Abstract
In humans, ingested inorganic arsenic is metabolized to monomethylarsenic (MMA) then to dimethylarsenic (DMA), although in most people this process is not complete. Previous studies have identified associations between the proportion of urinary MMA (%MMA) and increased risks of several arsenic-related diseases, although none of these reported on lung cancer. In this study, urinary arsenic metabolites were assessed in 45 lung cancer cases and 75 controls from arsenic-exposed areas in Cordoba, Argentina. Folate has also been linked to arsenic-disease susceptibility, thus an exploratory assessment of associations between single nucleotide polymorphisms in folate metabolizing genes, arsenic methylation, and lung cancer was also conducted. In analyses limited to subjects with metabolite concentrations above detection limits, the mean %MMA was higher in cases than in controls (17.5% versus 14.3%, p = 0.01). The lung cancer odds ratios for subjects with %MMA in the upper tertile compared to those in the lowest tertile was 3.09 (95% CI, 1.08–8.81). Although the study size was too small for a definitive conclusion, there was an indication that lung cancer risks might be highest in those with a high %MMA who also carried cystathionine β-synthase (CBS) rs234709 and rs4920037 variant alleles. This study is the first to report an association between individual differences in arsenic metabolism and lung cancer, a leading cause of arsenic-related mortality. These results add to the increasing body of evidence that variation in arsenic metabolism plays an important role in arsenic-disease susceptibility.
Keywords: arsenic, lung cancer, drinking water, metabolism
Inorganic arsenic (InAs) occurs naturally in the groundwater and surface water of many parts of the world, and tens of millions of people worldwide are exposed to drinking water containing this known carcinogen (Nordstrom, 2002). Based on epidemiologic evidence from several countries, the International Agency for Research on Cancer (IARC) has concluded that ingestion of inorganic arsenic causes cancer of the bladder, skin, and lung. Other evidence suggests that of all of the various malignant and non-malignant diseases linked to arsenic ingestion, lung cancer is the most common cause of arsenic-related mortality (Smith et al., 1998; IARC, 2002). The excess risks associated with drinking water arsenic may be quite high (Chen et al., 1992; Smith et al., 1992; NRC, 1999; Morales et al., 2000; NRC, 2001). The National Research Council has estimated that the excess cancer risk associated with lifetime exposures to arsenic at the US regulatory drinking water standard of 10 μg/L may be close to 1 in 300 (NRC, 2001). Risks may be even higher in susceptible subpopulations if they exist. These risks are about 30 to 300 times higher than the cancer risks estimated for exposure to all other known drinking water carcinogens at concentrations equal to their current US drinking water standard (Smith et al., 2002).
The primary metabolic pathway of ingested InAs in humans is methylation (Gebel, 2002; Styblo et al., 2002; Vahter, 2002). Once ingested, InAs is methylated to monomethylarsonic acid (MMA5) which is reduced to monomethylarsonous acid (MMA3). MMA3 is then methylated to dimethylarsinic acid (DMA5) which is reduced to dimethylarsinous acid (DMA3). In humans, this process is not complete, and some arsenic remains as InAs and MMA (MMA3 and MMA5). Almost all ingested arsenic is excreted through the urine and the relative distribution of arsenic metabolites in urine is commonly used as a biomarker of how well an individual can fully methylate ingested InAs (NRC, 1999). Typically, ingested InAs is excreted as 10–20% InAs, 10–15% MMA, and 60–75% DMA (Hopenhayn-Rich et al., 1993). However, large inter-individual variations exist (Vahter, 1999b).
Until recently, methylation of InAs was thought to be primarily a detoxification pathway since the methylated species most commonly found in human urine, MMA5 and DMA5, are more water soluble, more readily excreted, and less acutely toxic than InAs (Buchet et al., 1981a; Buchet et al., 1981b; Moore et al., 1997; Hughes and Kenyon, 1998; Gebel, 2002). MMA3 and DMA3 are highly unstable in human urine and so have been measured in only a few human studies. However, there is increasing evidence that MMA3 is much more toxic in vitro than its pentavalent form, and more toxic than InAs (Cullen et al., 1989; Styblo et al., 1997; Lin et al., 1999; Styblo et al., 1999; Petrick et al., 2000; Styblo et al., 2000; Lin et al., 2001; Mass et al., 2001).
Epidemiological studies have reported associations between individual methylation patterns, specifically the proportion of MMA in urine (%MMA), and the risks of several different arsenic-related diseases including bladder cancer, skin cancer, and arsenic-caused skin lesions (Del Razo et al., 1997; Hsueh et al., 1997; Yu et al., 2000; Chen et al., 2003a; Chen et al., 2003b; Tseng et al., 2005; Steinmaus et al., 2006; Wu et al., 2006; Ahsan et al., 2007; Huang et al., 2007; McCarty et al., 2007; Pu et al., 2007; Huang et al., 2008; Lindberg et al., 2008). These data provide a highly consistent body of evidence linking methylation capacity, and specifically high %MMA, to arsenic-related disease risks. However, to date no study has reported on the potential association between arsenic metabolism and lung cancer.
Several studies have also linked folate intake and folate metabolism to arsenic metabolism and arsenic-related disease risks (Gamble et al., 2005; Chen et al., 2007; Gamble et al., 2007; Huang et al., 2007; Kile and Ronnenberg, 2008). The results of these studies have led to the hypothesis that variants in genes that code for folate metabolizing enzymes could account for some inter-individual variation in arsenic metabolism and arsenic-related disease susceptibility. For this reason, we have also performed a preliminary investigation on whether certain polymorphisms in folate metabolizing genes, such as cystathionine B-synthase (CBS), might affect the relationship between arsenic metabolism and lung cancer relative risks.
Methods
The participants of this study were a subgroup of subjects from a case-control study of lung cancer and arsenic in drinking water (publication in progress). The study area for this investigation was the county of Unión in the Province of Córdoba, Argentina, where many private wells are contaminated with arsenic. All newly diagnosed incident cases of primary lung cancer, aged 20 to 80 who were living in Unión, were identified through rapid case ascertainment from 2000–2006 involving all pathologists and pulmonary medicine physicians in the county, and from radiographic services. In the original study, controls, individually matched to cases by sex and exact year of birth, were selected from computerized voter registration lists. The participants in the current study included all cases and controls in the original study who agreed to provide urine samples for arsenic metabolite measurements.
This study was approved by ethical review boards in the US and Argentina, and informed consent was obtained from all participants. All subjects were administered standardized questionnaires in their homes. Information sought included residential history, water sources at each current and past residence, smoking, and occupation. Buccal cell samples for DNA and a single first morning urine sample were also collected by study personnel during the home visits. A previous study has shown that a moderately strong correlation exists between arsenic concentrations in single first morning samples and samples collected over 24 hours (Calderon et al., 1999). Urine samples were kept frozen in the field laboratory at −20° and then transported on dry ice to the University of Washington, Seattle for analysis. The urinary concentrations of inorganic arsenic and its metabolites were measured using hydride generation atomic absorption spectroscopy (Crecelius, 1978). Details of the laboratory methods are described elsewhere (Chung et al., 2002). Detection limits for InAs, MMA, and DMA were 0.5, 1.0, and 2.0 μg/L, respectively. The corresponding replicate precisions were 15%, 17% and 11%. The MMA and DMA measured in this study are the sums of the trivalent and pentavalent forms. The trivalent forms, MMA3 and DMA3, are rapidly oxidized during storage and at the time of this study could not be reliably measured in field studies (Del Razo et al., 2001). Most samples were stored frozen for one to four months before analysis.
DNA was isolated from buccal samples using the PUREGENE™ DNA Purification Kit (Gentra Systems Inc., Minneapolis, MN) and quantified using PicoGreen dsDNA quantitation kits (Molecular Probes, Eugene, OR). All DNA samples were whole genome amplified using GenomiPhi DNA Amplification kits (Amersham BioSciences Corp., Piscataway, NJ). TaqMan® assays were obtained from the Assays-on-Demand service (Applied Biosystems, Foster City, CA) to genotype the SNPs listed below.
Polymorphisms in CBS rs234709 and rs4920037; methyltetrahydrofolate (MTHFR) rs1801133 and rs1801131; methionine synthase (MTR) rs1805087; thymidylate synthase (TYMS) rs16430; dihydrofolate reductase (DHFR) rs2618372; and serine hydroxymethyltransferase 1 (SHMT1) rs1979277 were selected a priori because they encode enzymes involved in folate metabolism. Polymorphisms in glutathione-S-transferase-1 (GSTO1) rs11509435 and rs4925 were assessed due to their modest associations with urinary %MMA seen in previous studies (Marnell et al., 2003; Meza et al., 2005; Lindberg et al., 2007; McCarty et al., 2007; Steinmaus et al., 2007). Polymorphisms were selected, especially those with non-synonymous amino acid changes, using the dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) and SNPper (http://snpper.chip.org/) databases. Genotyping was carried out using TaqMan® SNP Genotyping Assays (Applied Biosystems, Foster City, CA). Amplification reactions (95° C for 10 minutes, then 40 cycles of 95 °C for 15 seconds and 60° C for 1 minute) were performed on the ABI 9700 GeneAmp PCR system and a post-PCR read using the ABI 7700 SDS was performed to determine genotypes.
The proportion of arsenic in each species (%InAs, %MMA, and %DMA) was calculated by dividing the concentration of arsenic in each species by the sum of the concentrations of InAs, MMA, and DMA. At low metabolite concentrations, relatively small inaccuracies in laboratory measurements can cause relatively large errors when calculating metabolite proportions. In addition, the choice of methods used to assign values to subjects with metabolite concentrations below detection can also have large effects on metabolite proportion calculations. For example, in a subject with a total urinary arsenic (InAs + MMA + DMA) of 5 μg/L, assigning values for MMA to subjects with MMA concentrations below detection of either zero, the limit of detection divided by the square root of two, or at the limit of detection (1 μg/L) will give %MMA values of either 0%, 14%, or 20%, which would place the subject in either the lower, middle, or upper tertiles, respectively, of %MMA in this study. For these reasons, we excluded subjects who had InAs, MMA, and DMA concentrations below detection levels. The impact of this was assessed by performing separate analyses where all subjects were used and concentrations below detection were set at ½ the detection level.
Past arsenic exposure was assessed by linking information on residential water sources to arsenic water concentrations, obtained either through historic water records or from water samples we collected from as many current and past residences as possible. The focus of assessing past exposure was on well water, since previous research has shown that arsenic exposure in this area comes almost exclusively from wells (Bates et al., 2004). Arsenic concentrations in some wells used in the past could not be measured since some wells were closed or could not be located. All water samples collected were frozen at −20° C, transported to the United States on dry ice, and analyzed for arsenic content using graphite furnace atomic absorption spectroscopy, with a detection limit of 0.5 μg/L. Using these data, a year-by-year arsenic exposure profile was created for each subject. Since the focus was on well water, and since previous research suggests that arsenic-related cancer risks are more dependent on arsenic concentration than on cumulative exposure (Lubin et al., 2008), subjects were categorized based on whether or not they ever used well water in the study area and if so, the highest known well water arsenic concentration to which they were exposed.
Unconditional logistic regression was used to calculate lung cancer odds ratios (OR) comparing subjects with high and low proportions of all three arsenic metabolites (InAs, MMA, and DMA), with our focus being primarily on %MMA. The one to one case-control matching in the original case-control study was not retained since not all subjects in the original study provided urine samples. Category cutoff points for defining low, medium, and high %MMA were based on tertiles. Odds ratios were adjusted for age (≤ 65 versus > 65 years old), gender, smoking (ever versus never), and historical arsenic exposure in drinking water. This last variable was categorized as either (1) never used a well (and therefore presumed to have had low exposure), (2) used a well in the study area, but that well was closed or could not be found (“no measurement”) (3) used a well in the study area and the highest known arsenic concentration among all wells used was below 100 μg/L, or (4) used a well in the study area and the highest known arsenic concentration among all wells used was ≥ 100 μg/L. Entering highest known arsenic concentration in a greater number of categories or as a continuous variable had little impact on results.
Lung cancer ORs for genetic polymorphisms were calculated with logistic regression using the same methods described above. To assess whether a genetic polymorphism might affect the relationship between lung cancer and %MMA, lung cancer ORs were calculated for each percentage point increase in %MMA in analyses stratified by each genetic polymorphism. In preliminary analyses, for each SNP assessed, the associations between genotypes and %MMA, %DMA and %InAs were analyzed using multivariate linear regression, adjusted for age, gender, current smoking status, case-status, and total urinary arsenic. Mean %InAs, %MMA, %DMA levels in subjects with wildtype genotypes were compared to those with heterozygous and variant homozygous genotypes, separately and combined. Data on the impact of SNPs on arsenic methylation-lung cancer risks are only presented for CBS in this paper since this was the only gene related to arsenic metabolism (i.e. a linear regression p-value less than 0.05) (These p-values were not adjusted for multiple comparisons since these analyses were exploratory and based on a priori hypotheses). For the analyses presented in this paper, CBS heterozygotes and homozygous variants were combined into one stratum because of the small sample size. All data analyses were carried out using the SAS statistical program package (Version 8.0e, SAS Institute, Cary, NC). All p-values are two-sided.
Results
Overall, 141 cases and 252 controls were eligible for participation in the case-control study. Of these 109 (77%) of cases and 141 (56%) of the controls were interviewed and provided a urine sample. Eleven cases (8%) and 30 (12%) controls declined participation, and 21 cases (15%) and 81 controls (32%) could either not be located or were too ill to participate. Of those who provided a urine sample, 45 cases (41.3%) and 75 controls (53.2%) had levels of all three arsenic metabolites above the detection levels. Table 1 shows descriptive characteristics and arsenic exposure information of the study subjects. Cases were more likely to be current or former smokers (unadjusted OR = 4.31.; 95% confidence interval (CI), 2.26–8.20). In the analysis of all subjects in the original study (i.e. regardless of whether or not they provided urine samples), increased lung cancer risks were found for those with highest known water arsenic concentrations above 200 μg/L (OR=2.2), but with a wide confidence interval (95% CI, 0.7–7.3). Increased risks were more pronounced in those who smoked and who were exposed to arsenic in drinking water starting more than 40 years before diagnosis (details to be reported in a separate publication).
Table 1.
Sociodemographic characteristics of lung cancer cases and controls.
| All subjects
|
Subjects with all arsenic metabolites above detection levels
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases
|
Controls
|
Cases
|
Controls
|
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Total | 109 | 100 | 141 | 100 | 45 | 100 | 75 | 100 |
| Age (years) | ||||||||
| <65 | 43 | 39.4 | 57 | 40.4 | 17 | 37.8 | 35 | 46.7 |
| 65–75 | 40 | 36.7 | 45 | 31.9 | 21 | 46.7 | 23 | 30.7 |
| >75 | 26 | 23.9 | 39 | 27.7 | 7 | 15.6 | 17 | 22.7 |
| Gender | ||||||||
| Female | 19 | 17.4 | 24 | 17.0 | 4 | 8.9 | 10 | 13.3 |
| Male | 90 | 82.6 | 117 | 83.0 | 41 | 91.1 | 65 | 86.7 |
| Smoking | ||||||||
| Current | 41 | 37.6 | 35 | 24.8 | 18 | 40.0 | 25 | 33.3 |
| Former | 55 | 50.5 | 54 | 38.3 | 24 | 53.3 | 25 | 33.3 |
| Never | 13 | 11.9 | 52 | 36.9 | 3 | 6.7 | 25 | 33.3 |
| Past drinking water InAs | ||||||||
| Never used a wella | 54 | 49.5 | 66 | 46.8 | 21 | 46.7 | 36 | 48.0 |
| Used a well:a | ||||||||
| No measurementb | 33 | 30.3 | 44 | 31.2 | 9 | 20.0 | 20 | 26.7 |
| < 99 μg/Lc | 6 | 5.5 | 16 | 11.4 | 3 | 6.7 | 9 | 12.0 |
| 100–199 μg/Lc | 7 | 6.4 | 10 | 7.1 | 5 | 11.1 | 6 | 8.0 |
| ≥ 200 μg/Lc | 9 | 8.3 | 5 | 3.6 | 7 | 15.6 | 4 | 5.3 |
Drinking water arsenic exposure in the study area comes almost exclusively from well water, so the assessment of past arsenic exposure focused on the use of well water.
These are subjects who reported that they used water from a well in the study area, but an arsenic concentration could not be taken because the well was used in the past and was either closed or could not be located.
Highest known arsenic concentration among all measured wells used by the subject in the study area.
Table 2 shows the mean relative proportions of each arsenic species stratified by case status, gender, smoking, age, and urinary arsenic. %MMA was higher in cases than controls (17.5 vs. 14.3%, p = 0.01). The mean concentrations (and ranges) of urinary InAs, MMA, and DMA in all subjects with levels above detection were 3.8 μg/L (0.5–17.1 μg/L), 3.8 μg/L (1.0–20.2 μg/L), and 17.1 μg/L (2.0–78.2 μg/L) (not shown in tables). Table 3 displays the unadjusted and adjusted odds ratios for the association between lung cancer and urinary %MMA for those subjects with metabolite concentrations above the detection limits. The adjusted lung cancer OR for subjects with %MMA in the middle and upper tertiles compared to those in the lower tertile were 0.85 (95% CI, 0.29–2.51) and 3.09 (95% CI, 1.08–8.81), respectively. The logistic regression lung cancer OR for %MMA as a continuous variable was 1.106 (p = 0.008) suggesting that each one percentile increase in %MMA (e.g. a %MMA of 15.0% versus a %MMA of 14.0%) is associated with about a 10.6% increase in lung cancer risk. In the analysis using all subjects who provided urine and setting metabolite levels below detection at ½ the detection limit, the adjusted lung cancer OR comparing the upper and lower tertiles of %MMA was 2.14 (95% CI, 0.91–5.06) (not shown in tables). The lung cancer ORs for %DMA, %InAs, MMA/InAs, and DMA/MMA tertiles can be found at http://asrg.berkeley.edu/index.html. %DMA was less strongly associated with lung cancer than was %MMA. The adjusted lung cancer OR comparing subjects in the upper and lower tertiles of %DMA was 0.44 (95% CI, 0.16–1.23).
Table 2.
Univariate analyses of the mean proportions of each arsenic species (standard deviation).a
| Variable | N | % | %InAs | %MMA | %DMA |
|---|---|---|---|---|---|
| All | 120 | 100 | 15.7 (5.5) | 15.5 (6.7) | 68.7 (9.6) |
| Lung cancer | |||||
| Cases | 45 | 37.5 | 16.0 (6.7) | 17.5 (7.3) | 66.5 (11.1) |
| Controls | 75 | 62.5 | 15.7 (4.7) | 14.3 (5.9) | 70.1 (8.3) |
| p-valueb | 0.77 | 0.01 | 0.07 | ||
| Gender | |||||
| Women | 14 | 11.7 | 15.0 (5.9) | 14.5 (5.5) | 70.5 (9.8) |
| Men | 106 | 88.3 | 15.9 (5.5) | 15.6 (6.8) | 68.5 (9.5) |
| p-valueb | 0.57 | 0.54 | 0.45 | ||
| Smoking | |||||
| Current | 43 | 35.8 | 15.1 (5.4) | 14.7 (6.5) | 70.2 (10.3) |
| Ex-smokers | 49 | 40.8 | 16.2 (5.9) | 16.1 (6.3) | 67.7 (8.6) |
| Never | 28 | 23.3 | 16.1 (5.0) | 15.7 (7.6) | 68.2 (10.1) |
| p-valuec | 0.46 | 0.25 | 0.64 | ||
| Age (years) | |||||
| <65 | 52 | 43.3 | 15.9 (4.9) | 14.9 (6.5) | 69.2 (9.4) |
| 65–75 | 44 | 36.7 | 15.2 (6.2) | 16.1 (7.6) | 68.7 (10.7) |
| >75 | 24 | 20.0 | 16.5 (5.6) | 15.8 (5.3) | 67.7 (8.0) |
| p-valued | 0.80 | 0.36 | 0.62 | ||
| Total urinary arsenice | |||||
| Low tertile | 40 | 33.3 | 16.4 (4.7) | 16.2 (5.2) | 67.3 (8.0) |
| Medium tertile | 40 | 33.3 | 15.9 (6.5) | 14.7 (6.1) | 69.4 (9.5) |
| High tertile | 40 | 33.3 | 15.0 (5.3) | 15.5 (8.3) | 69.5 (11.0) |
| R (p-value)f | −0.11 (0.23) | −0.13 (0.17) | 0.14 (0.14) |
Includes only subjects with InAs, MMA, and DMA above detection levels
t-test for differences in means
t-test for differences in means comparing ever versus never smokers
t-test for differences in means comparing age < 65 years to age ≥ 65 years
Total urinary arsenic (μg/L) = InAs + MMA + DMA. Total urinary arsenic ranged from 4.8 to 112.3 μg/L, and the tertile cut-off points were 13.3 and 25.4 μg/L
Spearman correlation coefficient and associated p-value between total urinary arsenic as a continuous variable and the proportion of each metabolite as a continuous variable
Table 3.
Lung cancer odds ratios (OR) by tertile of %MMA in subjects with metabolite levels above detection limits.
| Crude
|
Adjusteda
|
||||||
|---|---|---|---|---|---|---|---|
| Cases | Control | OR | 95% CI | OR | 95% CI | p-valueb | |
| Low %MMAc | 12 | 28 | 1.00 | Reference | 1.00 | Reference | |
| Medium %MMA | 12 | 28 | 1.00 | 0.38–2.60 | 0.85 | 0.29–2.51 | |
| High %MMA | 21 | 19 | 2.58 | 1.03–6.46 | 3.09 | 1.08–8.81 | p-trend = 0.04 |
| %MMA continuousd | 1.078 | 1.015–1.145 | 1.106 | 1.026–1.191 | p = 0.008 | ||
Adjusted for age (≤ 65 versus > 65 years old), gender, smoking (ever versus never), and historic drinking water arsenic exposure (no well use, no measurement, 0–99 μg/L, ≥ 100 μg/L).
p-values for Mantel-Haenszel chi-square test for trend across lung cancer odds ratios by tertiles of %MMA or for the lung cancer odds ratio for %MMA as a continuous variable in the logistic regression analysis.
Low, medium and high are based on %MMA tertiles in all subjects with metabolite levels above the detection limit. The upper and lower tertiles are 17.2 and 11.8%. The range for the low, medium, and high %MMA tertiles are 3.8–11.81%, 11.84–17.22%, and 17.28–42.5%, respectively.
Logistic regression where the dependent variable is lung cancer case status and the independent variable is %MMA as a continuous variable. The values given are the odds ratio and its 95% confidence interval for each 1% increase in %MMA.
Data on CBS rs234709 and rs4920037 polymorphisms were available for 207 and 212 subjects, respectively. No association was seen between lung cancer and CBS rs234709 or rs4920037 polymorphisms: the lung cancer OR adjusted for age, gender, smoking, and highest known arsenic exposure comparing CBS rs234709 wildtypes to non-wildtypes was 0.89 (95% CI, 0.49–1.59). The corresponding OR for CBS rs4920037 was 0.92 (95% CI, 0.50–1.69) (data not shown in tables). No associations with lung cancer were seen with the other genetic polymorphisms we assessed.
Variant genotypes for CBS rs234709 and rs4920037 SNPs compared with wild-type homozygotes were associated with 24% (p = 0.01) and 26% (p = 0.02) increases, respectively, in mean %MMA. Table 4 shows the lung cancer odds ratios for each one percentage point increase in %MMA, stratified by the CBS polymorphism. All of these analyses involved small numbers of subjects and none of the results was statistically significant. However, for both CBS rs234709 and CBS rs4920037, lung cancer-%MMA associations appear somewhat greater in subjects with non-wildtype alleles than in subjects with wildtype alleles. The lung cancer odds ratios comparing subjects in the upper tertile of %MMA with those in the lower tertile were 0.33 (95% CI, 0.04–2.86) and 3.34 (95% CI, 0.53–20.9) for subjects with CBS rs234709 wildtype and non-wildtype genotypes, respectively (not shown in Tables). The corresponding odds ratios for subjects with CBS rs4920037 wildtype and non-wildtype genotypes were 1.43 (95% CI, 0.32–6.35) and 9.48 (95% CI, 0.20–448), respectively.
Table 4.
Lung cancer adjusted odds ratios (OR)a for each percentage point increase in %MMA, in analyses stratified by cystathionine synthase (CBS) polymorphism
| Polymorphism | N | ORb | 95% CI |
|---|---|---|---|
| CBS rs234709 CC (wildtype) | 45 | 0.89 | 0.74–1.07 |
| CBS rs234709 CT or TT (non-wildtype) | 60 | 1.10 | 0.99–1.22 |
| CBS rs4920037 GG (wildtype) | 68 | 1.04 | 0.94–1.15 |
| CBS rs4920037 GA and AA (non-wildtype) | 38 | 1.14 | 0.91–1.42 |
Logistic regression odds ratio where the dependent variable is lung cancer case status and the independent variable is %MMA as a continuous variable. The values given are the odds ratio and its 95% confidence interval for each 1% increase in %MMA.
Adjusted for age (≤ 65 versus > 65 years old), gender, smoking (ever versus never), and highest level of past known arsenic exposure (no well use, no measurement, 0–99 μg/L, ≥ 100 μg/L). Only includes subjects with arsenic metabolite concentrations above the detection limit.
Discussion
The lung cancer OR of 3.09 (95% CI, 1.08–8.81; p-trend = 0.04) comparing the upper tertile of %MMA to the lower tertile of %MMA, and the lung cancer OR of 1.106 (95% CI, 1.026–1.191; p-value = 0.008) for %MMA as a continuous variable are evidence that subjects who are less effective at methylating MMA to DMA are at greater risks of arsenic-related lung cancer than others. Although the number of subjects in this study is relatively small, the low p-values and the consistency of our results with other human, animal, and laboratory studies suggests that these findings are not due to chance and could represent real effects.
Data on associations between %MMA and increased relative risks of arsenic-related disease in humans are shown in Table 5. A few of the results in this table are for MMA/DMA ratio rather than %MMA. These were included because inter-individual variability in MMA/DMA ratios is more dependent on %MMA than %DMA since inter-individual variability in %MMA is generally much greater than inter-individual variability in %DMA (Buchet et al., 1984; Hopenhayn-Rich et al., 1996; Vahter, 1999a). Thus, variability in MMA/DMA ratios is more likely due to differences in %MMA than differences in %DMA. As seen in Table 5, in every study except for one the odds ratios for arsenic-related disease are higher in those with higher %MMA or higher MMA/DMA ratios. As a whole, these studies provide a fairly large and consistent body of evidence linking %MMA to arsenic-related disease risks.
Table 5.
Epidemiologic studies of arsenic metabolism and relative risks of arsenic-related disease.
| Low %MMA
|
High %MMA
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Study (Country) | Outcome | Definition | OR | 95% CI | Definition | OR | 95% CI | Strata |
| Current study (Argentina) | Lung cancer | %MMA < 11.8% | 1.00 | Ref | %MMA > 17.2% | 3.09 | 1.08–8.81 | All |
| Chen et al., 2003 (Taiwan) | Bladder cancer | MMA/DMA < 0.21 | 1.12 | 0.26–4.77a | MMA/DMA > 0.21 | 4.23 | 1.12–16.01 | CAE > 12 mg/L-yr |
| Steinmaus et al., 2006 (Argentina) | Bladder cancer | %MMA < 16.7% | 1.00 | Ref | %MMA ≥ 16.7% | 2.17 | 1.02–4.63 | Smokersb |
| Steinmaus et al., 2006 (US) | Bladder cancer | %MMA < 14.9% | 1.00 | Ref | %MMA ≥ 14.8% | 2.70 | 0.39–18.6 | Intake > 100 μg/day |
| Pu et al., 2007 (Taiwan) | Bladder cancer | %MMA ≤ 3.0% | 1.00 | Ref | %MMA > 9.2% | 2.8 | 1.6–4.8 | All |
| Huang et al., 2008 (Taiwan) | Bladder cancer | %MMA < 11.40% | 1.5 | 0.4–5.9a | %MMA ≥ 11.40% | 3.7 | 1.2–11.6 | CAE ≥ 20 mg/L-yr |
| Hsueh et al., 1997 (Taiwan) | Skin cancer | %MMA ≤ 26.7% | 8.35 | 1.07–65.0a | %MMA > 26.7% | 23.9 | 2.55–225 | CAE > 20 mg/L-yr |
| Yu et al., 2000 (Taiwan) | Skin cancer | %MMA ≤ 15.5% | 1.00 | Ref | %MMA > 15.5% | 5.50 | 1.22–24.81 | All |
| Chen et al., 2003 (Taiwan) | Skin cancer | MMA/DMA < 0.2 | 1.89 | 0.60–6.01a | MMA/DMA > 0.20 | 7.48 | 1.65–33.99 | CAE > 15 mg/L-yr |
| Ahsan et al., 2007 (Bangladesh) | Skin lesions | %MMA < 9.8% | 1.00 | Ref | %MMA > 16.4% | 1.57 | 1.10–2.26 | All |
| McCarty et al., 2007 (Bangladesh) | Skin lesions | na | 1.00 | Ref | 10X MMA/InAsc | 1.50 | 1.00–2.26 | All |
| Lindberg et al., 2008 (Bangladesh) | Skin lesions | %MMA ≤ 7.9% | 1.00 | Ref | %MMA > 12% | 2.8 | 1.9–4.2 | All |
| Tseng et al., 2005 (Taiwan) | PVD | %MMA ≤ 11.42 | 2.64 | 0.56–12.4a | %MMA > 11.42% | 4.57 | 1.01–20.61 | CAE > 0 |
| Wu et al., 2006 (Taiwan) | Atherosclerosis | %MMA < 13.4% | 1.7 | 0.6–4.5a | %MMA ≥ 13.4% | 2.7 | 1.0–7.8 | CAE > 1.7 mg/L-yr |
| Huang et al., 2007 (Taiwan) | Hypertension | %MMA < 8.14% | 1.00 | Ref | %MMA ≥ 15.55% | 1.04 | 0.66–1.62 | All |
Abbreviations: CAE, cumulative arsenic exposure; CI, confidence interval; OR, odds ratio; PVD, peripheral vascular disease; Ref, reference group
Reference groups (where OR = 1.00) are those with low %MMA or MMA/DMA ratio and low CAE.
No statistically significant effect seen in non-smokers.
Relative risk for a 10-fold increase in MMA/InAs ratio.
The study of hypertension by Huang et al. (2007) is the only published study of inter-individual differences in arsenic metabolism that did not find a clear association. The reason for this is unknown. It is possible that arsenic does not cause hypertension (the evidence linking arsenic to hypertension is not as strong as it is for the other outcomes assessed in Table 5) or that it causes hypertension by a mechanism that is different from other arsenic-related diseases, although the later is difficult to evaluate since the exact mechanisms of arsenic toxicity are unknown. Interestingly, in the Huang et al. study mean %MMA levels were somewhat higher in subjects with hypertension than in those without (14.32% versus 13.07%, n = 871, p = 0.03), and the unadjusted odds ratios for hypertension showed a statistically significant trend in people with low, medium, and high %MMA values [ORs = 1.00; 1.28 (95%CI, 0.90–1.81); 1.47 (95%CI, 1.04–2.07); respectively, p for trend = 0.02]. However, this trend was not seen after adjustment for age, gender, body mass index, smoking, triglycerides, and cumulative arsenic exposure.
In addition to the studies in Table 5, other data support the hypothesis that %MMA is related to arsenic-disease susceptibility. In Mexico, Del Razo et al. reported higher levels of %MMA in subjects with arsenic-related skin lesions than in those without lesions (14.3% versus 9.5%) (Del Razo et al., 1997). In one of the few studies involving MMA3, Valenzuela et al. reported higher %MMA3 levels in subjects with arsenic-caused skin lesions (mean %MMA3 = 7.7%, n = 55) than in those without these lesions (mean %MMA3 = 5.9%, n = 21, p = 0.072) (Valenzuela et al. 2005). (The Valenzuela and Del Razo et al. studies were not included in Table 5 because data were not presented as relative risks). In Finland, Maki-Paakkanen et al. found a positive association between lymphocyte chromosomal aberrations and MMA/InAs ratio (Maki-Paakkanen et al., 1998). Additional biologic plausibility comes from laboratory research where several studies have shown that MMA3 is more acutely toxic in vitro than MMA5, DMA, and InAs (Cullen et al., 1989; Styblo et al., 1997; Lin et al., 1999; Styblo et al., 1999; Petrick et al., 2000; Styblo et al., 2000; Lin et al., 2001; Mass et al., 2001). These data suggest that MMA, specifically MMA3, may be the primary toxic species of ingested inorganic arsenic. These findings, combined with epidemiologic evidence from Taiwan, Japan, Chile, and Argentina showing clear associations between InAs ingestion and lung cancer risks (IARC, 2002), all support the biologic plausibility of our results linking inter-individual differences in %MMA to increased lung cancer risks.
In our study, MMA was measured as total MMA, that is, MMA3 and MMA5 combined. At the time of this study, it was very difficult to accurately measure MMA3 separately in the field due to its instability in urine. If MMA3 is the primary toxic species, it may be that the total MMA is an accurate surrogate for MMA3, although currently this is unknown. If MMA3 truly is the toxic species, any inaccuracies involved in using total MMA as a surrogate for MMA3 would cause bias towards the null and true relative risks may actually be higher than those found in this study.
As in all of the studies in Table 5, the measurement of urinary methylation patterns was taken after disease diagnosis and assumed to be representative of subject’s past methylation patterns. Few studies have assessed changes in methylation patterns in the same individuals over time, but those that have suggest that these patterns remain fairly stable over time (Concha et al., 2002; Steinmaus et al., 2005b). Evidence suggests that stable genetic factors play a more important role in determining inter-individual differences in methylation patterns than do factors that are likely to have greater day to day variability such as diet or smoking (Chiou et al., 1997; Vahter, 1999a; Vahter, 1999b; Vahter, 2000; Chung et al., 2002; Vahter, 2002; Steinmaus et al., 2005a). It should also be noted that although intra-individual variability in methylation patterns could lead to misclassification of past methylation patterns, because we collected and analyzed metabolites from cases and controls using the same protocols, the resulting bias would be non-differential and likely towards the null, not towards the positive associations identified. Similar misclassification might occur as a result of DMA from arsenosugars in seafood. However, the study area is inland with relatively little seafood consumption, and the resulting bias would also likely be non-differential and towards the null.
In our main analyses, we used only samples with metabolite levels above detection limits. While this resulted in a smaller sample size and may have caused some reduction in study power, it likely improved the accuracy of our odds ratio estimates. This is because in samples with metabolite concentrations below detection, laboratory imprecision or inaccuracies in assigning values to samples below detection (e.g. zero, ½ the detection limit…) can cause relatively large errors when calculating metabolite proportions. These errors would most likely be non-differential and bias any true association towards the null. The decrease in the %MMA-lung cancer OR from 3.09 (95% CI, 1.08–8.81) to 2.14 (95% CI, 0.91–5.06) when we added subjects with metabolite levels below detection is consistent with this effect.
The assessment of methylation after cancer diagnosis also raises concerns about the temporal relationship between disease and methylation capacity. That is, the effects seen in our study and those in the other studies in Table 5 might not be due to the impact of methylation patterns on disease, but rather, due to the impact of disease or disease treatment on methylation patterns. Currently no data are available on the impact of severe chronic disease on arsenic metabolism. However, several of the studies linking %MMA to arsenic susceptibility involve non-melanoma skin cancer, benign skin lesions, or chromosomal aberrations, none of which would be expected to have significant systemic effects on metabolism. The consistency of our findings with these studies and other data on biologic plausibility suggest our results represent the effects of %MMA on lung cancer risks, although the possibility that lung cancer affects %MMA can not be completely ruled out. A longitudinal cohort study might be better able to establish temporality, although this type of study would be incredibly difficult given the 30 to 40 year (or longer) latency of arsenic-caused cancer.
Overall participation rates differed between the cases (77%) and controls (56%) in this study, but it is unlikely this difference had a major impact on our results since our primary exposure variable (%MMA) is probably not strongly related to participation. Most of the major factors that might be associated with both participation and %MMA were adjusted for in our analyses and had little impact on results (e.g. smoking, age, gender). Some dietary variables affect arsenic methylation, but the impacts are mostly small and thus unlikely to have caused the effects identified in this study (Steinmaus et al., 2005a; Li et al., 2008; Heck et al., 2007).
We found some evidence that the association between %MMA and lung cancer could be related to rs234709 and rs4920037, two intronic polymorphisms in the CBS gene. These SNPs may be functionally relevant or may be in linkage disequilibrium with some other functional SNP that may influence CBS activity. CBS is an important enzyme in the conversion of homocysteine to cystathionine, a precursor to cysteine and glutathione biosynthesis. CBS gene variants may influence CBS enzyme activity. The exact way this might affect arsenic toxicity is unknown although several possibilities exist (Selhub, 1999). (The relationship between arsenic and homocysteine metabolism is shown in Figure 1.) CBS enzyme deficiency can result in increased levels of homocysteine and s-adenosylhomocysteine (SAH), the later being a potent inhibitor of methylation reactions (De Kimpe et al., 1999; Selhub, 1999; Yi et al., 2000). If SAH selectively inhibits the methylation of MMA3 to DMA5, this might lead to increased levels of MMA3 (and a greater MMA3/MMA5 ratio) and thus higher risks of MMA3 associated toxicity. A previous study has identified associations between increased homocysteine and decreased %DMA and increased %MMA, although MMA3 was not specifically measured (Gamble et al., 2005). Another possible mechanism could be related to the involvement of CBS in glutathione production. CBS deficiencies might lead to decreased glutathione biosynthesis and inhibition of any potential detoxification pathway involving glutathione.
Figure 1.
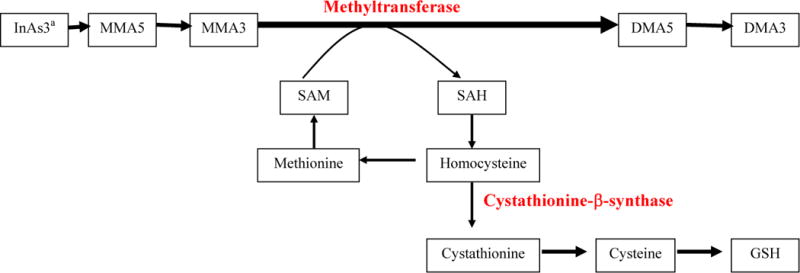
Involvement of cystathionine β-synthase and homocysteine in the metabolism of methylarsonous acid (MMA3) to dimethylarsinic acid (DMA5) and in glutathione biosynthesis.
Abbreviations: InAs, inorganic arsenic; MMA5, methylarsonic acid; MMA3, methylarsonous acid; DMA5, dimethylarsinic acid; DMA3, dimethylarsinous acid; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; GSH, glutathione
aCystathionine β-synthase and homocysteine are similarly involved in the conversion of InAs3 to MMA5
Overall, the results of this study suggest that the association between %MMA and arsenic-related lung cancer may be mediated by genetic variation in CBS. These results involve a small number of subjects and need to be confirmed. As such, our CBS findings should be viewed as preliminary exploratory results that may help guide researchers in selecting which genetic factors to be included in future studies.
In conclusion, millions of people are exposed to arsenic worldwide and these exposures may be associated with high cancer risks. Our results add to a gradually expanding body of evidence that inter-individual differences in arsenic metabolism play an important role in arsenic-related disease. Our results are the first to suggest that this may include lung cancer. Although the design of this study prevents us from confirming the temporal relationship between %MMA and lung cancer, the biologic plausibility of our results and their consistency with a variety of other research is evidence that our findings represent a true impact of MMA on lung cancer risks. Data such as these are important in identifying susceptible subpopulations that may need specific regulatory protection. These data may also help elucidate the mechanisms of arsenic-caused disease which are largely unknown. Further research is needed on the potential toxic effects of MMA3 in humans, the genetic and lifestyle factors that influence individual arsenic methylation, and the role of CBS genetic variants in arsenic toxicity.
Supplementary Material
Acknowledgments
Primary funding for this study was provided by the National Institute of Environmental Health Sciences (NIEHS) grants P42ES04705 and P30ES01896-22. Additional support was provided by the Northern California Center for Occupational and Environmental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None.
References
- Ahsan H, Chen Y, Kibriya MG, Slavkovich V, Parvez F, Jasmine F, Gamble MV, Graziano JH. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2007;16:1270–1278. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- Bates MN, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Kalman D, Steinmaus C, Smith AH. Case-control study of bladder cancer and exposure to arsenic in Argentina. Am J Epidemiol. 2004;159:381–389. doi: 10.1093/aje/kwh054. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Geubel A, Pauwels S, Mahieu P, Lauwerys R. The influence of liver disease on the methylation of arsenite in humans. Arch Toxicol. 1984;55:151–154. doi: 10.1007/BF00316119. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981a;48:71–79. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int Arch Occup Environ Health. 1981b;48:111–118. doi: 10.1007/BF00378431. [DOI] [PubMed] [Google Scholar]
- Calderon R, Hudgens E, Le XC, Schreinmachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect. 1999;107:663–667. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66:888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, van Geen A, Ahsan H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007;165:541–552. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Chao SC, Lee JY, Christiani DC. Arsenic methylation and skin cancer risk in southwest Taiwan. J Occup Environ Med. 2003a;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su HJ, Guo YL, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Christiani DC. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003b;14:303–310. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res. 1997;386:197–207. doi: 10.1016/s1383-5742(97)00005-7. [DOI] [PubMed] [Google Scholar]
- Chung JS, Kalman DA, Moore LE, Kosnett MJ, Arroyo AP, Beeris M, Mazumder DN, Hernandez AL, Smith AH. Family correlations of arsenic methylation patterns in children and parents exposed to high concentrations of arsenic in drinking water. Environ Health Perspect. 2002;110:729–733. doi: 10.1289/ehp.02110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G, Volger G, Nermell B, Vahter M. Intra-individual variation in the metabolism of inorganic arsenic. Int Arch Occup Environ Health. 2002;75:576–580. doi: 10.1007/s00420-002-0361-1. [DOI] [PubMed] [Google Scholar]
- Crecelius EA. Modification of the arsenic speciation technique using hydride generation. Anal Chem. 1978;50:826–827. [Google Scholar]
- Cullen WR, McBride BC, Manji H, Pickett AW, Reglinski J. The metabolism of methylarsine oxide and sulfide. Appl Organometal Chem. 1989;3:71–78. [Google Scholar]
- De Kimpe J, Cornelis R, Vanholder R. In vitro methylation of arsenite by rabbit liver cytosol: effect of metal ions, metal chelating agents, methyltransferase inhibitors and uremic toxins. Drug Chem Toxicol. 1999;22:613–628. doi: 10.3109/01480549908993171. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Vargas H, Albores A, Gonsebatt ME, Montero R, Ostrosky-Wegman P, Kelsh M, Cebrian ME. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol. 1997;71:211–217. doi: 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, Styblo M, Cullen WR, Thomas DJ. Determination of trivalent methylated arsenicals in biological matrices. Toxicol Appl Pharm. 2001;174:282–293. doi: 10.1006/taap.2001.9226. [DOI] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, Levy D, Alam S, Islam M, Parvez F, Ahsan H, Graziano JH. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86:1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebel TW. Arsenic methylation is a process of detoxification through accelerated excretion. Int J Hyg Environ Health. 2002;205:505–508. doi: 10.1078/1438-4639-00177. [DOI] [PubMed] [Google Scholar]
- Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, Baron JA, Howe GR, Ahsan H. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85:1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Smith AH, Goeden HM. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993;60:161–177. doi: 10.1006/enrs.1993.1024. [DOI] [PubMed] [Google Scholar]
- Hsueh YM, Chiou HY, Huang YL, Wu WL, Huang CC, Yang MH, Lue LC, Chen GS, Chen CJ. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:589–596. [PubMed] [Google Scholar]
- Huang YK, Huang YL, Hsueh YM, Yang MH, Wu MM, Chen SY, Hsu LI, Chen CJ. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19:829–839. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol. 2007;218:135–142. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM. Dose-dependent effects on the disposition of monomethylarsonic acid and dimethylarsinic acid in the mouse after intravenous administration. J Toxicol Environ Health Part A. 1998;53:95–112. doi: 10.1080/009841098159385. [DOI] [PubMed] [Google Scholar]
- IARC. Some drinking-water disinfectants and contaminants, including arsenic. Vol. 84. International Agency for Research on Cancer; Lyon: 2002. [Google Scholar]
- Kile ML, Ronnenberg AG. Can folate intake reduce arsenic toxicity? Nutr Rev. 2008;66:349–353. doi: 10.1111/j.1753-4887.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ekström EC, Goessler W, Lönnerdal B, Nermell B, Yunus M, Rahman A, El Arifeen S, Persson LA, Vahter M. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect. 2008;116:315–321. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Cullen WR, Thomas DJ. Methylarsenicals and arsinothiols are potent inhibitors of mouse liver thioredoxin reductase. Chem Res Toxicol. 1999;12:924–930. doi: 10.1021/tx9900775. [DOI] [PubMed] [Google Scholar]
- Lin S, Del Razo LM, Styblo M, Wang C, Cullen WR, Thomas DJ. Arsenicals inhibit thioredoxin reductase in cultured rat hepatocytes. Chem Res Toxicol. 2001;14:305–311. doi: 10.1021/tx0001878. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, Rudnai P, Leonardi G, Fletcher T, Vahter M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115:1081–1086. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL, Rahman M, Persson LA, Vahter M. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol Appl Pharmacol. 2008;230:9–16. doi: 10.1016/j.taap.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Moore LE, Fraumeni JF, Jr, Cantor KP. Respiratory cancer and inhaled inorganic arsenic in copper smelters workers: a linear relationship with cumulative exposure that increases with concentration. Environ Health Perspect. 2008;116:1661–1665. doi: 10.1289/ehp.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki-Paakkanen J, Kurttio P, Paldy A, Pekkanen J. Association between the clastogenic effect in peripheral lymphocytes and human exposure to arsenic through drinking water. Environ Mol Mutagen. 1998;32:301–313. doi: 10.1002/(sici)1098-2280(1998)32:4<301::aid-em3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Marnell L, Garcia-Vargas G, Chowdhury U, Zakharyan R, Walsh B, Avram D, Kopplin M, Cebrian ME, Silbergeld E, Aposhian HV. Polymorphisms in the human monomethylarsonic acid (MMAV) reductase/hGSTO1 gene and changes in urinary arsenic profiles. Chem Res Toxicol. 2003;16:1507–1513. doi: 10.1021/tx034149a. [DOI] [PubMed] [Google Scholar]
- Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD. Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol. 2001;14:355–361. doi: 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- McCarty KM, Chen YC, Quamruzzaman Q, Rahman M, Mahiuddin G, Hsueh YM, Su L, Smith T, Ryan L, Christiani DC. Arsenic methylation, GSTT1, GSTM1, GSTP1 polymorphisms, and skin lesions. Environ Health Perspect. 2007;115:341–345. doi: 10.1289/ehp.9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza MM, Yu L, Rodriguez YY, Guild M, Thompson D, Gandolfi AJ, Klimecki WT. Developmentally restricted genetic determinants of human arsenic metabolism: association between urinary methylated arsenic and CYT19 polymorphisms in children. Environ Health Perspect. 2005;113:775–781. doi: 10.1289/ehp.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MM, Harrington-Brock K, Doerr CL. Relative genotoxic potency of arsenic and its methylated metabolites. Mutat Res. 1997;386:279–290. doi: 10.1016/s1383-5742(97)00003-3. [DOI] [PubMed] [Google Scholar]
- Morales K, Ryan L, Kuo T, Wu M, Chen C. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–661. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- NRC. Arsenic in Drinking Water Subcommittee on Arsenic in Drinking Water. National Research Council; Washington, DC: 1999. pp. 229–250. [Google Scholar]
- NRC. Arsenic in Drinking Water 2001 Update. Subcommittee to Update the 1999 Arsenic in Drinking Water Report. National Research Council; Washington, DC: 2001. [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Aposhian VH. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pu YS, Yang SM, Huang YK, Chung CJ, Huang SK, Chiu AW, Yang MH, Chen CJ, Hsueh YM. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol Appl Pharmacol. 2007;218:99–106. doi: 10.1016/j.taap.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Smith A, Lopipero P, Bates M, Steinmaus C. Arsenic epidemiology and drinking water standards. Science. 2002;296:2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water [see comments] Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Bates MN, Yuan Y, Kalman D, Atallah R, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Hoang BK, Smith AH. Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. J Occup Environ Med. 2006;48:478–488. doi: 10.1097/01.jom.0000200982.28276.70. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005a;113:1153–1159. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Moore LE, Shipp M, Kalman D, Rey OA, Biggs ML, Hopenhayn C, Bates MN, Zheng S, Wiencke JK, Smith AH. Genetic polymorphisms in MTHFR 677 and 1298, GSTM1 and T1, and metabolism of arsenic. J Toxicol Environ Health A. 2007;70:159–170. doi: 10.1080/15287390600755240. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Atallah R, Smith A. Intra-individual variability in arsenic methylation in a US population. Cancer Epidemiol Biomarkers Prev. 2005b;14:919–924. doi: 10.1158/1055-9965.EPI-04-0277. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110(suppl 5):S767–S771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Serves S, Cullen W, Thomas D. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem Res Toxicol. 1997;10:27–33. doi: 10.1021/tx960139g. [DOI] [PubMed] [Google Scholar]
- Styblo M, Vega L, Germolec DR, Luster MI, Del Razo LM, Wang C, Cullen WR, Thomas DJ. Metabolism and toxicity of arsenicals in cultured cells. In: Chappell WR, Abernathy CO, Calderon RL, editors. Arsenic: Exposure and Health Effects. Elsevier Science Ltd; Amsterdam: 1999. pp. 311–324. [Google Scholar]
- Tseng CH, Huang YK, Huang YL, Chung CJ, Yang MH, Chen CJ, Hsueh YM. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206:299–308. doi: 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999a;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. Variation in human metabolism of arsenic. In: Chappell WR, Abernathy CO, Calderon RL, editors. Arsenic: Exposure and Health Effects. Elsevier; Amsterdam: 1999b. pp. 267–279. [Google Scholar]
- Vahter M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett. 2000;112–113:209–217. doi: 10.1016/s0378-4274(99)00271-4. [DOI] [PubMed] [Google Scholar]
- Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Hsueh YM, Hong CT, Su CL, Chang SF, Huang WL, Wang HT, Wang YH, Hsieh YC, Chen CJ. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol Appl Pharmacol. 2006;216:168–175. doi: 10.1016/j.taap.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–29323. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]
- Yu RC, Hsu KH, Chen CJ, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1259–1262. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


