mice displayed reduced fertility over time. The late infertility defect was attributed to the development of a POF phenotype. However, from an early time point ARKO mice displayed a reduction in litter size, smaller uteri, reduced uterine growth at estrous, abnormal placental development and placentamegaly [77]. While expression of AR and a physiological role in uterine function is evident in the human and mouse, its function in the regulation of uterine receptivity is still not fully understood.
6. Glucocorticoid Receptor
Glucocorticoids are important regulators of many physiological functions including metabolism, response to infection, stress response, blood pressure maintenance and electrolyte homeostasis [86]. Glucocorticoid secretion from the adrenal cortex is regulated by the hypothalamic-pituitary-adrenal axis and its activity is regulated by the local action of metabolizing enzymes [87-89]. Glucocorticoids bind the glucocorticoid receptor (NR3C1) to regulate glucocorticoid responsive gene expression [90]. GR was the first nuclear receptor to be cloned and sequenced [91]. GR is expressed in many tissues throughout development, including the placenta [92-94]. In humans, GR is highly expressed throughout the menstrual cycle in the fibroblasts, endothelial cells and lymphocytes in the stromal compartment, but absent in the glands [95]. In human endometrial stromal cells, glucocorticoids have been shown to inhibit the expression of PRL and stimulate the production of the PRL suppressing lipocortin-1 [96]. Additionally, glucocorticoids have been shown to regulate the production of prostaglandins, critical regulators of stromal vascular permeability during implantation [97]. However, GR null mice die shortly after birth due to respiratory insufficiency thus hindering the study of the role of GR in the adult uterus during implantation [98]. Generating a conditional GR knock-out model will be pivotal to elucidate the uterine specific role of GR in vivo.
7. Retinoic Acid Receptors (RAR and RXR)
Retinoic acid (RA) is one of the most potent and naturally-occurring metabolite of Vitamin A [99]. RA signaling is involved in embryonic development, growth and reproduction [100,101]. RAs regulate target gene expression by binding the retinoic acid receptor (RAR) and the retinoic X receptor (RXR) [101]. There are three known subtypes of the RA receptor subfamily, RAR-α (NR1B1), RAR-β (NR1B2) and RAR-γ (NR1B3). Similarly, there are three subtypes of the RXR, RXR-α (NR2B1), RXR-β (NR2B2) and RXR-γ (NR2B3) [102]. Immunohistochemical identification of the RAR and RXR though out the menstrual cycle in women revealed distinct patterns in endometrial compartment and subcellular localization. All RARs can be found in the nuclei of the luminal epithelium during the proliferative phase of the cycle. The staining for RAR in the nuclei was significantly decreased during the secretory phase, but not for RXR. Interestingly, RAR staining in the cytoplasm increased in the secretory phase. Nuclear export of RAR would suggest its activity is predominant during the proliferative phase, while RXR remains active throughout the menstrual cycle. A similar pattern expression for RAR and RXR can be observed in the stromal compartment during the proliferative and secretory phase [103].
The changes in RAR localization are positively correlated with serum estradiol and negatively correlated with progesterone serum levels [104]. Treatment of post-menopausal women with Premarin or a mixture of estrone and equilin sulfates for 3 months results in the up-regulation of retinaldehyde dehydrogenase (RALDH), critical enzyme in RA biosynthesis, RAR-α and RA-regulated genes. Interestingly, the premenopausal endometrium expresses high levels of these RA signaling components during the proliferative phase, while the RA catabolic enzymes retinoic acid 4-hydroxylase (CYP26A1) and tissue transglutaminase are increased in the secretory phase [105]. Similarly, expression of RALDH1, RALDH2 and CYP26A1 has been shown to fluctuate in the murine uterus throughout the estrous cycle and during implantation [54].
RA has been shown to inhibit the morphological transformation and suppress the expression of PRL and IGFBP1 in decidualizing HESC. The effect of RA appears to be partially mediated by the inhibition of cAMP signaling under this estrogen and medroxyprogesterone treatment and was only partially compensated by addition of cAMP. Treatment of cultured rat endometrial cells with estrogen increases the production of RA [106]. Additionally, treatment of rats with pregnant mare serum gonadotropin (PMSG), a pharmacological strategy to induce ovulation, results in the induction of RA by the estrogenized uterus. [54] Altogether, this evidence suggests a potential role for RA signaling in the regulation of estrogen-induced proliferation during the window of receptivity. Unfortunately, the double-null mice lacking RARα/RARγ or RARβ/RARγ die before or immediately after birth due to severe skeletal defects, hindering the study of the RAR during peri-implantation in the adult uterus [107,108].
8. Thyroid Hormone Receptor
Thyroid hormones (TH) are important in processes including growth, development, differentiation, metabolism and reproduction [109]. Hypothyroidism and hyperthyroidism have been known to affect the metabolism of sex hormones, ovarian function and have been associated with menstrual irregularities and infertility [110]. There are two major types of THs, thyroxine (T4), which is the main secretory product and the pro-hormone triiodothyronine (T3) [109]. T3 binds the two major isoforms of the thyroid hormone receptor (TR), TRα (NR1A1) and TRβ (NR1A2). There are two TRα isoforms, TRα1 and TRα2. The TRα2 isoform, however, cannot bind T3 and transactivate TH-responsive genes. Additionally, there are two TRβ isoforms, TRβ1 and TRβ2 [111]. Ablation of the TRβ1 in the mouse (Thrb−/−) results in a hyperactive goiter and elevated thyroid hormone levels.[112] In contrast, ablation of TRα1 in the mouse (TRa1−/−) results in mild hypothyroidism.[113] Unfortunately, disruption of the endocrine system in both of these mouse models hinders the ability to study the specific role of these TRs in endometrial biology.
It has been reported that TRα1, TRα2 and TRβ1 transcripts are regulated by progesterone in the endometrium. Treatment with the anti-progestin RU486 on day LH+8 of the menstrual cycle results in the down-regulation of TRα1 and TRα2 and the up-regulation of TRβ1 [114]. Both protein and message levels of these TRs are detected throughout the menstrual cycle in healthy women. TRα1 is higher in the midsecretory phase while TRα2 was highest in the proliferative phase. TRβ1 is highest in the luminal epithelium in the midsecretory phase and highest in the glandular epithelium in the mid to late secretory phase. In the stromal compartment, TRβ1 was expressed consistently throughout the menstrual cycle. Interestingly, TRβ2 was undetected at the message or protein level by immunostaining, but was present by Western blotting [115]. Collectively, these results underscore the potentially significant, yet largely unknown function of TRs in endometrial receptivity.
9. Peroxisome proliferator-activated receptor
The peroxisome proliferator-activated receptor (PPAR) family is composed of three forms, the PPARα (NR1C1) PPARδ, also referred to as PPARβ/δ, (NR1C2) and PPARγ (NR1C3) [116]. These receptors bind several natural and synthetic ligands to regulate lipid and glucose metabolism, energy homeostasis, inflammation and cell proliferation and differentiation [117,118]. PPAR expression is abundant in tissues like the liver or adipose, critical sites of metabolic regulation. Interestingly, PPARs can also be found in reproductive tissues inducing the hypothalamus, ovary, uterus and placenta in rodents [119]. PPARγ is expressed in the stroma at day 2.5, but subsequently decreases to a low level during implantation. In contrast, PPARα and PPARδ are constantly expressed in the glandular epithelium at days 0.5-6.5 of pregnancy. Both PPARs are present in the endometrial stroma of during days 4.5-6.5 of the rat pregnancy [120]. PPARδ has been observed in the stroma at the site of implantation in mouse and rat [121,122]. PPARδ has been suggested to play a role in placental function, intrauterine growth restriction (IUGR), inflammatory pathways and mediating COX2-derived prostacyclin (PGI2) signaling during pregnancy [123-126]. PPARα activation has been linked to the negative regulation of lipid peroxidation and nitric oxide (NO) production during placentation [127].
Genetic ablation of PPARα in the mice results in an increase in abortion rate. PPARα−/− mice display greater neonatal mortality, indicating an important role for PPARα in placental function [128]. Similarly, PPARδ null mice (PPARβ/δ−/−) display placental abnormalities and embryonic death during early pregnancy due to alteration in cyclo-oxygenase-2-derived prostacyclin mediated implantation [126,129]. PPARγ null embryos (PPARγ−/−) die very early in development due to defects in labyrinth formation during placentation [130]. Due to the early embryonic lethality of the PPARγ−/− mouse, the role of PPARγ in the adult endometrium remains unknown. It has been shown that the regulation of metabolism for energy homeostasis plays an important role in female reproduction, particularly in the function of the placenta. However, early events in pregnancy also require fine-tuning of metabolism to prepare the uterus for implantation and decidual transformation suggesting a role for the PPAR in the preparation of uterine receptivity. The mechanism by which PPARs regulate early implantation events remain to be fully elucidated.
10. The Orphan Nuclear Receptors
10.1 COUP-TFII
COUP-TFII (NR2F2), chicken ovalbumin upstream promoter-transcription factor II, is an orphan member of the nuclear receptor family best known for its role in the regulation of angiogenesis during mouse development, glucose metabolism and cancer progression [131]. COUP-TFII is expressed consistently throughout implantation in the endometrial stromal compartment. Conditional ablation of COUP-TFII in the endometrium using the PRCre/+ COUP-TFIIf/f (COUP-TFIId/d) bigenic mouse results in infertility due to a defect in blastocyst attachment and absent decidual response. The endometria of COUP-TFIId/d mice exhibited unopposed estrogen signaling in the luminal epithelium, a phenotype similar to that observed after endometrial ablation of Ihh [53]. Interestingly, Ihh was shown to be an upstream regulator of COUP-TFII expression [53]. Consistent with an increase in total ERα and its coactivator SRC-1, a proportional increase in activated phospho-ERα as well as the persistent expression of the estrogen target gene Ltf was observed in COUP-TFIId/d [132]. Furthermore, COUP-TFIId/d displayed a defect in epithelial maturation with a persistent expression of Muc1, glycocalyx and an inappropriate remodeling of the microvilli. Collectively these defects imposed a barrier to implantation and subsequent decidualization [132].
COUP-TFII plays a central role in the mechanism of stromal-epithelial crosstalk during implantation by regulating the WNT and bone-morphogenic-protein (BMP)-pathways. Administration of a pure ERα antagonist ICI 182,780 (ICI) rescued the expression of Bmp2 and Wnt4 in COUP-TFIId/d mice, molecules that are absolutely critical for stromal cell decidualization [133-135]. This resulted in the partial rescue the implantation defect and decidual response. Interestingly, the decidual defect in COUP-TFIId/d could also be rescued by the introduction of recombinant BMP2 into the uterine horn [132]. Similar to COUP-TFII, Bmp2 and Wnt4 are expressed in the stromal compartment during implantation. Conditional ablation of Bmp2 in the uterus using the PRCre/+ Bmp2f/f (Bmp2d/d) bigenic mouse model resulted in infertility. While attachment, proliferation and vascularization were normal in the Bmp2d/d model, endometrial stromal cells were unable to differentiate due to compromised Ptgs1 expression and a decrease in activating PR phosphorylations. The Bmp2 target, Wnt4, was also conditionally ablated in the murine endometrium using the PRcre/+ Wnt4f/f bigenic mouse model. Ablation of Wnt4 results in subfertility due to defects in stromal cell differentiation and progesterone responsiveness. Collectively, these results indicate a central role for COUP-TFII during implantation involving BMP and WNT signaling in the activation of progesterone signaling, regulation of epithelial maturation and stromal preparation for decidualization.
10.2 SF-1 and LRH-1
The steroidogenic factor-1 (NR5A1) and the liver receptor homolog-1 (NR5A2) are highly homologous orphan NRs involved in the regulation of steroidogenic genes. Steroidogenic factor-1 (SF-1) expression is predominantly observed in Leydig and Sertoli cells of the testis, granulosa and theca cells of the ovary, all the layers of the adrenal cortex, pituitary and hypothalamus [136,137]. SF-1 regulates the expression of the enzymes required for steroidogenesis including STAR, aromatase, CYP11A1 and CYP17A1. In the endometrium expression of SF-1 is silenced due to heavy promoter methylation. Therefore, SF-1 it is hypothesized not to play a role in blastocyst implantation. In endometriosis, aberrant demethylation of the SF-1 promoter in stromal cells results in the activation of SF-1 expression and consequently the up-regulation of steroidogenic enzymes. This condition is hypothesized to promote de novo estradiol synthesis and growth of endometriotic lesions [138]. It is known that COUP-TFII provides additional repression of the aromatase promoter in the endometrium [139]. Given that SF-1 is the stronger competitor in this process, is clear why SF-1 must remain silent in order to maintain endometrial homeostasis. The physiological and molecular consequences of activating SF-1 expression in the uterus remain unknown, but would provide insight into the role of SF-1 in endometrial pathologies.
Liver receptor homolog-1 (LRH-1) is known to be involved in liver bile acid metabolism, ovarian steroidogenesis and cell proliferation. It is expressed in the granulosa and luteal cells in the ovary where it was found to be critical for the regulation of cumulus expansion, luteinization and ovulation [140]. A recent study evaluated the role of LRH-1 in luteal function using the PRCre/+ NR5A2f/f (NR5A2d/d) bigenic mouse. These mice exhibited luteal insufficiently that resulted in implantation failure. Although implantation failure was rescued by progesterone supplementation, defects in implantation site spacing resulted in compromised placental formation, fetal growth retardation and fetal death. Furthermore, expression of preimplantation genes Hoxa10, Wnt4, Wnt5a, Ihh and Bmp2 was compromised in NR5A2d/d mice and was not rescued by progesterone supplementation. LRH-1 was also shown to be indispensable for mouse and human stromal cell decidualization [141]. Finally, it has been shown that both SF1 and LRH-1 cooperate to activate steroidogenesis in endometrial cancer cell lines, providing a possible mechanism for the observation that de novo estradiol synthesis seem to provide tumor cells with a growth advantage [142]. Collectively these results underscore the need to further evaluate the roles of LRH-1 and SF-1 in uterine function and disease.
10.3 Circadian Rhythm: ReverbA and RORC
Regulators of the circadian rhythm form part of the endogenous timing system of biological systems. These transcriptional regulators were originally identified in the suprachiasmatic nucleus in the brain but recent evidence demonstrated this system works in individual tissues, including reproductive tissues [143]. Recently, it was reported that progesterone might have a role in the regulation of this circadian biology. Overlapping PR binding with expression data after acute progesterone exposure of ovariectomized mice identified circadian rhythm biology and identified new gene target of PR including Clock, Npas2, Cry1 and Per1, Nr1d1, Rorc and Ppargc1a [59]. Rev-erbα (NR1D1) and RAR-related orphan receptor C (NR1F3) are orphan members of the NR superfamily. Together with the peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (Ppargc1a), these NRs negatively regulate Clock, Npas2, Cry1 and Per1. Interestingly, ReverbA and RORC are negatively regulated by acute progesterone stimulation, while Clock, Npas2, Cry1 and Per1 are up-regulated by progesterone [59]. This evidence underlines the previously unknown role of PR in the regulation of uterine circadian biology. Given that circadian rhythms are known to be important in hypothalamic-pituitary-gonadal axis, it is no surprise to find relationships between disrupted rhythm and menstrual irregularities as well as decrease pregnancy rates [144]. These observations open up a new field examining the role of circadian rhythm in blastocyst receptivity and fertility.
11. Conclusions and Future Directions
Implantation in mammals is a very complex process that requires intricate crosstalk between molecules within compartments of the endometrium for the tight regulation of receptivity (Figure 2.) The ovarian steroids estrogen and progesterone orchestrate the transcriptional programs required for changes in cell cycle progression and differentiation observed in the endometrium during implantation. It is clear that ER and PR play a central role in this process. Future studies should focus on delineating isoform specific targets and functions during implantation and disease. The roles of many of the NRs with endometrial expression have not been fully elucidated due to lack of appropriate in vivo systems. Tissue specific mouse models are currently the most efficient strategy for interrogating molecules that regulated the window of receptivity and have already defined the roles of several important regulators. (Summarized in Table 1.) Conditional mouse models utilizing PRcre, circumvent many of the issue associated with whole body knock-outs and will likely continue to shed light into the roles of NRs and downstream molecules. Significant advances in the understanding of paracrine crosstalk could also be achieved by utilizing compartment specific and inducible Cre models to answer questions about the spatiotemporal roles of these NRs and their effector molecules.
Figure 2. Model for Stromal-Epithelial Paracrine Signaling during Implantation.
Extensive crosstalk between the epithelial and stromal compartments in the endometrium is required for establishing the window of receptivity. Solid arrows (→) indicate activating interactions, (—/) indicate inhibition or repression and dashed arrows represent potential induction that remain to be elucidated.
Table 1. Summary of Implantation Phenotypes in Mouse Models.
| Target | Gene | Mouse Model | Mouse Uterine Phenotype |
|---|---|---|---|
| NR Ablation | |||
| Nr3a1 (Esr1) | ERαKO | Infertile, hypoplastic, no implantation, decidual response persists with progesterone priming. |
|
| Nr3a2 (Esr2) | ERβKO | Normal fertility uterine phenotype. Exaggerated estrogen responsiveness. |
|
| epithelial Esr1 | UTEpiαERKO | Infertile, loss of lactoferrin expression and enhanced uterine apoptosis. |
|
| Nr3c3 (Pgr) | PRKO, PRAKO, PRBKO |
Infertile, no implantation, absent decidual response, unopposed estrogen signaling. PRKO phenotype in PRAKO only. |
|
| epithelial Pgr | Wnt7aCre/+ PRf/f | Infertile, no implantation, absent decidual response, unopposed estrogen signaling. |
|
| Nr3c4 (AR) | ARKO | Reduced litter size, smaller uterus and reduced growth in response to hormones, abnormal placental development and placentamegaly. |
|
| Nr1c1 (PPARα) |
PPARα−/− | Increase in abortion rate and greater neonatal mortality. |
|
| Nr1c2 (PPARβ/δ) |
PPARβ/δ−/− | Placental abnormalities and embryonic death during early pregnancy due to alteration in cyclo-oxygenase-2-derived prostacyclin. |
|
| Nr1c3 (PPARγ) |
PPARγ−/− | Early death of embryo due to defects in labyrinth formation during placentation. |
|
| Nr2f2 (COUP-TFII) |
PRCre/+ COUP- TFIIf/f |
Infertile, no implantation, absent decidual response, unopposed estrogen signaling in luminal epithelium. |
|
| Nr5a2 (LRH-1) |
PRCre/+ NR5A2f/f | Infertile, luteal insufficiency, implantation failure, defect decidualization and placentation. |
|
| NR Target Ablation | |||
| Ihh | PRCre/+ Ihhf/f | Infertile, absent decidual response, unopposed estrogen signaling in luminal epithelium |
|
| Bmp2 | PRCre/+ Bmp2f/f | Infertile, decrease Cox2, absent decidual response. |
|
| Wnt4 | PRCre/+ Wnt4f/f | Subfertile, reduced number of glands, squamous cell metaplasia in response to prolonged estrogen treatment, defective decidualization and progesterone response. |
|
| Lif | Lif−/− | Infertile, no implantation, embryos remain dormant. |
|
| Hand2 | PRCre/+ Hand2f/f | Infertile, no implantation, altered progesterone responsiveness, persistent luminal epithelial proliferation. |
The use of ChIP-Seq in combination with expression profiling techniques such as microarray and RNA-Seq have been very powerful tools in the identification of chromatin interactions and direct transcriptional targets of NRs. It is no doubt the field of uterine biology will continue to reap the benefits of the advances in high content technology for the better understanding of NR action in blastocyst implantation. The ultimate goal of these efforts is to identify molecular markers of uterine receptivity. The knowledge of these will facilitate efforts in assisted reproductive clinics and improve the outlook of reproductively challenged couples. Additionally, many of the signaling pathways involved in regulating implantation are disrupted in gynecological malignancies such as endometriosis and endometrial cancer. A greater understanding of the role of hormone and nuclear receptor signaling in the regulation of proliferation and differentiation in the endometrium could identify more effective strategies for the treatment of these pathological conditions.
Highlights.
This review discusses the major nuclear receptors expressed in the endometrium during implantation and identifies important gaps that need to be addressed.
We delineate the known mechanisms that regulate receptivity to the implanting blastocyst with a focus on murine model studies.
We provide a model that describes the mechanism of stromal-epithelial crosstalk in the regulation of proliferation.
Acknowledgements
The authors are supported by NIH grant RO1 HD042311 (to F.J.D) and the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD007495, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.Yoshinaga K. Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. 1988;541:424–431. doi: 10.1111/j.1749-6632.1988.tb22279.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128:679–695. doi: 10.1530/rep.1.00340. [DOI] [PubMed] [Google Scholar]
- 4.Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Mouse models of implantation. Trends Endocrinol Metab. 2007;18:234–239. doi: 10.1016/j.tem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28:27–35. doi: 10.1055/s-0029-1242990. [DOI] [PubMed] [Google Scholar]
- 6.Dey SK, Lim H, Das SK, Reese J, Paria BC, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 7.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 8.Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics. 2001;7:135–148. doi: 10.1152/physiolgenomics.00061.2001. [DOI] [PubMed] [Google Scholar]
- 9.Groothuis PG, Dassen HH, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13:405–417. doi: 10.1093/humupd/dmm009. [DOI] [PubMed] [Google Scholar]
- 10.Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39:195–206. doi: 10.1530/jrf.0.0390195. [DOI] [PubMed] [Google Scholar]
- 11.McCormack JT, Greenwald GS. Evidence for a preimplantation rise in oestradiol-17beta levels on day 4 of pregnancy in the mouse. J Reprod Fertil. 1974;41:297–301. doi: 10.1530/jrf.0.0410297. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266:603–628. doi: 10.1002/jez.1402660610. [DOI] [PubMed] [Google Scholar]
- 13.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 14.Brenner RM, West NB, McClellan MC. Estrogen and progestin receptors in the reproductive tract of male and female primates. Biol Reprod. 1990;42:11–19. doi: 10.1095/biolreprod42.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67:393–409. doi: 10.1679/aohc.67.393. [DOI] [PubMed] [Google Scholar]
- 16.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 17.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, et al. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, et al. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 20.Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, et al. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proc Natl Acad Sci U S A. 2006;103:18350–18355. doi: 10.1073/pnas.0608861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Critchley HO, Brenner RM, Henderson TA, Williams K, Nayak NR, et al. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86:1370–1378. doi: 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- 23.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, et al. Research resource: whole-genome estrogen receptor alpha binding in mouse uterine tissue revealed by ChIP-seq. Mol Endocrinol. 2012;26:887–898. doi: 10.1210/me.2011-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghajanova L. Leukemia inhibitory factor and human embryo implantation. Ann N Y Acad Sci. 2004;1034:176–183. doi: 10.1196/annals.1335.020. [DOI] [PubMed] [Google Scholar]
- 26.Ernst M, Inglese M, Waring P, Campbell IK, Bao S, et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189–203. doi: 10.1084/jem.194.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 28.Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, et al. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141:4365–4372. doi: 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Yin Y, Zhao M, Hu L, Chen Q. The low expression of leukemia inhibitory factor in endometrium: Possible relevant to unexplained infertility with multiple implantation failures. Cytokine. 2013;62:334–339. doi: 10.1016/j.cyto.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Ferenczy A. Studies on the cytodynamics of human endometrial regeneration. I. Scanning electron microscopy. Am J Obstet Gynecol. 1976;124:64–74. doi: 10.1016/0002-9378(76)90013-2. [DOI] [PubMed] [Google Scholar]
- 31.Ferenczy A, Bertrand G, Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133:859–867. doi: 10.1016/0002-9378(79)90302-8. [DOI] [PubMed] [Google Scholar]
- 32.Griffith JS, Jensen SM, Lunceford JK, Kahn MW, Zheng Y, et al. Evidence for the genetic control of estradiol-regulated responses. Implications for variation in normal and pathological hormone-dependent phenotypes. Am J Pathol. 1997;150:2223–2230. [PMC free article] [PubMed] [Google Scholar]
- 33.Pollard JW, Pacey J, Cheng SV, Jordan EG. Estrogens and cell death in murine uterine luminal epithelium. Cell Tissue Res. 1987;249:533–540. doi: 10.1007/BF00217324. [DOI] [PubMed] [Google Scholar]
- 34.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 35.Martin L, Pollard JW, Fagg B. Oestriol, oestradiol-17beta and the proliferation and death of uterine cells. J Endocrinol. 1976;69:103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- 36.Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, et al. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 37.Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- 38.Finn CA, Martin L. Patterns of cell division in the mouse uterus during early pregnancy. J Endocrinol. 1967;39:593–597. doi: 10.1677/joe.0.0390593. [DOI] [PubMed] [Google Scholar]
- 39.Bigsby RM, Cunha GR. Effects of progestins and glucocorticoids on deoxyribonucleic acid synthesis in the uterus of the neonatal mouse. Endocrinology. 1985;117:2520–2526. doi: 10.1210/endo-117-6-2520. [DOI] [PubMed] [Google Scholar]
- 40.Bigsby RM, Cunha GR. Estrogen stimulation of deoxyribonucleic acid synthesis in uterine epithelial cells which lack estrogen receptors. Endocrinology. 1986;119:390–396. doi: 10.1210/endo-119-1-390. [DOI] [PubMed] [Google Scholar]
- 41.Greco TL, Duello TM, Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993;14:59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- 42.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- 45.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 46.Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–8364. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, et al. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 48.Moutsatsou P, Sekeris CE. Steroid receptors in the uterus: implications in endometriosis. Ann N Y Acad Sci. 2003;997:209–222. doi: 10.1196/annals.1290.024. [DOI] [PubMed] [Google Scholar]
- 49.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 50.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 52.Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–3505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- 53.Lee K, Jeong J, Kwak I, Yu CT, Lanske B, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 54.Vermot J, Fraulob V, Dolle P, Niederreither K. Expression of enzymes synthesizing (aldehyde dehydrogenase 1 and reinaldehyde dehydrogenase 2) and metabolizaing (Cyp26) retinoic acid in the mouse female reproductive system. Endocrinology. 2000;141:3638–3645. doi: 10.1210/endo.141.10.7696. [DOI] [PubMed] [Google Scholar]
- 55.Sapin V, Ward SJ, Bronner S, Chambon P, Dolle P. Differential expression of transcripts encoding retinoid binding proteins and retinoic acid receptors during placentation of the mouse. Dev Dyn. 1997;208:199–210. doi: 10.1002/(SICI)1097-0177(199702)208:2<199::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 56.Corthorn J, Valdes G. Variations in uterine kallikrein during cycle and early pregnancy in the rat. Biol Reprod. 1994;50:1261–1264. doi: 10.1095/biolreprod50.6.1261. [DOI] [PubMed] [Google Scholar]
- 57.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 58.Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357:18–29. doi: 10.1016/j.mce.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, et al. Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol. 2012;26:1428–1442. doi: 10.1210/me.2011-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Renzo GC, Giardina I, Clerici G, Mattei A, Alajmi AH, et al. The role of progesterone in maternal and fetal medicine. Gynecol Endocrinol. 2012;28:925–932. doi: 10.3109/09513590.2012.730576. [DOI] [PubMed] [Google Scholar]
- 61.Loutradis D, Beretsos P, Arabatzi E, Anagnostou E, Drakakis P. The role of steroid hormones in ART. J Steroid Biochem Mol Biol. 2008;112:1–4. doi: 10.1016/j.jsbmb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, et al. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20:2104–2117. doi: 10.1093/humrep/dei051. [DOI] [PubMed] [Google Scholar]
- 63.Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 64.Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, et al. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 65.Kao LC, Tulac S, Lobo S, Imani B, Yang JP, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 66.Evans GE, Martinez-Conejero JA, Phillipson GT, Simon C, McNoe LA, et al. Gene and protein expression signature of endometrial glandular and stromal compartments during the window of implantation. Fertil Steril. 2012;97:1365–1373. e1361–1362. doi: 10.1016/j.fertnstert.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagchi IC, Li Q, Cheon YP, Mantena SR, Kannan A, et al. Use of the progesterone receptor antagonist RU 486 to identify novel progesterone receptor-regulated pathways in implantation. Semin Reprod Med. 2005;23:38–45. doi: 10.1055/s-2005-864032. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das RM, Martin L. Progesterone inhibition of mouse uterine epithelial proliferation. J Endocrinol. 1973;59:205–206. doi: 10.1677/joe.0.0590205. [DOI] [PubMed] [Google Scholar]
- 71.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 72.Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A. 2006;103:14021–14026. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray S, Pollard JW. KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing. Proc Natl Acad Sci U S A. 2012;109:E1334–1343. doi: 10.1073/pnas.1118515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cloke B, Christian M. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol. 2012;358:166–175. doi: 10.1016/j.mce.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 78.Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod. 1992;7:1461–1466. doi: 10.1093/oxfordjournals.humrep.a137595. [DOI] [PubMed] [Google Scholar]
- 79.Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]
- 80.Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008;26:72–84. doi: 10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- 81.Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkila M, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- 83.McGrath M, Lee IM, Hankinson SE, Kraft P, Hunter DJ, et al. Androgen receptor polymorphisms and endometrial cancer risk. Int J Cancer. 2006;118:1261–1268. doi: 10.1002/ijc.21436. [DOI] [PubMed] [Google Scholar]
- 84.Kalantaridou SN, Calis KA, Vanderhoof VH, Bakalov VK, Corrigan EC, et al. Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2006;86:1475–1482. doi: 10.1016/j.fertnstert.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 85.Poukka H, Karvonen U, Janne OA, Palvimo JJ. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1) Proc Natl Acad Sci U S A. 2000;97:14145–14150. doi: 10.1073/pnas.97.26.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khulan B, Drake AJ. Glucocorticoids as mediators of developmental programming effects. Best Pract Res Clin Endocrinol Metab. 2012;26:689–700. doi: 10.1016/j.beem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 87.O’Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM. 2000;93:323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 88.Sheppard KE. Corticosteroid receptors, 11 beta-hydroxysteroid dehydrogenase, and the heart. Vitam Horm. 2003;66:77–112. doi: 10.1016/s0083-6729(03)01003-3. [DOI] [PubMed] [Google Scholar]
- 89.Nixon M, Upreti R, Andrew R. 5alpha-Reduced glucocorticoids: a story of natural selection. J Endocrinol. 2012;212:111–127. doi: 10.1530/JOE-11-0318. [DOI] [PubMed] [Google Scholar]
- 90.Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 91.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berger SA, Cole TJ, Schmid W, Schutz G. Molecular genetic analysis of glucocorticoid and mineralocorticoid signaling in development and physiological processes. Steroids. 1996;61:236–239. doi: 10.1016/0039-128x(96)00029-3. [DOI] [PubMed] [Google Scholar]
- 93.Blendy JA, Cole TJ, Montoliu L, Hummler E, Ganss R, et al. Molecular genetic analysis of cAMP and glucocorticoid signaling in development. Recent Prog Horm Res. 1995;50:97–108. doi: 10.1016/b978-0-12-571150-0.50009-3. [DOI] [PubMed] [Google Scholar]
- 94.Cole TJ, Blendy JA, Monaghan AP, Schmid W, Aguzzi A, et al. Molecular genetic analysis of glucocorticoid signaling during mouse development. Steroids. 1995;60:93–96. doi: 10.1016/0039-128x(94)00009-2. [DOI] [PubMed] [Google Scholar]
- 95.Bamberger AM, Milde-Langosch K, Loning T, Bamberger CM. The glucocorticoid receptor is specifically expressed in the stromal compartment of the human endometrium. J Clin Endocrinol Metab. 2001;86:5071–5074. doi: 10.1210/jcem.86.10.8101. [DOI] [PubMed] [Google Scholar]
- 96.Pihoker C, Feeney RJ, Su JL, Handwerger S. Lipocortin-I inhibits the synthesis and release of prolactin from human decidual cells: evidence for autocrine/paracrine regulation by lipocortin-I. Endocrinology. 1991;128:1123–1128. doi: 10.1210/endo-128-2-1123. [DOI] [PubMed] [Google Scholar]
- 97.Neulen J, Zahradnik HP, Flecken U, Breckwoldt M. The effect of cortisol on the synthesis of prostaglandins (PGF2 alpha, PGE2) by human endometrial fibroblasts in vitro with and without addition of estradiol-17 beta or progesterone. Prostaglandins. 1989;37:587–595. doi: 10.1016/0090-6980(89)90074-9. [DOI] [PubMed] [Google Scholar]
- 98.Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, et al. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol. 2001;173:193–202. doi: 10.1016/s0303-7207(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 99.Sporn MB, Roberts AB. Interactions of retinoids and transforming growth factor-beta in regulation of cell differentiation and proliferation. Mol Endocrinol. 1991;5:3–7. doi: 10.1210/mend-5-1-3. [DOI] [PubMed] [Google Scholar]
- 100.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 101.Sucov HM, Evans RM. Retinoic acid and retinoic acid receptors in development. Mol Neurobiol. 1995;10:169–184. doi: 10.1007/BF02740674. [DOI] [PubMed] [Google Scholar]
- 102.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 103.Kumarendran MK, Loughney AD, Prentice A, Thomas EJ, Redfern CP. Nuclear retinoid receptor expression in normal human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1996;2:123–129. doi: 10.1093/molehr/2.2.123. [DOI] [PubMed] [Google Scholar]
- 104.Fukunaka K, Saito T, Wataba K, Ashihara K, Ito E, et al. Changes in expression and subcellular localization of nuclear retinoic acid receptors in human endometrial epithelium during the menstrual cycle. Mol Hum Reprod. 2001;7:437–446. doi: 10.1093/molehr/7.5.437. [DOI] [PubMed] [Google Scholar]
- 105.Deng L, Shipley GL, Loose-Mitchell DS, Stancel GM, Broaddus R, et al. Coordinate regulation of the production and signaling of retinoic acid by estrogen in the human endometrium. J Clin Endocrinol Metab. 2003;88:2157–2163. doi: 10.1210/jc.2002-021844. [DOI] [PubMed] [Google Scholar]
- 106.Brar AK, Kessler CA, Meyer AJ, Cedars MI, Jikihara H. Retinoic acid suppresses in-vitro decidualization of human endometrial stromal cells. Mol Hum Reprod. 1996;2:185–193. doi: 10.1093/molehr/2.3.185. [DOI] [PubMed] [Google Scholar]
- 107.Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 108.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, et al. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 109.Song Y, Yao X, Ying H. Thyroid hormone action in metabolic regulation. Protein Cell. 2011;2:358–368. doi: 10.1007/s13238-011-1046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poppe K, Glinoer D, Tournaye H, Devroey P, Schiettecatte J, et al. Thyroid autoimmunity and female infertility. Verh K Acad Geneeskd Belg. 2006;68:357–377. [PubMed] [Google Scholar]
- 111.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 112.Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, et al. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 113.Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, et al. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Catalano RD, Critchley HO, Heikinheimo O, Baird DT, Hapangama D, et al. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol Hum Reprod. 2007;13:641–654. doi: 10.1093/molehr/gam021. [DOI] [PubMed] [Google Scholar]
- 115.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, et al. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95:230–237. 237 e231–232. doi: 10.1016/j.fertnstert.2010.06.079. [DOI] [PubMed] [Google Scholar]
- 116.Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod Toxicol. 2009;27:246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 117.Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, et al. PPAR expression and function during vertebrate development. Int J Dev Biol. 2002;46:105–114. [PubMed] [Google Scholar]
- 118.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 119.Yang J, Chen L, Zhang X, Zhou Y, Zhang D, et al. PPARs and Female Reproduction: Evidence from Genetically Manipulated Mice. PPAR Res. 2008;2008:723243. doi: 10.1155/2008/723243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishimura K, Yamauchi N, Chowdhury VS, Torii M, Hattori MA, et al. Expression of peroxisome proliferator-activated receptor isoforms in the rat uterus during early pregnancy. Cell Tissue Res. 2011;345:275–284. doi: 10.1007/s00441-011-1208-4. [DOI] [PubMed] [Google Scholar]
- 121.Ding NZ, Ma XH, Diao HL, Xu LB, Yang ZM. Differential expression of peroxisome proliferator-activated receptor delta at implantation sites and in decidual cells of rat uterus. Reproduction. 2003;125:817–825. doi: 10.1530/rep.0.1250817. [DOI] [PubMed] [Google Scholar]
- 122.Ding NZ, Teng CB, Ma H, Ni H, Ma XH, et al. Peroxisome proliferator-activated receptor delta expression and regulation in mouse uterus during embryo implantation and decidualization. Mol Reprod Dev. 2003;66:218–224. doi: 10.1002/mrd.10348. [DOI] [PubMed] [Google Scholar]
- 123.Mukherjee R, Jow L, Croston GE, Paterniti JR., Jr. Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARgamma2 versus PPARgamma1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem. 1997;272:8071–8076. doi: 10.1074/jbc.272.12.8071. [DOI] [PubMed] [Google Scholar]
- 124.Julan L, Guan H, van Beek JP, Yang K. Peroxisome proliferator-activated receptor delta suppresses 11beta-hydroxysteroid dehydrogenase type 2 gene expression in human placental trophoblast cells. Endocrinology. 2005;146:1482–1490. doi: 10.1210/en.2004-1357. [DOI] [PubMed] [Google Scholar]
- 125.Wang H, Xie H, Sun X, Tranguch S, Zhang H, et al. Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor delta signaling is critical to pregnancy success. J Biol Chem. 2007;282:37770–37782. doi: 10.1074/jbc.M706577200. [DOI] [PubMed] [Google Scholar]
- 126.Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martinez N, Kurtz M, Capobianco E, Higa R, White V, et al. PPARalpha agonists regulate lipid metabolism and nitric oxide production and prevent placental overgrowth in term placentas from diabetic rats. J Mol Endocrinol. 2011;47:1–12. doi: 10.1530/JME-10-0173. [DOI] [PubMed] [Google Scholar]
- 128.Yessoufou A, Hichami A, Besnard P, Moutairou K, Khan NA. Peroxisome proliferator-activated receptor alpha deficiency increases the risk of maternal abortion and neonatal mortality in murine pregnancy with or without diabetes mellitus: Modulation of T cell differentiation. Endocrinology. 2006;147:4410–4418. doi: 10.1210/en.2006-0067. [DOI] [PubMed] [Google Scholar]
- 129.Barak Y, Liao D, He W, Ong ES, Nelson MC, et al. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 131.Litchfield LM, Klinge CM. Multiple roles of COUP-TFII in cancer initiation and progression. J Mol Endocrinol. 2012;49:R135–148. doi: 10.1530/JME-12-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee KY, Jeong JW, Wang J, Ma L, Martin JF, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Franco HL, Dai D, Lee KY, Rubel CA, Roop D, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, et al. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995;92:10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 138.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300:104–108. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 139.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, et al. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang C, Large MJ, Duggavathi R, Demayo FJ, Lydon JP, et al. Liver receptor homolog-1 is essential for pregnancy. Nat Med. 2013 doi: 10.1038/nm.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dube C, Bergeron F, Vaillant MJ, Robert NM, Brousseau C, et al. The nuclear receptors SF1 and LRH1 are expressed in endometrial cancer cells and regulate steroidogenic gene transcription by cooperating with AP-1 factors. Cancer Lett. 2009;275:127–138. doi: 10.1016/j.canlet.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 143.Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- 144.Kennaway DJ, Boden MJ, Varcoe TJ. Circadian rhythms and fertility. Mol Cell Endocrinol. 2012;349:56–61. doi: 10.1016/j.mce.2011.08.013. [DOI] [PubMed] [Google Scholar]