Abstract
Visual information is crucial for goal-directed reaching. A number of studies have recently shown that motion in particular is an important source of information for the visuomotor system. For example, when reaching a stationary object, movement of the background can influence the trajectory of the hand, even when the background motion is irrelevant to the object and task. This manual following response may be a compensatory response to changes in body position, but the underlying mechanism remains unclear. Here we tested whether visual motion area MT+ is necessary to generate the manual following response. We found that stimulation of MT+ with transcranial magnetic stimulation significantly reduced a strong manual following response. MT+ is therefore necessary for generating the manual following response, indicating that it plays a crucial role in guiding goal-directed reaching movements by taking into account background motion in scenes.
Keywords: action, localization, manual following response, perception, pointing, TMS, visuomotor
Introduction
Visual motion is constantly produced as we move our eyes and head and as objects in the world move around us. The visuomotor system therefore faces a serious challenge in that it must register target as well as background motion and then segment these different sources of motion when directing actions to objects. Over the last 3 decades, a broad and expanding literature has examined how the visuomotor system processes and uses visual motion in goal-directed behavior.
Several different forms of visual motion are used by the visuomotor system, including motion of objects themselves and motion of the background. Goal-directed behavior clearly depends on the accuracy and efficiency with which the visual system codes object motion and position. Background motion, on the other hand, has a less understood role in visually guided action. Although there is a clear influence of background optic flow on posture, heading, and locomotion in general (Lee and Aronson 1974; Warren et al. 1988, 2001; Royden et al. 1992; Britten and van Wezel 1998), more recent studies have shown, surprisingly, that background motion also influences goal-directed reaching (Mohrmann-Lendla and Fleischer 1991; Brenner and Smeets 1997; Whitney et al. 2003; Saijo et al. 2005). The reason that background retinal motion influences reaching is debated; it is possible that the hand is dragged along with background motion in a manual following response (Saijo et al. 2005), akin to the well-known ocular following response (Miles et al. 1986). Alternatively, or in addition, the visual or visuomotor system might use background retinal motion to update (shift) the coded locations of targets, which causes subsequent deviations in the hand's trajectory (Whitney et al. 2003). The functional role of both mechanisms could be to help compensate for movements of the eye, head, or body (Whitney et al. forthcoming), and the end result of both hypotheses is a drift in the trajectory of reaching movements due to background retinal motion (a manual following response). Unfortunately, the neural locus of this process remains unknown.
Accumulating psychophysical evidence on the manual following response is consistent with the physiological responses of neurons in the MT complex (MT+, including MT and MST; Zeki 1974; Albright 1984; Newsome et al. 1986; Tanaka and Saito 1989; Orban 1998; Rees et al. 2000; Culham et al. 2001; Huk and Heeger 2002). For example, the manual following response is contrast-dependent and spatiotemporally tuned to higher velocities (Gomi et al. 2006), visual motion that covers larger portions of the visual field produces a stronger influence on reaching (Saijo et al. 2005), and visual motion in one region of the visual field causes a manual following response to targets in remote regions (Whitney et al. 2003; Whitney and Goodale 2005). All these factors are more consistent with the properties and tuning of neurons within the MT+ complex than with neurons in other visual areas (Tanaka and Saito 1989; Orban 1998). In addition, the manual following response correlates with the coherence of global motion (Saijo et al. 2005), similar to MT+ (Newsome et al. 1986; Rees et al. 2000). Finally, the manual following response occurs with a very short latency (under 150 ms; Brenner and Smeets 1997; Whitney et al. 2003; Saijo et al. 2005; Gomi et al. 2006), consistent with the brief neuronal latencies of MT+ responses (Ffytche et al. 1995; Schmolesky et al. 1998; Pascual-Leone and Walsh 2001). Together, the psychophysical evidence suggests that cortical motion areas within MT+ may play an important role in controlling reaching in the presence of background visual motion.
To directly test whether MT+ processing of background motion is used to control visually guided reaching, we measured reaching movements to stationary targets following visual motion adaptation while transcranial magnetic stimulation (TMS) was applied to MT+. The results showed that there was a manual following response after motion adaptation in a direction consistent with the motion aftereffect (MAE). Disruption of MT+, however, abolished this manual following response, demonstrating that MT+ processing influences visuomotor behavior. Sham and control conditions confirmed that MT+ stimulation selectively mitigated the manual following response. We conducted a second experiment to confirm that our stimulation of MT+ effectively disrupted a motion-induced perceptual illusion. Together, the experiments suggest that MT+ processes motion information that directly influences both manual localization (goal-directed reaching) and perceptual localization (perceived position).
Materials and Methods
Seven subjects participated in the visually guided reaching experiment, 3 of whom were naive as to the purpose of the study. Each subject had normal or corrected-to-normal visual acuity. The subject's head was placed in a chin rest 40 cm from a cathode ray tube (CRT; 800 × 600, 100 Hz), driven by a Macintosh G4 Powerbook. The adaptation stimulus consisted of a small drifting square-wave grating (8.49 × 4.25 deg, 0.12 cycles/deg, 4.1 Hz), presented within a stationary rectangular aperture in the right visual field (the precise location was determined separately for each subject; see below). Following adaptation, a small (0.43 × 0.85 deg) target was presented during the test phase for 20 ms vertically centered within the adapted region. The target could be presented in any one of 3 horizontal locations (within a range of ±1.7 deg around the center of the adapted region), determined randomly on each trial. To prevent subjects from memorizing the relative location of the target to the fixation point, the horizontal position of the fixation point was randomly jittered within a 1.7 deg window. Figure 1 shows a diagram of the stimulus. Following an initial adaptation period of 30 s, interleaved 3000 ± 100-ms adaptation and 20-ms test phases were presented (see Fig. 1 for event sequence). A 300-ms interstimulus interval separated each adaptation and test period. During each test phase, subjects were instructed to point as quickly as possible at the stationary target with their index finger (hitting the CRT with their finger). No feedback was provided about pointing accuracy. In separate sessions, subjects adapted to either rightward or leftward motion. Leftward and rightward motion was not interleaved because this prevents the buildup of motion adaptation. Within each session, there were 3 target positions and 10 repeated trials at each of these positions. Each session was repeated twice for each direction of motion for a total of 120 trials.
Figure 1.
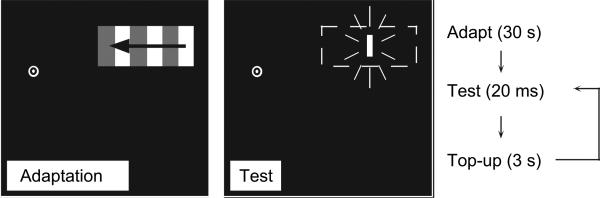
Stimulus and procedure in the first experiment. A drifting luminance-defined square-wave grating was presented within a stationary rectangular aperture during the adaptation periods. The direction of motion was leftward or rightward in separate conditions. Subjects always fixated at the bull's eye. During the test period, a flashed target was presented in a randomly determined position within the motion-adapted region (the dashed rectangle indicates the motion-adapted region and was not visible during the experiment). The stimuli are not drawn to scale.
An infrared marker was attached to the subject's index finger, and an Optotrak 3020 (NDI, Waterloo, Ontario, Canada) was used to track the subject's finger throughout the trial. The Optotrak collected the finger position at 250 Hz, with a spatial resolution of ~0.1 mm3. The trajectory of each reaching movement was recorded, as was the end point (landing position of the finger on the CRT). The measure of primary interest was the constant error (the distance between the end point reach position and actual target location), which was measured on each trial. The average constant error following leftward and rightward motion adaptation was compared using both within- and between-subjects non-parametric statistics.
On each trial, 100 ms before the target was presented, a trigger was sent from the Macintosh G4 to a Magstim™ SuperRapid magnetic stimulator (Whitland, South West Wales, UK), which generated the TMS. Repetitive TMS (rTMS) was delivered at 12 Hz for 500 ms and therefore covered the entire period of time during which the target was visible. The TMS was not delivered during motion adaptation. TMS was delivered in 3 separate conditions: the experimental site, MT+; a sham condition in which the TMS coil was placed adjacent to the subject, so the subject could hear the audible effect of stimulation but did not receive magnetic stimulation to the brain; and a control site that we do not believe mediates the manual following response (the vertex). In the sham and control conditions, we expected no influence of TMS on the resulting reaching movement. To be sure that we were, in fact, stimulating MT+, moving phosphenes were functionally localized in each subject, as these are known to be generated selectively in motion area MT+ and are a standard method of reliably localizing the motion-selective region (Walsh et al. 1998; Stewart et al. 1999; Pascual-Leone and Walsh 2001; Battelli et al. 2002; Theoret et al. 2002; McGraw et al. 2004; O'Shea et al. 2004; Silvanto et al. 2005). Data was collected in separate runs for each of the 3 stimulation conditions (120 trials per condition, as described above, for a grand total of 360 trials).
To localize moving phosphenes, we began by stimulating the subject's inions (primary visual areas) at 50-70% intensity using a 70-mm figure-of-eight coil while they viewed a blank CRT screen or closed their eyes. Each subject reported that they perceived small static phosphenes with stimulation at the inion. By moving laterally in incremental steps, subjects began to perceive moving phosphenes. Once subjects reported perceiving consistent moving phosphenes, they were instructed to indicate the location in the visual field in which the moving phosphenes were perceived. All subjects reported moving phosphenes that covered at least part of one quadrant of the visual field. The stimulation site was only considered valid if, after removing and repositioning the coil, the same phosphene location could be generated. We only stimulated the left hemisphere with the TMS coil placed tangential to the skull with the handle pointed backward, parallel to the horizontal and midsagittal plane. In each case, the TMS coil was held in place by the experimenter, and should any head movements take place, the coil was repositioned manually. Subjects perceived moving phosphenes exclusively in their right visual field. In one subject, we compared functional and structural magnetic resonance imaging to the stimulated location and confirmed that our stimulation site was centered on the MT+ region using Brain-Sight frameless stereotaxic software (Rogue Research, Montreal, Canada). The average location of the TMS-localized MT+ regions was 3.6 cm dorsal and 4.14 cm lateral to the inion (standard deviation ±0.92 cm lateral and ±1.1 cm dorsal). The identified location of MT+ in 2 of the subjects was consistent with that previously found for these subjects (Walsh et al. 1998).
Once MT+ was localized, the adaptation and test stimuli (described above, Fig. 1) were placed within the region of the visual field covered by the localized moving phosphene. This ensured that the motion-adapted region (dashed rectangle in Fig. 1) fell within a region of the visual field covered by TMS. During the experimental sessions, stimulation was 65% maximum stimulator intensity (1.3 T). Subjects reported that the moving phosphenes were not visible during the experimental session because they were attending to the task, and the high contrast test stimulus (above) was easily visible. Because the moving phosphenes were not visible and because the manual following response is strongly dependent on luminance contrast (Gomi et al. 2006), the phosphenes would not interact with or generate a manual following response.
In a second experiment, the methods above were used, with the exception that 3 subjects judged the relative position of the static flashed target, rather than reaching to it. Following adaptation to the stimulus above, 2 vertically aligned flashes were presented; one flash was the same target as in the reaching experiment and a second was a vernier comparison flash. The comparison flash was in all respects identical to the target except that it was presented below and outside the motion-adapted region. The comparison flash could be at one of 6 horizontally displaced locations relative to the original target (either to the left or right of the target). In a 2-alternative forced choice (binary choice) task, subjects reported whether the target (located in the motion-adapted region) appeared to the left or right of the vernier comparison flash. There were 15 trials for each of the 6 vernier offsets, for a total of 90 trials per session with an intertrial interval of 1 s. Each subject participated in 2 sessions, one with TMS stimulation of MT+ on each trial and the other with sham and control TMS stimulation (described above). The responses of each subject were fit with the logistic function f(x)= [1/(1 + exp[a(x-b])], where (b) estimates the physical misalignment between the flashes that creates an apparent alignment (the point of subjective equality, [PSE]; Finney 1971; McKee et al. 1985) and (a) indicates the slope of the function.
Results
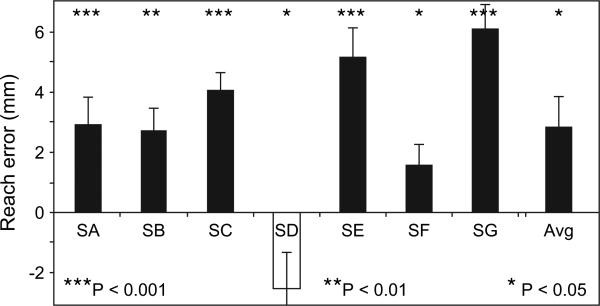
The manual following response—a deviation in the trajectory of reaching movements in the presence of background motion—has been reported several times (Fleischer 1991; Brenner and Smeets 1997; Yamagishi et al. 2001; Whitney et al. 2003; Mohrmann-Lendla and Saijo et al. 2005; Gomi et al. 2006) but has not been reported following exposure to motion (motion adaptation). Figure 2 shows that following adaptation to visual motion, there was a shift in the end point of reaching movements in a direction opposite that of the previously visible motion. Six of the 7 subjects displayed a consistent and significant deviation in their reaching movements (the least significant within-subject effect was for subject SF, Wilcoxon signed ranks test, Z = 2.5, P < 0.05). The average manual following response across all 7 subjects was 2.9 mm in a direction opposite that of the prior motion (Fig. 2; Z = 2.1, P < 0.05). Previous studies have found that visual motion present during a reach produces a manual following response (Mohrmann-Lendla and Fleischer 1991; Brenner and Smeets 1997; Yamagishi et al. 2001; Whitney et al. 2003; Saijo et al. 2005), and the present results extend this finding by showing that prior motion exposure (motion adaptation) can influence visuomotor control as well.
Figure 2.
The manual following response of reaching movements to a stationary object after visual motion adaptation in the sham and control TMS conditions. Positive values on the ordinate indicate a deviation in the end point of the reaching movement opposite the direction of motion adaptation. There was a significant average deviation (Z = 2.1, P < 0.05). Individually, 6 of the 7 subjects showed a significant positive error (consistent with the MAE, although no motion was perceived during the test period). Of the 6 subjects who showed a manual following response, the least significance was for subject SF (Wilcoxon signed ranks test, Z = 2.5, P < 0.05). These results indicate that prior exposure to motion influences goal-directed reaching. Error bars, 95% confidence intervals.
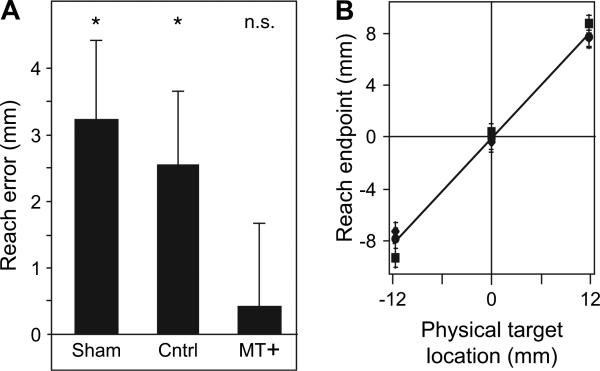
Our goal in the experiment was to measure whether the deviation in reaching movements (the manual following response) is due to processing in MT+. Figure 3A shows that in the sham and control conditions, when TMS was applied to the vertex or away from the subject's head, there was a significant shift in the average end point of the reaching movements. In the sham condition, in which TMS was delivered away from the head, the average deviation was 3.3 mm (Wilcoxon signed ranks test, Z = 2.05, P < 0.05); in the control condition, in which TMS was delivered to the vertex, the reach end point deviation was 2.5 mm (Z = 1.9, P < 0.05). There was not a significant difference in the manual following response between the sham and control conditions (Z = 0.85, P > 0.05). When rTMS was delivered to MT+, the end point deviation in the reaching movement was reduced to 0.45 mm (a significant reduction, Z = 2.08, P < 0.05) and was not significantly different from zero (Z = 0.17, P > 0.05). All 6 of the 7 subjects who showed a manual following response in Figure 2 showed a reduction in the error with TMS stimulation of MT+. Figure 3B confirms that the end points of each subject's reaching movements correlated with the 3 physical target locations; this indicates that subjects did not reach to random locations but were able to discriminate the 3 target positions (the least significant effect of target location was for subject SE, F2,357 = 644.9, P < 0.05).
Figure 3.
The effect of MT+ disruption on the manual following response. (A) In sham and control conditions, rTMS was delivered away from, or to the vertex of, each subject's head. There remained a significant manual following response in both conditions (Z = 2.05 and 1.9, respectively, P < 0.05, indicated by the asterisks). In the experimental condition, rTMS was directed to each subject's motion-sensitive cortical region MT+/V5 during the test period (but not during motion adaptation). In this condition, the manual following response was not significantly different from zero (Z = 0.17, P > 0.05). The results indicate that disruption of MT+ eliminates the manual following response and suggest that MT+ processes motion information for reaching movements to static objects. (B) Results for 3 representative subjects showing that reaching movements to the 3 targets were differentiable (subjects did not point to random locations). All 7 subjects showed a significant difference in end point reach position as a function of the physical target location (the least significant effect of physical target position was for subject SB, F2,357 = 644.9, P < 0.05). Error bars ± standard error of mean.
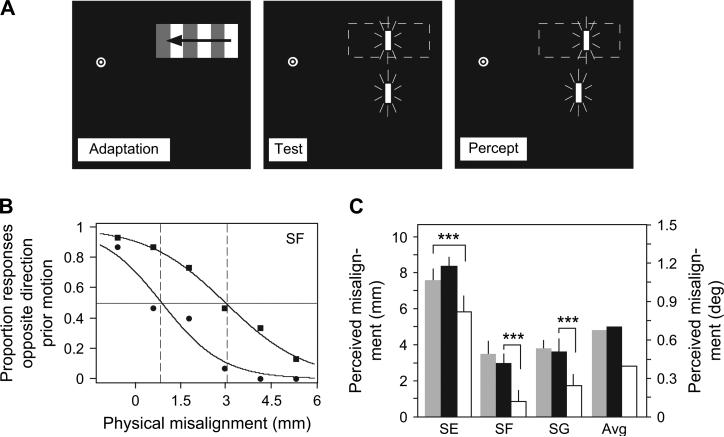
Figure 4 shows the results of the second experiment, which measured the perceived shift in the positions of the targets due to motion adaptation with and without stimulation of MT+ with rTMS. Without MT+ stimulation, subjects perceived a strong shift in the position of the target flashed within the motion-adapted region (weakest perceived shift was for subject SF, , P < 0.01). This is consistent with the results of several authors who have found that motion adaptation influences subsequent position judgments (Snowden 1998; Nishida and Johnston 1999; Whitaker et al. 1999; McGraw et al. 2002; Whitney and Cavanagh 2003). When MT+ was stimulated, however, the perceived shift was significantly reduced (the least significant reduction in the perceived shift was for subject SE; , P < 0.01). This confirms previous reports that TMS stimulation of MT+ reduces motion-induced position displacements (McGraw et al. 2004) and demonstrates that MT+ is involved in the assignment of a moving object's location (or a static object's location following previously exposed visual motion), perhaps via reentrant or feedback connections (Shipp and Zeki 1989; Pascual-Leone and Walsh 2001).
Figure 4.
Stimuli and results of the second experiment. (A) The stimulus in the second experiment was identical to that in the first experiment, with the exception that an additional reference target was flashed below the motion-adapted region. In the test period, subjects made a vernier alignment judgment. Following motion adaptation, subjects perceived physically aligned targets to appear misaligned, consistent with previous reports (Whitney and Cavanagh 2003). (B) Psychometric functions for subject SF revealing the perceived misalignment between the flashed test stimuli with sham TMS (squares) and with TMS of MT+ (circles). The ordinate shows the proportion of flashed targets in the motion-adapted region that appeared shifted opposite the direction of previously viewed motion. The abscissa shows the relative misalignment between the 2 flashed targets; positive values indicate that the target in the motion-adapted region was physically shifted in the direction of prior motion. The PSE on the psychometric function (PSE, indicated by 50% point on the psychometric function) is the physical misalignment between the flashes that created an apparent alignment between them (i.e., the point at which the illusion in A was nulled). For the psychometric functions in (B), the perceived misalignment in the sham condition was 3 mm and the perceived misalignment with stimulation of MT+ was 0.9 mm, a significant reduction (maximum likelihood ratio test, , P < 0.001). (C) rTMS of MT+ significantly reduced the perceived misalignment between the flashed targets for all 3 subjects. The white bars indicate TMS of MT+; the black and gray bars indicate sham and control TMS, respectively. The least significant reduction occurred in the control versus MT+ stimulation conditions for subject SE (, P < 0.01). Within each subject, all pairwise comparisons (MT+ vs. control or sham stimulation) were significant at the 0.01 level (X2 test, Bonferroni corrected for multiple comparisons), indicated by the asterisks. Error bars ± standard error of the logistic curve fit.
Discussion
The results of the first experiment demonstrated that visual motion processed in MT+ influences goal-directed reaching. Several previous reports that background visual motion influences reaching (Mohrmann-Lendla and Fleischer 1991; Brenner and Smeets 1997; Whitney et al. 2003; Saijo et al. 2005; Whitney and Goodale 2005) are therefore likely to be mediated by a necessary contribution of MT+. This is consistent with the finding that lesions to area MT+ in the human or monkey not only impair motion perception (Zihl et al. 1983; Newsome et al. 1985; Newsome and Pare 1988; Zeki 1991; Stoner and Albright 1992; Marcar et al. 1997; Vaina 1998) but also limit these subjects’ abilities to interact with moving objects (Schenk et al. 2000). Similarly, stimulating MT+ with TMS impairs reaching to moving objects (Schenk et al. 2005). The results of the present experiment show that MT+ is not only crucial for acting on moving objects but also plays an important role in reaching to stationary objects following exposure to background motion.
The fact that prior exposure to motion (i.e., motion adaptation) produced a manual following response, or deviation in the trajectory of the hand, suggests that the output of motion opponent mechanisms may be used by the visuomotor system. The same mechanisms of motion opponency that allow the perceptual system to maintain equilibrium or calibrate (e.g., as revealed by motion adaptation and aftereffects) may afford the visuomotor system a similar benefit. For example, by adapting to the state of motion in the environment, the visuomotor system—like the perceptual system—may enhance its sensitivity to changes in visual motion; this, in turn, may provide a better or more precise measure of ego motion. This sort of similar opponent motion processing for perception and visuomotor behavior could help bridge studies that have found an influence of visual (and illusory) motion on reaching (Mohrmann-Lendla and Fleischer 1991; Brenner and Smeets 1997; Brouwer et al. 2002; Whitney et al. 2003; Saijo et al. 2005; Whitney and Goodale 2005; Gomi et al. 2006) with studies that suggest that MT+ is the neural locus of the MAE (Tootell et al. 1995; He et al. 1998; Culham et al. 1999; Theoret et al. 2002).
Previous research on the manual following response has used continuously visible motion during the reaching movement (Mohrmann-Lendla and Fleischer 1991; Brenner and Smeets 1997; Whitney et al. 2003; Saijo et al. 2005; Gomi et al. 2006). Some studies have varied motion onset (Brenner and Smeets 1997; Saijo et al. 2005; Gomi et al. 2006), duration (Whitney et al. 2003; Saijo et al. 2005), or the timing of motion reversals (Whitney et al. 2003). In the present study, however, we wanted to avoid having a strongly visible motion signal during the reaching phase of the trial and we wanted to avoid having any other visible stimulus when the target was presented. The result was that subjects perceived visual motion neither during the target presentation (Whitney and Cavanagh 2003) nor during the reaching phase of the trial. We can therefore safely conclude that it was the internal state of motion adaptation that caused the manual following response and that MT+ mediated this response.
It has been suggested that background visual (i.e., retinal) motion is used by the visuomotor system as a source of feedback for gauging and compensating for body movements (Whitney et al. 2003). Several facts lend credence to this hypothesis, including the fact that visual motion is rapidly processed (both neural and psychophysically measured latencies are extremely brief; Tynan and Sekuler 1982; Allik and Dzhafarov 1984; Schmolesky et al. 1998; Jancke et al. 2004) and visual motion influences posture (Lee and Aronson 1974; Lee 1980), heading and locomotion (Britten and van Wezel 1998; Warren et al. 2001; Srinivasan and Zhang 2004), and eye movements (Yee et al. 1983; Collewijn and Tamminga 1984; Keller and Khan 1986; Miles et al. 1986; Howard and Marton 1992; Masson et al. 1995; Mohrmann and Thier 1995; Niemann and Hoffmann 1997; Schwarz and Ilg 1999). The results here suggest that if visual motion serves a functional role—helping to compensate for body movements during reaching (Whitney et al. 2003)—then MT+ plays an integral part in this process. Further, the results here raise the intriguing possibility that without area MT+, we might not only be impaired at intercepting moving objects (Schenk et al. 2005), but also have deficits interacting with static objects as we move around the world.
Acknowledgments
Notes Thanks to Dr Daniel Wolpert for providing testing space and the use of an Optotrak, to Dr Rob McIntosh for providing analysis software, and to Dr Juha Silvanto for help collecting pilot data. Thanks also to Dr Hiroaki Gomi, Dr Alan Johnston, and Dr Ikuya Murakami for helpful discussions. This work was supported by grants from the National Institutes of Health (DW), the Leverhulme Trust (DM), and Canadian Institutes of Health Research (MG).
Funding to pay the Open Access publication charges for this article was provided by the University of California, Davis.
Footnotes
Conflict of Interest: None declared.
References
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- Allik J, Dzhafarov EN. Reaction time to motion onset: local dispersion model analysis. Vision Res. 1984;24:99–101. doi: 10.1016/0042-6989(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Battelli L, Black KR, Wray SH. Transcranial magnetic stimulation of visual area V5 in migraine. Neurology. 2002;58:1066–1069. doi: 10.1212/wnl.58.7.1066. [DOI] [PubMed] [Google Scholar]
- Brenner E, Smeets JB. Fast responses of the human hand to changes in target position. J Mot Behav. 1997;29:297–310. doi: 10.1080/00222899709600017. [DOI] [PubMed] [Google Scholar]
- Britten KH, van Wezel RJ. Electrical microstimulation of cortical area MST biases heading perception in monkeys. Nat Neurosci. 1998;1:59–63. doi: 10.1038/259. [DOI] [PubMed] [Google Scholar]
- Brouwer AM, Brenner E, Smeets JB. Hitting moving objects: is target speed used in guiding the hand? Exp Brain Res. 2002;143:198–211. doi: 10.1007/s00221-001-0980-x. [DOI] [PubMed] [Google Scholar]
- Collewijn H, Tamminga EP. Human smooth and saccadic eye movements during voluntary pursuit of different target motions on different backgrounds. J Physiol. 1984;351:217–250. doi: 10.1113/jphysiol.1984.sp015242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham J, He S, Dukelow S, Verstraten FA. Visual motion and the human brain: what has neuroimaging told us? Acta Psychol. 2001;107:69–94. doi: 10.1016/s0001-6918(01)00022-1. [DOI] [PubMed] [Google Scholar]
- Culham JC, Dukelow SP, Vilis T, Hassard FA, Gati JS, Menon RS, Goodale MA. Recovery of fMRI activation in motion area MT following storage of the motion aftereffect. J Neurophysiol. 1999;81:388–393. doi: 10.1152/jn.1999.81.1.388. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Guy CN, Zeki S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain. 1995;118:1375–1394. doi: 10.1093/brain/118.6.1375. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. University Press; Cambridge (UK): 1971. [Google Scholar]
- Gomi H, Abekawa N, Nishida S. Spatiotemporal tuning of rapid interactions between visual-motion analysis and reaching movement. J Neurosci. 2006;26:5301–5308. doi: 10.1523/JNEUROSCI.0340-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Cohen ER, Hu X. Close correlation between activity in brain area MT/V5 and the perception of a visual motion aftereffect. Curr Biol. 1998;8:1215–1218. doi: 10.1016/s0960-9822(07)00512-x. [DOI] [PubMed] [Google Scholar]
- Howard IP, Marton C. Visual pursuit over textured backgrounds in different depth planes. Exp Brain Res. 1992;90:625–629. doi: 10.1007/BF00230947. [DOI] [PubMed] [Google Scholar]
- Huk AC, Heeger DJ. Pattern-motion responses in human visual cortex. Nat Neurosci. 2002;5:72–75. doi: 10.1038/nn774. [DOI] [PubMed] [Google Scholar]
- Jancke D, Erlhagen W, Schoner G, Dinse HR. Shorter latencies for motion trajectories than for flashes in population responses of cat primary visual cortex. J Physiol. 2004;556:971–982. doi: 10.1113/jphysiol.2003.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Khan NS. Smooth-pursuit initiation in the presence of a textured background in monkey. Vision Res. 1986;26:943–955. doi: 10.1016/0042-6989(86)90152-5. [DOI] [PubMed] [Google Scholar]
- Lee DN. The optic flow field: the foundation of vision. Philos Trans R Soc Lond B Biol Sci. 1980;290:169–179. doi: 10.1098/rstb.1980.0089. [DOI] [PubMed] [Google Scholar]
- Lee DN, Aronson E. Visual proprioceptive control of standing in human infants. Percept Psychophys. 1974;15:529–532. [Google Scholar]
- Marcar VL, Zihl J, Cowey A. Comparing the visual deficits of a motion blind patient with the visual deficits of monkeys with area MT removed. Neuropsychologia. 1997;35:1459–1465. doi: 10.1016/s0028-3932(97)00057-2. [DOI] [PubMed] [Google Scholar]
- Masson GS, Proteau L, Mestre DR. Effects of stationary and moving textured backgrounds on the visuo-oculo-manual tracking in humans. Vision Res. 1995;35:837–852. doi: 10.1016/0042-6989(94)00185-o. [DOI] [PubMed] [Google Scholar]
- McGraw PV, Walsh V, Barrett BT. Motion-sensitive neurones in V5/MT modulate perceived spatial position. Curr Biol. 2004;14:1090–1093. doi: 10.1016/j.cub.2004.06.028. [DOI] [PubMed] [Google Scholar]
- McGraw PV, Whitaker D, Skillen J, Chung ST. Motion adaptation distorts perceived visual position. Curr Biol. 2002;12:2042–2047. doi: 10.1016/s0960-9822(02)01354-4. [DOI] [PubMed] [Google Scholar]
- McKee SP, Klein SA, Teller DY. Statistical properties of forced-choice psychometric functions: implications of probit analysis. Percept Psychophys. 1985;37:286–298. doi: 10.3758/bf03211350. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J Neurophysiol. 1986;56:1321–1354. doi: 10.1152/jn.1986.56.5.1321. [DOI] [PubMed] [Google Scholar]
- Mohrmann H, Thier P. The influence of structured visual backgrounds on smooth-pursuit initiation, steady-state pursuit and smooth-pursuit termination. Biol Cybern. 1995;73:83–93. doi: 10.1007/BF00199058. [DOI] [PubMed] [Google Scholar]
- Mohrmann-Lendla H, Fleischer AG. The effect of a moving background on aimed hand movements. Ergonomics. 1991;34:353–364. doi: 10.1080/00140139108967319. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Mikami A, Wurtz RH. Motion selectivity in macaque visual cortex. III. Psychophysics and physiology of apparent motion. J Neurophysiol. 1986;55:1340–1351. doi: 10.1152/jn.1986.55.6.1340. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann T, Hoffmann KP. The influence of stationary and moving textured backgrounds on smooth-pursuit initiation and steady state pursuit in humans. Exp Brain Res. 1997;115:531–540. doi: 10.1007/pl00005723. [DOI] [PubMed] [Google Scholar]
- Nishida S, Johnston A. Influence of motion signals on the perceived position of spatial pattern. Nature. 1999;397:610–612. doi: 10.1038/17600. [DOI] [PubMed] [Google Scholar]
- Orban GA. Visual processing in macaque area MT/V5 and its satellites (MSTd and MSTv). In: Rockland KS, Kaas JH, Peters A, Gross CG, Schiller PH, Rose MG, Bullier J, Roe AW, editors. Cerebral cortex: extrastriate cortex in primates. Plenum Publishing; Boston: 1998. pp. 359–434. [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci. 2004;16:1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Royden CS, Banks MS, Crowell JA. The perception of heading during eye movements. Nature. 1992;360:583–585. doi: 10.1038/360583a0. [DOI] [PubMed] [Google Scholar]
- Saijo N, Murakami I, Nishida S, Gomi H. Large-field visual motion directly induces an involuntary rapid manual following response. J Neurosci. 2005;25:4941–4951. doi: 10.1523/JNEUROSCI.4143-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk T, Ellison A, Rice N, Milner AD. The role of V5/MT+ in the control of catching movements: an rTMS study. Neuropsychologia. 2005;43:189–198. doi: 10.1016/j.neuropsychologia.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Schenk T, Mai N, Ditterich J, Zihl J. Can a motion-blind patient reach for moving objects? Eur J Neurosci. 2000;12:3351–3360. doi: 10.1046/j.1460-9568.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schwarz U, Ilg UJ. Asymmetry in visual motion processing. Neuroreport. 1999;10:2477–2480. doi: 10.1097/00001756-199908200-00008. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. The organization of connections between areas V5 and V1 in macaque monkey visual cortex. Eur J Neurosci. 1989;1:309–332. doi: 10.1111/j.1460-9568.1989.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cereb Cortex. 2005;9:9. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Snowden RJ. Shifts in perceived position following adaptation to visual motion. Curr Biol. 1998;8:1343–1345. doi: 10.1016/s0960-9822(07)00567-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan MV, Zhang S. Visual motor computations in insects. Annu Rev Neurosci. 2004;27:679–696. doi: 10.1146/annurev.neuro.27.070203.144343. [DOI] [PubMed] [Google Scholar]
- Stewart L, Battelli L, Walsh V, Cowey A. Motion perception and perceptual learning studied by magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1999;51(Suppl):334–350. [PubMed] [Google Scholar]
- Stoner GR, Albright TD. Neural correlates of perceptual motion coherence. Nature. 1992;358:412–414. doi: 10.1038/358412a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Saito H. Analysis of motion of the visual field by direction, expansion/contraction, and rotation cells clustered in the dorsal part of the medial superior temporal area of the macaque monkey. J Neurophysiol. 1989;62:626–641. doi: 10.1152/jn.1989.62.3.626. [DOI] [PubMed] [Google Scholar]
- Theoret H, Kobayashi M, Ganis G, Di Capua P, Pascual-Leone A. Repetitive transcranial magnetic stimulation of human area MT/V5 disrupts perception and storage of the motion aftereffect. Neuropsychologia. 2002;40:2280–2287. doi: 10.1016/s0028-3932(02)00112-4. [DOI] [PubMed] [Google Scholar]
- Tootell R, Reppas J, Dale A, Look R, Sereno M, Malach R, Brady T, Rosen B. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375:139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- Tynan PD, Sekuler R. Motion processing in peripheral vision: reaction time and perceived velocity. Vision Res. 1982;22:61–68. doi: 10.1016/0042-6989(82)90167-5. [DOI] [PubMed] [Google Scholar]
- Vaina LM. Complex motion perception and its deficits. Curr Opin Neurobiol. 1998;8:494–502. doi: 10.1016/s0959-4388(98)80037-8. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A. Task-specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc Biol Sci. 1998;265:537–543. doi: 10.1098/rspb.1998.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WH, Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4:213–216. doi: 10.1038/84054. [DOI] [PubMed] [Google Scholar]
- Warren WH, Jr, Morris MW, Kalish M. Perception of translational heading from optical flow. J Exp Psychol Hum Percept Perform. 1988;14:646–660. doi: 10.1037//0096-1523.14.4.646. [DOI] [PubMed] [Google Scholar]
- Whitaker D, McGraw PV, Pearson S. Non-veridical size perception of expanding and contracting objects. Vision Res. 1999;39:2999–3009. doi: 10.1016/s0042-6989(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Whitney D, Cavanagh P. Motion adaptation shifts apparent position without the motion aftereffect. Percept Psychophys. 2003;65:1011–1018. doi: 10.3758/bf03194830. [DOI] [PubMed] [Google Scholar]
- Whitney D, Goodale MA. Visual motion due to eye movements helps guide the hand. Exp Brain Res. 2005;162:394–400. doi: 10.1007/s00221-004-2154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D, Murakami I, Gomi H. Forthcoming. The utility of visual motion for goal directed reaching. In: Nijhawan R, editor. Problems of Space and Time in Perception and Action. Cambridge University Press; Cambridge (UK): [Google Scholar]
- Whitney D, Westwood DA, Goodale MA. The influence of visual motion on fast reaching movements to a stationary object. Nature. 2003;423:869–873. doi: 10.1038/nature01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Anderson SJ, Ashida H. Evidence for dissociation between the perceptual and visuomotor systems in humans. Proc R Soc Lond B Biol Sci. 2001;268:973–977. doi: 10.1098/rspb.2001.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee RD, Daniels SA, Jones OW, Baloh RW, Honrubia V. Effects of an optokinetic background on pursuit eye movements. Invest Ophthalmol Vis Sci. 1983;24:1115–1122. [PubMed] [Google Scholar]
- Zeki S. Cerebral akinetopsia (visual motion blindness). Brain. 1991;114(Pt 2):811–824. doi: 10.1093/brain/114.2.811. A review. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J, von Cramon D, Mai N. Selective disturbance of movement vision after bilateral brain damage. Brain. 1983;106:313–340. doi: 10.1093/brain/106.2.313. [DOI] [PubMed] [Google Scholar]