Abstract
The ineffectiveness of small molecule drugs against cancer has generated significant interest in more potent macromolecular agents. Gelonin, a plant-derived toxin that inhibits protein translation, has attracted much attention in this regard. Due to its inability to internalize into cells, however, gelonin exerts only limited tumoricidal effect. To overcome this cell membrane barrier, we modified gelonin, via both chemical conjugation and genetic recombination methods, with low molecular weight protamine (LMWP), a cell-penetrating peptide (CPP) which was shown to efficiently ferry various cargos into cells. Results confirmed that gelonin-LMWP chemical conjugate (cG-L) and recombinant gelonin-LMWP chimera (rG-L) possessed N-glycosidase activity equivalent to that of unmodified recombinant gelonin (rGel); however, unlike rGel, both gelonin-LMWPs were able to internalize into cells. Cytotoxicity studies further demonstrated that cG-L and rG-L exhibited significantly improved tumoricidal effects, with IC50 values being 120-fold lower than that of rGel. Moreover, when tested against a CT26 s.c. xenograft tumor mouse model, significant inhibition of tumor growth was observed with rG-L doses as low as 2 μg/tumor, while no detectable therapeutic effects were seen with rGel at 10-fold higher doses. Overall, this study demonstrated the potential of utilizing CPP-modified gelonin as a highly potent anticancer drug to overcome limitations of current chemotherapeutic agents.
Keywords: Gelonin, LMWP, Cell penetrating peptide, Cancer, Ribosome-inactivating protein, Toxin
1. Introduction
Current anti-cancer drug therapies primarily utilize small molecule agents. While some have shown to be efficacious, most of these small molecule drugs have suffered from a poor therapeutic index – a ratio of the concentration required for efficacy versus that for toxicity [1]. This issue becomes most apparent in the treatment of cancers, where side-effects often limit the amount of drug dosing; which, subsequently, results in accumulation of sub-optimal drug concentrations at the tumor target. With unmatched potency and selectivity, macromolecules have drawn significant interest over the past few decades for their potential to overcome the limitations of small molecule drugs [2-4]. Clinical translation of macromolecular drugs, however, has largely been limited due to low bioavailability, instability in physiological environment and, in many cases, poor intracellular transport of these agents [2, 3, 5, 6]. A typical example is the plant-derived ribosome-inactivating protein (RIP) toxins. Since the initial discovery of ricin from castor oil plants [7], more than 50 different RIPs have now been identified [8, 9]. RIPs are extremely potent inhibitors of protein synthesis, and thus have drawn considerable interest for potential use as anticancer drugs [10]. Gelonin, which belongs to this RIP family, is a 30-kDa single chain glycoprotein extracted from seeds of Gelonium multiflorum and inactivates ribosomes by the cleavage of a single adenine residue (A4324) in the 28S ribosomal RNA [11]. Because of the high substrate specificity, non-stoichiometric mode of action, and repetitive reaction mechanism, the potential therapeutic efficacy of gelonin cannot be matched by any of the existing anti-tumor agents [11]. It has even been postulated that a single molecule of gelonin toxin is sufficient to completely kill one cancer cell, if the drug could reach the ribosome [12, 13]. Yet, this unparalleled therapeutic potency has not been realized clinically, primarily due to the inability of gelonin to cross the cell membrane barrier [8, 11]. A means to deliver gelonin into the intracellular compartment therefore becomes an essential element to utilize this extremely potent N-glycosidase activity for cancer treatment.
The 1988 discovery by Frankel and Pabo that TAT (transactivator of transcription) protein derived from HIV-1 virus could internalize into cells [14] led to the identification of a class of peptides with unique and unprecedented cell-penetrating activity [15-17]. Later studies demonstrated that these peptides, so-called “cell penetrating peptides (CPPs)”, were also able to efficiently translocate the attached cargos such as protein molecules or nano-scale drug carriers into cells [15-17]. Although the mechanism of cell entry remains unclear and not unified, it now appears that the interaction of the cationic CPP with negatively charged glycosaminoglycans on the cell surface is an essential prerequisite as addition of extracellular heparin, heparan or dextran sulfate completely neutralizes the cell-internalizing function of these CPPs [18, 19].
Low molecular weight protamine (LMWP) is a 14-mer peptide (VSRRRRRRGGRRRR), previously developed by Yang and coworkers, that exhibits CPP-like cell-penetrating behavior [20]. Both in vitro and in vivo studies demonstrated that LMWP could transduce proteins, genes and even nano-scale drug carriers like liposomes into living cells without perturbing the cell membranes [20-24]. Aside from this cell penetrating ability, LMWP also possesses a number of other significant advantages over other CPPs, including: 1) the capability for efficient mass production via a simple 1-step enzymatic digestion of protamine, while most of the CPPs are only available by means of biological or peptide synthesis; 2) a thoroughly investigated toxicological and immunological profiles which clarified that LMWP is neither toxic nor immunogenic; and 3) demonstrated in vivo safety as an antidote for heparin reversal [20, 25-27]. Based on these findings, we hypothesized that modification of gelonin with LMWP could effectively and safely deliver gelonin into tumor cells, thereby drastically enhancing gelonin's cytotoxic effects against tumors. The feasibility of this CPP-based approach for enhancing intracellular delivery of native gelonin (nGel) has been, in fact, shown by our research group [20]. However, obvious limitations to use of nGel (e.g, low economic feasibility of commercial nGel and poor conjugation efficiency) were also clearly recognized and, indeed, discouraged further studies. To address these problems, we have attempted to synthesize gelonin-LMWP utilizing recombinant gelonin (rGel) as an alternative to nGel, based on its potential for large-scale production by Escherichia coli (E. coli) [28].
In this study, we present in vitro findings of both chemically and biologically modified rGel. For chemical modification, LMWP was covalently attached to rGel using a heterobifunctional polyethylene glycol (PEG) cross-linker. For recombinant modification, gene encoding LMWP was inserted at the C-terminus of the gelonin gene, and the resulting fusion protein was then expressed in E. coli. The inhibitory activity on protein translation by both chemically synthesized and recombinant gelonin-LMWP conjugates, abbreviated as cG-L and rG-L respectively, was assessed using a cell-free translational system. In addition, the cell penetrating ability and potency against tumor cells were examined in a variety of cancer cell lines. Furthermore, preliminary in vivo investigation of the inhibition on tumor growth was conducted in a CT26 xenograft tumor mice model.
2. Materials and Methods
2.1. Materials
The pET28a-gelonin vector (pET-Gel) was used for overexpression of recombinant gelonin (rGel). Competent E. coli cells (TOP10, BL21star (DE3) and BL21-CodonPlus), pEXP-5-NT/TOPO TA expression kit, AcTEV™ protease, LB broth, fetal bovine serum albumin (FBS), PBS (pH 7.4), Dulbecco's Modified Eagle Medium (DMEM), RPMI1640 and Hoechst 33342 trihydrochloride, trihydrate were purchased from Invitrogen (Carlsbad, CA). Carbenicillin, kanamycin, and isopropyl-β-thiogalactopyranoside (IPTG) were purchased from Fisher Scientific (Pittsburg, PA). Heparin sulfate, rhodamine B isothiocyanate, Traut's reagent (2-iminothiolane; 2-IT) and DTNB (5, 5'-dithiobis-(2-nitrobenzoic acid)) were purchased from Sigma-Aldrich (St. Louis, MO). DNA primers were purchased from Integrated DNA Technologies Inc. (Coralville, IA). DNA restriction endonucleases (NdeI, NheI-HF, EcoRI-HF and XhoI) and T4 DNA ligase were purchased from New England Biolabs (Ipswich, MA). BCA protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). Rabbit reticulocyte lysate assay system, luciferase assay system and recombinant RNasin® ribonuclease inhibitor were purchased from Promega Corporation (Madison, WI). Cell proliferation kit II (XTT) was purchased from Roche Applied Science (Indianapolis, IN). Native gelonin extracted from the seeds of Gelonium Multiflorum (nGel) was purchased from Enzo Life Sciences Inc. (Farmingdale, NY). LMWP was obtained from ISTN (Lancaster, PA) and heterobifunctional PEG (NHS-PEG-PDP; 2 kDa) was purchased from JenKem Technology USA Inc. (Allen, TX).
2.2. Expression and Purification of Recombinant Gelonin (rGel)
The pET-Gel vector was transformed into E. coli strain (BL21 (DE3)), and rGel was produced following the method described by Hossann et al [28]. Briefly, a single colony of pET-Gel transformed BL21 (DE3), grown on LB agar plate with 80 μg/mL kanamycin, was picked and inoculated into 200 mL of LB medium. This starter culture was incubated overnight at 37°C with shaking at 250 rpm and then used to inoculate 5 L of fresh LB medium, which was incubated at 37°C with shaking at 250 rpm. When the optical density at 600 nm reached 1, IPTG inducer was added to a final concentration of 0.5 mM. The culture was further incubated under the same conditions for 6 hr. Cells were then harvested by centrifugation at 4000 rpm for 20 min at 4°C. The pellet was suspended in 30 mL of 20 mM phosphate buffer saline (PBS, 300 mM NaCl, pH 7) and the cells were lysed by sonication (4 × 30 sec, with 50% output in ice bath). The cell lysate was centrifuged at 20,000 rpm for 30 min and the supernatant was loaded onto HisPure® Ni-NTA resin (Bio-Rad Laboratories, Hercules, CA) pre-equilibrated with 20 mM PBS (300 mM NaCl, pH 7). The impurities were washed with 200 mL of PBS and then rGel was eluted with 20 mM PBS containing imidazole (300 mM NaCl, 400 mM imidazole, pH 7). For further purification, the eluent from the Ni-NTA resin was loaded onto a cation exchange column (HiTrap Sepharose CM-FF, GE Healthcare Bio-Sciences, Pittsburgh, PA) connected to a HPLC (Alltech 526 HPLC pump, Deerfield, IL) and rGel was purified by elution with a salt gradient (0 to 2 M NaCl at a rate of 0.02 M/min, flow rate: 1 mL/min).
2.3. Preparation and Purification of Chemically-Conjugated Gelonin-LMWP (cG-L)
Chemical conjugation of rGel with LMWP was accomplished using Traut's reagent and a heterobifunctional PEG (NHS-PEG-PDP, 2 kDa) as the cross-linker. The NHS group on one side of the PEG chain was amine reactive while the PDP group at the other end was thiol reactive. The conjugation scheme is shown in Fig. 1. Briefly, thiol groups were first introduced to rGel (5 mg/mL in 2 mL of 10 mM PBS, 50 mM triethanolamine, 2 mM EDTA, pH 8) by incubation with 10 molar excess of Traut's reagent for 1 hr at room temperature. Unreacted Traut's reagent was removed by ultrafiltration using a centrifugal filtration device (molecular weight cut-off: 10 kDa, Amicon® Centricon® Centrifugal Filter Devices, Millipore Corporation, Billerica, MA) and the generated thiol groups on rGel were quantified by Ellman's assay.
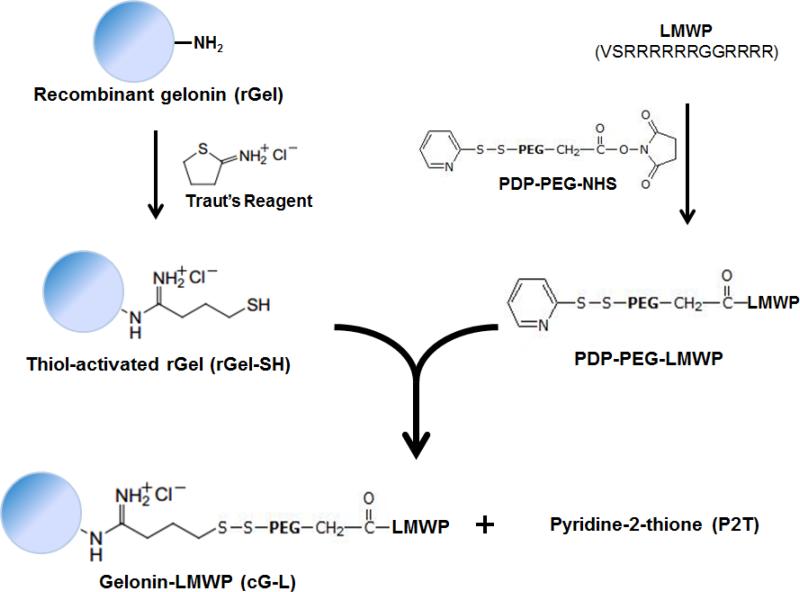
Fig. 1.
Scheme of gelonin-LMWP chemical conjugation via a disulfide bond using heterobifunctional PEG as the cross-linker. (cG-L: gelonin-LMWP chemical conjugate)
Next, the amine group on the LMWP peptide (10 mg/mL in 20 mM PBS with 0.15 M NaCl, pH 7.4) was reacted with 3-fold molar excess of NHS-PEG-PDP for 4 hr at room temperature with shaking to introduce LMWP with the thiol-reactive PDP group. Unreacted PEG was removed by loading the reaction mixture onto a heparin column (HiTrap Heparin HP, GE Healthcare Bio-Sciences, Pittsburgh, PA) and washing with 50 mM phosphate buffer (pH 7.4). Purified LMWP-PEG-PDP was then eluted with 2 M NaCl (50 mM PBS, 2 M NaCl, pH 7.4) at a flow rate of 1 mL/min. LMWP-bound PDP groups were quantified by the pyridine-2-thione (P2T) assay. Following the preparation of both thiolated-rGel (rGel-SH) and LMWP-PEG-PDP, they were mixed together at a molar ratio of 1:5 (rGel-SH:LMWP-PEG-PDP) and incubated overnight at 4°C. The final cG-L product was purified from unreacted rGel by using a heparin column and elution with a salt gradient (1st step: 0.4 M NaCl for 20 min, 2nd step: 0.4 to 1.6 M NaCl at a rate of 0.015 M/min; flow rate: 1 mL/min). Any unreacted LMWP and LMWP-PEG-PDP which might be present in the cG-L peak fraction was further removed by centrifugal filtration (Eppendorf Centrifuge 5702R), using membranes with a 10 kDa molecular weight cut-off pore size. The purified cG-L was stored at 4°C until further use.
2.4. Preparation and Purification of the Recombinant Gelonin-LMWP Chimera (rG-L)
2.4.1. Construction of Gelonin-LMWP Genes
The gelonin-LMWP gene was constructed by inserting a PCR fragment encoding the LMWP gene into the pET-Gel vector (pET28a-Gel). Briefly, double stranded DNA fragments containing partial C-terminal gelonin and LMWP encoding codons (646 bp) were prepared by PCR using pET-Gel vector as a template. The primers used for the PCR reaction were as follows: 1) the forward primer was 5’-GGA GCT CGA ATT CTT ATT AAC GAC GAC GAC GAC CAC CAC GAC GAC GAC GAC GAC GGC TTA CAC CTT TCG GAT CTT TGT CG-3’ and 2) the backward primer was 5’-AAC GAT AAC GGC CAG CTA GCG GAA ATT GC-3’. The PCR product was purified by 1% agarose gel electrophoresis and inserted into a pEXP-5-NT/TOPO vector using the vendor's protocol (Invitrogen, Carlsbad, CA). Both the pET-Gel vector and the pEXP-5-NT/TOPO vector encompassing the partial gelonin and LMWP genes were then double digested with NheI & EcoRI-HF. The open digested pET-Gel vector and the DNA insert were purified by 1% agarose gel electrophoresis, ligated by T4 ligase reaction and then transformed into TOP10 competent cells. The prepared pET28a-Gel-LMWP vectors were submitted for DNA sequencing analysis.
For expression of rG-L with N-terminal thioredoxin-6×His tag (rTRX-G-L), the pET-Gel-LMWP vector (pET22b-TRX-Gel-LMWP) was prepared utilizing the pET28a-Gel-LMWP vector. The full length gelonin-LMWP gene was digested from pET28a-Gel-LMWP vector by NdeI & XhoI restriction enzymes and, after purification by 1% agarose gel electrophoresis, it was inserted into the pET22b-TRX vector which contains the gene encoding for thioredoxin-6×His tag and TEV protease cleavable peptide. The prepared pET-Gel-LMWP vector was submitted for DNA sequencing analysis. The schematic design of the pET-Gel-LMWP vector and partial DNA sequencing analysis result is depicted in Fig. S1 (see online supplementary data). In addition, amino acid sequence of rTRX-G-L and schematic peptide images of rGel, rTRX-G-L and rG-L are shown in Fig. S2.
2.4.2. Expression and Purification of rG-L
Prior to large scale (5L) production, the expression of rG-L was tested in a small culture (6 mL) under various conditions, including different media (L.B, 2xYT and TB), temperatures (37°C, 25°C and 16°C) and final IPTG concentrations (0.1, 0.5 and 1 mM), using both pET28a-Gel-LMWP and pET-Gel-LMWP vectors. To express the rG-L, vectors were separately transformed into BL21star (DE3) E. coli strains. For the pET28a-Gel-LMWP vector, a different E. coli strain (BL21-CodonPlus) was also used to test the expression. Similar procedures used for expression of rGel were employed for expression of rG-L. After expression and cell lysis, both the supernatant and the pellet of the cell lysate were investigated for rG-L expression via SDS-PAGE analysis. The insoluble pellet fraction of rG-L expression was solubilized in 1% SDS solution with boiling and sonication, before separation with SDS-PAGE. Separate batches with no IPTG induction served as controls. The success of the expression was determined by the presence of the expected rG-L or rTRX-G-L band in the SDS-PAGE results.
Based on the expression study results, the pET-Gel-LMWP vector was adopted for large scale production of rG-L. The expression and Ni-NTA resin purification procedures applied for production of rGel were identical to those used for rTRX-G-L and thus would not be reiterated here. After expression and purification, rTRX-G-L was incubated with TEV protease to remove the thioredoxin-6×His tag following the vendor's protocol (AcTEV™ protease, Invitrogen, Carlsbad CA). The cleaved product was loaded onto a heparin column, and the final rG-L was acquired by salt gradient elution (1st step: no salt for 10 min, 2nd step: 0 to 1.4 M NaCl at a rate of 0.02 M/min; flow rate: 1 mL/min).
2.5. Protein Assays
The products of rGel, cG-L and rG-L were monitored by SDS-PAGE on 10% Tris-HCl gel. Specifically, the expression and purification of rTRX-G-L was confirmed by western blot assay. Briefly, the resolved protein bands were electrotransferred to a nitrocellulose membrane and blocked non-specific binding with superblock T20 blocking buffer (Thermo Scientific) for 1 hr at room temperature. After blocking, the membrane was washed three times with TBS-T buffer (50 mM Tris, 0.15 M NaCl, 0.1% Tween 20) and then incubated with primary antibodies (mouse anti-6×His tag, Abcam, 1:000 dilution of stock) at 4°C overnight. After incubation, the membrane was washed with TBS-T buffer three times and incubated with secondary antibodies (alkaline phosphatase (AP)-conjugated anti-mouse IgG, Sigma-Aldrich, 1:10,000 dilution of stock) at room temperature for 1 hr. The membrane was then washed with TBS-T buffer three times and the protein bands were developed with the Nitroblue tetrazolium chloride/5-bromo-4-chloro-3' indolyphosphate p-toluidine salt substrate (NBT/BCIP, Roche) in a buffer containing 0.1M Tris-HCl, 0.1M NaCl, and 0.05M MgCl2 at pH 9.5. Purity of the proteins was assessed by performing densitometry analysis (ImageJ software, National Institutes of Health, Bethesda, MD) on the gels. Protein concentration was determined by the BCA protein assay using native gelonin (nGel; Enzo Life Sciences Inc.) as the standard.
2.6. Assessment of the Inhibition of Protein Translation by cG-L and rG-L
The ability of nGel, rGel, cG-L and rG-L to inhibit protein translation was evaluated in a cell-free translational system using rabbit reticulocyte lysate and luciferase mRNA. Briefly, in separate eppendorf tubes, 5 μL of either nGel, rGel, cG-L or rG-L of different concentrations (10−12 - 10−7 M) were mixed with 35 μL of rabbit reticulocyte lysate, 1 μL of amino acid without methionine, 1 μL of amino acid without leucine, 1.4 μL of potassium chloride, 1 μL of luciferase control mRNA, 1 μL of RNasin® ribonuclease inhibitor and 4.6 μL of ultrapure water (total reaction volume: 50 μL). The reaction mixture was incubated at 30°C for 90 min, and the amount of translated luciferase was then measured by a chemiluminescent assay using the luciferase assay system (Promega Corp., Madison, WI). Briefly, 2.5 μL of the reaction mixture was added to 50 μL of luciferase substrate and the luminescence intensity was measured by a plate reader (BioTEK® Synergy™ BioTEK, Co., Winooski, VT) following the vendor's protocol. The relative luminescence intensities (R.L.I.), defined here as the measured luminescence intensities divided by the mean luminescence intensity of the blank control (which had no addition of gelonin samples), were plotted against gelonin concentrations and the concentration required to inhibit 50% luciferase translation (IC50) was calculated by nonlinear regression using Prism software (Prism version 5.0, GraphPad, San Diego, CA).
2.7. Cell Cultures
CT26 murine adenocarcinoma cells, LS174T human adenocarcinoma cells, 9L rat glioma cells and PC-3 human prostate cancer cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA). The cells were cultured in 75 cm2 flasks at 37°C in a 95% air/5% CO2 containing humidified incubator. CT26 cells were maintained in RPMI1640 medium with 1% (v/v) penicillin-streptomycin, and 10% FBS. Both LS174T and 9L cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 2 mM L-glutamine, high glucose, 1% (v/v) penicillin-streptomycin, and 10% FBS. PC-3 cells were cultured in 50% RPMI1640 and 50% DMEM with 1% (v/v) penicillin-streptomycin, and 10% FBS. Culture media in the flask was changed every other day. After reaching confluency, cells were transferred into new culture flasks by detaching with 0.25% Trypsin-EDTA and reseeding at a 1:3 split ratio for the continuous cultures.
2.8. Evaluation of LMWP-Mediated Cellular uptake of cG-L and rG-L
The rGel, cG-L and rG-L proteins were each labeled with rhodamine dye by mixing the sample (2 mg/mL in 0.1 M sodium bicarbonate buffer, pH 9.3) with rhodamine B isothiocyanate at a molar ratio of 1:5, and then incubating at room temperature for 4 hr. After incubation, unreacted excess rhodamine dye was removed by applying the reaction solution to dye removal resin following the vendor's protocol (Bio-Rad Laboratories, Hercules CA). The protein to dye ratio was determined by measuring the optical density at 280 nm and 520 nm for protein and the dye, respectively.
Prior to the cell uptake study, CT26 cells were seeded onto a 24 well plate with 5×104 cells/well and incubated for 24 hr in complete RPMI1640 medium with 10% FBS. When cell confluency reached approximately 50%, rhodamine-labeled rGel, cG-L and rG-L were added to the cells (~ 5 μM final concentrations with identical fluorescence intensities among the samples) and incubated for 3 hr at 37°C in a humidified CO2 incubator. The cells were washed three times with heparin/PBS (10 mg/mL heparin in 50 mM phosphate buffer, 0.15 M NaCl, pH 7.4), followed by the addition of the Hoechst 33342 solution (1:1000 dilution of 16.2 mM stock solution) to counter-stain the nuclei of these cells. After 30 min incubation with Hoechst 33342, cells were washed three times with PBS. Images of the live cells were then taken using a Nikon TE2000S epifluorescence microscope equipped with a standard mercury bulb, a charge-coupled device camera (Roper Scientific, Tucson, AZ), a 20× objective (Nikon Plan Fluor ELWD 20) and a triple-pass DAPI/FITC/TRITC filter set (Chroma Technology Corp., Brattleboro, VT). Cell images were acquired and analyzed by Metamorph software (Molecular Devices Corporation, Sunnyvale, CA).
2.9. Anti-tumor Activities of cG-L and rG-L
The anti-tumor activities of rGel, cG-L and rG-L were determined in various cancer cell lines (e.g. CT26, LS174T, 9L and PC-3 cells) by XTT assay. Briefly, cells were detached using trypsin, re-suspended in complete medium and then dispensed into 96-well plates at a density of 104 cells per well. After incubation for 24 hr, gelonin samples were added to the wells at varying final concentrations (10−10 - 10−5 M) and incubated for 48 hr. Relative cell proliferation was measured by XTT assay following the vendor's protocol (Roche Applied Science, Indianapolis IN).
2.10. In Vivo Evaluation of Inhibition on Tumor Growth by rG-L
Six-week-old male athymic nude mice with an average weight ranging from 22 - 25 g were purchased from Charles River Laboratories (Raleigh, NC). These mice were housed in animal facilities and fed with standard chow diet. Three days after arrival, mice were randomly divided into 5 groups and treated, separately, with: 1) PBS; 2) rGel (injected dose: 20 μg); 3) rG-L (2 μg); 4) rG-L (4 μg); and 5) rG-L (20 μg). Animal experiments were conducted according to protocols approved by the University of Michigan Committee on Use and Care of Animals (UCUCA; protocol No. 08945). Briefly, at day 0 (3 days after arrival of animals), CT26 cells were harvested and implanted to the left hind region of the mice leg (5×106 cells in 50 μL). Test samples were administered by intra-tumor injection on day 7, when the tumor size reached about 100 mm3, and also on day 10. Tumor size was measured daily with a vernier caliper and the tumor volume (mm3) was calculated as V = (a2 × b)/2, where a is the width and b is the length of the tumor [29].
2.11. Statistical Analysis
All data were presented as mean ± standard deviation. Statistically significant differences among groups were determined using the 1-way ANOVA and Tukey's multiple comparison test as post-hoc test (Prism version 5.0, GraphPad, San Diego, CA). Results that yielded p-values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Expression and Purification of Recombinant Gelonin (rGel)
The rGel protein with N-terminal 6×His tag was successfully over-expressed as a soluble protein from E. coli and purified using a Ni-NTA metal affinity column. rGel, which selectively bound to the resin via the 6×His tag, was eluted with 400 mM imidazole. When the eluent was further loaded onto a cation exchange column (CM-FF HP column), rGel was retained in the column, presumably due to its basic nature (pI = 9.1), and then eluted as a single peak using 0.4 M NaCl. According to the results (data not shown) from densitometry analysis of the SDS-PAGE gels, the average purity of rGel was ≥ 95%. The total amount of expressed rGel in a 5-L culture, as determined by the BCA protein assay, was estimated to be approximately 5 mg (i.e. ~1 mg/L culture).
3.2. Synthesis and Purification of gelonin-LMWP chemical conjugate (cG-L)
The cG-L was successfully synthesized by coupling rGel with LMWP via a disulfide bond. Results from the Ellman's assay indicated that an average of 6 active thiol groups was introduced to each rGel molecule following activation with Traut's reagent. On the other hand, one thiol-active PDP group was introduced to each LMWP molecule through conjugation with NHS-PEG-PDP. Since PEG itself did not have a strong affinity for heparin, unreacted PEG could be readily removed by passing the reaction mixture through a heparin column. Results from the P2T assay showed that approximately 40% of the LMWP eluent from the heparin column contained PEG. Although non-reacted LMWP could also be present in this LMWP fraction, no further purification was deemed necessary, simply because these LMWP molecules lacked the reactive PDP groups and thus would not interact with the above activated rGel. After activation of both rGel and LMWP, disulfide bonds were allowed to form between the thiol groups on rGel and the PDP group on LMWP, yielding the ultimate cG-L.
After synthesis, the cG-L was purified using a heparin column. An initial major peak correlating to the elution of rGel was found to come out at 0.4 M NaCl, with a retention time of 5 min (data not shown). A second major peak representing the cG-L conjugate was eluted at above 1 M NaCl and a significantly extended retention time (~ 70 min), presumably due to presence of the heparin-binding LMWP moiety in the conjugate.
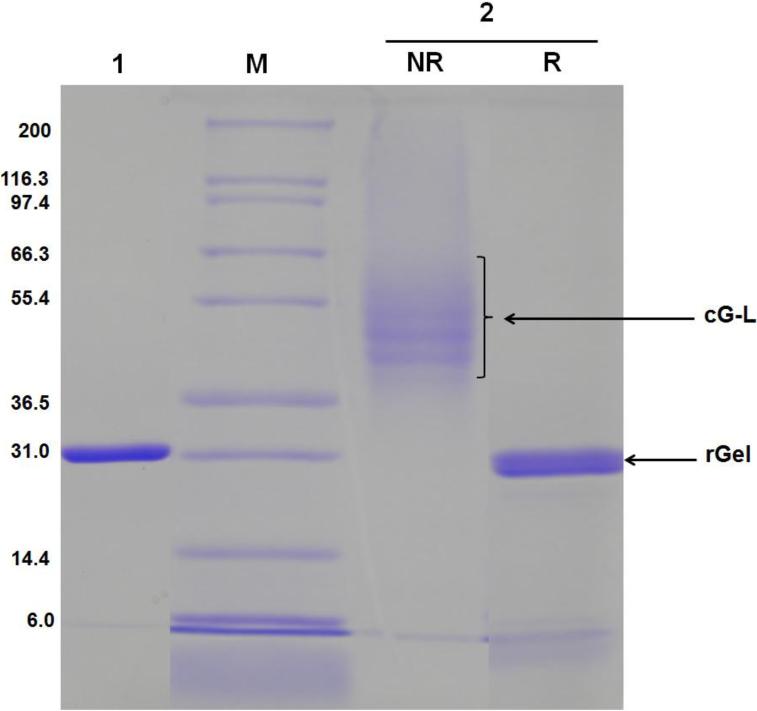
Successful synthesis and purification of cG-L was further confirmed by SDS-PAGE. As seen in Fig. 2, whereas the unreacted rGel was visible as a single band at the position corresponding to the molecular weight of gelonin (~ 31 kDa; Lane 1), the cG-L conjugate produced multiple bands with molecular weights slightly higher than that of gelonin under non-reducing conditions (Lane 2). Assessment from the molecular weight distribution of these bands suggested the presence of a heterogeneous mixture in the final cG-L product containing 2 to 5 LMWP peptides per gelonin molecule. The disulfide linkage between LMWP and rGel in the cG-L conjugate was also confirmed by comparison of the gel results under reducing (Lane 2R) and non-reducing conditions (Lane 2NR). In the presence of reducing agent, DTT, the disulfide bond between rGel and LMWP was detached and, as a consequence, a single band corresponding to the size of gelonin (31 kDa) was again observed. The yield of the final cG-L product prepared by chemical synthesis was about 35% (3.5 mg cG-L from initially 10 mg rGel).
Fig. 2.
SDS-PAGE results of cG-L purified by a heparin column. Lane 1: 1st elution peak fraction, Lane M: markers of the protein molecular weight standard (Invitrogen), Lane 2: 2nd elution peak fraction (NR: non-reducing condition, R: reducing condition). rGel migrated at its expected molecular weight of 31 kDa whereas cG-L migrated at higher molecular weight under non-reducing (NR) conditions; suggesting approximately 2 - 5 LMWP molecules were conjugated to each rGel. Disulfide bond formation between rGel and LMWP-PEG-PDP was confirmed by the size reduction of cG-L to gelonin size under reducing (R) conditions. (rGel: recombinant gelonin, cG-L: gelonin-LMWP chemical conjugate)
3.3. Expression and Purification of Recombinant Gelonin-LMWP Chimera (rG-L)
Successful preparation of pET28a-Gel-LMWP and pET-Gel-LMWP (pET22b-TRX-Gel-LMWP) vectors containing the gelonin-LMWP gene was confirmed by DNA sequencing analysis (Fig. S1B). Test expression of rG-L using the pET28a-Gel-LMWP vector in a small culture displayed very low levels of rG-L, with no soluble protein being observed. In contrast, the pET-Gel-LMWP vector allowed obvious expression of rG-L, with a significant portion being identified as soluble proteins (data not shown). The pET-Gel-LMWP vector was therefore selected for the subsequent large scale expression of rG-L.
The recombinant gelonin-LMWP chimera containing N-terminal thioredoxin-6×His tag (rTRX-G-L) was produced as a soluble protein from E. coli in a 5 L culture, and was purified using a Ni-NTA column and eluted with imidazole (400 mM). As shown in the SDS-PAGE (Fig. 3A), the rTRX-G-L was clearly identified by the presence of an intense band at 44 kDa (Lane 1) and further confirmed by the western blot assay results (Lane W) (See Fig. S3 for detailed information on the expression and purification of rTRX-G-L). Following incubation with the TEV protease, the thioredoxin-6×His tag was clearly removed (displayed by the appearance of the 31-kDa band in Lane T of Fig. S3).
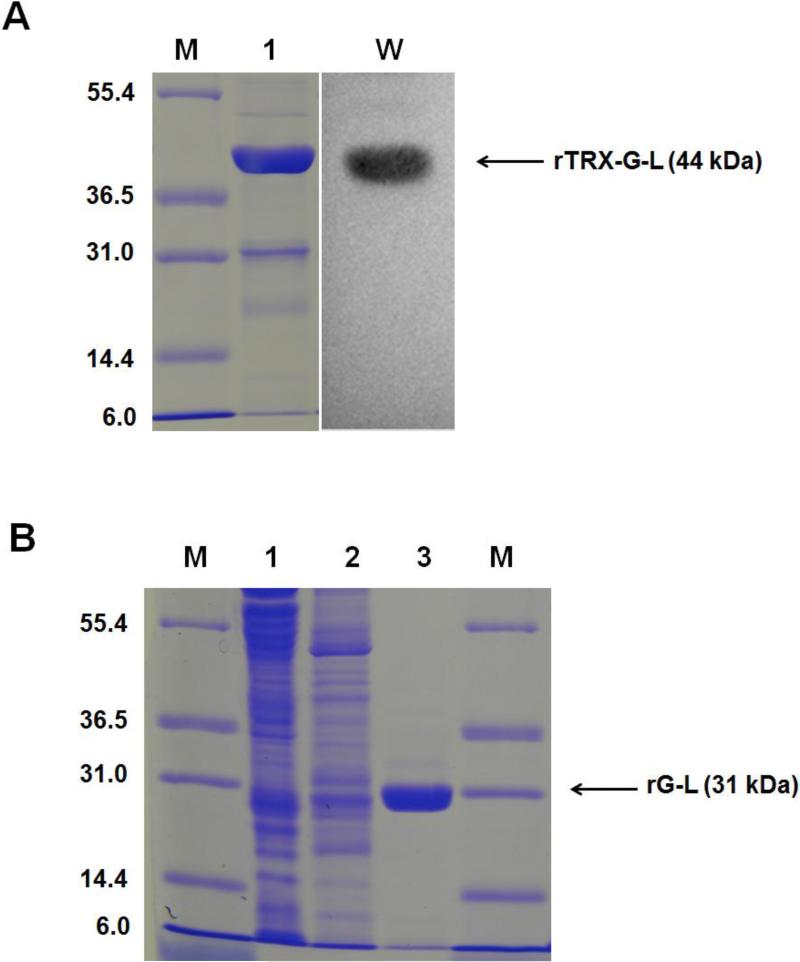
Fig. 3.
Expression and purification of rG-L. (A) SDS-PAGE and western blot assay results for rTRX-G-L. Lane M: markers of the protein molecular weight standard (Invitrogen). Lane 1 represents rTRX-G-L purified by Ni-NTA column. Lane W: western blot assay result for the rTRX-G-L; (B) SDS-PAGE analysis of the fractions from the heparin column. Eluent from the Ni-NTA column containing rTRX-G-L was incubated with TEV protease to cleave the thioredoxin-6×His tag and then applied to a heparin column with NaCl gradient. Lane M: markers of the protein molecular weight standard (Invitrogen). Lane 1, 2 and 3 represented results from the three peak fractions (1, 2, and 3, respectively). Chromatogram is shown in Fig. S4. Results showed that rG-L was eluted from the 3rd peak at 0.8 M NaCl. (rTRX-G-L: recombinant N-terminal 6×His tagged gelonin-LMWP chimera)
Three major peaks were observed when the rG-L was further purified using a heparin column (see Fig. S4). Anionic (Fraction 1) and slightly cationic (Fraction 2) endogenous bacterial proteins that bound nonspecifically to the Ni-NTA resins and eluted together with rTRX-G-L were eluted at 0 and 0.1 M NaCl, respectively. The rG-L product was eluted as a single peak (Fraction 3) by 0.8 M NaCl and with the longest retention time (50 min), presumably due to the presence of the heparin-binding LMWP moiety in the conjugate. Results from SDS-PAGE on these elution fractions were consistent with the above findings (Fig. 3B). As shown, Fraction 1 and 2 displayed multiple bands representing various sizes of bacterial endogenous proteins, while the Fraction 3 yielded a single band with a MW of 31 kDa. According to densitometry analysis of the gel bands, the purity of rG-L was above 95%. The total yield of rG-L from a 5 L cell culture was about 1.5 mg.
3.4. Inhibition of Protein Translation by cG-L and rG-L
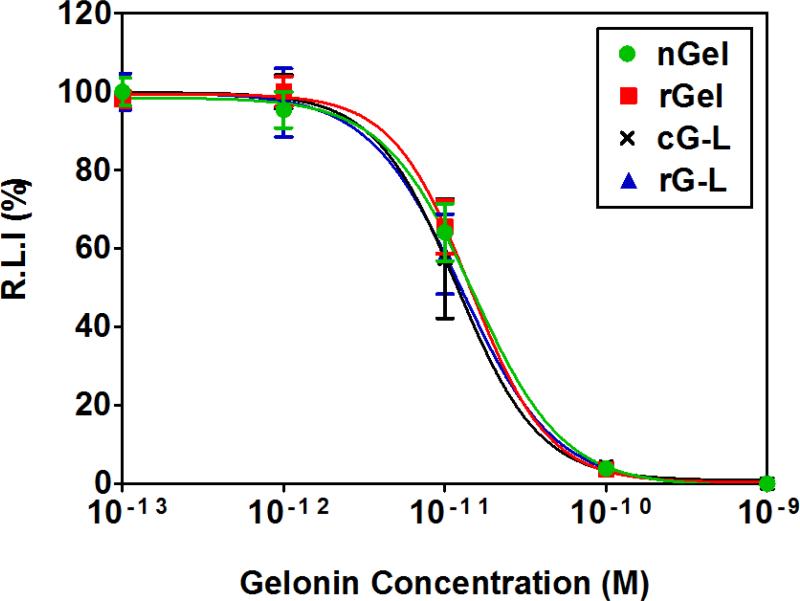
The potency of nGel, rGel, cG-L and rG-L on the inhibition of protein translation were examined in a cell-free translational system. In the presence of varying concentrations of gelonin samples, inhibition of luciferase translation was assessed using a chemiluminescent assay to determine the relative amount of translated luciferase. In the absence of actual cells, all of the four gelonin samples displayed almost identical profiles for the inhibition of luciferase translation (Fig. 4). Accordingly, IC50 values calculated from these inhibition profiles (nGel: 15 ± 3.4 pM; rGel: 14.4 ± 3.6 pM; cG-L: 13.5 ± 4.9 pM; rG-L: 12.9 ± 3.1 pM) displayed no statistically significant differences among the four gelonin samples. It should be noted the IC50 value of rGel determined from our experiments was in good accordance to that reported by Hossann et al [28]. These results demonstrated that neither chemical conjugation nor biological insertion of LMWP to gelonin altered the N-glycosidase activity of gelonin on its inhibition of protein translation in a cell-free system.
Fig. 4.
Inhibition of protein translation by native (commercial) gelonin (nGel; circle), recombinant gelonin (rGel; square), gelonin-LMWP chemical conjugate (cG-L; cross), or recombinant gelonin-LMWP chimera (rG-L; triangle) using a cell-free translational system and luciferase as the marker. The quantity of the translated luciferase was measured by chemiluminescent assay (Promega) (N=3). The relative luminescence intensity (R.L.I)-versus-gelonin concentrations curves were fitted by applying the nonlinear regression model to the plots using Prism software (GraphPad).
3.5. Cellular Uptake of cG-L and rG-L
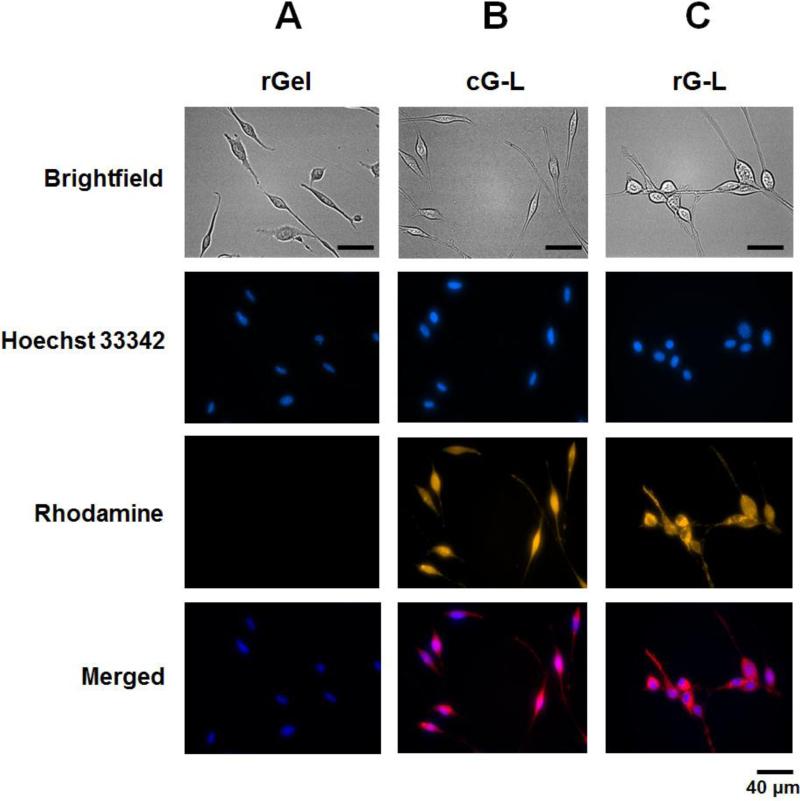
Cell-internalizing function of rGel, cG-L and rG-L was examined by uptake studies in CT26 cells utilizing rhodamine-labeled gelonin samples. Fig. 5 depicted the fluorescence microscopic images of CT26 cells taken after incubation with the gelonin samples and Hoechst 33342 counter-stain solution. While only minimal fluorescence intensity was observed in rGel-treated cells (Fig. 5A), strong fluorescence signals were clearly visible inside the cells that were treated with either cG-L or rG-L (Fig. 5B and 5C, respectively). Moreover, the merged images in Fig. 5B and 5C suggested an even distribution of cG-L and rG-L throughout the entire cell, rather than being confined in certain specific sub-cellular compartments such as endosomes.
Fig. 5.
LMWP-mediated cellular uptake by tumor cells of: (A) recombinant gelonin (rGel), (B) gelonin-LMWP chemical conjugate (cG-L), or (C) recombinant gelonin-LMWP chimera (rG-L). CT26 cells were treated with rhodamine-labeled gelonin samples for 3 hr at 37°C in 5% CO2 humidified incubator. After stringent wash with 10 mg/mL heparin/PBS solution, nuclei were counter-stained with Hoechst 33342. Images of the cells were captured by different channels (brightfield (gray), Hoechst 33342 (blue) and rhodamine (gold)) by Nikon epifluorescence microscope. Merged images were obtained by overlapping images taken with Hoechst 33342 and rhodamine channels.
3.6. Cell Culture Analysis of the Anti-tumor Activities of cG-L and rG-L
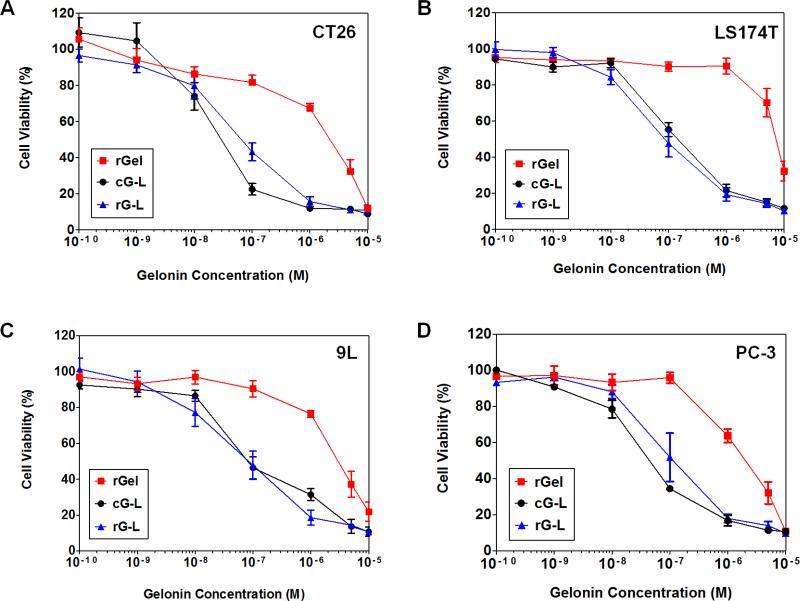
To evaluate whether LMWP-mediated cell internalization would enhance the anti-tumor effects of gelonin, the rGel, cG-L and rG-L samples were tested against four different cancer cell lines (CT26, LS174T, 9L and PC-3). As seen in Fig. 6, against the four cancer cell lines tested, rGel displayed cytotoxic effects only at concentrations above the micro-molar level. This toxicity may be attributed to the uptake of gelonin via fluid phase pinocytosis [11, 30]. In a sharp contrast, both cG-L and rG-L yielded significantly magnified cytotoxicity against all of the tested cancer cell lines. The IC50 values, estimated from the curves in Fig. 6 and summarized in Table 1, were in full agreement with the above findings. As seen, the IC50 values of both cG-L and rG-L were about 20- to 120-fold lower than that of rGel against the four tested cancer cell lines. It is interesting to note that there was basically no significant difference in cytotoxicity between cG-L and rG-L across all of the tested cancer cell lines.
Fig. 6.
Anti-tumor effects of recombinant gelonin (rGel), gelonin-LMWP chemical conjugate (cG-L) and recombinant gelonin-LMWP chimera (rG-L) against (A) CT26, (B) LS174T, (C) 9L and (D) PC-3 cell lines. Cells were plated onto 96 well plates (104 cells/well) and cytotoxicity was measured using the XTT assay (N=3). Both cG-L and rG-L displayed significantly higher cytotoxicity against all of the tested cancer cell lines than that of rGel, confirming the event of LMWP-mediated uptake in tumor cells.
Table 1.
Cytotoxicity levels (IC50) of rGel, cG-L and rG-L in various cancer cell lines (CT 26, LS174T, 9L and PC-3).
| Samples | CT 26a | LS174Ta | 9La | PC-3a |
|---|---|---|---|---|
| rGel | 1630 ± 510 | 5870 ± 1030 | 3100 ± 420 | 3430 ± 1140 |
| cG-L | 37.3 ± 15.7*** | 123.1 ± 26.9*** | 1062 ± 22.7*** | 57.8 ± 23.4** |
| rG-L | 79.1 ± 21.4*** | 67.8 ± 13.7*** | 61.3 ± 12.2*** | 83.6 ± 28.3** |
IC50 values are displayed as nM.
P < 0.001
P < 0.0001.
For all experiments, N=3. (rGel: recombinant gelonin, cG-L: gelonin-LMWP chemical conjugate, rG-L: recombinant gelonin-LMWP chimera)
3.7. In Vivo Evaluation of the Inhibition on Tumor Growth by rG-L
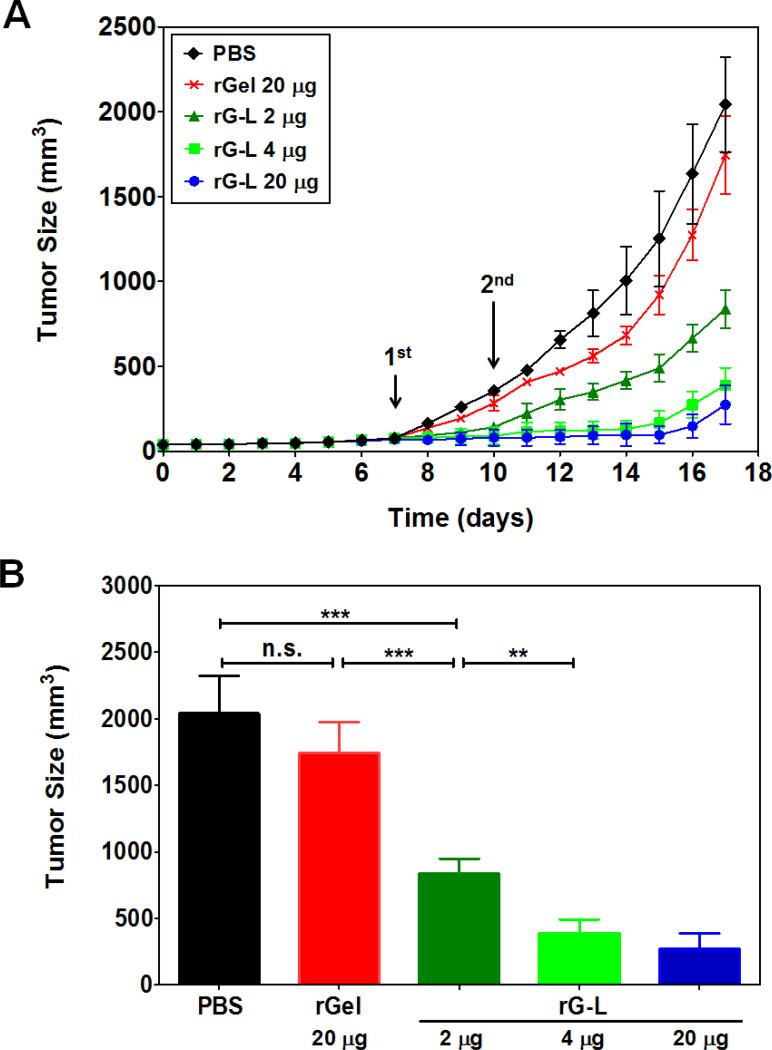
Preliminary animal studies using the CT26 s.c. xenograft tumor mouse model were conducted to assess the in vivo anti-tumor effects of the rG-L. To limit other pharmacokinetic factors, intra-tumor injection was selected for drug administration. As illustrated in Fig. 7, mice treated with 20 μg rGel displayed a slight (14%) reduction in the measured tumor size at day 17, when comparing with the control of PBS-treated animals. As described earlier, this minor cytotoxic effect by the cell-impermeable rGel was probably attributed to gelonin's uptake via the fluid phase pinocytosis mechanism [11, 30]. In sharp contrast, animals treated with 2, 4 or 20 μg of rG-L, exhibited significant, dose-dependent inhibition on tumor growth, with the measured tumor size being reduced considerably by 58, 80 and 86%, respectively. These finding provided a “proof-of-concept” to our hypothesis that incorporation of the cell-penetrating LMWP would significantly augment the anti-tumor effects of gelonin.
Fig. 7.
In vivo tumoricidal effects of recombinant gelonin-LMWP chimera (rG-L) in a CT26 s.c. xenograft tumor mouse model. (A) Inhibition of tumor growth by intra-tumor injection of PBS solution (control; diamond), recombinant gelonin (rGel; cross) and 2 μg (triangle), 4 μg (square), or 20 μg (circle) of rG-L (N=5). Treatment was carried out at Day 7 and 10 after tumor implantation. Tumor size was measured daily using a vernier caliper right after tumor inoculation (Day 0). Tumor volume (mm3) was calculated by using the following equation: V = (a2×b)/2, where a represented the width and b represented the length of the tumor. (B) Comparison of average tumor sizes at Day 17 (study terminated after average tumor volume of the PBS-treated group exceeded 2000 mm3). **P < 0.001, ***P < 0.0001, n.s.: not significant.
4. Discussion
While macromolecular drugs have drawn significant recognition as the next generation of anticancer agents due to their unmatched reaction efficiency and the repetitive mode of action, their inability to cross the membrane barrier of tumor cells remains as a bottleneck challenge in the potential clinical applications, as most of the machineries for tumor cytotoxicity are present in the cell cytosol. In this study, we made a rational hypothesis that modification of the macromolecular drug, such as the protein toxin gelonin, with the cell-penetrating peptide, LMWP, would enable the transduction of gelonin into tumor cells, thereby drastically augmenting its anti-tumor efficacy in vivo. To prove this concept, gelonin-LMWP conjugates were synthesized via both chemical conjugation and recombinant methods (the products were termed as cG-L and rG-L, respectively), utilizing recombinant gelonin (rGel). Although both conjugation methods proved feasible, they both possessed several advantages and pitfalls. For chemical conjugation, one of the benefits was that LMWP and gelonin were linked with a disulfide bond that would be automatically cleaved once entering the cells, due to the presence of a reducing condition in the cytosol by the elevated concentrations of glutathione and reductase [31]. Detachment of LMWP from gelonin would allow the delivered gelonin to be entrapped in the cytosol, eliminating the possibility of trafficking into the nucleus, which was reported to be the destiny of many CPPs [32-34]. The other benefit was that, although remained unproven, many investigators were speculating that the CPP-mediated protein translocation was a reversible process, implicating the probability that the ferried protein cargos could be fluxed back from the cells. The use of a cytosol-cleavable disulfide bond would alleviate this concern.
The primary drawback from chemical conjugation was that the final cG-L product was a mixture of gelonin conjugates containing various numbers of LMWPs per gelonin molecule; as demonstrated by our SDS-PAGE results in Fig. 2. Although many studies reported that a single CPP was sufficient to transduce a large protein into cells [35, 36], it was nevertheless postulated that extra CPP chains on the protein cargo might increase the extent of cell transduction. Hence, the heterogeneous nature of the cG-L would not only affect the batch-to-batch reproducibility of the product, but also the uptake results of these conjugates. In addition, chemical synthesis practically was not really suitable for mass production of the conjugates, therefore hindering its potential for clinical applications.
On the contrary, the benefits and shortcomings of the recombinant approach were exactly the opposite to those of the chemical conjugation method. Recombinant engineering would allow synthesis of a homogeneous 1:1 gelonin-LMWP protein chimera, and also expression of rG-L from E. coli was one of the most efficient and economic means for recombinant production of heterologous proteins, thereby being suitable for mass-scale production; both were the pitfalls of the chemical method. However, recombinant engineering lacked the ability to create a protein chimera through the disulfide linkage, thereby being unable to enjoy the afore-mentioned benefits of the chemical method resulting from formation of the cytosol-cleavable –S-S- bond between gelonin and LMWP.
For chemical synthesis of the gelonin-LMWP conjugate, two criteria must be met concerning the selection of a cross-linking method: 1) preservation of gelonin activity after conjugation, and 2) external-exposure of LMWP on the conjugate thereby fully retaining its cell-penetrating activity. In the previous study, to maintain gelonin activity, LMWP modification of glycosylated native gelonin (nGel) was accomplished by strategically conjugating LMWP to the carbohydrate moiety [20]. Recombinant gelonin (rGel), however, as with many other recombinant proteins, is devoid of any sugar residue, and thus it was necessary to activate the peptide body of the rGel [28]. Herein we selected the Traut's reagent to achieve thiol-activation of the gelonin molecule because, when comparing with other conventional activating agents to produce a reactive –SH group, such as N-succinimidyl 3-(2-pyridyldithio)-propionate (SPDP), N-succinimidyl iodoacetate, or 4-(iodoacetamido)-l-cyclohexenyl-1,2-dicarboxylic acid anhydride, etc., Traut's reagent was reported to not impair gelonin's biological activity [37, 38]. In addition, the good aqueous solubility and slow hydrolysis rate (half-life: 1 hr in 50mM triethanolamine buffer at pH 8) of the Traut's reagent rendered it a more favorable choice [39]. As shown from our results in Section 3.2., thiol groups were successfully introduced to gelonin using the Traut's reagent, confirmed by the Ellman's assay [40]. Alternatively, to ensure an external exposure of LMWP, a short PEG chain containing heterobifunctional activated groups on both ends was employed as the cross-linker to produce the cG-L conjugate. Our results on the binding of cG-L to the heparin column (Section 3.2) indeed confirmed the exposure of LMWP on cG-L after chemical conjugation and, more importantly, SDS-PAGE findings in Fig. 2 clearly demonstrated the formation of a disulfide linkage between gelonin and LMWP. It is noteworthy that, in this study, remarkably high conjugation yield (35%) was accomplished for synthesis of the cG-L, which was presumably due to thiol activation of multiple amine groups on gelonin (average 6 thiols per gelonin) by Traut's reagent and, moreover, during conjugation reaction, effective prevention of any aggregation between LMWP and gelonin by the heterobifunctional PEG.
Regarding recombinant synthesis, LMWP gene was initially inserted to the C-terminus of the gelonin-encoding gene (pET-Gel vector) to produce the pET28a-Gel-LMWP vector. Despite that rGel was successfully expressed using the pET-Gel vector, there was almost no expression of the rG-L under tested conditions. This poor expression of rG-L was likely due to inefficient translation of the LMWP gene caused by codon usage bias, a finding previously reported by Lee and other investigators [33, 41]. Indeed, LMWP was known to consist of abundant arginine residues that was translated by the rarest codons in E. coli [33], hence severely limiting its expression level. To this regard, the poor translation of LMWP appeared to significantly impair the overall expression of the ultimate rG-L. On the other hand, the use of the BL21-CodonPlus E. coli strain, which contained extra copies of genes encoding the tRNAs for rare amino acids, also did not provide any enhancement on rG-L expression.
Aside from the low expression, the low solubility of rG-L also presented a concern. When eukaryotic proteins were expressed by prokaryotic E. coli cells, improper folding of proteins often occurred, resulting in insoluble aggregates called inclusion bodies [42, 43]. This seemed to be the major hurdle that must be overcome in order to succeed in the production of rG-L, since the total expression level of rG-L was already very low. A strategy often used to improve the expression of so-called “difficult-to-produce” proteins was by inclusion and co-expression with a highly expressible fusion partner. Thioredoxin (TRX), for instance, is a small 12-kDa redox protein that could be overexpressed to an extraordinary high level (accumulate up to 40% of the total cellular proteins) in E. coli and still remain soluble [42]. Based on this principle, we inserted the full length gelonin-LMWP gene into the pET-TRX vector containing the TRX gene to create the pET-Gel-LMWP vector (pET22b-TRX-Gel-LMWP) (Fig. S1A). Results shown in Fig. 3A clearly demonstrated the plausibility of this strategy, as the N-terminal thioredoxin-6×His tagged-gelonin-LMWP protein chimera (rTRX-G-L) was successfully produced in large quantities as a soluble protein in E. coli.
Maintenance of the functions of both cG-L and rG-L to inhibit protein translation was a major initial concern, since it was demonstrated in the literatures that even a slight conformational change of the 3-D structure could result in a significant alteration on the biological activities of proteins [44, 45]. Nevertheless, data obtained using a cell-free translational system confirmed that all of the rGel, cG-L and rG-L products possessed activities equivalent to that of the native gelonin (nGel), displaying no significant difference in the measured IC50 values. Although the rGel results were somewhat expected, it was a little surprising to notice that there was virtually no loss in activity for both the cG-L and rG-L chimeras. Nevertheless, gelonin was known to be extremely stable due to its specific structure [46-48], which consequently could contribute to the retention of its activity even after modification with LMWP.
While the results showing retention of the biological activity of both cG-L and rG-L was truly encouraging, intracellular transport of gelonin was also required to enable its access to the ribosomes of cancer cells. With the incorporation of the cell-penetrating LMWP peptide, fluorescence microscopy data in Fig. 5 clearly demonstrated that both cG-L and rG-L were able to internalize into cells, while little, if any, of the impermeable rGel was found inside the cytosol of the test tumor cells. More importantly, the transduced cG-L and rG-L appeared to be evenly distributed within the cytosol, enabling them to maximize their cytotoxic effects. Comparison of the IC50 values of both cG-L (32 - 113.4 nM) and rG-L (55.4 - 95.4 nM) with that of rGel (1630 - 5870 nM) summarized in Table 1 yielded a solid support to our initial crucial hypothesis that modification of gelonin with LMWP would lead to a much improved anti-tumor activity. Notably, this greatly enhanced cytotoxic activity of gelonin appeared to be indiscriminative to cancer types, as all of the four tested cancer cell lines yielded similar responses. This phenomenon may be accounted for in terms of the universal cell transduction mechanism of most CPPs, which, in principle and practice, suggests that all cell types including brain cells and erythrocytes are transducible [19, 23, 49]. This universal (or nonselective) fashion of cell transduction is, indeed, a significant and unique merit of the CPPs, as it widens their application to a variety of diseases. Nevertheless, it should be also noted that this non-selectivity may, on the other hand, raise toxicity concerns for clinical use of the CPP-modified drugs, especially if the drug has no cell selectivity. In fact, both cG-L and rG-L displayed enhanced cytotoxicity not only against cancerous cell lines, but also on non-cancerous cell lines (i.e., 293 Human Embryonic Kidney cells and Madin-Darby Canine Kidney cells) (data not shown). Therefore, it will be necessary to exploit a drug delivery system (DDS) for effective yet safe use of the LMWP-modified gelonins. Also, noteworthy was that both cG-L and rG-L yielded almost identical cytotoxic activities toward cancer cells. This was somewhat unexpected, considering the fact that the two major factors affecting tumor-killing capability of the LMWP-gelonin conjugates lied in the enzymatic activity and cell-penetrating ability. Although the intrinsic activities to inhibit protein translation were shown to be identical for cG-L and rG-L (Fig. 4), the cG-L actually possessed a higher ratio of the CPP moieties (2-5 LMWP chains per gelonin molecule) comparing to that (1 LMWP chain per gelonin molecule) of rG-L. This result seemed to suggest that, despite the possibility for greater cellular uptake of cG-L than rG-L, one well-exposed LMWP would be sufficient to effectively facilitate the cell entry of gelonin; consistent with findings reported by other investigators [20, 23, 33]. Also, considering the extraordinary potency of gelonin, which only requires a few molecules to induce apoptosis, any difference in the extent of uptake of cG-L and rG-L into cells may have not significantly affected their anti-cancer effects.
Albeit that cG-L and rG-L exhibited equivalent IC50 values and equally promising in vitro cell culture cytotoxicity, rG-L was nevertheless selected for subsequent preliminary animal studies, simply because of its homogeneity, batch-to-batch manufacturing consistency, and, most critically, the possibility for mass production to satisfy the need of large quantities for animal investigation. In a CT26 s.c. xenograft tumor mouse model, the rG-L product displayed a significantly enhanced tumoricidal activity over controls administered with either PBS solution or non-modified gelonin (i.e. rGel) (Fig. 7). A dose-dependent reduction on tumor growth was observed for rG-L, with a total gelonin dose being as low as only 2 μg. On the contrary, non-modified rGel displayed virtually no effect on tumor growth, even at a total gelonin dose of 20 μg. Previously, Park et al. reported, by using the same animal tumor model, that intra-tumor administration of gelonin at a dose as high as 100 μg did not yield visible reduction on tumor size [20]. Overall, both in vitro and in vivo findings provided strong evidence to support our hypothesis; i.e. modification of gelonin with the cell-penetrating LMWP would drastically enhance gelonin's clinical potential for cancer treatment.
5. Conclusions
Despite decades of efforts, a cure to the vast majority of cancers remains elusive. Highly potent and specific macromolecular drugs have shown promise to overcome the limitations of traditional small molecule drugs. Effective intracellular delivery of these large drugs, however, continues to be the main hurdle to clinical realization of these drugs. Recent discovery of the cell-penetrating peptides offers hope to finally solve this intracellular delivery problem. In this study, we reported the “first” synthesis, via both chemical conjugation and genetic engineering methods, of recombinant gelonin (rGel) modified with a potent yet non-toxic cell-penetrating peptide, LMWP. A novel coupling method based on the use of the Traut's reagent and a heterobifunctional PEG cross-linker was developed to synthesize the gelonin-LMWP chemical conjugate (cG-L), whereas an innovative strategy based on the co-expression with a highly expressible fusion partner, thioredoxin (TRX), was adopted to produce the recombinant gelonin-LMWP chimera (rG-L) containing the “difficult-to-produce” LMWP sequence with abundant arginine residues that are translated by the rarest codons in E. coli. In vitro cell culture studies revealed that cG-L and rG-L not only retained the protein translation-inhibiting activity and cell-penetrating capability, but also yielded 20- to 120-fold lower IC50 values than that of the unmodified rGel. Preliminary in vivo studies using a xenograft tumor mouse model showed that while intra-tumor injection of the cell-impermeable rGel resulted in virtually no inhibition on tumor growth, both of the gelonin-LMWP conjugates exhibited significantly enhanced anti-tumor effects. Overall, our investigation shed light of the possibility to realize clinical application of the potent protein toxin drugs for cancer treatment. Based on the success of this study, development of a drug delivery system (DDS) for tumor selective delivery of CPP-modified macromolecular drugs, such as LMWP-modified gelonin, is currently underway in our research group.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health R01 Grants CA114612 and NS066945. It was also partially supported by National Key Basic Research Program of China (2013CB932502). Furthermore, this work was partially sponsored by Grant R31-2008-000-10103-01 from the World Class University (WCU) project of South Korea. Victor C. Yang is currently a participating faculty member in the Department of Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, South Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blagosklonny MV. The power of chemotherapeutic engineering: arresting cell cycle and suppressing senescence to protect from mitotic inhibitors. Cell Cycle. 2011;10:2295–2298. doi: 10.4161/cc.10.14.16819. [DOI] [PubMed] [Google Scholar]
- 2.Kreitman RJ. Immunotoxins. Expert Opin Pharmacother. 2000;1:1117–1129. doi: 10.1517/14656566.1.6.1117. [DOI] [PubMed] [Google Scholar]
- 3.Devi GR. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 4.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 5.Foged C, Nielsen HM. Cell-penetrating peptides for drug delivery across membrane barriers. Expert Opin Drug Deliv. 2008;5:105–117. doi: 10.1517/17425247.5.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res. 2004;21:389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- 7.Stirpe F. On the action of ribosome-inactivating proteins: are plant ribosomes species-specific? Biochem J. 1982;202:279–280. doi: 10.1042/bj2020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri M, Kaur I, Perugini MA, Gupta RC. Ribosome-inactivating proteins: current status and biomedical applications. Drug Discov Today. 2012;17:774–783. doi: 10.1016/j.drudis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004;44:371–383. doi: 10.1016/j.toxicon.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Tumer NE, Li XP. Interaction of ricin and Shiga toxins with ribosomes. Curr Top Microbiol Immunol. 2012;357:1–18. doi: 10.1007/82_2011_174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980;255:6947–6953. [PubMed] [Google Scholar]
- 12.Atkinson SF, Bettinger T, Seymour LW, Behr JP, Ward CM. Conjugation of folate via gelonin carbohydrate residues retains ribosomal-inactivating properties of the toxin and permits targeting to folate receptor positive cells. J Biol Chem. 2001;276:27930–27935. doi: 10.1074/jbc.M102825200. [DOI] [PubMed] [Google Scholar]
- 13.Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 14.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 15.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Magzoub M, Graslund A. Cell-penetrating peptides: [corrected] from inception to application. Q Rev Biophys. 2004;37:147–195. doi: 10.1017/s0033583505004014. [DOI] [PubMed] [Google Scholar]
- 18.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 19.Kwon YM, Li YT, Liang JF, Park YJ, Chang LC, Yang VC. PTD-modified ATTEMPTS system for enhanced asparaginase therapy: a proof-of-concept investigation. J Control Release. 2008;130:252–258. doi: 10.1016/j.jconrel.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park YJ, Chang LC, Liang JF, Moon C, Chung CP, Yang VC. Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: in vitro and in vivo study. FASEB J. 2005;19:1555–1557. doi: 10.1096/fj.04-2322fje. [DOI] [PubMed] [Google Scholar]
- 21.Choi JK, Jang JH, Jang WH, Kim J, Bae IH, Bae J, Park YH, Kim BJ, Lim KM, Park JW. The effect of epidermal growth factor (EGF) conjugated with low-molecular-weight protamine (LMWP) on wound healing of the skin. Biomaterials. 2012;33:8579–8590. doi: 10.1016/j.biomaterials.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 22.Xia H, Gao X, Gu G, Liu Z, Zeng N, Hu Q, Song Q, Yao L, Pang Z, Jiang X, Chen J, Chen H. Low molecular weight protamine-functionalized nanoparticles for drug delivery to the brain after intranasal administration. Biomaterials. 2011;32:9888–9898. doi: 10.1016/j.biomaterials.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Kwon YM, Chung HS, Moon C, Yockman J, Park YJ, Gitlin SD, David AE, Yang VC. L Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL) J Control Release. 2009;139:182–189. doi: 10.1016/j.jconrel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YS, Huang Y, Park YJ, David AE, White L, He H, Chung HS, Yang VC. Specific down regulation of 3T3-L1 adipocyte differentiation by cell-permeable antisense HIF1alpha- oligonucleotide. J Control Release. 2010;144:82–90. doi: 10.1016/j.jconrel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang LC, Lee HF, Yang Z, Yang VC. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (I): preparation and characterization. AAPS PharmSci. 2001;3:E17. doi: 10.1208/ps030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang LC, Liang JF, Lee HF, Lee LM, Yang VC. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (II): in vitro evaluation of efficacy and toxicity. AAPS PharmSci. 2001;3:E18. doi: 10.1208/ps030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee LM, Chang LC, Wrobleski S, Wakefield TW, Yang VC. Low molecular weight protamine as nontoxic heparin/low molecular weight heparin antidote (III): preliminary in vivo evaluation of efficacy and toxicity using a canine model. AAPS PharmSci. 2001;3:E19. doi: 10.1208/ps030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hossann M, Li Z, Shi Y, Kreilinger U, Buttner J, Vogel PD, Yuan J, Wise JG, Trommer WE. Novel immunotoxin: a fusion protein consisting of gelonin and an acetylcholine receptor fragment as a potential immunotherapeutic agent for the treatment of Myasthenia gravis. Protein Expr Purif. 2006;46:73–84. doi: 10.1016/j.pep.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Urva SR, Yang VC, Balthasar JP. Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci. 2010;99:1582–1600. doi: 10.1002/jps.21918. [DOI] [PubMed] [Google Scholar]
- 30.Madan S, Ghosh PC. Interaction of gelonin with macrophages: effect of lysosomotropic amines. Exp Cell Res. 1992;198:52–58. doi: 10.1016/0014-4827(92)90148-2. [DOI] [PubMed] [Google Scholar]
- 31.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 32.Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- 33.Lee TY, Park YS, Garcia GA, Sunahara RK, Woods JH, Yang VC. Cell permeable cocaine esterases constructed by chemical conjugation and genetic recombination. Mol Pharm. 2012;9:1361–1373. doi: 10.1021/mp200623w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. 2011;22:888–893. doi: 10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Mae M, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006;6:509–514. doi: 10.1016/j.coph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Lambert JM, Senter PD, Yau-Young A, Blattler WA, Goldmacher VS. Purified immunotoxins that are reactive with human lymphoid cells. Monoclonal antibodies conjugated to the ribosome inactivating proteins gelonin and the pokeweed antiviral proteins. J Biol Chem. 1985;260:12035–12041. [PubMed] [Google Scholar]
- 38.McIntyre GD, Scott CF, Jr., Ritz J, Blattler WA, Lambert JM. Preparation and characterization of interleukin-2-gelonin conjugates made using different cross-linking reagents. Bioconjug Chem. 1994;5:88–97. doi: 10.1021/bc00025a012. [DOI] [PubMed] [Google Scholar]
- 39.Traut RR, Bollen A, Sun TT, Hershey JW, Sundberg J, Pierce LR. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973;12:3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- 40.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 41.Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:494–500. doi: 10.1016/0958-1669(95)80082-4. [DOI] [PubMed] [Google Scholar]
- 42.LaVallie ER, DiBlasio-Smith EA, Collins-Racie LA, Lu Z, McCoy JM. Thioredoxin and related proteins as multifunctional fusion tags for soluble expression in E. coli. Methods Mol Biol. 2003;205:119–140. doi: 10.1385/1-59259-301-1:119. [DOI] [PubMed] [Google Scholar]
- 43.Singh SM, Panda AK. Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng. 2005;99:303–310. doi: 10.1263/jbb.99.303. [DOI] [PubMed] [Google Scholar]
- 44.Ebert RF, Spryn LA. Immunotoxin construction with a ribosome-inactivating protein from barley. Bioconjug Chem. 1990;1:331–336. doi: 10.1021/bc00005a006. [DOI] [PubMed] [Google Scholar]
- 45.Battelli MG, Barbieri L, Stirpe F. Toxicity of, and histological lesions caused by, ribosome inactivating proteins, their IgG-conjugates, and their homopolymers. APMIS. 1990;98:585–593. doi: 10.1111/j.1699-0463.1990.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 46.Hosur MV, Nair B, Satyamurthy P, Misquith S, Surolia A, Kannan KK. X-ray structure of gelonin at 1.8 A resolution. J Mol Biol. 1995;250:368–380. doi: 10.1006/jmbi.1995.0383. [DOI] [PubMed] [Google Scholar]
- 47.Mujoo K, Cheung L, Murray JL, Rosenblum MG. Pharmacokinetics, tissue distribution, and in vivo antitumor effects of the antimelanoma immunotoxin ZME-gelonin. Cancer Immunol Immunother. 1995;40:339–345. doi: 10.1007/BF01519635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Qu Y, Li H, Yuan J. Truncations of gelonin lead to a reduction in its cytotoxicity. Toxicology. 2007;231:129–136. doi: 10.1016/j.tox.2006.11.074. [DOI] [PubMed] [Google Scholar]
- 49.Goncalves E, Kitas E, Seelig J. Binding of oligoarginine to membrane lipids and heparan sulfate: structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry. 2005;44:2692–2702. doi: 10.1021/bi048046i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.