Abstract
Aim
To evaluate the safety and efficacy of robotic gastrectomy versus open gastrectomy for gastric cancer.
Methods
A comprehensive search of PubMed, EMBASE, Cochrane Library, and Web of Knowledge was performed. Systematic review was carried out to identify studies comparing robotic gastrectomy and open gastrectomy in gastric cancer. Intraoperative and postoperative outcomes were also analyzed to evaluate the safety and efficacy of the surgery. A fixed effects model or a random effects model was utilized according to the heterogeneity.
Results
Four studies involving 5780 patients with 520 (9.00%) cases of robotic gastrectomy and 5260 (91.00%) cases of open gastrectomy were included in this meta-analysis. Compared to open gastrectomy, robotic gastrectomy has a significantly longer operation time (weighted mean differences (WMD) =92.37, 95% confidence interval (CI): 55.63 to 129.12, P<0.00001), lower blood loss (WMD: -126.08, 95% CI: -189.02 to -63.13, P<0.0001), and shorter hospital stay (WMD = -2.87; 95% CI: -4.17 to -1.56; P<0.0001). No statistical difference was noted based on the rate of overall postoperative complication, wound infection, bleeding, number of harvested lymph nodes, anastomotic leakage and postoperative mortality rate.
Conclusions
The results of this meta-analysis suggest that robotic gastrectomy is a better alternative technique to open gastrectomy for gastric cancer. However, more prospective, well-designed, multicenter, randomized controlled trials are necessary to further evaluate the safety and efficacy as well as the long-term outcome.
Introduction
Minimally invasive surgery has become widely applied in the field of general surgery including gastric cancer [1]. In 1997, robotic surgery systems were introduced as an effort to overcome technical disadvantages of laparoscopic surgery [2]. Robotic systems have 3-D imaging, tremor filter, and articulated EndoWrist (Intuitive Surgical Inc., Sunnyvale, CA, USA). With these advanced equipments, robotic surgery is superior to conventional laparoscopic surgery due to its significant improvements in visibility and manipulation [3]. Moreover, robotic gastrectomy (RG) can precisely perform lymph node dissection for gastric cancer and provide a convenient and comfortable environment for surgeons [4].
A variety of reports have demonstrated the safety and feasibility of this approach [5,6]. However, the feasibility and safety between RG and open gastrectomy (OG) in treating gastric cancer is not well elucidated. Previous reports were all based on single-institutional experience, and evidence in the context of randomized controlled trial is not available. The aim of this study is to perform a systematic review and meta-analysis of studies comparing the safety and efficacy of RG versus OG in treating gastric cancer.
Materials and Methods
A comprehensive search was conducted by two authors (LGX and CJR) in May 3, 2013 and updated in July 3, 2013, without restriction to regions and the date of publication. Relevant articles comparing RG and OG for gastric cancer were identified by searching PubMed, EMBASE, Web of Knowledge databases and the Cochrane Library. The following search terms were employed: robotic surgery, da Vinci, gastric cancer, gastrectomy. Gastrectomy included distal gastrectomy, proximal gastrectomy and radical gastrectomy. We excluded conference abstracts, reviews, case reports, non-comparative studies, non-relevant topic papers, non-English papers and animal studies. Relevant data from included studies were extracted and summarized by two independent authors. Any disagreements were resolved though discussions among the author group.
Results
The outcomes that were analyzed and compared between robotic and open approaches to gastrectomy included operative time, blood loss, overall postoperative complication rate, postoperative hospital stays, numbers of harvested lymph nodes and postoperative mortality. In addition, in terms of postoperative complication, anastomotic leakage, bleeding, as well as wound infection were also analyzed.
Quality Assessment
The methodological quality of retrospective studies was assessed by the modified Newcastle-Ottawa scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The quality of the studies assessment consisted of three items: patient selection, comparability of RG and OG groups, and exposure according to a previous meta-analysis [7].
Statistical analysis
We performed statistical analysis by Revman software, version 5.2 (Cochrane Collaboration, Oxford, UK).Continuous and dichotomous variables were analysis by weighted mean differences (WMD) and odds ratios (OR), respectively. A 95% confidence interval (CI) was recorded. Heterogeneity among the studies was assessed using the χ2 test and I2. A fixed effect model was applied when I2 <50%, and a random effect model when I2 was greater than 50%. P values of less than 0.05 were considered to indicate statistical significance. Publication bias was analyzed by funnel plots and evaluated by the Begg's and Egger's test.
Study Characteristics
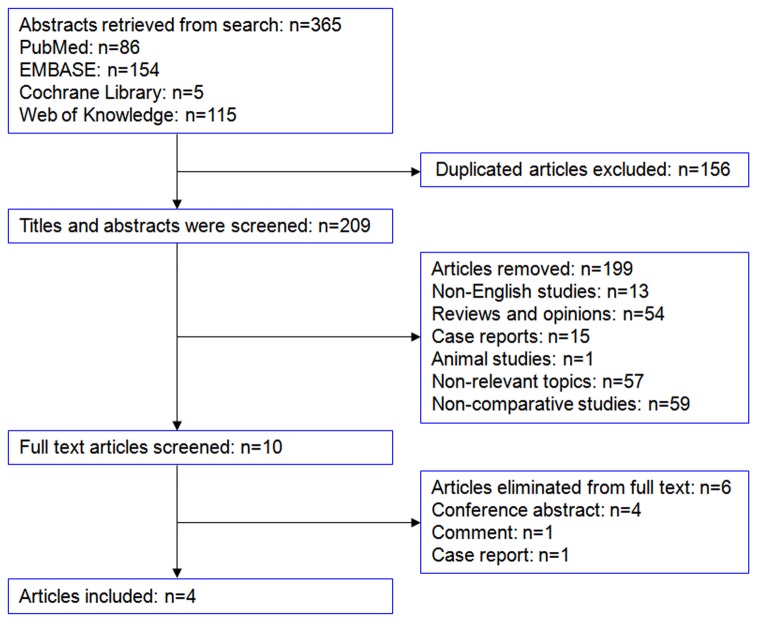
A total of 365 abstracts were identified from searching in PubMed, EMBASE, Cochrane Library, and Web of Knowledge electronic database. 156 duplicates were removed by using the Endnote software. After reviewing 209 titles and abstracts, 199 studies were excluded. One comment, one case report and four conference abstracts were screened among the remaining 10 studies by full articles review. Finally, four retrospective studies [4,8–10] with 5780 cases were included in our meta-analysis (Figure 1). The baseline characters of the include studies and quality assessment were listed in Table 1.
Figure 1. Flow chart of selection.
Table 1. Baseline characters of include studies and quality assessment (mean ± SD).
| Author | Year | country | Study type | group | N | Sex | BMI | Age | Quality |
|---|---|---|---|---|---|---|---|---|---|
| (m/f) | (mean±SD) | (mean±SD) | assessment | ||||||
| Caruso S[8] | 2011 | Italy | retrospective | RG | 29 | 18/11 | 27±3 | 64.8±12.4 | 6 stars |
| study | OG | 120 | 65/55 | 28±4 | 65.1±11 | ||||
| Huang KH[4] | 2012 | China | retrospective | RG | 39 | 19/20 | 24.2±3.7 | 65.1±15.9 | 5 stars |
| study | OG | 586 | 406/180 | 23.7±3.6 | 67.9±30.1 | ||||
| Kim KM[9] | 2012 | Korea | retrospective | RG | 436 | 265/171 | 23.6±3.1 | 54.2±12.5 | 5 stars |
| study | OG | 4542 | 3008/1534 | 23.8±8.0 | 57.7±11.8 | ||||
| Kim MC[10] | 2010 | Korea | retrospective | RG | 16 | 10/6 | 21.3±3.4 | 53.8±15.6 | 6 stars |
| study | OG | 12 | 9/3 | 25.2±1.9 | 56.0±12.4 |
Operation time
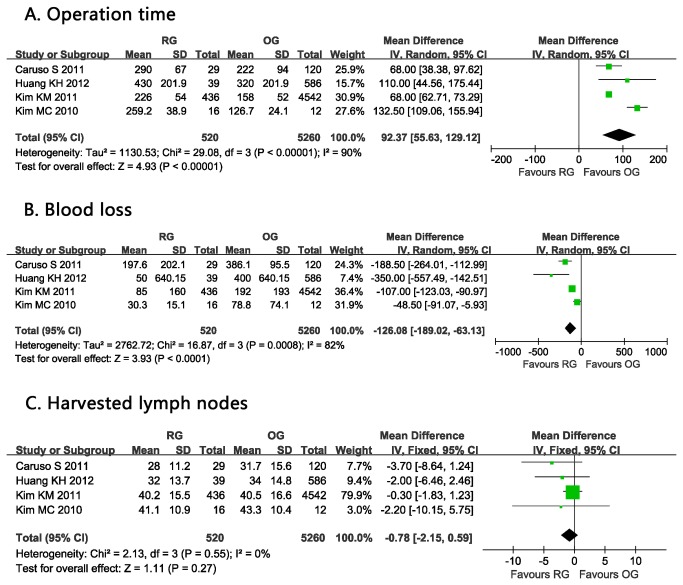
Operation time was significantly longer with RG than OG reported in all included studies [4,8–10]. Pooled analysis of operation time had a significant difference between RG and OG in this regard and with a significant heterogeneity (WMD: 92.37 min, 95% CI: 55.63 to 129.12 min, P <0.00001, I2=90%) (Figure 2A).
Figure 2. Forest plot showing a meta-analysis for robotic gastrectomy versus open gastrectomy on A. Operation time; B. Blood loss; C.
Harvested lymph nodes.
Blood loss
A statistical difference of blood loss was observed between these two approaches [4,8–10]. The estimated intraoperative blood loss was significantly lower in the RG group than in the OG group. (WMD:-126.08 ml, 95% CI: -189.02 to -63.13 ml, P <0.0001, I2=82%) (Figure 2B).
Harvested lymph nodes
The pooled data from these four studies showed no difference in the number for the harvested lymph nodes between RG and OG [4,8–10]. (WMD = -0.78; 95% CI -2.15 to 0.59; P =0.27) (Figure 2C).
Postoperative hospital stay
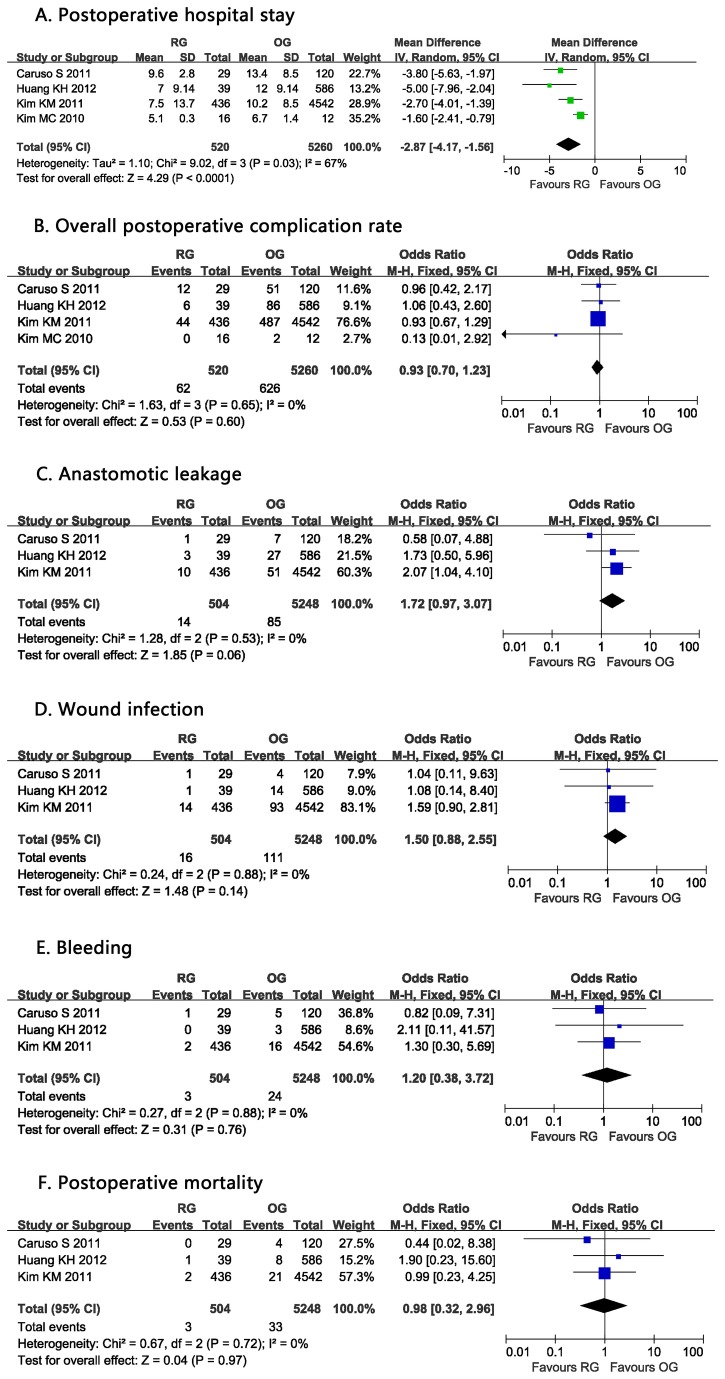
Postoperative hospital stay was shorter with RG [4,8–10]. Compared to OG, RG reduced postoperative stay by a mean of 2.87 days. (WMD = -2.87 d; 95% CI -4.17 to -1.56 d; P <0.0001), with high heterogeneity among these studies (I2 = 67%) (Figure 3A).
Figure 3. Forest plot showing a meta-analysis for robotic gastrectomy versus open gastrectomy on A. Postoperative hospital stay; B. Overall postoperative complication rate; C. Anastomotic leakage; D. Wound infection; E. Bleeding; F. Postoperative mortality.
Overall postoperative complication rate
All four included studies reported postoperative complication rate [4,8–10]. The overall postoperative complication morbidity was 11.92% (62/520) in RG and 11.90% (626/5260) in OG. Meta-analysis found no significant difference (OR: 0.93, 95% CI: 0.70 to 1.23, P =0.60, I2=0%) (Figure 3B).
Anastomotic leakage
The rate of anastomotic leakage was described in three studies [4,8,9]. No difference was observed in pooled analysis between 2.78% (14/504) for RG and 1.62% (85/5248) for OG (OR:1.72, 95% CI: 0.97 to 3.07, P =0.06, I2=0%) (Figure 3C).
Wound infection
Three studies described postoperative wound infection [4,8,9] and there were no significant differences between RG and OG. (OR: 1.50, 95% CI: 0.88 to 2.55, P=0.14, I2=0%) (Figure 3D).
Bleeding
The incidence of bleeding was 0.6% in RG group and 0.4% in OG group. No differences were observed in three studies [4,8,9] (OR: 1.20, 95% CI: 0.38 to 3.72, P =0.76, I2=0%) (Figure 3E).
Postoperative mortality
Postoperative mortality was mentioned in three of the studies. The study by Caruso S et al. found no difference between RG and OG on 30-day mortality [8], which was similar to the results reported by Huang KH et al. [4] and Kim KM et al. [9]. Pooled analysis revealed no statistical difference with no heterogeneity (OR=0.98, 95% CI: 0.32 to 2.96, P=0.97, I2=0%) (Figure 3F).
Publication bias
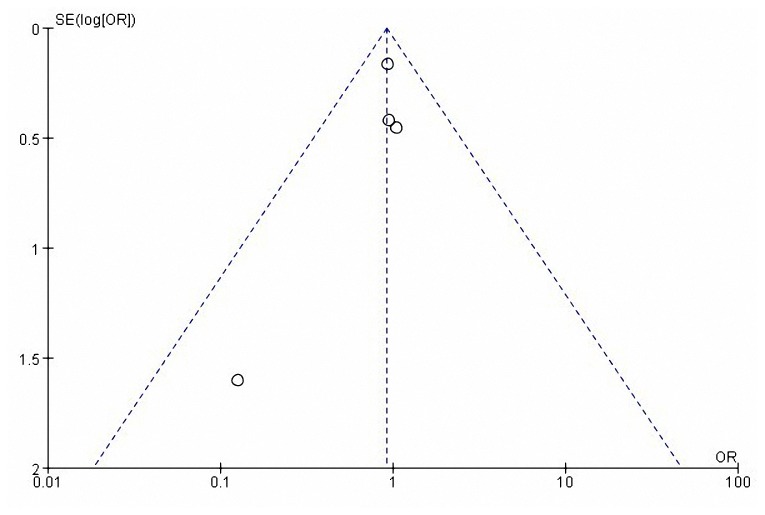
The complication rate was evaluated with a standard-error based funnel plot using fix effect size between RG and OG. The outcomes of all the studies were within the 95% CIs and were slightly unsymmetrical (Figure 4). No evidence of publication bias was revealed among these studies from statistical tests (Begg's test P =0.734; Egger's test P =0.309).
Figure 4. Funnel plot of overall postoperative complication rate in patients between RG and OG.
OR, odds ratio; SE, standard error.
Sensitivity analysis
Sensitivity analysis was performed by excluding the study reported by Kim MC et al. [10] in which the total sample size was less than 50. All variables were conducted for sensitivity analysis. The index would be excluded for further sensitivity if there were not enough available studies (less than 2). The results were not significantly influenced by sensitivity analysis as shown in Table 2.
Table 2. Sensitivity analysis of outcomes.
| Outcomes | Number of Studies | Patients | WMD/OR | 95% CI | P | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| Operative time (min) | 3[4,8,9] | RG=504 | 68.26 | 63.07, 73.45 | <0.00001 | 0 | 0.46 |
| OG=5428 | |||||||
| Postoperative hospital stay (d) | 3[4,8,9] | RG=504 | -3.29 | -4.30, -2.29 | <0.00001 | 15 | 0.31 |
| OG=5428 | |||||||
| Estimated blood loss (ml) | 3[4,8,9] | RG=504 | -173.88 | -270.68, -77.08 | 0.0004 | 79 | 0.009 |
| OG=5428 | |||||||
| Total postoperative complication | 3[4,8,9] | RG=504 | 0.95 | 0.71, 1.26 | 0.72 | 0 | 0.97 |
| OG=5248 | |||||||
| Harvested lymph nodes | 3[4,8,9] | RG=504 | -0.73 | -2.13,0.66 | 0.30 | 0 | 0.37 |
| OG=5248 | |||||||
Discussion
With the development of technology, robot-assisted laparoscopy has been widely performed in the field of urology [11], gynecology [12] and general surgery [13], and has become an attractive option for surgeons. RG has been considered as a potentially feasible and safe technique, which has been widely reported by many studies. This meta-analysis was conducted in an attempt to evaluate the currently available evidence for the role of robotic versus open gastrectomy for gastric cancer and to identify whether the use of robotic surgery can be practically beneficial. Four studies involving 5780 patients with 520 (9.00%) cases of robotic gastrectomy and 5260 (91.00%) cases of open gastrectomy were included in this meta-analysis.
The operation time was significantly longer with RG than OG (P <0.00001). This could be attributed to the docking time and preparation time for RG. A previous study has reported that the mean docking time in RG was 63.3 minutes [5]. With experience gained in robotic surgery, the docking time could be reduced by half an hour [4]. Another explanation was that RG needed a learning curve in order to be proficient [14], cases with initial experience of RG may take longer than the subsequent cases due to less skilled performance. Operation time would be strikingly reduced by experience accumulated surgeons [15]. However, some studies included in this analysis also obtained cases with initial experience of RG [4]. Moreover, the operation time can be reduced by the upgraded robotic instruments.
The most striking finding was the reduction of blood loss in RG versus OG, with statistical significance (P <0.0001). Due to the benefits of dexterity of scale motion and 3D image, robotic surgery can perform in a precise way while minimizing blood loss [10]. The median volume of blood loss was 30ml when performing RG reported by a previous study [16]. The lower blood loss indicated a lower transfusion rate. In addition, the amount of blood loss and the need for transfusions had a positive correlation with perioperative mortality and morbidity [17,18] . Studies have reported that a lower blood loss may result in a lower recurrence and thus, may improve the quality of life of gastric patients [19].
RG was associated with significantly shorter hospital stay (P <0.0001). This might be attributed to the advantages of robotic surgery systems. Robotic surgery is a minimally invasive technique [20] which contributes to reduced pain, quicker return to oral intake, as well as avoiding the long abdominal incision of open surgery and reducing tissue injury.
There was no significant difference on overall postoperative complication rate. The incidence of postoperative complication for RG (11.92%) was similar to OG (11.90%). Besides, no significant difference was observed in terms of postoperative mortality. These results demonstrated that RG is a safer and a more feasible alternative technique to OG.
Anastomotic leakage is a major complication after gastric cancer surgery [21]. The rate of anastomotic leakage ranged from 1%-10% according to previous reports [22,23]. However, according to this meta-analysis, the incidence of leakage was not significantly different between these two groups. The anastomotic leakage rate was 2.78% (14/504) for RG and 1.62% (85/5248) for OG (P=0.06). Yoon HM et al. reported there was no anastomotic leakage when performing RG in 36 patients [24]. As anastomotic leakage was associated with morbidity and mortality, more attention should be paid to this issue and more effort should be done to prevent leaks when performing RG. In addition, the safety of RG should be further investigated by well designed randomized controlled trials and the application of this novel approach should be with caution considering the high rate of leak when performing RG.
No statistically difference was observed between RG and OG regarding to wound infection and bleeding.
The prognosis of gastric cancer is poor, lymph node metastasis is considered to be an important prognostic factor [25]. Previous studies reported the incidence of lymph nodes metastases in early gastric cancer ranged from 3% to 25% and the rate varied from 3%-5% in mucosa cancers and 16%-25% in submucosal tumors respectively [26]. Thus, extended lymph nodes dissection and the number of harvested lymph nodes could be used to evaluate the oncologic adequacy. For most resectable gastric cancer, the recommended standard surgery is total and distal gastrectomy with D2 lymphadenectomy [27] .Therefore, D2 lymphadenectomy is a critical part of the minimally invasive gastrectomy procedure. However, laparoscopic D2 gastrectomy entails the removal of node stations along the celiac trunk, left gastric artery, and hepatic pedicle. The technical difficulty of D2 gastrectomy has limited its pervasive application [28] .With the technical advantages, robotic surgery can achieve meticulous dissection, even in difficult lymphatic stations around major vessels or in difficult area [8]. Analysis of the pooled data revealed that the number of harvested lymph nodes was similar between RG and OG, which indicated RG could be performed safely. Several studies have also demonstrated robotic lymph node dissection was feasible and safe [29,30]. In addition, with advantages of clear 3D image and dexterity, RG could carry out a safe and effective lymphadenectomy with less blood loss [31].
Several limitations should be considered in this meta-analysis. Firstly, all the included studies are retrospective studies which are non-randomized instead of randomized controlled trials. However, according to a previously published study, well designed non-randomized comparative studies of surgical techniques can reach available results as randomized controlled trials [32]. Secondly, as is known to all, surgical parameters might be influenced by surgeon’s learning curve. In this meta-analysis, the robotic cohorts from most if not all of these institutions represented their initial experiences, which could introduce a bias against the robotic outcomes. Thirdly, high heterogeneity was existed in terms of operation time, blood loss and postoperative hospital stay. Since it was difficult to match baseline characters in all selected studies, we used a random effected model to evaluate these parameters. Fourthly, the long-term outcomes cannot be accessed because of the insufficient data. The long-term outcomes after gastrectomy were reported in only one study [8] with the follow-up time ranged from 4-53 months for RG and from 1-115months for OG. The result indicated no significant difference in survival rate between RG and OG. Finally, the cost effective between RG and OG was not compared in this meta-analysis due to insufficient data. Thus, further comparison studies addressing cost effective are needed to clarify this issue.
In conclusion, RG is safe and efficient. RG is associated with a longer operation time, less blood loss, and shorter hospital stay compared to those of OG. There is no difference on overall postoperative complication, wound infection, bleeding, anastomotic leakage rate and harvested lymph nodes. RG may be a more practical and feasible alternative technique to OG. However, more prospective, well-designed, multicenter, randomized controlled trials are necessary to further address the safety and efficacy as well as the long-term outcome of RG.
Supporting Information
PRISMA Checklist.
(DOCX)
Acknowledgments
We gratefully acknowledge Kyna Chen, who is from University of California Davis (UC Davis), for helping proofread this article.
Funding Statement
This work was supported by a Natural Science Foundation of China grant (81272508) and key applied and basic projects of Guangzhou science and technology program (11C22120714). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4: 146-148. PubMed: 8180768. [PubMed] [Google Scholar]
- 2. Cadière GB, Himpens J, Germay O, Izizaw R, Degueldre M et al. (2001) Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg 25: 1467-1477. PubMed: 11760751. [DOI] [PubMed] [Google Scholar]
- 3. Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20: 1521-1525. doi: 10.1007/s00464-005-0855-5. PubMed: 16897284. [DOI] [PubMed] [Google Scholar]
- 4. Huang KH, Lan YT, Fang WL, Chen JH, Lo SS et al. (2012) Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg 16: 1303-1310. doi: 10.1007/s11605-012-1874-x. PubMed: 22450954. [DOI] [PubMed] [Google Scholar]
- 5. Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH et al. (2009) Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 249: 927-932. doi: 10.1097/01.sla.0000351688.64999.73. PubMed: 19474671. [DOI] [PubMed] [Google Scholar]
- 6. Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK et al. (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16: 1480-1487. doi: 10.1245/s10434-009-0435-3. PubMed: 19290486. [DOI] [PubMed] [Google Scholar]
- 7. Xiong B, Ma L, Zhang C (2012) Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol 21: 274-280. doi: 10.1016/j.suronc.2012.05.004. PubMed: 22789391. [DOI] [PubMed] [Google Scholar]
- 8. Caruso S, Patriti A, Marrelli D, Ceccarelli G, Ceribelli C et al. (2011) Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot 7: 452-458. doi: 10.1002/rcs.416. PubMed: 21984205. [DOI] [PubMed] [Google Scholar]
- 9. Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ et al. (2012) Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 99: 1681-1687. doi: 10.1002/bjs.8924. PubMed: 23034831. [DOI] [PubMed] [Google Scholar]
- 10. Kim MC, Heo GU, Jung GJ (2010) Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 24: 610-615. doi: 10.1007/s00464-009-0618-9. PubMed: 19688399. [DOI] [PubMed] [Google Scholar]
- 11. Montorsi F (2007) A plea for integrating laparoscopy and robotic surgery in everyday urology: the rules of the game. Eur Urol 52: 307-309. doi: 10.1016/j.eururo.2007.05.005. PubMed: 17531375. [DOI] [PubMed] [Google Scholar]
- 12. Tinelli A, Malvasi A, Gustapane S, Buscarini M, Gill IS et al. (2011) Robotic assisted surgery in gynecology: current insights and future perspectives. Recent Pat Biotechnol 5: 12-24. doi: 10.2174/187220811795655913. PubMed: 21517747. [DOI] [PubMed] [Google Scholar]
- 13. Marano A, Priora F, Lenti LM, Ravazzoni F, Quarati R et al. (2013) Application of Fluorescence in Robotic General Surgery: Review of the Literature and State of the Art. World J Surg, 37: 2800–11. PubMed: 23645129. [DOI] [PubMed] [Google Scholar]
- 14. Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK et al. (2012) Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer 12: 156-163. doi: 10.5230/jgc.2012.12.3.156. PubMed: 23094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K et al. (2011) Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 146: 1086-1092. doi: 10.1001/archsurg.2011.114. PubMed: 21576595. [DOI] [PubMed] [Google Scholar]
- 16. D'Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P et al. (2011) Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res 166: e113-e120. doi: 10.1016/j.jss.2010.11.881. PubMed: 21227455. [DOI] [PubMed] [Google Scholar]
- 17. Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM et al. (2010) Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg 252: 11-17. doi: 10.1097/SLA.0b013e3181e3e43f. PubMed: 20505504. [DOI] [PubMed] [Google Scholar]
- 18. Spence RK, Carson JA, Poses R, McCoy S, Pello M et al. (1990) Elective surgery without transfusion: influence of preoperative hemoglobin level and blood loss on mortality. Am J Surg 159: 320-324. doi: 10.1016/S0002-9610(05)81227-9. PubMed: 2305940. [DOI] [PubMed] [Google Scholar]
- 19. Vamvakas EC (1995) Perioperative blood transfusion and cancer recurrence: meta-analysis for explanation. Transfusion 35: 760-768. doi: 10.1046/j.1537-2995.1995.35996029162.x. PubMed: 7570938. [DOI] [PubMed] [Google Scholar]
- 20. Robinson TN, Stiegmann GV (2004) Minimally invasive surgery. Endoscopy 36: 48-51. doi: 10.1055/s-2004-814113. PubMed: 14722855. [DOI] [PubMed] [Google Scholar]
- 21. Sand J, Luostarinen M, Matikainen M (1997) Enteral or parenteral feeding after total gastrectomy: prospective randomised pilot study. Eur J Surg 163: 761-766. PubMed: 9373227. [PubMed] [Google Scholar]
- 22. Ichikawa D, Kurioka H, Yamaguchi T, Koike H, Okamoto K et al. (2004) Postoperative complications following gastrectomy for gastric cancer during the last decade. Hepatogastroenterology 51: 613-617. PubMed: 15086217. [PubMed] [Google Scholar]
- 23. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A et al. (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359: 453-462. doi: 10.1056/NEJMoa0707035. PubMed: 18669424. [DOI] [PubMed] [Google Scholar]
- 24. Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW et al. (2012) Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc 26: 1377-1381. doi: 10.1007/s00464-011-2043-0. PubMed: 22083338. [DOI] [PubMed] [Google Scholar]
- 25. Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y et al. (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3: 219-225. doi: 10.1007/PL00011720. PubMed: 11984739. [DOI] [PubMed] [Google Scholar]
- 26. Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA et al. (2005) Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 241: 27-39. PubMed: 15621988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11: 439-449. doi: 10.1016/S1470-2045(10)70070-X. PubMed: 20409751. [DOI] [PubMed] [Google Scholar]
- 28. Shehzad K, Mohiuddin K, Nizami S, Sharma H, Khan IM et al. (2007) Current status of minimal access surgery for gastric cancer. Surg Oncol 16: 85-98. doi: 10.1016/j.suronc.2007.04.012. PubMed: 17560103. [DOI] [PubMed] [Google Scholar]
- 29. Pugliese R, Maggioni D, Sansonna F, Ferrari GC, Forgione A et al. (2009) Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma. Analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol 35: 281-288. doi: 10.1016/j.ejso.2008.02.001. PubMed: 18342480. [DOI] [PubMed] [Google Scholar]
- 30. Jacob BP, Gagner M (2003) Robotics and general surgery. Surg Clin North Am 83: 1405-1419. doi: 10.1016/S0039-6109(03)00159-2. PubMed: 14712875. [DOI] [PubMed] [Google Scholar]
- 31. Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A et al. (2008) Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc 22: 2753-2760. doi: 10.1007/s00464-008-0129-0. PubMed: 18813994. [DOI] [PubMed] [Google Scholar]
- 32. Abraham NS, Byrne CJ, Young JM, Solomon MJ (2010) Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 63: 238-245. doi: 10.1016/j.jclinepi.2009.04.005. PubMed: 19716267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOCX)