Abstract
Most patients with chronic myeloid leukemia (CML) in chronic phase (CP) treated with tyrosine kinase inhibitors (TKI) achieve complete cytogenetic response (CCyR). An increasing number of patients also achieve deep molecular responses (MR). We determined the frequency and significance of deep MR after TKI therapy for CML in CP. MR included: major molecular response (MMR), MR4, MR4.5, and undetectable transcripts (UND), ie BCR-ABL/ABL of ≤0.1%, ≤0.01%, ≤0.0032%, and undetectable transcripts, respectively. 483 patients received imatinib 400mg/day (IM400, 71, July 2000-April 2001), imatinib 800mg/day (IM800, 204, June 2001-July 2005), nilotinib (NILO, 106, July 2005 to date), or dasatinib (DASA, 102, November 2005 to date). UND rates at 36 months were 18.1%, 30.6%, 29.2%, and 28.6%, respectively. Patients achieving UND have superior transformation-free survival (TFS) and overall survival (OS) versus those obtaining ≤MMR, but not other MR levels. At the 18- and 24-month landmark analysis patients achieving UND have no advantage in TFS and OS compared to those achieving a lesser degree of MR. Among patients achieving MR4.5, those who maintain it for ≥2 years (susMR4·5) have no additional benefit in TFS or OS. Most patients with early CP CML receiving TKI achieve MMR. BCR-ABL transcripts become undetectable in a significant fraction of them. Deeper MR at 18 or 24 months are not associated with a benefit in TFS or OS. Furthermore, achieving susMR4·5 does not appear to further reduce the risk of transformation or death.
Keywords: chronic myeloid leukemia, tyrosine kinase inhibitors, BCR-ABL, molecular response
Introduction
Tyrosine Kinase inhibitors (TKI) have dramatically changed the treatment landscape of chronic myeloid leukemia (CML) improving the rates of complete cytogenetic response (CCyR), minimizing transformation to accelerated phase (AP) or blastic phase (BP), and prolonging patient survival.1,2 Most patients with CML in early chronic phase (CP) achieve CCyR with imatinib at standard or higher doses,3,4 or with second generation TKIs (nilotinib5,6 or dasatinib).7,8 The achievement of CCyR correlates with improved event-free survival (EFS), transformation-free survival (TFS), and overall survival (OS) across all Sokal risk categories9.
Molecular testing with reverse-transcriptase (RT)-polymerase chain reaction (PCR) for the BCR-ABL fusion transcript shows persistent CML in the majority of patients at the time of CCyR.10,11 By molecular analysis, a CCyR corresponds approximately to a 2-log reduction in the BCR-ABL transcript levels, or BCR-ABL transcripts of 1% in the International Scale (IS). Major molecular response (MMR) was initially defined in the International Randomized Study of Interferon vs. STI571 (IRIS) as a 3-log reduction of the transcript levels from baseline12 and subsequently standardized by the IS as transcripts of ≤0.1%.13 MMR achieved at 18 months has been associated with improved EFS.12,14-18 However, no benefit in OS has been demonstrated of achieving MMR among patients that achieve CCyR.
With longer follow-up and the use of more potent TKI modalities, an increasing number of patients achieve and maintain deeper levels of molecular response (MR), including undetectable BCR-ABL transcripts (UND). Moreover, a fraction of patients who maintain a BCR-ABL/ABL ratio (%BCR-ABL) ≤0.0032, also referred to as MR4.5, for at least 2 years on imatinib, may not relapse upon treatment discontinuation.19,20 However, whether deeper responses confer long-term survival or other clinical benefit, beyond the potential for treatment discontinuation, is not fully defined.
We analyzed patients with early CP CML treated with 4 different TKI modalities to assess the frequency and prognostic impact of different levels of MR. We also studied the clinical significance and predictive factors for the achievement of a MR4.5 maintained for ≥2 years.
Methods
Patients
Patients with CML in early CP enrolled in consecutive or parallel clinical studies of TKI therapy, all conducted at MD Anderson Cancer Center (MDACC), were included in this analysis. Treatments and study periods were: imatinib 400mg/day (IM400) from July 2000 until April 2001,21 imatinib 800mg/day (IM800) from June 2001 to July 2005,3,4 nilotinib 400 mg twice daily (NILO) from July 2005 to date5 and dasatinib (DASA) 100mg/day from November 2005 to date.7 We included only patients enrolled on or before November 2011 to allow enough follow-up for this analysis. All studies were approved by the MDACC institutional review board and conducted in accordance with the declaration of Helsinki. Eligibility criteria for each study have been previously described.3-5,7,21.
Molecular Testing and Outcome Measures
The level of MR was assessed by real-time quantitative (RQ)-PCR. A qPCR assay developed in the clinical molecular diagnostic laboratory at MDACC was used to monitor patients for the presence of BCR-ABL fusion transcripts as described previously.22 Briefly, 2.85μg of total RNA at 100ng/μl concentration was reverse transcribed in a 60μl final volume using Superscript II reverse transcriptase enzyme (Life Technologies, Carlsbad, CA). A multiplex qPCR was then performed using 5μl of cDNA in a single tube to detect BCR-ABL transcripts e1a2, e13a2 (b2a2), and e14a2 (b3a2) along with ABL for normalization. BCR-ABL and ABL transcript levels are detected simultaneously and quantitative results are expressed as the percent ratio of BCR-ABL to ABL transcript levels. The BCR-ABL transcript type(s) were determined by subsequent capillary electrophoretic separation of the fluorochrome-labeled products.22 Test characteristics including sensitivity were established according to Clinical Laboratory Improvement Amendments and College of American Pathologists guidelines and the assay can detect 1 BCR-ABL1 fusion transcript in 100,000 ABL copies. The assay was standardized to the IS and all samples were tested in the diagnostic laboratory as part of routine clinical work up of patients. The following levels of MR were studied: MMR, MR4, MR4.5, and UND corresponding, respectively, to transcript levels of ≤0.1%, ≤0.01%, ≤0.0032%, and undetectable. To compare the frequencies of and outcomes associated with different levels of MR, patients were divided in groups according to their best level of %BCR-ABL as follows: no-MR: >0.1%; MMR group: 0.01%<MMR≤0.1%; MR4 group: 0.0032%<MR4≤0.01%; MR4.5 group: UND<MR4.5≤0.0032%; UND: undetectable transcripts. TFS was defined as the time interval between treatment start and transformation to AP/BP or death. OS was defined as time to death from any cause at any time or last follow-up.
Statistical Analysis
Patient characteristics were summarized using median (range) for continuous and frequency (percentage) for categorical variables. Kaplan-Meier method was used to estimate the probability of TFS and OS, and log-rank tests were performed to compare the differences among different MR groups. Landmark analyses were conducted to assess the association between MR and survival outcomes. Univariate and multiple logistic regression models were fit to assess the association between patient characteristics or treatment received and the achievement of sustained MR4.5 for ≥2 years (i.e., susMR4.5). All analyses were conducted in SAS 9.3 and Splus 8.2.
Results
Patient populations
From March 2000 to November 2011 483 consecutive patients with CML in early CP were treated on TKI clinical trials. Of these, 71 received IM400, 204 IM800, 106 NILO, and 102 DASA. Median age was 48 years (15-86). Approximately 10% of patients had a high-risk Sokal score. The distribution of baseline patient- and CML-related characteristics was similar among the 4 treatment groups. Median time from diagnosis to TKI initiation was 26 days (0–365) (Table I).
Table I. Patient and Disease Characteristics at the Time of Treatment Start.
| Characteristic | All patients (N = 483) | IM400 (N = 71) | IM800 (N = 204) | NILO (N = 106) | DASA (N = 102) |
|---|---|---|---|---|---|
| Median age, years [range] | 48 [15-86] | 48 [15-79] | 48 [17-85] | 50 [17-86] | 48 [19-83] |
| Male: female (%) | 283 (59):200 (41) | 38 (53):33 (46) | 124 (61):80 (39) | 64 (60):42 (40) | 57 (56):45 (44) |
| Median WBC, ×109/L [range] | 28.3 [0.8–342.5] | 20.7 [1.6-277.0] | 27.5 [2.2-283.0] | 41.5 [1.4–342.5] | 27.1 [0.8–294.7] |
| Median hemoglobin, g/dL [range] | 12.3 [6.2–16.7] | 12.7 [7.9–15.7] | 12.4 [6.2–16.7] | 12.3 [8.0–15.8] | 11.9 [6.7–16.2] |
| Median platelets, ×109/L [range] | 343 [52-2,954] | 386 [126-1,425] | 342 [96-1,458] | 319 [52-2,954] | 347 [108-1,782] |
| Median PB blasts, % [range] | 0 [0-12] | 0 [0-2] | 0 [0-12] | 0 [0-7] | 0 [0-5] |
| Median PB basophils, % [range] | 3 [0-19] | 3 [0-16] | 3 [0-19] | 3 [0-13] | 3 [0-19] |
| Median BM blasts, % [range] | 2 [0-14] | 1 [0-6] | 2 [0-14] | 2 [0-8] | 2 [0-8] |
| Median BM basophils, % [range] | 2 [0-15] | 2 [0-9] | 3 [0-15] | 2 [0-13] | 2 [0-12] |
| Median spleen size*, cm [range] | 0 [0-21] | 0 [0-17] | 0 [0-21] | 0 [0-20] | 0 [0-20] |
| Clonal Evolution, n (%) | 14 (3) | 1 (1) | 5 (2) | 2 (2) | 6 (6) |
| Sokal risk group, n (%) | |||||
| Low | 297 (61) | 46 (65) | 112 (55) | 67 (63) | 72 (71) |
| Intermediate | 138 (29) | 20 (28) | 66 (32) | 29 (27) | 23 (22) |
| High | 48 (10) | 5 (7) | 26 (13) | 10 (10) | 7 (7) |
IM400, imatinib 400 mg daily IM800, imatinib 800 mg daily; NILO, nilotinib 400 mg twice daily; DASA, dasatinib 100 mg daily; WBC, white blood cell. PB, peripheral blood; BM, bone marrow.
From the costal arch
Response to TKI therapy
Follow-up time for the entire population was 75.1 months (2.5–140.3). Since clinical trials of second generation TKI were initiated more recently, follow-up of patients treated with NILO and DASA is significantly shorter [30.4 (2.5–77.5) and 36.4 (2.5–72.5) months, respectively] compared to that of patients receiving standard or high-dose imatinib [128.7 (16.4–140.3) and 103.5 (3.7–132.2) months, respectively] (p< 0.0001). The cumulative rate of CCyR was 89% for the entire cohort and 84.5%, 89.7%, 89.6% and 90.2% for IM400, IM800, NILO, and DASA, respectively. Among patients that achieved CCyR the rates of best MR were: no-MR in 7.7% of patients, MMR in 14.2%, MR4 in 5.6%, MR4.5 in 22.4%, and UND in 50.1%. Since the cumulative rate of deeper MR is time-dependent, in order to compare MR rates across treatment groups with different follow-up times we analyzed the frequency of the various levels of MR at 36 months. The percentages of patients achieving UND at 36 months were similar among patients treated with IM800, NILO, or DASA (30.6%, 29.2%, and 28.6%, respectively) but lower in those receiving IM400 (18.1%). Also, 27.3% of patients treated with IM400 who achieved CCyR failed to achieve at least a MMR at this time point, a rare occurrence in the other groups (Table II). Median time to reach MMR was 9.3, 5.9, 5.5, and 5.8 months for patients treated with IM400, IM800, NILO, and DASA, respectively, while time to achieve UND was 32.8, 24.3, 17.4, and 27.1 months, respectively. The cumulative rates of different MR in the 4 treatment groups are shown in Fig. 1.
Table II. Rates of different levels of MR among 430 patients who achieved CCyR at any time.
| Treatment Group | Evaluable | No MR | MMR | MR4 | MR4·5 | UND | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| N. | N | % | N | % | N | % | N | % | N | % | |
| Best response (at any time) | |||||||||||
|
| |||||||||||
| IM400 | 60 | 7 | 12 | 8 | 13 | 7 | 12 | 10 | 16 | 28 | 47 |
| IM800 | 182 | 9 | 5 | 16 | 9 | 9 | 5 | 38 | 21 | 110 | 60 |
| NILO | 95 | 9 | 10 | 20 | 21 | 7 | 7 | 24 | 25 | 35 | 37 |
| DASA | 92 | 8 | 9 | 17 | 18 | 1 | 1 | 24 | 26 | 42 | 46 |
| All patients | 429* | 33 | 8 | 61 | 14 | 24 | 6 | 96 | 22 | 215 | 50 |
|
| |||||||||||
| At 36 months | |||||||||||
|
| |||||||||||
| IM400 | 44 | 12 | 27 | 8 | 18 | 5 | 12 | 11 | 25 | 8 | 18 |
| IM800 | 147 | 8 | 5 | 26 | 18 | 20 | 14 | 48 | 33 | 45 | 30 |
| NILO | 48 | 4 | 8 | 8 | 17 | 5 | 10 | 17 | 36 | 14 | 29 |
| DASA | 56 | 5 | 9 | 15 | 27 | 1 | 2 | 19 | 34 | 16 | 28 |
| All patients | 295 | 29 | 10 | 57 | 19 | 31 | 11 | 95 | 32 | 83 | 28 |
MR, molecular response; CCyR, complete cytogenetic response; MMR, major molecular response; UND, undetectable BCR-ABL transcripts; IM400, imatinib 400 mg daily (N = 71); IM800, imatinib 800 mg daily (N = 204); NILO, nilotinib 400 mg twice daily (N = 106); DASA=dasatinib 100 mg daily (N = 102).
One patient who achieved CCyR did not have follow-up RQ-PCR
Figure 1.
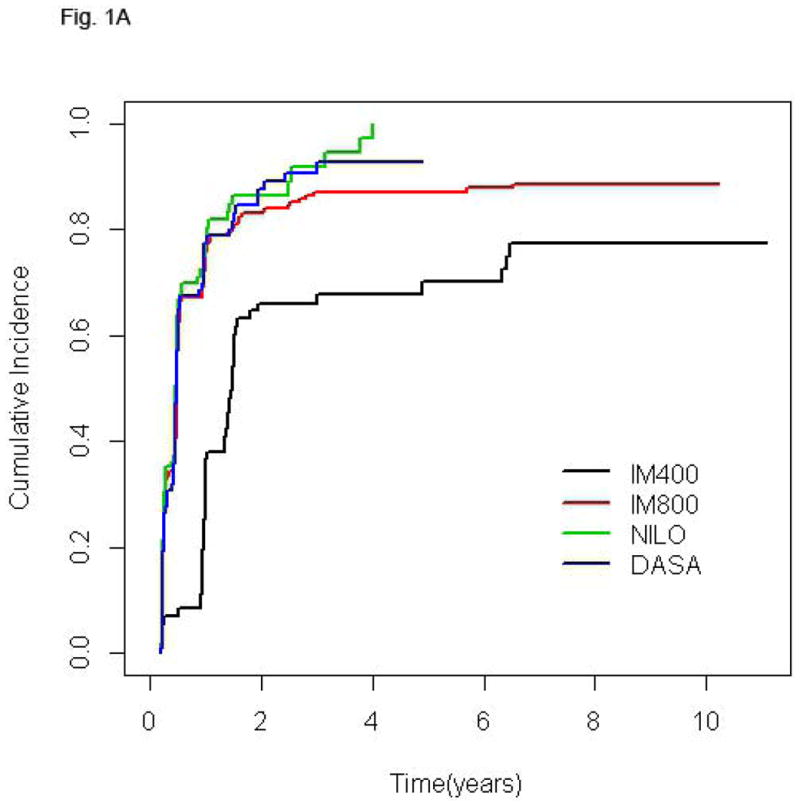
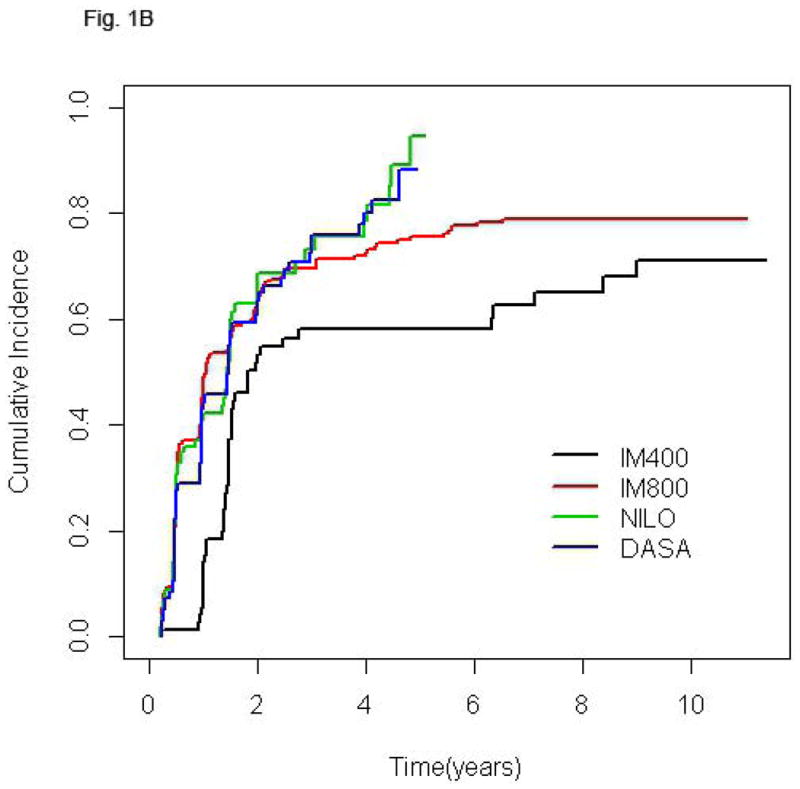
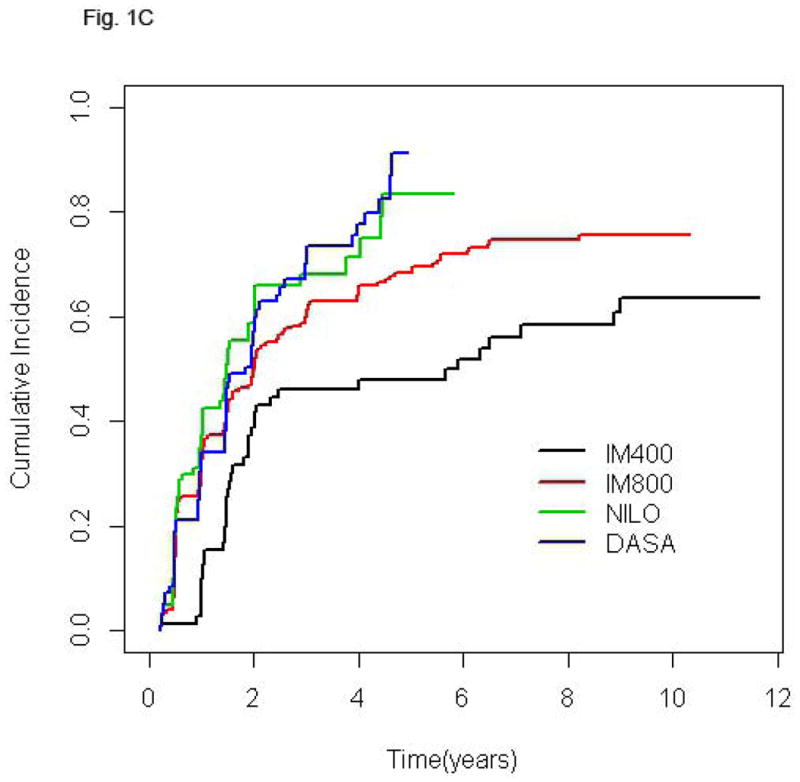
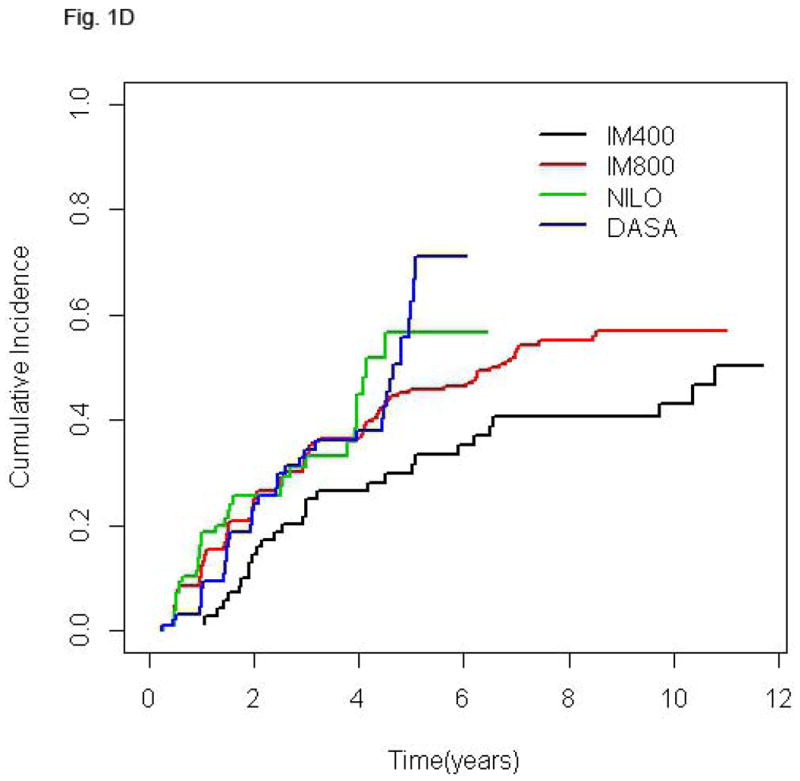
Cumulative incidence of MMR (A), MR4 (B), MR4.5 (C), and UND (D) according to treatment group. In each plot, for a given treatment group, the cumulative incidence step-function curves increase with each event, with the height of the curve representing the actual proportion of patients who had achieved response by that time point, and the curves continuing until the last response was achieved. IM400, n=71; IM800, n=204; NILO, n=106; DASA, n=102.
Clinical outcome
Because the prognostic significance of CCyR is well established in patients receiving frontline TKI therapy for CP CML, we analyzed clinical outcome according to the depth of MR only in patients who achieved CCyR. The resulting population included 430 patients, 429 of whom were molecularly evaluable. The depth of MR inversely correlated with the risk of losing CCyR (39%, 21%, 21%, 15%, and 3% in patients with <MMR, MMR, MR4, MR4.5, and UND, respectively) or MMR (25%, 33%, 19%, and 3%, respectively). Very few patients that had achieved CCyR progressed to AP or BP. That notwithstanding, an advantage in TFS was observed in UND patients compared to the no-MR or MMR cohorts (Fig. 2A), with most transformations occurring within the first 24 months of therapy. The 6-years OS of patients achieving UND was superior compared to that of patients in the no-MR or MMR group, but not to that of patients achieving MR4 or MR4.5 (Fig. 2B). Notably, transformation accounted for a minority of deaths in all categories.
Figure 2.
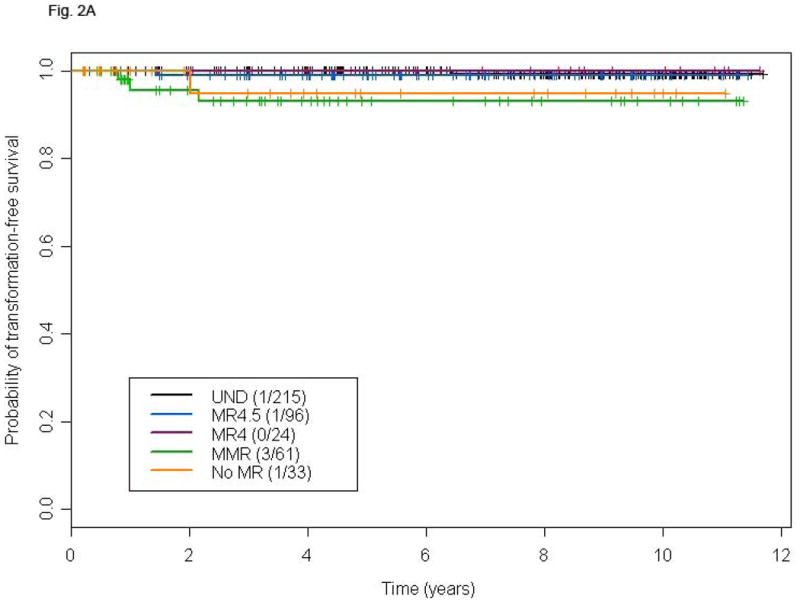
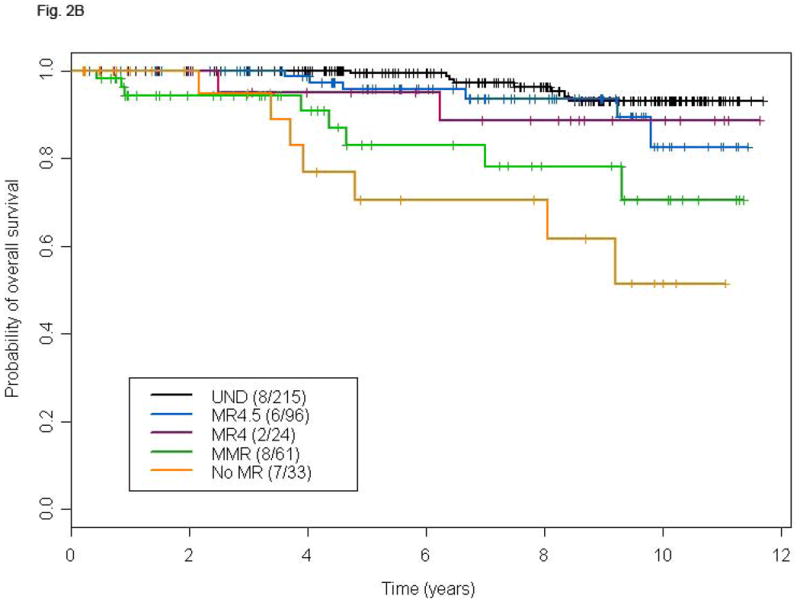
TFS (A), and OS (B) according to different levels of molecular response in patients achieving complete cytogenetic remission at any time. P-values for the comparison MR4.5, MR4, MMR, and no-MR versus UND are: 0.49, 0.74, 0.001, and 0.02, respectively (A), and 0.16, 0.28, <0.0001, and <0.0001, respectively (B).
Landmark Analyses
The median time to achieve UND was approximately 24 months for the entire population. To account for this lead-time, we performed landmark analyses in patients who achieved CCyR and were still on study at 18 or 24 months. Patients who achieved UND at 18 or 24 months had no advantage in terms of TFS compared to those with a lesser degree of MR at both landmarks (Figs. 3A and B). Similarly, OS was not different among patients with different levels of MR at these same landmarks (Figs. 3C and D). A total of 15 deaths were recorded in the 18 months landmark population and 12 deaths in the 24 months one. Only one death was due to transformation on or outside the study period.
Figure 3.
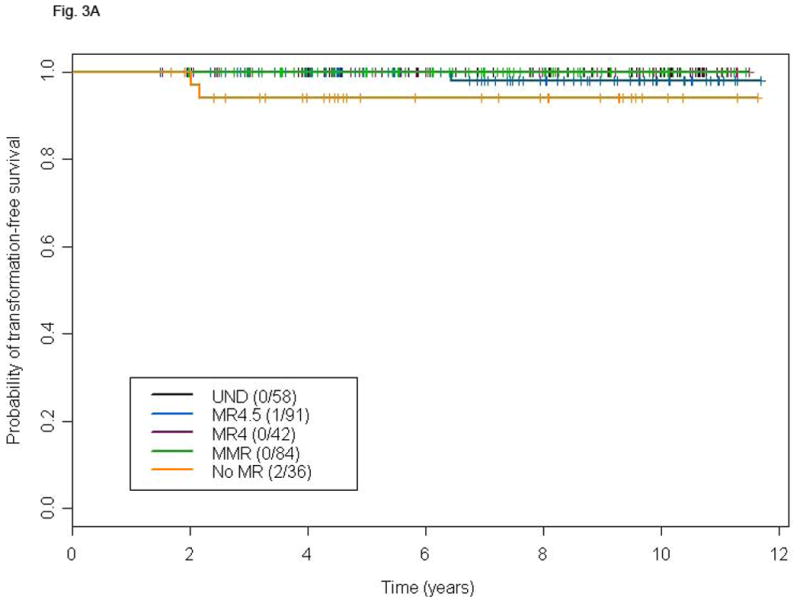
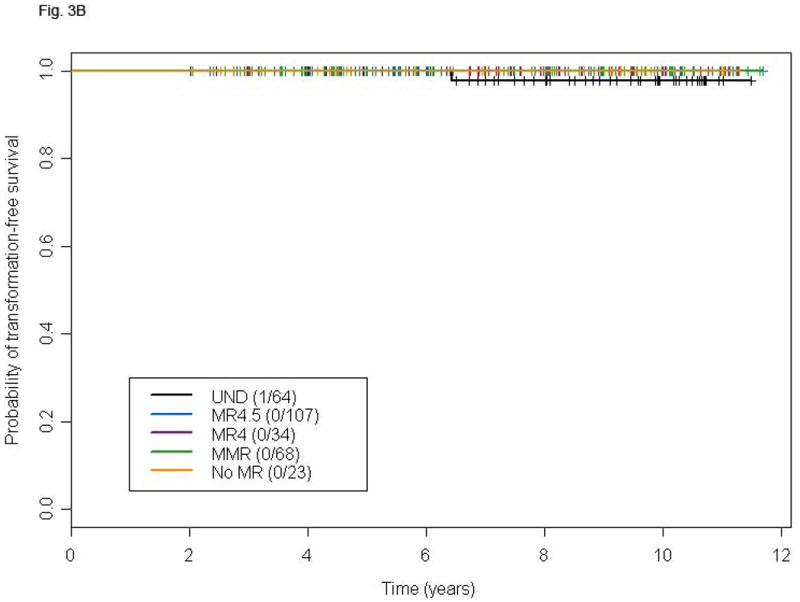
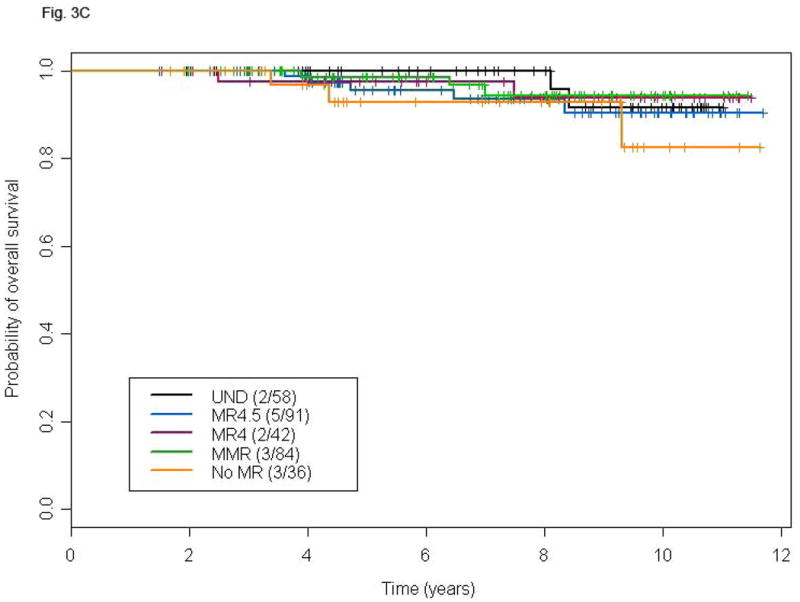
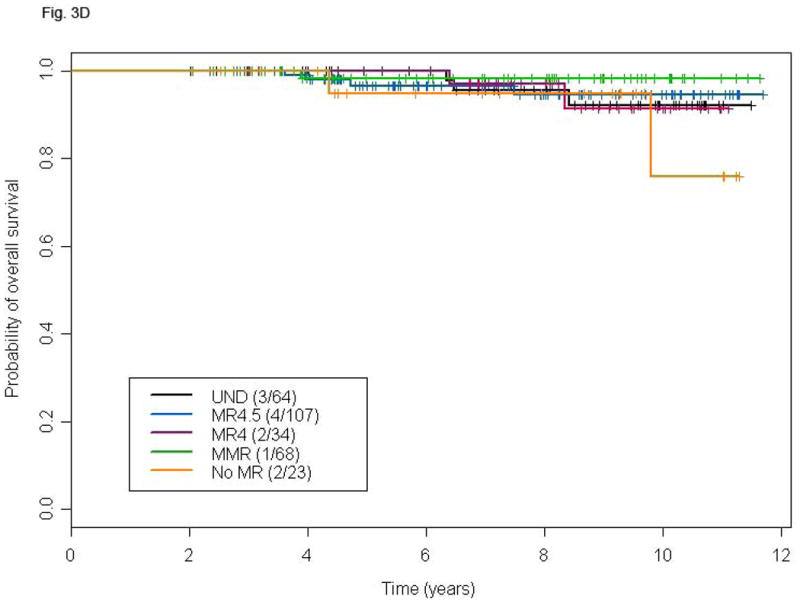
Landmark analysis at 18 and 24 months for TFS (A and B) and OS (C and D) according to different levels of molecular response in patients with CCyR. P-values for the comparison MR4.5, MR4, MMR, and no-MR versus UND are: 0.03, 0.25, 0.37 and 0.15, respectively (A), 0.16, 0.30, 0.61 and 0.16 respectively (B), 0.48, 0.86, 0.89 and 0.24, respectively (C), and 0.94, 0.99, 0.38, and 0.44, respectively (D).
susMR4.5
We analyzed the frequency and prognostic consequences of achieving a susMR4.5. In this analysis we included patients who achieved an MR4.5 at any time and identified within such group those with susMR4.5. To allow sufficient time to achieve and maintain an MR4.5, we only included the 348 patients with minimum follow-up of 40 months. Among them 261 patients achieved MR4.5, 70 (26.8%) unsustained and 191 (73.2%) susMR4.5. We observed no significant difference in terms of TSF or OS (Figs. 4A and B) between the two groups. A total of 14 deaths were recorded, 5 (7.1%) in patients with unsustained MR4.5 (Parkinson's disease, 1, post-transplant complications, 1, cardiovascular, 1, other cancers, 1, unknown, 1) and nine (4.7%) in those with susMR4.5 (transformation, 1, cardio/cerebrovascular, 3, other cancers, 2, bowel obstruction, 1, suicide, 1, unknown in remission, 1). Factors associated with the probability of achieving a susMR4.5 in univariate analysis included baseline age, hemoglobin level, platelet counts, PB basophils, spleen size, IS %BCR-ABL, TKI modality, and 3- and 6-months CCyR. Of these, older age, higher platelet counts, treatment with second generation TKIs (versus imatinib), and achievement of CCyR at 3 or 6 months were independently predictive in multivariable analysis (Table III).
Figure 4.
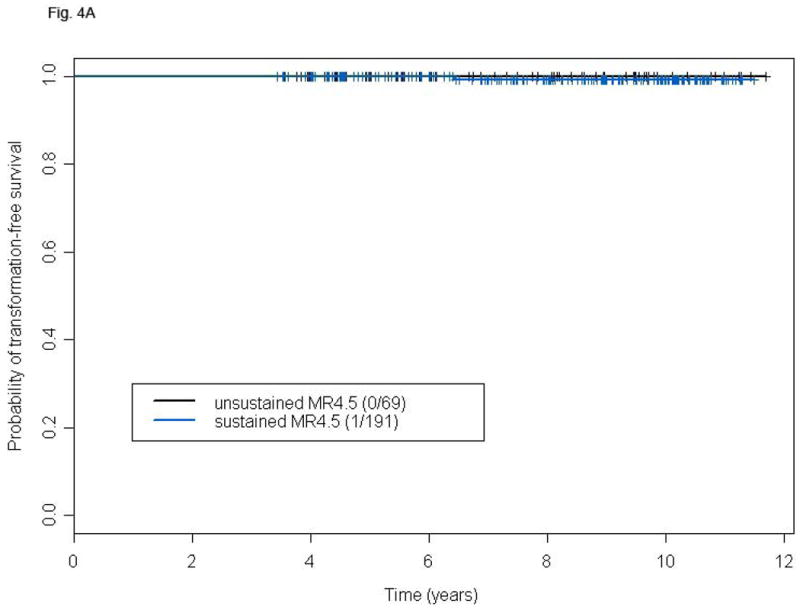
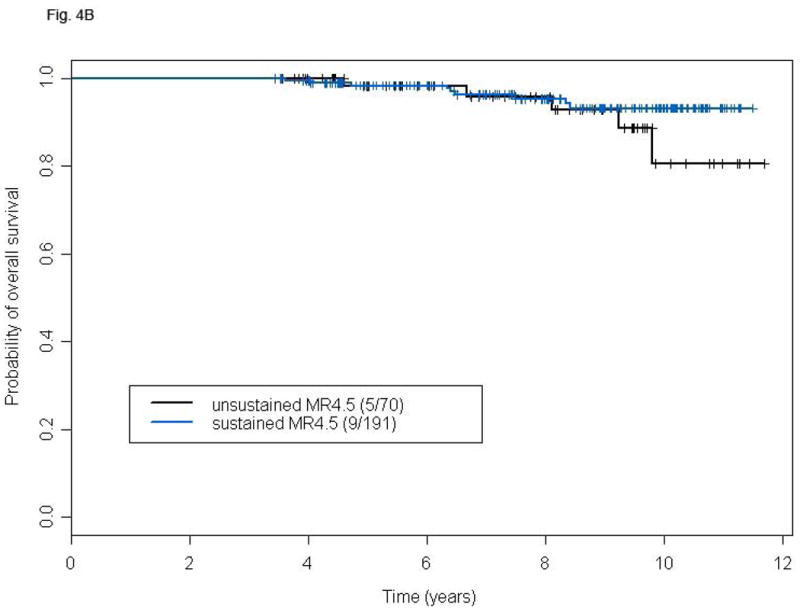
TFS (A) and OS (B) of patients with susMR4.5 compared to those with MR4.5 not sustained for at least 2 years after a minimum follow-up of 48 months. P-values for the comparison are: 0.58 (A) and 0.30 (B).
Table III. Multivariable Logistic Regression Model for the Achievement of susMR4.5.
| Variable | Odds Ratio | (95% CI) | P |
|---|---|---|---|
| TKI modality | |||
| IM800 (vs. IM400) | 1.68 | (0.86 – 3.28) | 0.13 |
| NILO (vs. IM400) | 0.39 | (0.18 – 0.86) | 0.02 |
| DASA (vs. IM400) | 0.45 | (0.21 – 0.98) | 0.04 |
| Age* | 1.02 | (1.01 – 1.04) | 0.008 |
| Platelet count* | 1.73 | (1.20 – 2.49) | 0.004 |
| Response to TKI therapy | |||
| CCyR at 3 months | 2.14 | (1.28 – 3.59) | 0.004 |
| CCyR at 6 months | 3.70 | (1.76 – 7.75) | 0.0005 |
IM400, imatinib 400 mg daily; IM800, imatinib 800 mg daily; NILO, nilotinib 400 mg twice daily; DASA; dasatinib 100 mg daily; TKI, tyrosin kinase inhibitor; CCyR, complete cytogenetic response.
Age and platelet count are included as continuous variables
Discussion
In the present analysis we showed that BCR-ABL transcripts may become undetectable in a substantial proportion of patients with early CP CML treated with TKI. The cumulative incidence of deep MR and the fraction of patients achieving UND is similar with IM800 or second generation TKIs, but lower with standard-dose imatinib. We and others reported high rates of deep MR with the use of IM800.3,4 While a formal comparison between high-dose imatinib and second-generation TKIs in terms of quality of MR was beyond the scope of our study, our data suggest that high-dose imatinib may be a valid treatment option if the aim is to achieve deep MR.
We observed a lower risk of losing CCyR, progression to AP or BP, or death in patients with deeper MR. The achievement of lower levels of BCR-ABL transcripts with interferon therapy23 or imatinib12,14-18 has been associated with durable cytogenetic responses. However, the probability of loss of CCyR was not different between patients with MMR or CMR in prior studies.15 Achieving MMR by 18 months in patients with early CP CML in CCyR on imatinib has also been shown to confer an advantage in EFS and, to a lesser extent, TFS. However, OS was not shown to be significantly different.18 Other series have shown different results. In a series of 269 CP CML patients treated upfront with imatinib at our institution, MR at various time points predicted for survival (mainly PFS), but not independently of the degree of cytogenetic response.24 Similarly, among 224 patients with CP CML investigators at Hammersmith found that in patients in CCyR the achievement of MMR at 12 or 18 months did not translate into a 5-year PFS or OS benefit.17 More recently we reproduced these observations in patients treated upfront with second-generation TKIs. In that analysis there was no difference in CCyR duration or EFS among patients achieving CCyR with or without MMR at all landmarks between three and 18 months.25.
In this analysis, among patients that achieved CCyR, those achieving UND at any time showed superior TFS and OS compared to patients obtaining no better than MMR. This is undoubtedly influenced by the longer time needed to achieve UND resulting in a lead-time bias as patients who achieve UND by definition have not had earlier events. When adjusting for the time to achieve the best MR, the clinical benefit of achieving UND vanished, with no differences observed in TFS or OS even between the best (UND) and the worst (no-MR) MR category. This likely reflects the fact that most events (or failures) represent loss of response, which can be frequently salvaged with subsequent TKIs or other options, as opposed to AP and, particularly, BP that are more frequently irreversible but fortunately occur rarely once patients achieve CCyR. A frequent clinical question is whether a patient that achieves CCyR but not MMR or deeper MR should consider a change in therapy. Based on these observations, the risk of transformation or death is minimal for such patients and a change of therapy cannot be justified at the present time in the absence of a prospective study demonstrating not only the achievement of deeper responses, but, more importantly, an improved long-term outcome after such change. Interestingly, the 33 (7%) patients who never achieved a MMR in our population were equally distributed among the 4 treatment groups and only one of them belonged to the high-risk Sokal category.
Given the excellent clinical outcome of CML patients achieving and maintaining deep MR, TKI discontinuation has become a pressing clinical question. A consensus requirement for considering discontinuation is the achievement of MR4.5 for two consecutive years.19,20 In the Australian CML8 study19 and the French STop IMatinib (STIM) trial20 patients with undetectable transcripts for ≥2 years on imatinib were discontinued and closely followed with RQ-PCR. Forty-four percent of patients in the former and 38% of patients with >12 months follow-up in the latter relapsed molecularly.
It is unclear how often the criteria for sustained UND are met in clinical practice. In the present analysis around 75% of patients treated for ≥40 months (n=348) achieve MR4.5. Three quarters of them maintain such response for ≥2 years. Assuming that approximately 40% of patients will not relapse after treatment discontinuation, only about 15% of all patients in our series could potentially maintain their response after stopping TKI therapy. Approaches to improve these ratios should be pursued. Marin et al. suggested that achievement of the deepest responses is strongly correlated with adherence to therapy.26 Adherence was not assessed in our studies other than by patient diaries, which are known to be inaccurate. Also, further studies should investigate whether a different, perhaps longer, period of susMR4.5 should be considered as the minimum requirement for TKI discontinuation. Mathematical models can be applied to define patient-specific optimal MR4.5 durations before discontinuing therapy.27.
An important practical question is how to identify patients who are likely to achieve a susMR4.5 early after therapy initiation. Recently, Branford et al. reported a cumulative MR4.5 rate of 43% at 8 years and found female sex and lower %BCR-ABL at 3 months to predict for the achievement of stable MR4.5 for ≥2 years.28 In our analysis we confirmed the predictive value of early response to TKI therapy, but also found older age, higher platelet count, and initial treatment with second-generation TKI to be independently associated with the achievement of susMR4.5. Early response to TKI has been shown to correlate with the achievement of deeper MR and excellent long-term survival.18 We previously observed that adolescent/young adult CML patients have lower rates of CCyR and MMR compared to older patients, perhaps due to poorer adherence to TKI therapy.29 Finally, the use of second-generation TKI has resulted in faster and higher rates of high-quality MR.6,8 The relationship between higher platelet counts and the achievement of susMR4.5 needs further confirmation and study.
Our analysis has two possible limitations. First, the 4 treatment groups considered were part of separate studies in different time periods, and not a single, randomized trial. However, all patients were consecutively enrolled and eligibility criteria for all studies were similar. Characteristics at study entry were not different across groups and timing of surveillance and patient management policies have been homogeneous throughout the study periods. Thus, we believe these results are representative of what can be expected in routine clinical practice. Second, in the landmark analyses only a small number of transformations or deaths were recorded overall. Therefore the present analysis may be underpowered to detect significant differences in outcome between MR categories. Whether, and to what extent, such potential statistical difference may also have a clinical significance is even less clear.
In conclusion, with TKI therapy, MR4.5 can be achieved in a significant fraction of patients with CML CP. The achievement of deeper MR can minimize the risk of loss of CCyR or MMR. However, no additional benefit is observed in terms of TFS or OS in patients already with stable CCyR. Sustained deep MR were not associated with an additional benefit in TFS and OS, suggesting that patients with CCyR an residual detectable disease still have an excellent long-term outcome with minimal risk for transformation. The clinical value of treatment changes to aim for deeper MR needs to be tested prospectively.
Acknowledgments
This research has been supported in part by MD Anderson Cancer Center Support (Grant CA016672) and the National Institute of Health (Grant P01 CA049639).
Footnotes
Authorship Contributions: L.F., J.E.C., and H.M.K. designed research. H.K.M., A.Q., S.O., E.J.J., F.R., G.B., G.G., S.V., J.A.B., and J.E.C. treated patients, collected, analyzed and interpreted data. L.F. and D.V. collected analyzed and interpreted data. X.W. performed statistical analysis, analyzed and interpreted data. R.L. analyzed and interpreted data. All authors wrote and approved the final version of the manuscript.
Disclosure of Conflicts of Interest: H.K.M. has received research funding from Bristol-Myers Squibb, Ariad, Pfizer, and Novartis. E.J.J. has received honoraria from Pfizer, Novartis, and Bristol-Myers Squibb. F.R. has received research funding from Bristol-Myers-Squibb and has received honoraria from Bristol-Myers Squibb, Novartis, and Pfizer. J.E.C.: is consultant for Ariad, Pfizer, and Teva and has received research funding from Ariad, Bristol-Myers Squibb, Novartis, Chemgenex, and Pfizer. L.F., X.W., D.V., S.O., G.B., A.Q., G.G., S.V., and J.A.B. declare no conflicts of interest.
References
- 1.Druker BJ, Guilhot F, O'Brien SG, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. The New England Journal of Medicine. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, O'Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–1987. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-Dose Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia: High Rates of Rapid Cytogenetic and Molecular Responses. Journal of Clinical Oncology. 2009;27(28):4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, Randomized, Open-Label Study of Daily Imatinib Mesylate 400 mg Versus 800 mg in Patients With Newly Diagnosed, Previously Untreated Chronic Myeloid Leukemia in Chronic Phase Using Molecular End Points: Tyrosine Kinase Inhibitor Optimization and Selectivity Study. Journal of Clinical Oncology. 2010;28(3):424–430. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Jones D, O'Brien S, et al. Nilotinib As Front-Line Treatment for Patients With Chronic Myeloid Leukemia in Early Chronic Phase. Journal of Clinical Oncology. 2010;28(3):392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia. The New England Journal of Medicine. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Jones D, O'Brien S, et al. Results of Dasatinib Therapy in Patients With Early Chronic-Phase Chronic Myeloid Leukemia. Journal of Clinical Oncology. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. The New England Journal of Medicine. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, Hochhaus A, Hughes T, Kantarjian H. Front-Line and Salvage Therapies With Tyrosine Kinase Inhibitors and Other Treatments in Chronic Myeloid Leukemia. Journal of Clinical Oncology. 2011;29(5):524–531. doi: 10.1200/JCO.2010.31.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller MC, Gattermann N, Lahaye T, et al. Dynamics of BCR-ABL mRNA expression in first-line therapy of chronic myelogenous leukemia patients with imatinib or interferon α/ara-C. Leukemia. 2003;17(12):2392–2400. doi: 10.1038/sj.leu.2403157. [DOI] [PubMed] [Google Scholar]
- 11.Branford S, Rudzki Z, Harper A, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;17(12):2401–2409. doi: 10.1038/sj.leu.2403158. [DOI] [PubMed] [Google Scholar]
- 12.Hughes TP, Kaeda J, Branford S, et al. Frequency of Major Molecular Responses to Imatinib or Interferon Alfa plus Cytarabine in Newly Diagnosed Chronic Myeloid Leukemia. The New England Journal of Medicine. 2003;349(15):1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 13.Branford S, Cross NCP, Hochhaus A, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20(11):1925–1930. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 14.Paschka P, Müller MC, Merx K, et al. Molecular monitoring of response to imatinib (Glivec®) in CML patients pretreated with interferon alpha. Low levels of residual disease are associated with continuous remission. Leukemia. 2003;17(9):1687–1694. doi: 10.1038/sj.leu.2403033. [DOI] [PubMed] [Google Scholar]
- 15.Cortes J, Talpaz M, O'Brien S, et al. Molecular Responses in Patients with Chronic Myelogenous Leukemia in Chronic Phase Treated with Imatinib Mesylate. Clinical Cancer Research. 2005;11(9):3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 16.Iacobucci I, Saglio G, Gianantonio Rosti, et al. Achieving a Major Molecular Response at the Time of a Complete Cytogenetic Response (CCgR) Predicts a Better Duration of CCgR in Imatinib-Treated Chronic Myeloid Leukemia Patients. Clinical Cancer Research. 2006;12(10):3037–3042. doi: 10.1158/1078-0432.CCR-05-2574. [DOI] [PubMed] [Google Scholar]
- 17.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116(19):3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–1724. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 20.Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncology. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian HM, Cortes J, O'Brien S, et al. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: high incidence of early complete and major cytogenetic responses. Blood. 2003;101(1):97–100. doi: 10.1182/blood-2002-02-0545. [DOI] [PubMed] [Google Scholar]
- 22.Luthra R, Medeiros L. TaqMan® Reverse Transcriptase-Polymerase Chain Reaction Coupled With Capillary Electrophoresis for Quantification and Identification of bcr-abl Transcript Type. Methods in Molecular Biology. 2006;335:135–145. doi: 10.1385/1-59745-069-3:135. [DOI] [PubMed] [Google Scholar]
- 23.Kurzrock R, Estrov Z, Kantarjian H, Talpaz M. Conversion of Interferon-Induced, Long-Term Cytogenetic Remissions in Chronic Myelogenous Leukemia to Polymerase Chain Reaction Negativity. Journal of Clinical Oncology. 1998;16:1526–1531. doi: 10.1200/JCO.1998.16.4.1526. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian H, O'Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia. Cancer. 2008;112(4):837–845. doi: 10.1002/cncr.23238. [DOI] [PubMed] [Google Scholar]
- 25.Jabbour E, Kantarjian HM, O'Brien S, et al. Front-Line Therapy With Second-Generation Tyrosine Kinase Inhibitors in Patients With Early Chronic Phase Chronic Myeloid Leukemia: What Is the Optimal Response? Journal of Clinical Oncology. 2011;29(32):4260–4265. doi: 10.1200/JCO.2011.36.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marin D, Bazeos A, Mahon FX, et al. Adherence Is the Critical Factor for Achieving Molecular Responses in Patients With Chronic Myeloid Leukemia Who Achieve Complete Cytogenetic Responses on Imatinib. Journal of Clinical Oncology. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn M, Glauche I, Mu¨ ller MC, et al. Model-based decision rules reduce the risk of molecular relapse after cessation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Blood. 2013;121(2):378–384. doi: 10.1182/blood-2012-07-441956. [DOI] [PubMed] [Google Scholar]
- 28.Branford S, Yeung DT, Ross DM, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121(19):3818–3824. doi: 10.1182/blood-2012-10-462291. [DOI] [PubMed] [Google Scholar]
- 29.Pemmaraju N, Kantarjian H, Shan J, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97(7):1029–1035. doi: 10.3324/haematol.2011.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]


