Abstract
Overexpressed Human epidermal growth factor receptor 2 (HER2) drives the biology of 20% breast cancer and is a prediction of a poor prognosis for patients. HER2-targeted therapies significantly improve outcomes for HER2-positive patients. Traditional Chinese herbs/medicines have been used to treat breast cancer patients including HER2-positive patients in Asia for decades. Although the traditional medicines demonstrate efficacy in clinics for HER2-positive patients, the mechanism is largely unknown. In this article, we screened a 10,000 natural product library in 6 different cell lines representing breast cancer, and assessed the ability of each drug to cause cytotoxicity through a high-throughput screening approach. We have identified eight natural compounds that selectively inhibit the proliferation of HER2-positive cells. Two of the hit compounds, peonidin-3-glucoside and cyaniding-3-glucoside, are both extracts from black rice. They inhibit the phospho-HER2 and phospho-AKT and were confirmed to induce HER2-psotive breast cancer cells apoptosis both in vitro and in vivo. Peonidin-3-glucoside and cyaniding-3-glucoside treatments significantly reduced the tumor size and volume in vivo compared to the control group. There is no significant difference of antitumorgenic effects between peonidin-3-glucoside and cyaniding-3-glucoside treatments.
Introduction
Breast cancer is a serious and sometimes life-threatening disease. An estimated 232,340 new cases of invasive breast cancer are expected to be diagnosed among women in the US during 2013 according to American Cancer Society. Breast cancer has also become common to Chinese women in recent years possibly due to the change of environments, growth patterns, diet and aging. Based on the model generated by Linos et al., in 2021, the estimated breast cancer incidence rate would be 85.3 to 87.8 per 100,000 woman in China [1]. Evidently, there is a clear need for the development of new therapeutic agents.
HER2 overexpression occurs in ∼20% of patients with breast cancer and is associated with aggressive disease and decreased survival. A number of therapeutic approaches have been developed against HER2 worldwide including tyrosine kinase inhibitors, monoclonal antibodies such as Trastuzumab [2]. The mechanism has been largely studied and since then, the disease-free survival and overall survival of patients have all been improved significantly [2]–[5]. In China, there are reports of successful novel therapeutic approaches using traditional medicine for breast cancer patients [6], 7. Traditional Chinese Herbs/Medicines have developed into a mature system for more than three thousand years. Although thousands of Traditional Chinese Medicines have been proved to be effective clinically, the mechanisms of the drug actions are largely unclear. With the modern technology, researchers successfully purified and identified numerous extracts that have not been well defined before. With the rich prior human experiences, we proposed to screen a natural product library which contains 10,000 extracts against representative breast cancer cells and tried to identify compounds that selectively inhibit HER2-positive breast cancer cells.
Materials and Methods
Compound Library
The natural product library contains 10,000 natural products with a minimum of 98% purity confirmed by NMR and HPLC (Pharmanic, Chengdu, Sichuan, China). Briefly, compounds were extracted by supercritical CO2 extraction (SFE-CO2) and the residues after SFE-CO2 extraction were then refluxed with 80% ethanol and the ethanol extracts were spray-dried to obtain the extracts. Then the extracts samples were compared to the reference chemical standards purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) by Pharmanic. The conditions of the solvent gradient elution were 8-20% (A) in 0–20 min, 20–40% (A) in 25–30 min, 40–70% (A) in 30–45 min, 70–90% (A) in 55–60 min at a flow-rate of 1.0 ml/min. Detection was conducted with different wavelengths of 230, 240, 270, 262, and 420 nm with the reference wavelength of 550 nm at room temperature. Compounds were present at 10 mmol/L in DMSO. Afatinib (BIBW2992) was gifted from the Pharmacology department of Chengdu Medical College with a >98% purity.
Cell Culture
All cell lines were obtained from the American Type Culture Collection (ATCC) except SUM190 ( Table 1 ). SUM190 cells were gifted from Chengdu Medical College bio-core facility [8]. MCF-7, MDA-MB-453 and MDA-MB-231 cells were maintained in DMEM supplemented with 2 mmol/L L-glutamine, 10% fetal bovine serum and 1% penicillin/streptomycin. BT474 cells were maintained in DMEM: Ham's F12 medium (1∶1 mixture) supplemented with 2 mmol/L L-glutamine, 5 µg/ml insulin, 10% fetal bovine serum and 1% penicillin/streptomycin. HCC1569 cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cells were maintained in a 5% CO2 atmosphere at 37°C.
Table 1. Breast cancer cell lines used in HTS and their molecular classifications [4], [38].
| Cell line | Classification | Immunoprofile |
| MCF-7 | Luminal A | ER+, PR+/−, HER2− |
| BT474 | Luminal B | ER+, PR+/−, HER2+ |
| MDA-MB-453 | Luminal | ER−, PR−, HER2+ |
| SUM190 | Basal | ER−, PR−, HER2− |
| MDA-MB-231 | Basal B | ER−, PR−, HER2− |
| HCC1569 | Basal A | ER−, PR+/−, HER2+ |
High-throughput Screening Natural Compounds for Activity in Breast Cancer Cells
To identify compounds that might have inhibitory effect on breast cancer cell proliferation, we used a high-throughput drug screen experimental approach to assess the activity of 10,000 natural compounds. Prior to the screen, the cell viability assay was miniaturized to a 96-well, low-volume, black, flat-bottom polystyrene microplates (Corning, Shanghai, China) format to accelerate assay throughput. Cells were grown to 80% confluence, harvested and aliquoted into 96-well plates at concentrations of 1,000 cells per well in a total volume of 90 µL/well. The outer wells were inoculated with medium to minimize evaporation from the sample wells. Cells were allowed to attach overnight and 10 µl culture medium that containing either vehicle or drug at a 100 µmol/L concentration were added to give a final concentration of 10 µmol/L. Cell proliferation was evaluated using 20 µL Alamar-Blue reagent per well according to manufacturer's instructions after 72-h incubation. The plates were then incubated at 37°C for an additional 4 h and the fluorescent signal was measured. Fluorescence was measured with an excitation at 530 nm and emission at 590 nm on ZS-2 plate reader. After background subtraction, cell viability values were normalized to vehicle controls and expressed as percentage of the mean of the relative vehicle controls. Compounds which showed a percentage of inhibition against cell proliferation more than 50% are defined as “hit compounds” for each cell line. Vehicle only wells served as intra-plate controls on each plate were uniformly distributed throughout the screen. Background plates containing vehicle only served as inter-plate controls were inserted throughout the screen. The controls were used to calculate the background levels/noises of the assay and to evaluate assay response as well as performance. Performance of the assay was assessed using Z′ factors.
Quantitative Compounds Screen Assay
Cells were grown to 80% confluence, harvested and aliquoted into 96 well plates at concentrations of 1,000 cells per well in a total volume of 90 µL/well. Hit compounds or vehicle (DMSO) were then loaded to each well to give a final doses range from 50 µmol/L to 0.03 µmol/L (serial four-fold dilutions) in 100 µL total volume. Cells were cultured for 72 hours at 37°C in a 5% CO2 atmosphere. Aliquots of 20 µL Alamar-Blue reagent were added directly to each well, the plates were incubated at 37°C for 3 h and the fluorescent signal was measured with an excitation at 530 nm and emission at 590 nm on ZS-2 plate reader. Data were normalized as percentage inhibition relative to vehicle control. IC50 values were estimated using a four parameter logistic curve model by SigmaPlot 12.0 (Systat Software, INC).
Western Blotting
Cells were grown to 70–80% confluence, harvested and aliquoted into 100 mm dishes. Media was removed and replaced by media supplied with different doses of compounds the next day. Dished were incubated for an additional 6 h. Cells were washed with PBS and cell lysates were prepared by scraping cells into RIPA buffer with protease inhibitor and phosphatase inhibitor. The homogenates were centrifuged. The supernatants were transferred to fresh tubes and protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Inc.) per manufacturer's instructions. Equal amounts of total protein (25 µg) were subjected to electrophoresis (10% tris-HCl, 1.0 mm gels) and transferred to PVDF membranes. Membranes were blocked in TBS containing 0.05% Tween 20 (TBST) and 5% nonfat milk or 5% BSA for 1 hour at room temperature. Primary antibodies were diluted per manufacturer's instructions and incubated in TBST/5% nonfat milk or TBST/5% BSA for 2 h at room temperature or overnight at 4°C. Secondary antibodies were diluted (1∶10,000) and incubated with PVDF membranes for 1 h at room temperature. Proteins were detected by ECL plus western blotting detection system (GE healthcare). The following antibodies were used: anti-phospho-HER2 (Tyr1248), anti-HER2, anti-phospho-AKT (Thr308 or Ser473), anti-AKT, anti-phospho-p42/44MAPK, anti-p42/44MAPK, and anti-β-actin antibodies (Rui Biological Ltd., Shanghai, China).
Annexin V-FITC assay
Cells were treated with hit compounds for 48 h at 37°C, pooled and washed with PBS for three times, and re-suspended in binding buffer. Cells were then stained with annexin-V-FITC antibody supplied by the Chengdu Medical College Flow-cytometry core facility. Propidium iodide (PI) (final concentration 1 g/ml) was added immediately prior to analysis. Bivariant analysis of FITC fluorescence and PI fluorescence gave different cell populations, where FITC (−) and PI (−) were designated as viable cells, FITC (+) and PI (−) as apoptotic cells, and FITC (+) and PI (+) as late apoptotic or necrotic cells.
Caspase 3/7 activity assay
Cells were grown to 70–80% confluence, harvested and aliquoted into 96-well plates. Different doses of compounds were added to the plates the next day. Plates were incubated for an additional 48 h at 37°C. Aliquots of Alamar-Blue reagent were added directly to each well, the plates were incubated at 37°C for 3 h and the fluorescent signal was measured with an excitation at 530 nm and emission at 590 nm on ZS-2 plate reader. Then equal volume of caspase 3/7 activity assay reagent (Promega, China) was added to each well and the luminescence signal was measured on ZS-2 plate reader. Data were normalized as luminescence relative to fluorescence.
In vivo efficacy in xenograft models
In vivo experiments were carried out under pathogen-free conditions at the animal facility in accordance with the institutional guidelines of the Chengdu Medical College Institutional Animal Care and Use Committee (IACUC). All protocols were reviewed and approved by IACUC and HER2-positive breast cancer cell line MDA-MB-453 cells were resuspended to 2×106 cells/100 µl in PBS and implanted subcutaneously into the flank region of 6–7-week-old female nude mice weighing 18 to 22 gram. When tumors reached 50 to 60 mm3 in volume, animals were randomly assigned to 3 groups, receiving either saline (control) or peonidin-3-glucoside (6 mg/kg) and cyaniding-3-glucoside (6 mg/kg) as oral gavage 7 times a week for a total of 25 days. Tumors were measured every 5 days with a caliper and tumor volume (in mm3) was calculated using the following formula: V = 4/3π[(w+l)/4]3, where V = volume (mm3), w = width (mm), l = length (mm). Once the control tumors reached 1000 mm3, the animals were euthanized due to ethical requirements. After 25 days treatment, all animals were euthanized using overdosed CO2, and the tumor tissues were extracted for immunostaining and weighing. All values are expressed as the mean ± SEM.
Statistical analysis
In vitro data were reported as mean ± SD, each treatment performed in duplicate or triplicate. Data were log-transformed to stabilize variances for proliferation assays. In vivo data were reported as mean ± SEM. Values were analyzed using the Student's t test or with one-way ANOVA when three groups were present. Statistical significance was considered as p≤0.05.
Results
High-throughput Screen (HTS) development and assay performance measurements
For optimization of assay performance, the number of cells/well and the length of the incubation period, and the volume of Alamar-Blue reagent were empirically determined ( Table 2 ). To identify compounds that might have inhibitory effect against HER2 positive cell proliferation, we used a three-step screen experimental approach to assess the activity of 10,000 natural compound library. First step, a one dose (10 µmol/L) HTS was performed using three HER2-positive breast cancer cell liens including BT474, MDA-MB-453, and HCC1569. Compounds that exhibited precipitation were excluded from the following screen. We have identified 45 compounds that inhibited cell proliferation by more than 50% at 10 µmol/L after a 72-hour incubation. Second step, a one dose (10 µmol/L) screen was performed using three HER2-negative breast cancer cell lines including MCF-7, SUM190, and MDA-MB-231 using hit compounds from first step. The assay steps were summarized in Table 3 . Controls present in each assay plate consisted of 0.2% DMSO (vehicle control) or afatinib (positive control). Combined with the result from first two steps, we have identified 8 compounds that selectively inhibited HER2-positive breast cancer cell proliferation by more than 50% at 10 µmol/L after a 72-hour incubation (step 1) compare to no anti-proliferation activity against HER2-negative breast cancer cells (step 2). The protocol of the preliminary screen study is summarized in Table 3 . A total of 543 plates were screened. The assay performed well over the entire course of the screen as demonstrated by the average of Z′ values (0.58) [9].
Table 2. Screen assay protocol for step 1 and 2.
| Step | Event | Parameter | Description | Notes |
| 1 | Add reagent | 90 µL | 1,000 cells/well | |
| 2 | Add library compounds or DMSO | 10 µL | 10 µmol/L | |
| 3 | Incubate | 72 hrs | 5% CO2/37°C incubation | |
| 4 | Add reagent | 20 µl | Alamar-blue reagents | |
| 5 | Incubate | 4 hrs | 5% CO2/37°C incubation | |
| 6 | Read | Fluorescence | 530 nm Ex/590 nm Em, gain = 60. | Instrument:Zs-2 plate reader |
Plates lidded until read.
Table 3. FP assay performance and post-screen analysis summary.
| Category | Parameters | Descriptions |
| Assay | Nature of the assay | Cell-based proliferation assay |
| Assay strategy | Detection of cell proliferation using Alamar-blue reagents | |
| Reagents and sources | See materials and Methods | |
| Assay protocol | Key steps outlined in Table 2 | |
| Library screened | Nature of the library | Fractions derived from natural Chinese medicine |
| Size of the library | 10,000 natural compounds arrayed in 96-well plates as single compounds at 10 mmol/L in DMSO | |
| Source | Pharmanic, China | |
| Quality control | All compounds assured by the company as >98% pure on HPLC with QC data | |
| Concentration tested | 10 µmol/L in 0.2% DMSO (v/v) for step 1 and 2. Serial dilutions for step 3. | |
| Format | 96-well plate | |
| Plate controls | Positive control: afatinib; negative control: 0.2% DMSO | |
| Plate number and duration | Step 1: 540 plates, 5 monthStep 2: 3 plates, 2 weeksStep 3: 10 plates, 2 weeks | |
| Output, detector, analysis software | ZS-2 detector; Fixed endpoint; Fluorescence value, SigmaPlot | |
| Normalization | % inhibition = 100 x (sample result – average of vehicle treated control)/average of vehicle treated control | |
| Performance | Z′ = 0.58 | |
| Post-screen analysis | Selection of actives | Actives were selected from the primary screen using a threshold of better than 50% inhibition |
| Retesting of initial actives | Original samples retested using screening assay condition; compounds with triplicated activity tested in dose-response mode (8 half dilutions) | |
| List of validated compounds | Table 4 |
Hit compounds inhibit the growth of HER2-postive cancer cell lines
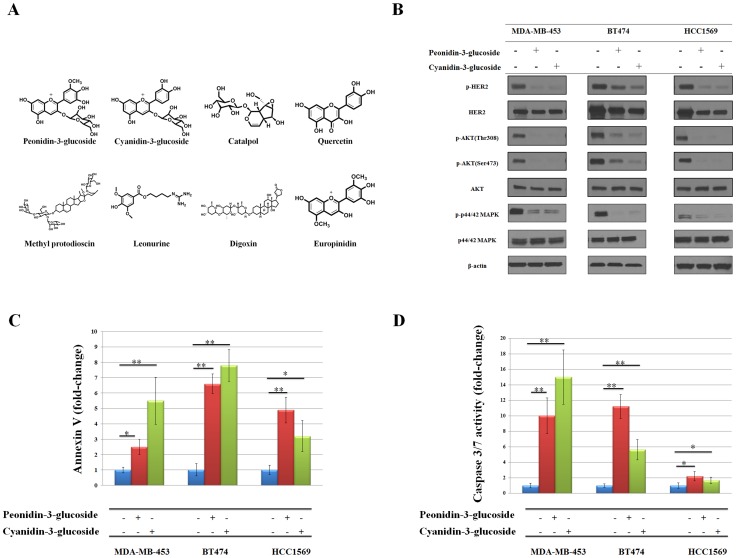
Third step, hit drugs that only inhibit HER2-positive cell line proliferation were chosen for a quantitative screening to generate IC50 values as described in the Materials and Methods section. The eight candidates's structure were summarized in Figure 1A and were verified and their IC50 values were confirmed ( Table 4 ). All eight compounds showed a dose-dependent inhibition of BT474, HCC1569, and MDA-MB-453 cell proliferation with less than 10 µmol/L IC50 values ( Table 4 ). Based on the activities and safety profiles of the hit compounds, we prioritized to validate the top two hits: peonidin-3-glucoside and cyaniding-3-glucoside.
Figure 1. In vitro antitumorigenic activity of peonidin-3-glucoside or cyaniding-3-glucoside.
(A). Structures of hit compounds. (B) Effect of peonidin-3-glucoside and cyanidin-3-glucoside on HER2 and downstream signaling. BT474, MDA-MB-453, and HCC1569 cells were treated with or without peonidin-3-glucoside or cyanidin-3-glucoside for 6 h and whole-cell extracts were analyzed by western blotting with the indicated antibodies. (C) Peonidin-3-glucoside and cyanidin-3-glucoside treatments induce apoptosis in MDA-MB-453, BT474, and HCC1569 cells. Cells were treated with or without peonidin-3-glucoside or cyanidin-3-glucoside for 48 h and annexin V positive cells were counted using flow cytometry. (D) Peonidin-3-glucoside and cyanidin-3-glucoside treatments increase caspase 3/7 activity in MDA-MB-453, BT474, and HCC1569 cells. Cells were treated with or without peonidin-3-glucoside or cyanidin-3-glucoside for 48 h and cell viability and caspase 3/7 activity was measured as per the manufacturer's instructions. Data were normalized as caspase 3/7 activity divided by cell viability. Data represent mean ± SEM. *p<0.05, **p<0.01.
Table 4. Summary of hit compounds.
| Number | Compound | Derived from | IC50 (µM) | ||
| BT474 | HCC1569 | MDA-MB-453 | |||
| 1 | Peonidin-3-glucoside | Black rice | 5.2±0.8 | 4.3±0.6 | 1.2±0.5 |
| 2 | Cyanidin-3-glucoside | Black rice | 1.8±0.5 | 2.6±0.8 | 1.5±0.3 |
| 3 | Catalpol | Rehmannia glutinosa | 2.4±0.4 | 1.9±0.7 | 2.7±0.6 |
| 4 | Quercetin | Cuscuta chinensis Lam | 4.4±0.3 | 4.9±1.1 | 4.0±0.6 |
| 5 | Methyl protodioscin | Cochinchinese Asparagus Root | 3.5±0.6 | 3.2±0.8 | 4.2±0.5 |
| 6 | Leonurine | Rehmannia glutinosa | 3.6±0.8 | 4.5±0.9 | 4.6±0.8 |
| 7 | Digoxin | Digitalis lanata | 4.2±0.7 | 3.6±0.8 | 4.7±0.4 |
| 8 | Europinidin | Plumbago zeylanica | 5.8±1.5 | 7.0±1.8 | 8.0±1.2 |
Hit compounds inhibit HER2 in HER2-postive cancer cell lines
We first determined the phosphorylation status of the HER2 protein and its downstream mediator AKT to further confirm the anti-proliferation activities of the hit drugs are due to selective inhibition of HER2 protein. To evaluate the response of the cell lines to hit drugs, BT474, MDA-MB-453 and HCC1569 cells were treated with drugs (10 µmol/L) for 6 h. Western blotting show that both peonidin-3-glucoside and cyanidin-3-glucoside significantly reduce the phospho-HER2, phospho-AKTs, and phopspho-p44/42MAPK levels compared to control cells ( Fig. 1B ).
Hit compounds induce apoptosis in HER2-postive cancer cell lines
To further confirm the hit compounds activity, we performed an Annexin V staining assay and a caspase 3/7 activity assay to detect apoptosis events. Our result show in Figure 1C&D supported the cell viability assay results ( Table 4 ). Three HER2-positive breast cancer cell lines, MDA-MB-453, BT474, and HCC1569 were treated with or without hit compounds (5 µmol/L) for 48 h. Both peonidin-3-glucoside and cyaniding-3-glucoside significantly induced apoptosis in all tested lines compared to their controls.
Antitumor activity of hit compounds in vivo
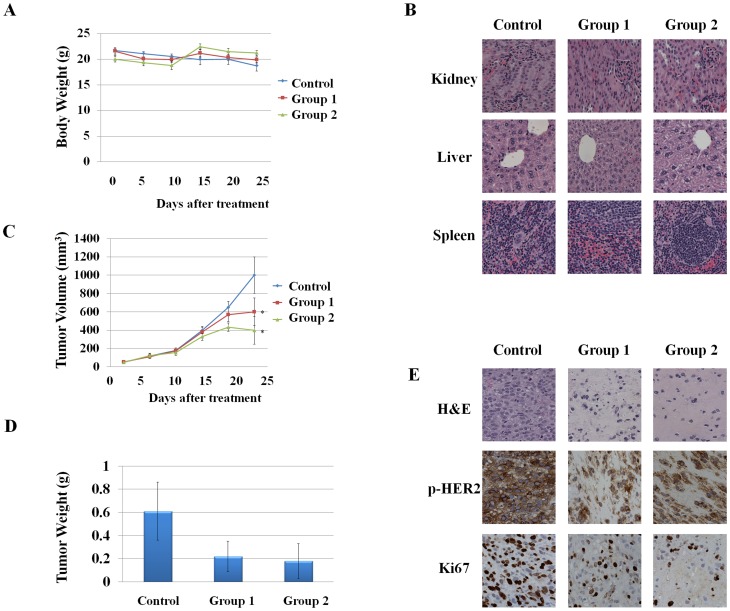
To assess the antitumor effect of peonidin-3-glucoside and cyaniding-3-glucoside in a xenograft model of HER2-positive breast cancer, MDA-MB-453 cells were used in female nude mice. After 25 days of peonidin-3-glucoside (6 mg/kg) and cyaniding-3-glucoside (6 mg/kg) treatment, animal weight stayed stable during the treatments in each group ( Fig. 2A ). Animals treated with drugs did not exhibit signs of organ damage ( Fig. 2B ). Tumor growth rates were significantly different from control group ( Fig. 2C ). Accordingly, final tumor volumes ( Fig. 2C ) and weights ( Fig. 2D ) were suppressed in the peonidin-3-glucoside (6 mg/kg) and cyaniding-3-glucoside groups compared to the control group. This suppression was accompanied by decreased phospo-HER2 levels and decreased proliferation marker Ki67 levels ( Fig. 2E ).
Figure 2. Antitumorigenic activity of peonidin-3-glucoside or cyaniding-3-glucoside in xenografted nude mice.
(A) Body weight of animals. (B) H&E staining for kidney, liver, and spleen. (C and D) Effects on tumor volume and tumor weight. Nude mice bearing MDA-MB-453 cells as xenografts were treated with control (saline), or group 1 (peonidin-3-glucoside (6 mg/kg/day)) or group 2 (cyaniding-3-glucoside (6 mg/kg/day)). Values are means ± SE (n = 10). *P<0.05. (E) H & E staining, expression of phospho-HER2 and Ki67. Control: saline; Group 1: peonidin-3-glucoside (6 mg/kg/day); group 2: cyaniding-3-glucoside (6 mg/kg/day).
Discussion
Identification and characterization of new pharmacological activities from existing Chinese Traditional Medicines represents an effective way to accelerate the translation of discoveries at the bench to clinical applications partially due to the known clinical effects of the drugs. To date, a number of interesting hits have been identified.
In the present study, we performed a three-step screen for searching anti-HER2 positive breast cancer agents. In step 1, we screened the entire 10,000 Chinese Traditional Medicine extracts library against three HER2-positive cell lines. In step 2, we screened the hit compounds from step 1 against three HER2-negative cell lines. In step 3, we generated the IC50 values for the hit compounds which selectively inhibit HER2-positive cell proliferation.
Peonidin-3-glucoside and cyaniding-3-glucoside are anthocyanin pigments in colored rice cultivars [10]. They have been reported to have anti-cancer properties [11]-[16]. Anthocyanins have been used as medicine or supplements for many years and it has been reported that depending on the nutrition habits, the daily intake of anthocyanins in humans could be up to 150 mg/day [14]. Catalpol is an iridoid glucoside and exhibits anti-inflammatory and anti-diabetes activities [17]–[19]. Quercetin is a natural flavonoid widely distributed in plants that acts as a neuroprotective, anti-cancer, anti-ROS formation agent [6], [20]–[23]. Methyl protodioscin is one of the main bioactive extracts from the Traditional Chinese Medicine Dioscorea collettii var hypoglauca. Studies show that methyl protodioscin has anti-proliferative effects on cancer cells due to the induction of cell cycle arrest and/or apoptosis [13], [24]–[29]. Leonurine exerted cardioprotective, antioxidant, and anti-inflammation properties [30]–[33]. Digoxin is a purified cardiac glycoside extracted from Digitalis lanata. Digoxin is widely used in the treatment of various heart conditions including atrial fibrillation, atrial flutter and heart failure that cannot be controlled by other medication [34]–[36]. Digoxin is a very potent Na+/K+ ATPase pump inhibitor with a very narrow therapeutic index. Therefore extra cautious is needed when use this drug [35], [37]. Europinidin is a water soluble, bluish red plant dye derived from Plumbago zeylanica. There is no citable reference for this compound regarding its activities.
We prioritized to study peonidin-3-glucoside and cyaniding-3-glucoside and showed significant anti-tumor activity in vitro. To further evaluate the efficacy of these two compounds, we conducted in vivo anti-tumor experiments using immune-compromised mice model. It has been reported that depending on the nutrition habits, the daily intake of anthocyanins in humans could be up to 150 mg/day [14]. We conservatively choose the safe human dose as 30 mg/day to convert the dosage to the mouse equivalent does using the recommended conversion of animal doses to human equivalent does based on body surface area by US Food and Drug Administration (2005). We calculated the equivalent doses of human (30 mg/day) is equal to 6 mg/kg/day to mouse assuming the human body weight is 60 kg and the mouse body weight is 0.020 kg. The formula listed below has been used:
Animal equivalent dose = (30 mg/60 kg) x 12.3
Both peonidin-3-glucoside and cyaniding-3-glucoside showed significant anti-tumor activity in vivo, however, there is no significant difference between the two treatments.
Conclusions
In this study, we screened a 10,000 Chinese Medicine extracts library against three representative HER2-positive breast cancer cell lines for anti-proliferation drugs and identified 45 hits in step 1. In step 2, the hits identified from step 1 were profiled against three representative HER2-negative breast cancer cell lines. We identified eight compounds as selective anti-proliferation agents of HER2-positive breast cancer cells. In step 3, those eight compounds have been validated to generate IC50 values. Very interestingly, there are two compounds, peonidin-3-glucoside and cyaniding-3-glucoside were extracted from the same source (Black rice). To initiate the validation, we also tested the ability of these drugs to induce the early apoptosis and further confirmed the anti-proliferation abilities. Studies using nude mice bearing MDA-MB-453 cells confirmed the antitumorgenic effects of peonidin-3-glucoside and cyaniding-3-glucoside treatments in vivo. In conclusion, the discovery of HER2 selective anti-proliferation of breast cancer cells by Chinese Traditional Medicines have important implications in the development of these drugs as new anti-breast cancer agents.
Acknowledgments
We would like to thank the Chengdu Medical College flow-cytometry core facility and the animal core facility for helping conducting the in vitro and in vivo studies.
Funding Statement
This work is supported by Sichuan Province Health Bureau (110465) and by the National Natural Science Foundation of China (81273074). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ziegler RG, Anderson WF, Gail MH (2008) Increasing breast cancer incidence in China: the numbers add up. J Natl Cancer Inst 100: 1339–1341. [DOI] [PubMed] [Google Scholar]
- 2. Vrbic S, Pejcic I, Filipovic S, Kocic B, Vrbic M (2013) Current and future anti-HER2 therapy in breast cancer. J buon 18: 4–16. [PubMed] [Google Scholar]
- 3. Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, et al. (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23: 4265–4274. [DOI] [PubMed] [Google Scholar]
- 4. Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, et al. (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 6. Huang C, Lee SY, Lin CL, Tu TH, Chen LH, et al. (2013) Co-treatment with Quercetin and 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose Causes Cell Cycle Arrest and Apoptosis in Human Breast Cancer MDA-MB-231 and AU565 Cells. J Agric Food Chem 61: 6430–6445. [DOI] [PubMed] [Google Scholar]
- 7. Wu XQ, Shao SJ, Qu WC (2012) [Effects of ru'ai shuhou recipe on the matrix metalloproteinases and the inhibitive factors in the recurrence and metastasis of HER2 positive breast cancer]. Zhongguo Zhong Xi Yi Jie He Za Zhi 32: 1526–1530. [PubMed] [Google Scholar]
- 8. Liang M, Liu X, Cheng D, Liu G, Dou S, et al. (2010) Multimodality nuclear and fluorescence tumor imaging in mice using a streptavidin nanoparticle. Bioconjug Chem 21: 1385–1388. [DOI] [PubMed] [Google Scholar]
- 9. Inglese J, Shamu CE, Guy RK (2007) Reporting data from high-throughput screening of small-molecule libraries. Nature chemical biology 3: 438–441. [DOI] [PubMed] [Google Scholar]
- 10. Chen XQ, Nagao N, Itani T, Irifune K (2012) Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem 135: 2783–2788. [DOI] [PubMed] [Google Scholar]
- 11. Fernandes I, Faria A, Azevedo J, Soares S, Calhau C, et al. (2010) Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J Agric Food Chem 58: 3785–3792. [DOI] [PubMed] [Google Scholar]
- 12.Bunea A, Rugina D, Sconta Z, Pop RM, Pintea A, et al.. (2013) Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry. [DOI] [PubMed]
- 13. Fernandes I, Marques F, de Freitas V, Mateus N (2013) Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem 141: 2923–2933. [DOI] [PubMed] [Google Scholar]
- 14. Heinonen M (2007) Antioxidant activity and antimicrobial effect of berry phenolics—a Finnish perspective. Mol Nutr Food Res 51: 684–691. [DOI] [PubMed] [Google Scholar]
- 15. Iriti M, Varoni EM (2013) Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients 5: 2564–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamenickova A, Anzenbacherova E, Pavek P, Soshilov AA, Denison MS, et al. (2013) Effects of anthocyanins on the AhR-CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines. Toxicol Lett 221: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong Z, Chen CX (2013) Effect of catalpol on diabetic nephropathy in rats. Phytomedicine 20: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Liu Z, Peng Y, Zhang L, Ju P, et al.. (2013) Validated LC-MS method for simultaneous quantitation of catalpol and harpagide in rat plasma: application to a comparative pharmacokinetic study in normal and diabetic rats after oral administration of Zeng-Ye-Decoction. Biomed Chromatogr. [DOI] [PubMed]
- 19. Pungitore CR, Leon LG, Garcia C, Martin VS, Tonn CE, et al. (2007) Novel antiproliferative analogs of the Taq DNA polymerase inhibitor catalpol. Bioorg Med Chem Lett 17: 1332–1335. [DOI] [PubMed] [Google Scholar]
- 20.Calero CI, Beltran Gonzalez AN, Gasulla J, Alvarez S, Evelson P, et al.. (2013) Quercetin antagonism of GAB receptors is prevented by ascorbic acid through a redox-independent mechanism. Eur J Pharmacol. [DOI] [PubMed]
- 21.Hu Y, Wang S, Wu X, Zhang J, Chen R, et al.. (2013) Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma. J Ethnopharmacol. [DOI] [PubMed]
- 22.Lee DE, Chung MY, Lim TG, Huh WB, Lee HJ, et al.. (2013) Quercetin Suppresses Intracellular ROS Formation, MMP Activation, and Cell Motility in Human Fibrosarcoma Cells. J Food Sci. [DOI] [PubMed]
- 23. Wang G, Wang JJ, Chen XL, Du SM, Li DS, et al. (2013) The JAK2/STAT3 and mitochondrial pathways are essential for quercetin nanoliposome-induced C6 glioma cell death. Cell Death Dis 4: e746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali Z, Smillie TJ, Khan IA (2013) Two spirostan steroid glycoside fatty esters from Dioscorea cayenensis. Nat Prod Commun 8: 323–326. [PMC free article] [PubMed] [Google Scholar]
- 25. Cao X, Yao Z, Shao M, Chen H, Ye W, et al. (2010) Pharmacokinetics of methyl protodioscin in rats. Pharmazie 65: 359–362. [PubMed] [Google Scholar]
- 26. Hu K, Dong A, Yao X, Kobayashi H, Iwasaki S (1997) Antineoplastic agents. II. Four furostanol glycosides from rhizomes of Dioscorea collettii var. hypoglauca. Planta Med 63: 161–165. [DOI] [PubMed] [Google Scholar]
- 27. Hu K, Yao X (2003) The cytotoxicity of methyl protodioscin against human cancer cell lines in vitro. Cancer Invest 21: 389–393. [DOI] [PubMed] [Google Scholar]
- 28. Wang G, Chen H, Huang M, Wang N, Zhang J, et al. (2006) Methyl protodioscin induces G2/M cell cycle arrest and apoptosis in HepG2 liver cancer cells. Cancer Lett 241: 102–109. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Zhou J, Ju Y, Zhang H, Liu M, et al. (2001) Effects of two saponins extracted from the polygonatum Zanlanscianense pamp on the human leukemia (HL-60) cells. Biol Pharm Bull 24: 159–162. [DOI] [PubMed] [Google Scholar]
- 30. Liu H, Zhang X, Du Y, Ji H, Li S, et al. (2012) Leonurine protects brain injury by increased activities of UCP4, SOD, CAT and Bcl-2, decreased levels of MDA and Bax, and ameliorated ultrastructure of mitochondria in experimental stroke. Brain Res 1474: 73–81. [DOI] [PubMed] [Google Scholar]
- 31. Liu X, Pan L, Wang X, Gong Q, Zhu YZ (2012) Leonurine protects against tumor necrosis factor-alpha-mediated inflammation in human umbilical vein endothelial cells. Atherosclerosis 222: 34–42. [DOI] [PubMed] [Google Scholar]
- 32. Liu XH, Pan LL, Deng HY, Xiong QH, Wu D, et al. (2013) Leonurine (SCM-198) attenuates myocardial fibrotic response via inhibition of NADPH oxidase 4. Free Radic Biol Med 54: 93–104. [DOI] [PubMed] [Google Scholar]
- 33. Norata GD, Catapano AL (2012) Leonurine: a new comer in the natural compounds affecting atherosclerosis. Atherosclerosis 224: 37–38. [DOI] [PubMed] [Google Scholar]
- 34.Kapoor S (2013) Digoxin and its Antineoplastic Properties: An Evolving Role in Oncology. J Pediatr Hematol Oncol. [DOI] [PubMed]
- 35.Kubesova HM, Weber P, Meluzinova H, Bielakova K, Matejovsky J (2013) Benefits and pitfalls of cardiovascular medication in seniors. Wien Klin Wochenschr. [DOI] [PubMed]
- 36.Sand CR, Leiria TL, Kalil RA (2013) Assessment of the adherence of cardiologists to guidelines for the treatment of atrial fibrillation. Arq Bras Cardiol. [DOI] [PMC free article] [PubMed]
- 37.Moffett BS, Valdes SO, Kim JJ (2013) Possible digoxin toxicity associated with concomitant ciprofloxacin therapy. Int J Clin Pharm. [DOI] [PubMed]
- 38. Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]