Abstract
Echolocating organisms represent their external environment using reflected auditory information from emitted vocalizations. This ability, long known in various non-human species, has also been documented in some blind humans as an aid to navigation, as well as object detection and coarse localization. Surprisingly, our understanding of the basic acuity attainable by practitioners—the most fundamental underpinning of echoic spatial perception—remains crude. We found that experts were able to discriminate horizontal offsets of stimuli as small as ~1.2° auditory angle in the frontomedial plane, a resolution approaching the maximum measured precision of human spatial hearing and comparable to that found in bats performing similar tasks. Furthermore, we found a strong correlation between echolocation acuity and age of blindness onset. This first measure of functional spatial resolution in a population of expert echolocators demonstrates precision comparable to that found in the visual periphery of sighted individuals.
Keywords: Echolocation, Perception, Crossmodal perception, Blindness
Introduction
When vision is unavailable or insufficient for perception, other sensory modalities often take precedence in sampling the environment. In the case of echolocation—used, for example, by many bats and some marine mammals as a mechanism for navigation, object perception, hunting, and social communication (Thomas et al. 2004)—this sampling is active, taking the form of self-generated auditory pulses whose reflected echoes are then interpreted to generate surface and object percepts. To a limited extent, active echolocation has also been demonstrated in some humans, whose putative “facial vision” or “obstacle sense” was found to be auditory, not tactile in nature (Supa et al. 1944; Worchel and Dallenbach 1947); whose performance was aided by active sounds versus passive hearing (Supa et al. 1944; Teng and Whitney 2011); and who were able to detect, localize, and discriminate some stimuli under various conditions (Kellogg 1962; Thaler et al. 2011; Hausfeld et al. 1982; Rice 1967; Rice and Feinstein 1965; Rice et al. 1965; Schenkman and Nilsson 2010). However, the behavioral envelope of human echolocation, and thus its potential mechanisms and range of utility, remains poorly understood. In particular, it is a reasonable working hypothesis that the spatial acuity of echolocation is critical to the object recognition and navigation tasks performed on a daily basis by highly trained, blind expert practitioners. Some echo tasks have shown localization using detection (Rice 1969) or lateralization paradigms (Dufour et al. 2005; Thaler et al. 2011); despite this, no standardized measure exists by which to assess echoic spatial resolution. In vision, the Snellen chart (Snellen 1863) is a common tool for acuity assessments; more powerful measures, probing finer scales of spatial discrimination, include Vernier acuity—a relative position judgment of two objects (Kniestedt and Stamper 2003; Westheimer 1979). In this study, therefore, we investigated the spatial resolution of echoic object localization in highly trained blind experts using an auditory analogue to the Vernier task.
Methods
Subjects
We recruited a sample of seven blind human echolocators with the aid of World Access for the Blind, a non-profit organization devoted to teaching echolocation techniques (Table 1). All participants gave verbal and written informed consent, and all were compensated for their time in accordance with guidelines set forth by the Committee for Protection of Human Subjects at the University of California, Berkeley. One participant was excluded from analysis by our criterion defining highly trained echolocators as those who, by self-report, have at least 10,000 h of echolocation training experience, including both formal training and daily normal use. Six highly trained echolocators remained in the sample, with estimates of training time (combining formal training and daily use) ranging from approximately 12,000 h to over 200,000 h. No participants but one had any light perception abilities, and all reported using echolocation frequently as an aid in their daily lives.
Table 1.
| Sex | Age of blindness onset (years) | Cause of blindness | Age when tested (years) | Vernier threshold (deg) | |
|---|---|---|---|---|---|
| S02 | M | 0 | Glaucoma | 28 | 1.22 |
| S03 | M | 5 | Retinitis pigmentosa, juvenile macular degeneration | 25 | 3.52 |
| S04 | M | 14 | Optic nerve atrophy | 27 | 7.58 |
| S05 | M | 1.8 | Retinoblastoma | 33 | 1.94 |
| S06 | M | 17 | Familial exudative vitreoretinopathy | 22 | 4.93 |
| S07 | M | 0.75 | Retinoblastoma | 41 | 1.36 |
Stimuli and procedure
Subjects’ echo vocalizations were generated via trains of single-pulse tongue clicks with a typical clicking frequency of approximately 1–3 Hz. By emitting these self-generated tongue clicks from a fixed distance, subjects evaluated the relative positions of two identical, circular flat plastic disks, arranged vertically on a frame and separated horizontally by varying amounts (Fig. 1a). The disks, 20.3 cm in diameter and 6.35 mm thick, were coated with a thin layer of primer to ensure a uniform, matte reflecting surface. The frame consisted of a wooden base and two wooden dowels supporting two crossbars separated vertically by 27.5 cm. The hooks on the disks’ rear surfaces allowed rapid removal and replacement in different configurations, and the crossbars were marked with 1-cm gradations. Participants were seated 50 cm from the disks, measured orthogonally from the ears to the plane formed by the disks, at a height such that their ears were halfway between the crossbars supporting the rods. Due to accessibility considerations, sessions were conducted in participants’ homes or similar quiet testing spaces. A large sound-absorbent foam surface, approximately 193 cm tall by 203 cm wide, was mounted on a frame approximately 1 m behind the testing apparatus to provide a consistent acoustic background behind the stimuli across different testing sites. Participants wore close-fitting eye masks for consistency across subjects and to control for the residual light perception retained by one participant. In each trial (see detailed procedure below), the experimenter placed one disk on each crossbar at a specific position to the left or right of center. The top disk was always displaced an equal and opposite distance from center as the bottom disk. The distance of the disks from the center on each trial was pseudorandomized. The horizontal center-to-center separation between disks was the independent variable manipulated in the experiment and subtended 1.1°–13.2° auditory angle; to avoid ceiling effects, the three participants with the best performance were seated farther away from the apparatus, their ears 100 cm from the disk plane, such that the disks subtended 0.57°–3.4° (thus making the task more difficult). A shoulder tap signaled the beginning of the trial for the subject.
Fig. 1.
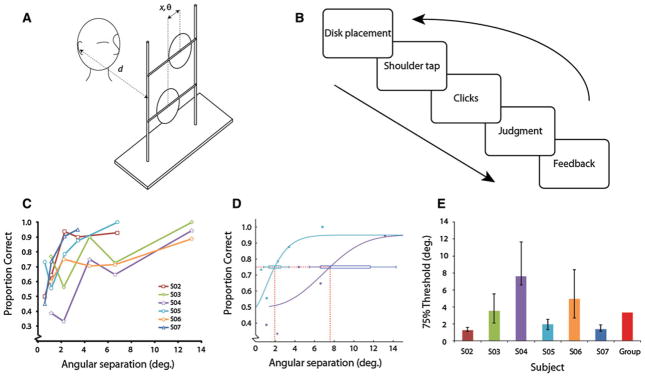
a Experimental setup for vernier echolocation discrimination. The stimulus frame was situated such that the normal distance between the plane of the disks and the ears was d. For 3 participants, d = 50 cm, and for 3, d = 100 cm. The auditory angle subtended, θ, was calculated by using the center-to-center horizontal separation between disks, x. At a distance of 50 cm, θ was between 1.1° and 13.2°; at 100 cm, it ranged from 0.57° to 3.4°. x was always between 1 and 12 cm. b Structure of one trial. While experimenter placed the disks at the appropriate separations on the frame, a barrier prevented auditory cues from informing subjects about the location of the disks. The barrier was removed, and a shoulder tap signaled the subject to begin clicking. The subject then made a judgment and received feedback. Sessions consisted of 80 trials and five stimulus separations. c Performance versus angular separation between disks for six subjects. Chance performance was 50%. d Calculation of 75% performance thresholds. Two representative psychometric functions are shown, from subjects S04 and S05. Confidence intervals were generated with psignifit’s BCa bootstrapping method, running 10,000 simulations. e Thresholds plotted for each subject and group average. Individual subjects’ error bars are bootstrapped standard deviations; group average is the median of the distribution of individual subjects’ bootstrapped and averaged thresholds (see “Methods” for details)
The task, a two-alternative-forced choice (2AFC) discrimination task using the method of constant stimuli, was to determine whether the top disk was positioned to the right or left of the bottom disk (Fig. 1b), using only clicking. We conducted two sessions with participants, each consisting of 80 trials and lasting approximately 1–2 h. During the first (practice) session, subjects gave two responses per trial: an immediate response without clicking and then a response after producing clicks. The immediate responses served as a control to establish that subjects were unable to use ambient sound to perform the task, that is, that the clicks were necessary to make the judgments. The same dual-response method with one expert and a larger pool of sighted blindfolded participants had confirmed the utility of the clicks in a previous study, with subjects remaining at chance levels of performance without clicking. (Teng and Whitney 2011). Thus, in the second session, participants made only a clicking judgment. During repositioning of the stimuli between trials, a padded foam and cardboard screen shielded the stimulus frame, and any associated auditory cues that might have been generated, from the participant. While clicking, participants were allowed to translate vertically, but monitored to ensure a constant distance from the stimulus rig. For one participant (S07), data were collected as part of our earlier study, but reanalyzed here, with an 80-trial session in which four angular separations subtended 0.57°–3.4°. For this participant, the two-response procedure was used in the second session as well as the first. One participant (S03) was confused about the task during the first 20 trials, so those trials were excluded from analysis; including these trials or removing this subject from the group analysis had no influence on the overall pattern of results. Feedback regarding accuracy and the actual displacement from center of the disks (in cm) was given for each trial.
Functionally, echolocation for blind practitioners is similar to vision in that it serves to generate a spatial representation of the environment. We therefore found it appropriate to make a direct comparison, using the same apparatus, of the discrimination performance achievable by auditory (echoic) and visual modalities. Using the same apparatus described above (Fig 3a), four sighted, psychophysically naïve volunteers with normal (n = 2) or corrected-to-normal (n = 2, eyeglasses) vision made visual judgments of the disks’ relative positions in a single session of 120 trials. Though not compensated, each gave informed consent to participate according to UC Berkeley human subject research protection protocols. Subjects sat 50 cm from the plane of the disks, with the left eye covered by an eye patch, and fixated monocularly straight ahead. The frame was situated 35° right of the midline (see Fig. 3a) against a matte black background under normal laboratory lighting conditions, such that the white disks were visible at high contrast (96.7%). In each trial, as in the echo experiment, subjects judged whether the top disk was to the left or right. Between trials, the rig was occluded by a handheld screen while the experimenter replaced the disks. The disks were situated at a large eccentricity because visual acuity varies greatly across the visual field: central vision is extremely fine, with minimum angles under 5 s of arc for foveal vernier judgments, (e.g., Levi et al. 1985) and 1 min of arc for reliable recognition of visual objects such as letters (Kniestedt and Stamper 2003). Due to this magnification factor, we presented visual targets at 35° eccentricity (Anstis 1974), which is expected to yield visual acuity measures that approximate the acuity measured in the echoic vernier acuity experiment (see “Results”).
Fig. 3.
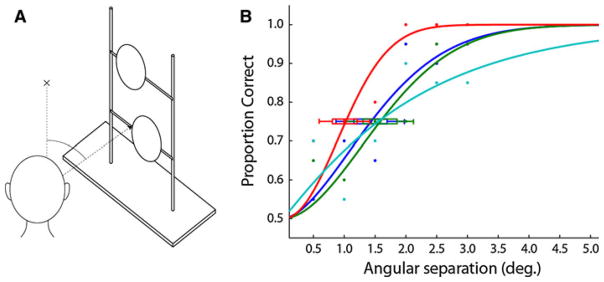
Visual vernier thresholds from 4 sighted naïve subjects at 57 cm distance and 35° retinal eccentricity. The same stimulus rig from the echolocation experiment (Fig. 1) was used to present pairs of disks with vernier offsets ranging from 0.5° to 3.0°, in 0.5° increments. Sessions consisted of 120 trials—20 at each of six separation conditions, randomly interleaved. Between trials, a screen occluded the stimulus frame. Subjects fixated a point and viewed the stimulus monocularly. One experimenter placed disks during each trial; another monitored subjects’ gaze to ensure fixation. The group average 75% threshold was 1.4°
Analysis
We fitted psychometric curves to results from individual sessions. The curve fitting procedure, implemented in the psignifit toolbox for Matlab 7.1 (The Mathworks, Natick, MA), utilized a maximum-likelihood method and the Weibull function for generating the underlying shape of the fitted curve (Wichmann and Hill 2001a). Performance was measured as percent correct and constrained to range from 50% to 95%, with the 75% intercept chosen as the threshold. Confidence intervals were generated based on 10,000 simulations using psignifit’s BCa bootstrapping method (Wichmann and Hill 2001b). A similar curve fitting procedure was used in the visual vernier comparison experiment; however, performance for this comparison was allowed to range from 50 to 100%.
In calculating correlations between age of blindness onset, blindness duration, and echolocation acuity, we used a non-parametric test (Spearman’s rho) to avoid violating normality, linearity, and homoscedasticity assumptions due to the small sample size. We then bootstrapped each subject’s vernier threshold 10,000 times and fit a regression line to each iterated set of six thresholds. The values of the resulting 10,000 regression slopes yielded significance estimates for the correlation (Fig. 2): fewer than 0.5% were negative. Additionally, group averages were obtained by computing the mean of each of 10,000 sets of individual subjects’ bootstrapped thresholds and finding the median value of the resultant distribution.
Fig. 2.
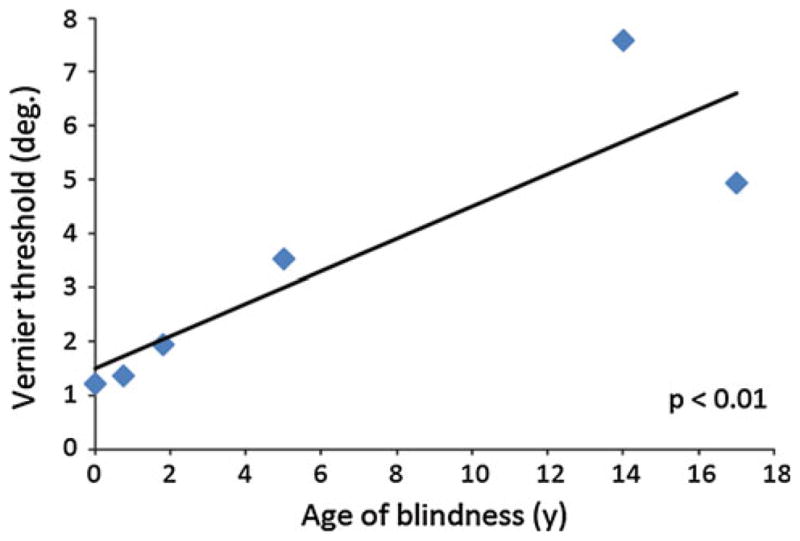
Correlation between vernier echo acuity and age of blindness onset, in years. N = 6, Spearman ρ = 0.94. Bootstrapped confidence intervals (see “Methods”) yield P <0.01
Results
The six participants performed robustly above chance, with a mean bootstrapped 75% threshold of 3.46° auditory angle subtended, as measured from the coronal plane of the ears. The three best performers exhibited performance thresholds of less than 2°, superior to previous reports of blindfolded sighted (Teng and Whitney 2011) subjects performing the same task and blind individuals performing other tasks of spatial localization (Rice 1969; Thaler et al. 2011; Kellogg 1962; Rice et al. 1965). Interestingly, there were substantial and systematic individual differences between our subjects, raising the question of whether early blindness might predict performance. Confirming this, we found a significant correlation between age of blindness onset and echolocation acuity (n = 6, Spearman ρ = 0.94, P <0.01; see Fig. 2). A correlation between blindness duration and acuity did not reach significance; nor did a correlation between acuity and estimated training time. Of course, age and duration of blindness covary, so future studies are necessary to disentangle whether age or experience is the critical factor for the extraordinary precision of echolocation found here.
In our monocular visual vernier comparison experiment, 4 psychophysically naïve, sighted participants achieved a group mean 75% threshold of 1.4° at an eccentricity of 35°. This is comparable to the echo-acuity thresholds obtained by some of the blind subjects.
Discussion
The results here demonstrate remarkably precise spatial acuity of echolocation in expert practitioners. From a pragmatic standpoint, it is worth noting that even the later blind echolocators in our group achieved a relatively high degree of spatial precision and reported using echolocation functionally on a daily basis. Thus, individual differences in echolocation (or visual) acuity do not preclude the use of echolocation (or vision) in daily life. The spatial resolution of the best human echolocators we tested exceeds that of various previous human echolocation studies (Kellogg 1962; Rice 1969, 1965; Thaler et al. 2011) and compares favorably to several tests of artificial echolocation devices (De Volder et al. 1999; Hughes 2001), though variation in methods makes direct comparison difficult. Additionally, it is comparable to that of other species known to rely heavily on echolocation for spatial object perception and navigation behaviors (Moss and Surlykke 2010; Pack and Herman 1995). In a previous investigation into echo acuity in the big brown bat, Eptesicus fuscus, the angular separation between brass rods was manipulated (Simmons et al. 1983). The 75% performance threshold of the bats in that experiment was approximately 1.5° horizontal angle when measured in a comparable fashion (2AFC localization) (Simmons et al. 1983), compared to the 1.2°–1.9° thresholds of the three best performers in our study. Thus, the subset of human echolocators who were blind from an early age shows spatial resolution comparable to that observed in a species with specializations for echolocation.
Although based on a small group of participants, the correlation in Fig. 2 suggests that early blindness plays a role in achieving the most precise levels of echolocation. This would be consistent with previous evidence of superior passive auditory performance in the blind compared to the sighted, early- versus late-blind individuals (Gougoux et al. 2005; Lessard et al. 1998; Muchnik et al. 1991; Röder et al. 1999), and recent echolocation results in 2 participants (Thaler et al. 2011), though the interplay of experience, practice, and age of blindness remains open to further study.
Our results indicate both superior echolocation acuity in blind expert practitioners compared to previous studies, as well as a strong tendency toward higher precision with earlier blindness onset. This pattern of results may have several causes; for example, participants who had been blind from an early age, or for an extended duration, could plausibly have been proficient at the detection of echo cues, but might lack the additional benefit of intensive, specific training in active echolocation. The novel vernier offset task could also have provided a more precise testbed for spatial echolocation acuity than prior tasks. Indeed, the vernier task analogue in the visual modality is a standard method of precisely and reliably measuring the acuity of vision; likewise, we believe that our vernier acuity measure of echolocation ability can provide a standard measure of the spatial resolution of echolocation acuity. Although our measurements were conducted in the medial plane, where passive spatial hearing acuity is known to be at its finest (Blauert and Allen 1997; Middlebrooks and Green 1991), this is also true of many previous human and non-human studies (Kellogg 1962; Rice and Feinstein 1965; Rice et al. 1965). Studies in which some form of echolocalization was conducted at large eccentricities off the medial plane indicate increased difficulty and decreased performance at those eccentricities (Rice 1969; Thaler et al. 2011). Because the echolocation pulse is directional, it is likely that echoic vernier thresholds at greater eccentricities would be higher than those reported here.
The underlying cues mediating human echolocation performance remain unclear and are likely task-dependent; possibilities include binaural (interaural level and time difference) cues, monaural spectral features, and interference “ripples” (Bassett and Eastmond 1964; Carlson-Smith and Wiener 1996; Rice 1967; Simmons et al. 1983; Arias and Ramos 1997). Regardless of the particular cues involved, however, our implementation of a standardized approach for measuring spatial resolution has revealed that humans possess a remarkably precise spatial resolution of echolocation—more precise, for example, than would be predicted by simply calculating the wavelengths sampled by the peak-energy frequencies of typical echolocation clicks (Rice 1967; Rojas et al. 2009).
The resolution attained in our group of volunteers corresponds approximately to typical visual acuity at a retinal eccentricity of 35° (Anstis 1974), measured here using the same stimuli and procedure, but with monocular visual judgments made by sighted observers (Fig. 3b). This comparison is instructive, because it indicates that the spatial scale of the information afforded by vision to make the vernier judgment is comparable to the spatial scale of the information afforded by echolocation to make the same judgment. Our results, therefore, suggest that the usefulness of visual vernier misalignments at 35° eccentricity may extend to echolocation as well; they raise the intriguing possibility that traditionally visual functions—including object and scene recognition, visually guided behavior, and navigation—that occur in the peripheral visual field might also be available, with spatial resolution as high as vision, based on echolocation cues.
Although, at present, few blind individuals in the world are known to have formal training in the use of active echolocation, it is clear that this skill could be taken advantage of much more extensively within the blind community, with practitioners achieving much higher resolution than previously recognized, potentially subserving fine object discrimination in addition to navigation. In light of that, the vernier acuity method presented here may provide a useful operational measure of the spatial resolution of echolocation.
Acknowledgments
We thank all participants for their cooperation and World Access for the Blind for facilitating communication.
Footnotes
Conflict of interest The authors declare no competing financial interests.
Contributor Information
Santani Teng, Email: steng@berkeley.edu, Department of Psychology, The University of California at Berkeley, 3210 Tolman Hall, Berkeley, CA 94720, USA.
Amrita Puri, Department of Psychology, Hendrix College, Conway, AR, USA.
David Whitney, Department of Psychology, The University of California at Berkeley, 3210 Tolman Hall, Berkeley, CA 94720, USA.
References
- Anstis SM. Letter: a chart demonstrating variations in acuity with retinal position. Vis Res. 1974;14(7):589–592. doi: 10.1016/0042-6989(74)90049-2. [DOI] [PubMed] [Google Scholar]
- Arias C, Ramos OA. Psychoacoustic tests for the study of human echolocation ability. Appl Acoust. 1997;51:399–419. [Google Scholar]
- Bassett IG, Eastmond EJ. Echolocation: measurement of pitch versus distance for sounds reflected from a flat surface. J Acoust Soc Am. 1964;36(5):911–916. [Google Scholar]
- Blauert J, Allen JS. Spatial hearing: the psychophysics of human sound localization. MIT Press; Cambridge: 1997. Review edition. [Google Scholar]
- Carlson-Smith C, Wiener WR. The auditory skills necessary for echolocation: a new explanation. J Vis Impair Blind. 1996;90(1):21–35. [Google Scholar]
- De Volder AG, Catalan-Ahumada M, Robert A, Bol A, Labar D, Coppens A, Michel C, Veraart C. Changes in occipital cortex activity in early blind humans using a sensory substitution device. Brain Res. 1999;826(1):128–134. doi: 10.1016/s0006-8993(99)01275-5. [DOI] [PubMed] [Google Scholar]
- Dufour A, Despres O, Candas V. Enhanced sensitivity to echo cues in blind subjects. Exp Brain Res. 2005;165(4):515–519. doi: 10.1007/s00221-005-2329-3. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005;3(2):e27. doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausfeld S, Power RP, Gorta A, Harris P. Echo perception of shape and texture by sighted subjects. Percept Mot Skills. 1982;55(2):623–632. doi: 10.2466/pms.1982.55.2.623. [DOI] [PubMed] [Google Scholar]
- Hughes B. Active artificial echolocation and the nonvisual perception of aperture passability. Hum Mov Sci. 2001;20(4–5):371–400. doi: 10.1016/s0167-9457(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Kellogg WN. Sonar system of the blind. Science. 1962;137:399–404. doi: 10.1126/science.137.3528.399. [DOI] [PubMed] [Google Scholar]
- Kniestedt C, Stamper RL. Visual acuity and its measurement. Ophthalmol Clin N Am. 2003;16(2):155v–170v. doi: 10.1016/s0896-1549(03)00013-0. [DOI] [PubMed] [Google Scholar]
- Lessard N, Pare M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395(6699):278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vis Res. 1985;25(7):963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Ann Rev Psychol. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Moss CF, Surlykke A. Probing the natural scene by echolocation in bats. Front Behav Neurosci. 2010:4. doi: 10.3389/fnbeh.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchnik C, Efrati M, Nemeth E, Malin M, Hildesheimer M. Central auditory skills in blind and sighted subjects. Scand Audiol. 1991;20(1):19–23. doi: 10.3109/01050399109070785. [DOI] [PubMed] [Google Scholar]
- Pack AA, Herman LM. Sensory integration in the bottlenosed dolphin: immediate recognition of complex shapes across the senses of echolocation and vision. J Acoust Soc Am. 1995;98(2 Pt 1):722–733. doi: 10.1121/1.413566. [DOI] [PubMed] [Google Scholar]
- Rice CE. Hum Echo Percept. Science. 1967;155(763):656–664. doi: 10.1126/science.155.3763.656. [DOI] [PubMed] [Google Scholar]
- Rice CE. Perceptual enhancement in the early blind? Psychol Rec. 1969;19(1):1–14. [Google Scholar]
- Rice CE, Feinstein SH. Sonar system of the blind: size discrimination. Science. 1965;148:1107–1108. doi: 10.1126/science.148.3673.1107. [DOI] [PubMed] [Google Scholar]
- Rice CE, Feinstein SH, Schusterman RJ. Echo-detection ability of the blind: size and distance factors. J Exp Psychol. 1965;70:246–255. doi: 10.1037/h0022215. [DOI] [PubMed] [Google Scholar]
- Röder B, Teder-Salejarvi W, Sterr A, Rosler F, Hillyard SA, Neville HJ. Improved auditory spatial tuning in blind humans. Nature. 1999;400(6740):162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- Rojas JAM, Hermosilla JA, Montero RS, Espi PLL. Physical analysis of several organic signals for human echolocation: oral vacuum pulses. Acta Acust Unit Acust. 2009;95:325–330. doi: 10.3813/aaa.918155. [DOI] [Google Scholar]
- Schenkman BN, Nilsson ME. Human echolocation: blind and sighted persons’ ability to detect sounds recorded in the presence of a reflecting object. Perception. 2010;39(4):483–501. doi: 10.1068/p6473. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Kick SA, Lawrence BD, Hale C, Bard C, Escudié B. Acuity of horizontal angle discrimination by the echolocating bat, Eptesicus fuscus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1983;153(3):321–330. doi: 10.1007/bf00612586. [DOI] [Google Scholar]
- Snellen HMD. Art. XXIV.-Test-types for the determination of the acuteness of vision. Am J Med Sci. 1863;44(92):520. [Google Scholar]
- Supa M, Cotzin M, Dallenbach KM. “Facial vision”: the perception of obstacles by the blind. Am J Psychol. 1944;57(2):133–183. [Google Scholar]
- Teng S, Whitney D. The acuity of echolocation: spatial resolution in sighted persons compared to the performance of an expert who is blind. J Vis Impair Blind. 2011;105(1):20–32. [PMC free article] [PubMed] [Google Scholar]
- Thaler L, Arnott SR, Goodale MA. Neural correlates of natural human echolocation in early and late blind echolocation experts. PLoS ONE. 2011;6(5):e20162. doi: 10.1371/journal.pone.0020162PONE-D-11-04391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Moss CF, Vater M. Echolocation in bats and dolphins. University of Chicago Press; Chicago: 2004. [Google Scholar]
- Westheimer G. The spatial sense of the eye. Proctor lecture. Investig Ophthalmol Vis Sci. 1979;18(9):893–912. [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling and goodness-of-fit. Percept Psychophys. 2001a;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001b;63(8):1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Worchel P, Dallenbach KM. “Facial vision”: perception of obstacles by the deaf-blind. Am J Psychol. 1947;60(4):502–553. [PubMed] [Google Scholar]


