Abstract
Prostacyclin is an endogenous lipid metabolite with properties of vasodilation and anti-platelet aggregation. While the effects of prostacyclin on the vascular protection have been well-documented, the role of this eicosanoid in the central nervous system has not been extensively studied. Recently, a transgenic mouse containing a hybrid enzyme, of cyclooxygenase-1 linked to prostacyclin synthase, was developed that produces elevated levels of prostacyclin in vivo. The goal of this study was to investigate whether increased prostacyclin biosynthesis could affect behavioral phenotypes in mice. Our results uncovered that elevated levels of prostacyclin broadly affect both cognitive and non-cognitive behaviors, including decreased anxiety-like behavior and improved learning in the fear-conditioning memory test. This study demonstrates that prostacyclin plays an important, but previously unrecognized, role in central nervous system function and behavior.
Keywords: Prostacyclin, eicosanoid, behavior, memory, anxiety
1. Introduction
Prostacyclin (PGI2) is synthesized in vascular endothelial cells when arachidonic acid (AA) is converted to PGI2 through a three-step catalytic reaction by two enzymes, cyclooxygenases-1 or 2 (COX-1, COX-2) and PGI2 synthase (PGIS). Prostacyclin mediates biological actions through its G-protein-coupled receptor (IP receptor), where it is known to cause vasodilation and anti-platelet aggregation as one of the major vasoprotectors [1, 2] and the endocrine systems [3, 4], and is used as a vascular protective agent and treatment option in pulmonary hypertension [5, 6]. Although PGI2 is known to be generated in cerebral arteries [7, 8], the consequences of increased PGI2 secretion within central nervous system (CNS) have not been extensively characterized.
Early studies on PGI2 indicated a reduction in cerebral blood flow after PGI2 intravenous infusion in normotensive humans and rats [9–12], implicating a possible role in the maintenance of cerebrovascular tone. This is probably of importance since in cases of brain injury, PGI2 may potentially be therapeutic. For example, 48 hours following fluid percussion brain injury in rats, PGI2 treatment reversed blood flow reductions in the injured cortex of rats [13], indicating an improvement in cortical perfusion. Additionally, administration of PGI2 agonists TTC-909 [14] and ONO-1301 [15] both reduce infarct volume after middle cerebral artery occlusion (MCAO) in rats, with ONO-1301 reducing neurological deficits. TTC-909 was also shown to reduce memory impairments in rats with cerebral embolism [16], supporting the notion that PGI2 may play a role in cognitive functions.
Interestingly, there is evidence for a possible prostacyclin receptor isoform within the CNS, IP2, which is pharmacologically distinguished from the peripheral receptor, IP1 [17, 18]. IP2 receptor expression has been identified in the cerebral cortex, striatum, thalamus, and hippocampus of the rat brain [18]. Additionally, activation of the IP2 receptor by the novel ligand, 15R-TIC, was found to be neuroprotective after MCAO in monkeys. 15R-TIC significantly reduced infarct volume and increased the cerebral metabolic rate of oxygen, which was shown to not be as a result of increased cerebral blood flow to the damaged regions [19].
The discovery of IP2 receptor expression in the brain supports the possibility that PGI2 directly acts in the CNS and could potentially contribute to cognitive functioning. CP-Tg mice overexpress the TriCat enzyme [20, 21], a hybridized enzyme linking COX-1 and PGIS via a transmembrane domain. Overexpression of this hybrid results in a three-fold increase in anabolism of PGI2 in cell lines and shows an increased ability to efficiently convert AA into PGI2 [22]. CP-TG mice expressing this enzyme express show resistance to induced thrombotic stroke and cardiac arrest [21]. Our aim in this study was to investigate whether PGI2 could beneficially affect cognitive-like behaviors in PGIS-overexpressing mice.
2. Materials and methods
2.1. Animals
Heterozygous CP-Tg, containing a hybrid enzyme of COX-1 linked to PGIS (COX-1-10aa-PGIS; N = 20), and non-transgenic (NTg; N = 9), mice used in experiments were maintained in the Animal Care Facility at the University of Houston. They were kept in groups of one to five mice per cage in a room with a 12 hour light cycle (lights on at 6:00AM and off at 6:00PM) at 23C with food and water available ad libitum. Male mice and female mice were randomized across groups. 3–6 months were used for all behavioral assays. All experiments were conducted in accordance with the University of Houston IACUC guidelines using approved protocols. CP-Tg mice were genotyped using PGIS-specific primers (BD2442-1:GGATTCCAAGTCTCCACCC; BD2442-2: GCAGTCACACTGGTAGCGG) [21].
2.2. PGI2 Activity Assay
PGI2 activity in CNS tissue was quantified as previously reported [23]. To determine the activities of the enzyme that converted arachidonic acid (AAins were homogenized with cold PBS (50mg / 100 µl). 0.1 µl of [14C] AA (1 µM) was added to the homogenized samples in a total reaction volume of 0.1 mL. After a 5 minute incubation, the reaction was terminated by adding 200 µl of solvent containing 0.1% acetic acid in 35% acetonitrile and 65% deionized H2O (solvent A). After centrifugation at 13,000 rpm for 10 minutes, the supernatant was injected into a reverse phase C18 column (Varian Microsorb-MV 100-5, 4.5×250 mm) using the solvent A with a gradient from 35% to 100% of acetonitrile for 40 min at a flow-rate of 1.0 mL/min. The [14C]-labeled AA metabolites, including [14C]-6-keto-PGF1a (degraded PGI2) were monitored directly by a flow scintillation analyzer (Packard 150TR). A positive control for PGI2 production was performed using cell lysates containing 100,000 HEK293 cells transiently transfected with COX-1-10aa-PGIS cDNA; production of PGI2 and metabolites were analyzed by HPLC, as previously reported [22].
2.3. Open Field test
Anxiety-like and non-cognitive behavior was assessed by the open field test at three and six month periods for all mice. Each mouse was allowed to explore a novel environment in a clear chamber for 30 min while being monitored (OptoMax, Columbus Instruments) with standard room lighting conditions [24].
2.4. Rotarod test
Motor learning and coordination were evaluated by an accelerating cylindrical drum Rotamex Rotarod machine (Columbus Instruments; Columbus, OH) at three and six months of age. Each mouse was placed on a horizontal accelerating rod (4–40 rpm) and subjected to 4 trials a day for two days with 15 min. intervals between each trial. A trial ended when the mouse fell off the rod, time elapsed 200 seconds, or became inverted twice in the same trial without falling.
2.5. Light dark (LD) exploration
Mice were subjected to light-dark exploration test to evaluate anxiety-like behavior at three and six months of age. The light-dark box consisted of a light compartment (27 cm × 27 cm × 27 cm) and a dark compartment (27 cm × 18 cm × 27 cm) separated by a partition with a single opening (7 cm × 7 cm) to allow passage between compartments as previously described [25, 26]. Each trail lasted 5 min and time was measured manually as previously described [24].
2.6. Elevated-plus maze
Anxiety-like behavior was measured using the Elevated plus maze at three and six months of age. The test consists of an X-shaped apparatus with two arms closed off from observation on the sides and end by large blinders, and two arms open to light with a central area between the arms not enclosed. Standard lighting was used during testing. The mice were placed in the center area facing an open arm and were recorded by a video camera for 5 minutes. Time and number of transitions were documented manually.
2.7. Fear conditioning
Contextualized and Cued fear conditioning to test for short and long-term memory and learning was conducted at six months as previously described [27, 28].
2.8. Statistical analyses
Quantitative results were expressed as mean ± standard error of mean (SEM). For contextualized and cued fear conditioning trials was analyzed using repeated measures ANOVA (p≤0.05 was considered statistically significant). All other data was analyzed using one-way ANOVA and Fisher LSD post hoc test to determine significant differences between groups (p≤0.05 was considered statistically significant).
Results
3.1. Analysis of PGI2 Production in Brain
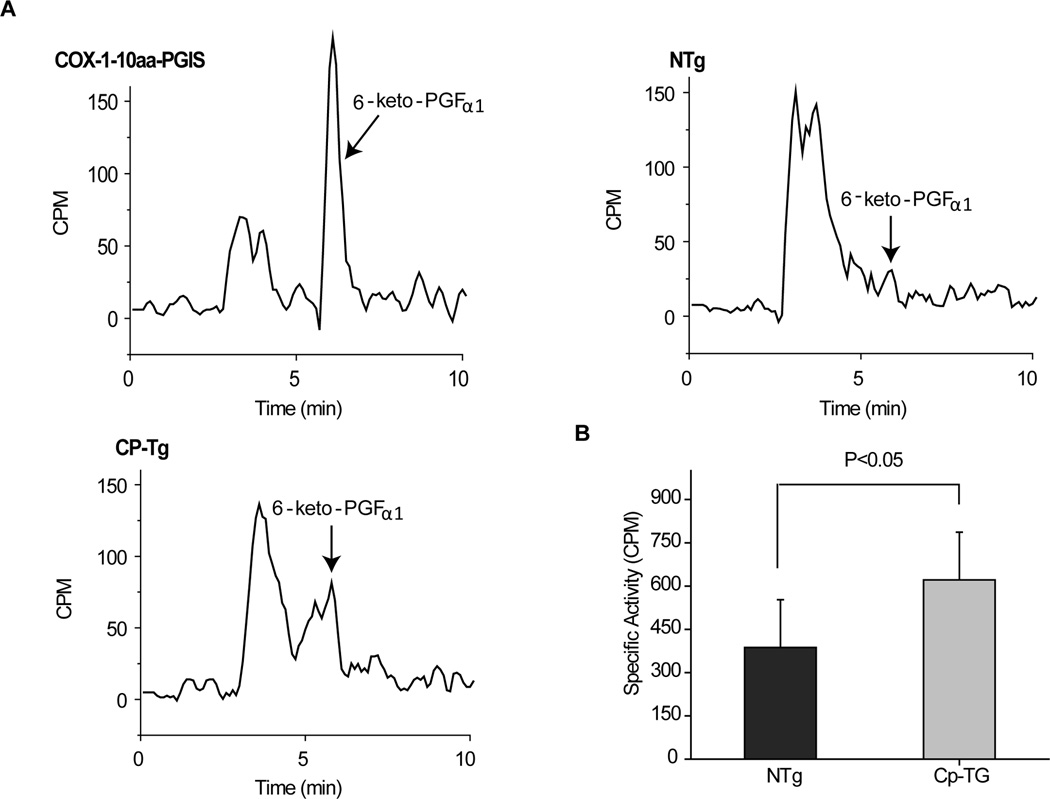
A functional activity assay was used to demonstrate enhanced PGI2 production in the CP-Tg mouse line. As shown in Figure 1A, incubation of arachidonic acid resulted in a peak of 6-Keto-PGF1α at ~6 minutes in HPLC in CP-Tg mice, and a notable enhancement in 6-Keto-PGF1α production compared to the NTg control. Brain tissue homogenates from Ntg and CP-Tg mouse cortex showed a mean 60.4% increase in PGI2 production in the CP-Tg line, compared to control (n=3, p<0.05).
Figure 1. Measurement of PGI2 production in brain.
(A) Examples showing 6-Keto-PGF1α detected using HPLC, using a positive control (COX-1-10aa-PGIS positive cell lysate), non-transgenic mouse, and TG-Cp mouse brain tissue. (B) This figure demonstrates that CP-Tg mouse tissue exhibits enhanced conversion of arachidonic acid to PGI2 (n=3, for each group, p<0.05), compared with control.
3.2. Effect of PGIS overexpression on non-cognitive behavior
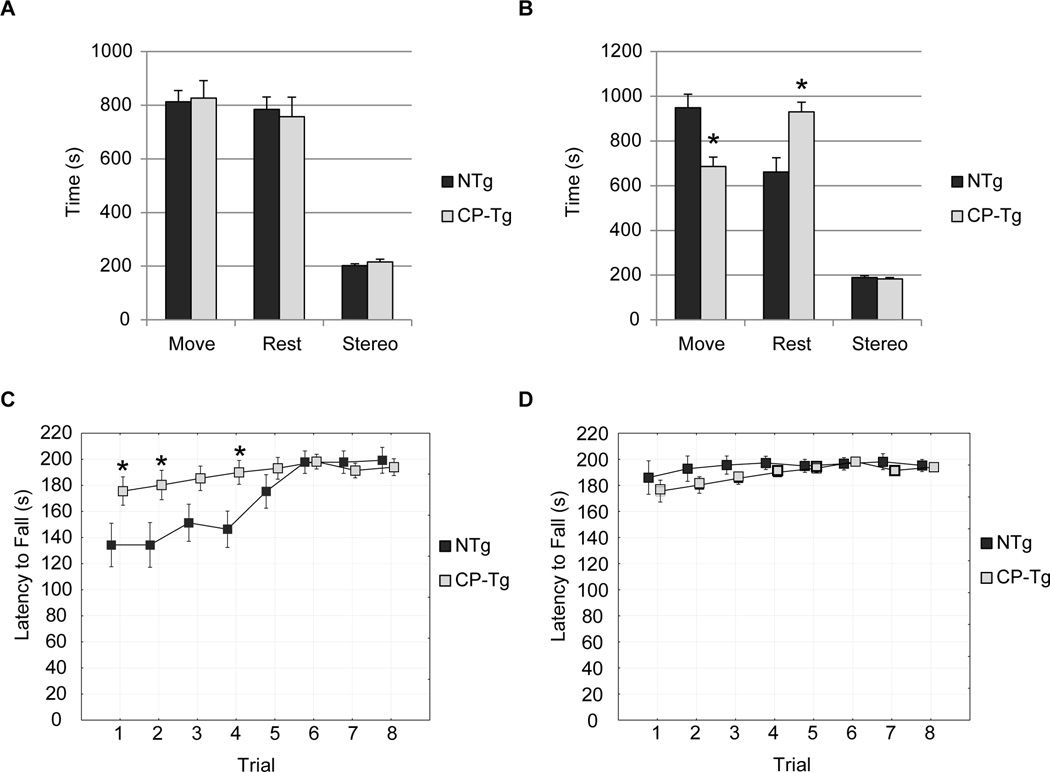
Motor functions were assessed by the open-field activity and a motorized rotarod. PGIS overexpression had no effect on spontaneous activity at 3 months (Fig. 2A), but at 6 months displayed hypo-activity (Fig. 2B, p < 0.05). At 3 months of age PGIS mice had an increased latency to fall in trials 1, 2, and 3 indicating increased coordination and balance (Fig. 2C, p < 0.05). At six months of age PGIS mice had normal motor coordination, balance and learning (Fig. 2D).
Figure 2. PGIS overexpression had significant effect on non-cognitive behavior.
(A) Exploratory behavior measured by the open field activity in 3 month old mice shows no significant differences. (B) At 6 months of age, exploratory behavior measured in CP-Tg mice is significantly decreased compared to NTg mice. (MOVE) = Total time moving, (REST) = time at rest, (STEREO) = time moving in stereotypic fashion. (C). In 3 month old mice, CP-Tg showed improved coordination on the rotarod, compared with NTg mice on trials 1, 2, and 4. (D) At 6 months of age, there was no significant difference between groups. * p < 0.05.
3.3. Analysis of anxiety-like behavior in open field assay
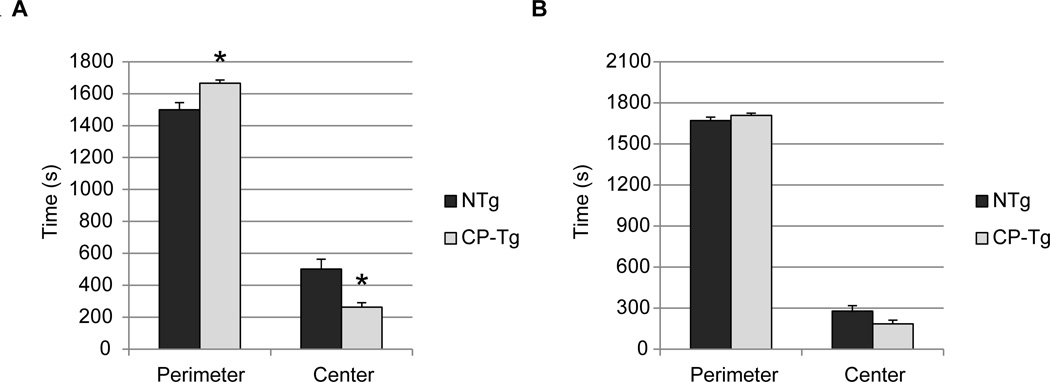
To assess anxiety-like behavior in mice time spent in the center and the perimeter was measured. At 3 months of age, CP-Tg mice showed a significant increase in time spent in the perimeter with a concomitant decrease in in time spent in the center)\, an indication of anxiety-like behavior in rodents (Fig. 3A, p < 0.05). There were no differences observed at 6 months (Fig. 3B).
Figure 3. Effect of PGIS on anxiety-like behavior measured by the open field activity.
(A) Three month old CP-Tg mice exhibit greater time spent on the periphery of the open field, compared with NTg. (B) At 6 months of age, there is no significant difference between NTg and CP-Tg mice between the perimeter and thecenter of the open field). * p < 0.05.
3.4. Light Dark test
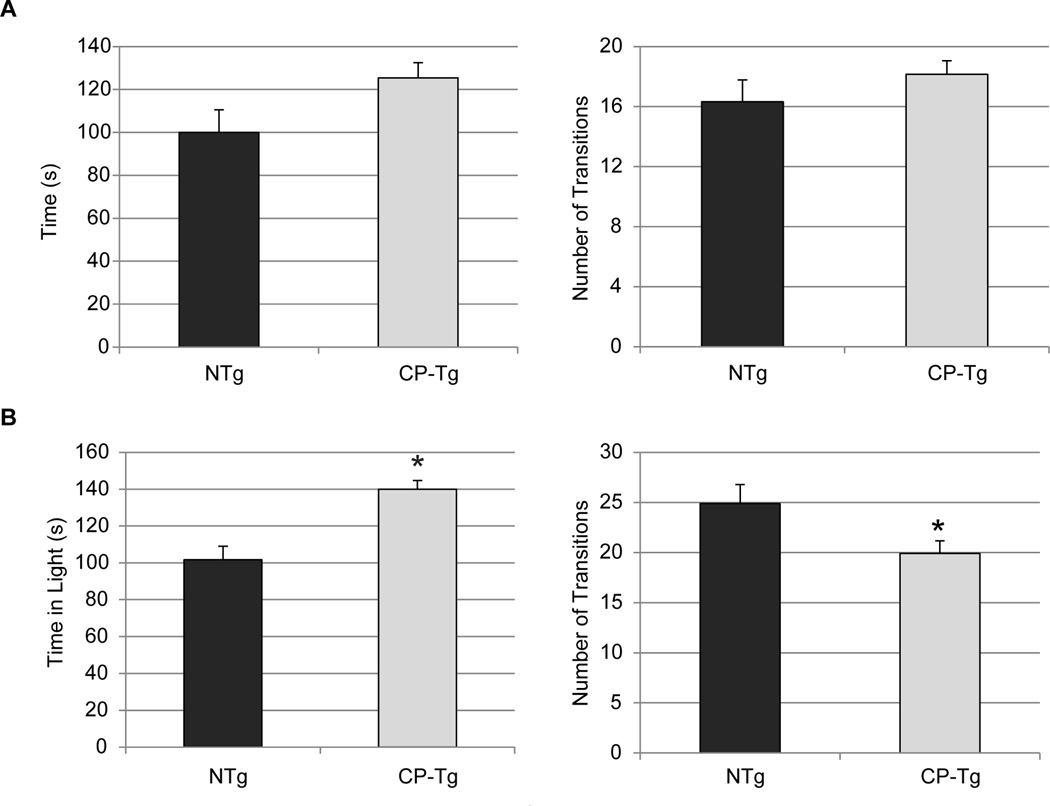
The light dark (LD) test was used to further assess anxiety-like behavior by observing the naturalistic conflict between the tendency to explore a novel environment rather than the aversive brightly lit open-field. At 3 months, there were no significant differences in either the time spent in the lit area or number of transitions (Fig. 4A and 4B). At six months of age PGIS mice significantly spent more time in the lit are and made fewer transitions (Fig. 4C and 4D, p < 0.05).
Figure 4. PGIS overexpression decreased anxiety-like behavior at six months in light dark exploration.
(A) At three months of age, there was no significant difference in time or number of transitions between the two groups. (B) At six months of age, CP-Tg mice spent a significantly greater time in light, and had a reduced number of transitions, compared to NTg mice. * p < 0.05.
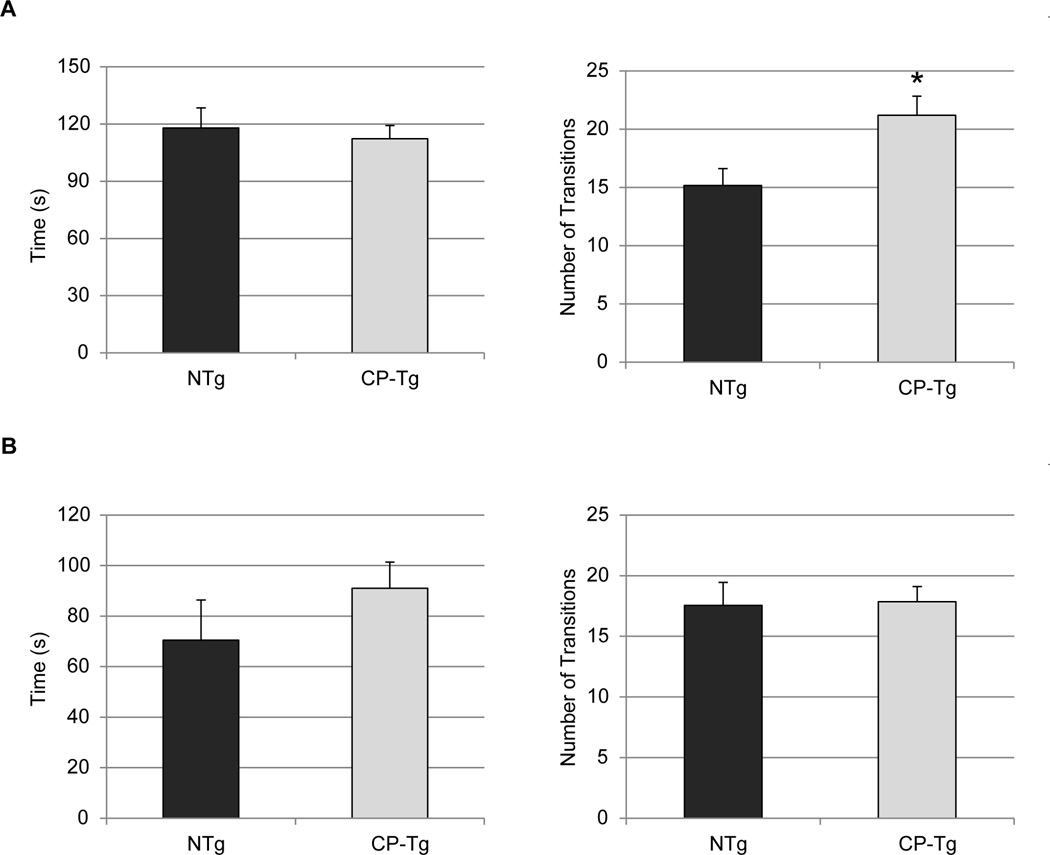
3.5. Elevated Plus Maze
To further assess anxiety-like behavior in the CP-Tg mice the elevated plus maze (EPM) was used in addition to the LD test. This test introduces height and openness in the arms which are not present in the LD. There were no significant differences in time spent in the open arms, but a significant increase in number of transitions at 3 months (Fig. 5A and 5B, p < 0.05). There were no significant differences in time spent in the open arms or number transitions at 6 months (Fig. 5C and 5D).
Figure 5. Anxiety-like behavior measured by elevated plus maze.
(A) Time spent in open arms is similar between CP-Tg and NTg lines in three month old mice, but CP-Tg mice show significantly more transitions than NTg mice. (B) In six month old mice, the time spent in open arms and the number of transitions is similar between the two groups. * p < 0.05.
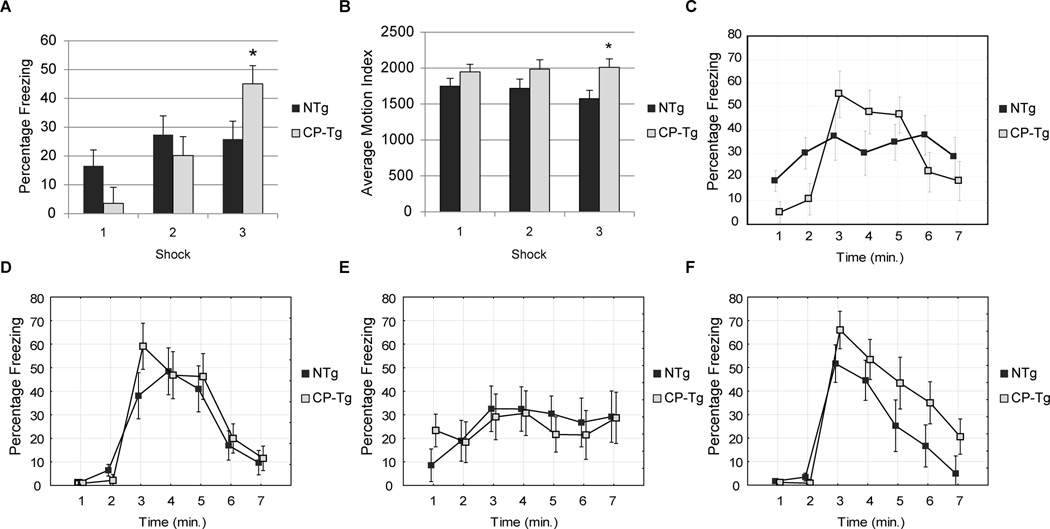
3.6. PGIS and Fear Conditioning
All mice responded to the electric shocks during the training phase. Compared to the NTg group, CP-Tg mice showed a higher percentage freezing during the third shock compared to NTg mice during the training trial (Fig. 6A, p < 0.05). There were no differences in total percent freezing time during the contextual fear conditioning trial (Fig. 6B). CP-Tg mice exhibited an abnormal freezing pattern, as indicated by within subject significance (Fig. 6B, p < 0.004), with elevated percentage freezing from 3–6 minutes, in contrast to NTg mice that showed a relatively sustained freezing pattern during the duration of the whole trial.
Figure 6. PGIS overexpression had a significant effect on associative learning.
(A) CP-Tg has a significant increase in percentage freezing time during the third shock of the training trial. (B) CP-Tg mice exhibited an increased average motion index compared with NTg mice. Mice were tested in both cue and context trials. (C) CP-Tg exhibited an enhanced response in the short-term memory component in the contextual fear conditioning (p < 0.05). (D) No significant differences were seen in long term memory contextual fear conditioning. (E) No significant differences were seen in short term cue fear conditioning. (F) No significant differences were observed in long term cue fear conditioning.
4. Discussion
This report is the first characterization of the effect of PGIS overexpression on cognitive and non-cognitive behaviors in mice. Prostacyclin synthase is abundantly expressed peripherally and throughout the CNS, within the cerebrovasculature, in neurons and in glia cells [29]. PGI2 has long been recognized as a vasoprotective agent and it has been well-established that PGI2 plays an important role in inflammation [1, 2], but the question of whether PGI2 might exert a significant influence on CNS function has not been addressed. Previous work has shown that loss of the peripheral IP1 prostacyclin receptor appears to reduce both inflammatory and pain responses [1, 30].
We found that the increased levels of PGI2 in CP-Tg mice did not result in any significant differences in body weight or alterations in general development. Additionally, increased PGI2 did not appear to be lethal, as all animals survived the duration of the study. In CP-Tg mice transgenic expression of the hybrid enzyme, COX-1-10aa-PGIS, occurs in all major organ systems, including the CNS [21]. Consequently, it is possible that the changes in cognitive and non-cognitive behaviors we observed in CP-Tg mice could result from increased PGI2 production from both within the CNS and peripherally.
Assessments of motor coordination indicated that 3 month PGIS mice were more coordinated than wild-type mice, while at 6 months there was no difference, an indication that PGIS overexpression did not negatively impact motor coordination. In terms of general exploratory activity, open field testing revealed that PGIS overexpression resulted in significantly decreased exploratory behavior at six months. No differences were observed at 3 months. While a reduction in these behaviors could be interpreted as impaired locomotor activity, our results from rotarod testing indicated otherwise. Additionally, increased amount of time spent at the perimeter versus the center of the open field at 3 months was observed, indicative of anxiety-like behavior [31]. However, at 6 months no effect on anxiety-like behavior in the open field was observed.
Since one behavioral assay may not completely capture anxiety-like behavior in rodents [32], we tested the effect of the PGIS genotype on two other widely-used anxiety assays: elevated-plus maze and the light/dark box. Interestingly, in the elevated-plus maze there were no significant differences between CP-Tg mice (3- and 6-month old) and the controls in time spent in the open arm, an indication that the PGI2 had no effects on anxiety-like behavior in this assay. However, in the light/dark box assay, PGIS expression resulted in a decrease in anxiety-associated behavior at 6 months, with more time spent in the light, but there was no significant effect at 3 months, raising the possibility that PGI2 could act to mediate anxiety. Collectively, the tests on anxiety show that the effects of elevated PGI2 are task-dependent, and that elevated endogenous PGI2 levels may be anxiogenic in young mice, while being anxiolytic in older mice.
Several areas of the cortex have been shown to be involved in anxiety including prelimbic and infralimbic cortices [33, 34], orbitofrontal cortex [35] and prefrontal cortex [36–38]. Additionally, the hippocampus [36, 39–41] and striatum [42–44] are thought to also regulate anxious behaviors in rodents. Since the PGI2 receptor, IP2, is expressed in the cerebral cortex, hippocampus, and striatum [18], this certainly raises the possibility that increased activation or desensitization of the IP2 receptor (due to elevated PGI2 levels) in the cortex, hippocampus and striatum could mediate anxious-like behavior in mice.
Our work also shows that elevated PGI2 levels impact associative learning. We introduced CP-Tg mice to cued and contextual fear conditioning to assess memory of a conditioned stimulus (cue) and spatial memory of the environment (context). Our results indicated that testing of contextual-associated memory after one hour of training was significantly increased in 6-month-old CP-Tg mice, while at 24 hours post-training the performance of CP-Tg mice was identical to their wild-type counterparts. Short-term memory was improved, however, long-term memory remained unchanged, PGI2 is likely to play a role in short-term memory. Since IP2 receptor expression has been found in the hippocampus [18], and contextual fear conditioning is hippocampal-dependent [45–48], it is conceivable that PGI2 could be involved in hippocampal-dependent memory acquisition and/or consolidation. These observations complement the recent findings by Muramatsu et al [49] who reported that prostacyclin elevates cAMP levels through activation of the IP receptor on cultured neurons in vitro, promoting axonal remodeling.
To our knowledge, this is the first evidence of the involvement of PGI2 in anxiety and contextual fear conditioning. Considering the limited degree of understanding of the role of PGI2 and its associated receptor action within the CNS, the findings in this study warrant further attention. Future work will corroborate the behavioral findings presented here with histological and molecular confirmation of CNS IP2 receptor expression and PGI2 levels in PGIS mice, as well as understanding some of the potential underlying mechanisms of PGI2-induced anxiolytic behavior and facilitation of short-term memory. Elucidating the role of PGI2 in cognitive functioning will broaden our understating of its role in the CNS.
Highlights.
Prostacyclin-overexpressing mice display enhanced motor coordination at 3 months of age.
Prostacyclin-overexpressing mice exhibit alterations in anxiety-associated behavior
Six-month old transgenic-mice displayed improved short-term memory in the contextual fear-conditioning test.
Our observations suggest that prostacyclin expression can modulate anxiety and memory
Acknowledgements
This work was supported in part by the following grants: NIH 1R15AG039008 (JLE), RO1HL56712 (for KHR); RO1 HL079389 (KHR) and RC1HL100807 (RD and KHR) and American Heart Association grant 10GRNT4470042 (KHR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorris SL, Peebles RS., Jr PGI2 as a regulator of inflammatory diseases. Mediators of inflammation. 2012;2012:926968. doi: 10.1155/2012/926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stitham J, Midgett C, Martin KA, Hwa J. Prostacyclin: an inflammatory paradox. Frontiers in pharmacology. 2011;2:24. doi: 10.3389/fphar.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurgul-Convey E, Lenzen S. Protection against cytokine toxicity through endoplasmic reticulum and mitochondrial stress prevention by prostacyclin synthase overexpression in insulin-producing cells. J Biol Chem. 2010;285:11121–11128. doi: 10.1074/jbc.M109.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taggart MJ, Europe-Finner GN, Mitchell BF. Possible dual roles for prostacyclin in human pregnancy and labor. J Clin Invest. 2008;118:3829–3832. doi: 10.1172/JCI37785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DK, Higenbottam TW, Wallwork J. Treatment of primary pulmonary hypertension intravenous epoprostenol (prostacyclin) British heart journal. 1987;57:270–278. doi: 10.1136/hrt.57.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan CH, Dixon RA, Willerson JT, Ruan KH. Prostacyclin therapy for pulmonary arterial hypertension. Texas Heart Institute journal / from the Texas Heart Institute of St Luke's Episcopal Hospital, Texas Children's Hospital. 2010;37:391–399. [PMC free article] [PubMed] [Google Scholar]
- 7.Hagen AA, White RP, Robertson JT. Synthesis of prostaglandins and thromboxane B2 by cerebral arteries. Stroke. 1979;10:306–309. doi: 10.1161/01.str.10.3.306. [DOI] [PubMed] [Google Scholar]
- 8.Santhanam AV, Smith LA, Katusic ZS. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke. 2010;41:350–356. doi: 10.1161/STROKEAHA.109.564492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MM, Pickles H. Effect of epoprostenol (prostacyclin, PGI2) on cerebral blood flow in man. J Neurol Neurosurg Psychiatry. 1982;45:1033–1036. doi: 10.1136/jnnp.45.11.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook PJ, Maidment CG, Dandona P, Hutton RA, James IM. The effect of intravenous epoprostenol (prostacyclin, PGI2) on cerebral blood flow and cardiac output in man. Br J Clin Pharmacol. 1983;16:707–711. doi: 10.1111/j.1365-2125.1983.tb02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickles H, Brown MM, Thomas M, Hewazy AH, Redmond S, Zilkha E, et al. Effect of indomethacin on cerebral blood flow, carbon dioxide reactivity and the response to epoprostenol (prostacyclin) infusion in man. J Neurol Neurosurg Psychiatry. 1984;47:51–55. doi: 10.1136/jnnp.47.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raczka E, Quintana A. Effects of intravenous administration of prostacyclin on regional blood circulation in awake rats. Br J Pharmacol. 1999;126:1325–1332. doi: 10.1038/sj.bjp.0702426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bentzer P, Venturoli D, Carlsson O, Grande PO. Low-dose prostacyclin improves cortical perfusion following experimental brain injury in the rat. J Neurotrauma. 2003;20:447–461. doi: 10.1089/089771503765355522. [DOI] [PubMed] [Google Scholar]
- 14.Karasawa Y, Komiyama H, Yoshida S, Hino N, Katsuura Y, Nakaike S, et al. Effect of TTC-909 on cerebral infarction following permanent occlusion of the middle cerebral artery in stroke prone spontaneously hypertensive rats. J Pharmacol Sci. 2003;91:305–312. doi: 10.1254/jphs.91.305. [DOI] [PubMed] [Google Scholar]
- 15.Hazekawa M, Sakai Y, Yoshida M, Haraguchi T, Uchida T. Single injection of ONO-1301-loaded PLGA microspheres directly after ischaemia reduces ischaemic damage in rats subjected to middle cerebral artery occlusion. J Pharm Pharmacol. 2012;64:353–359. doi: 10.1111/j.2042-7158.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama-Tsuyuki Y, Kawashima K, Araki H, Otomo S. Prostacyclin analogue TTC-909 reduces memory impairment in rats with cerebral embolism. Pharmacol Biochem Behav. 1995;52:555–559. doi: 10.1016/0091-3057(95)00139-n. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura K, Watanabe Y, Onoe H. Prostacyclin receptor in the brain and central terminals of the primary sensory neurons: an autoradiographic study using a stable prostacyclin analogue [3H]iloprost. Neuroscience. 1995;65:493–503. doi: 10.1016/0306-4522(94)00505-y. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe Y, Matsumura K, Takechi H, Kato K, Morii H, Bjorkman M, et al. A novel subtype of prostacyclin receptor in the central nervous system. J Neurochem. 1999;72:2583–2592. doi: 10.1046/j.1471-4159.1999.0722583.x. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, Takamatsu H, Kakiuchi T, Ohba H, Kataoka Y, Yokoyama C, et al. Neuroprotection by a central nervous system-type prostacyclin receptor ligand demonstrated in monkeys subjected to middle cerebral artery occlusion and reperfusion: a positron emission tomography study. Stroke. 2006;37:2830–2836. doi: 10.1161/01.STR.0000245088.60282.22. [DOI] [PubMed] [Google Scholar]
- 20.Ruan KH, Deng H, So SP. Engineering of a protein with cyclooxygenase and prostacyclin synthase activities that converts arachidonic acid to prostacyclin. Biochemistry. 2006;45:14003–14011. doi: 10.1021/bi0614277. [DOI] [PubMed] [Google Scholar]
- 21.Ruan KH, Mohite AJ, So SP. Resistant to thrombosis, induced stroke and heart arrest by incorporation of a single gene of PGI-synthesizing COX-1-PGIS in vivo: Implication against human heart disease. International journal of cardiology. 2013 doi: 10.1016/j.ijcard.2013.03.114. [DOI] [PubMed] [Google Scholar]
- 22.Ruan KH, So SP, Cervantes V, Wu H, Wijaya C, Jentzen RR. An active triple-catalytic hybrid enzyme engineered by linking cyclo-oxygenase isoform-1 to prostacyclin synthase that can constantly biosynthesize prostacyclin, the vascular protector. FEBS J. 2008;275:5820–5829. doi: 10.1111/j.1742-4658.2008.06703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan KH, So SP, Wu H, Cervantes V. Large-scale expression, purification, and characterization of an engineered prostacyclin-synthesizing enzyme with therapeutic potential. Arch Biochem Biophys. 2008;480:41–50. doi: 10.1016/j.abb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010;1359:178–185. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Elhardt M, Martinez L, Tejada-Simon MV. Neurochemical, behavioral and architectural changes after chronic inactivation of NMDA receptors in mice. Neurosci Lett. 2010;468:166–171. doi: 10.1016/j.neulet.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez LA, Klann E, Tejada-Simon MV. Translocation and activation of Rac in the hippocampus during associative contextual fear learning. Neurobiol Learn Mem. 2007;88:104–113. doi: 10.1016/j.nlm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Siegle I, Klein T, Zou MH, Fritz P, Komhoff M. Distribution and cellular localization of prostacyclin synthase in human brain. J Histochem Cytochem. 2000;48:631–641. doi: 10.1177/002215540004800507. [DOI] [PubMed] [Google Scholar]
- 30.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 31.Bailey KR, Crawley JN. In: Anxiety-Related Behaviors in Mice. Buccafusco JJ, editor. Boca Raton (FL): Methods of Behavior Analysis in Neuroscience; 2009. [Google Scholar]
- 32.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta psychiatrica Scandinavica Supplementum. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 34.Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- 35.Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- 36.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- 39.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Bertoglio LJ, Joca SR, Guimaraes FS. Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav Brain Res. 2006;175:183–188. doi: 10.1016/j.bbr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Favilla C, Abel T, Kelly MP. Chronic Galphas signaling in the striatum increases anxiety-related behaviors independent of developmental effects. J Neurosci. 2008;28:13952–13956. doi: 10.1523/JNEUROSCI.4986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KS, Lee KW, Baek IS, Lim CM, Krishnan V, Lee JK, et al. Adenylyl cyclase-5 activity in the nucleus accumbens regulates anxiety-related behavior. J Neurochem. 2008;107:105–115. doi: 10.1111/j.1471-4159.2008.05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlak CR, Ho YJ, Schwarting RK, Bauhofer A. Relationship between striatal levels of interleukin-2 mRNA and plus-maze behaviour in the rat. Neurosci Lett. 2003;341:205–208. doi: 10.1016/s0304-3940(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 45.Chen C, Kim JJ, Thompson RF, Tonegawa S. Hippocampal lesions impair contextual fear conditioning in two strains of mice. Behav Neurosci. 1996;110:1177–1180. doi: 10.1037//0735-7044.110.5.1177. [DOI] [PubMed] [Google Scholar]
- 46.Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- 47.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 48.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muramatsu R, Takahashi C, Miyake S, Fujimura H, Mochizuki H, Yamashita T. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat Med. 2012;18:1658–1664. doi: 10.1038/nm.2943. [DOI] [PubMed] [Google Scholar]