Abstract
Mice lacking the serotonin receptor 1A [Htr1aknock-out (Htr1aKO)] display increased innate and conditioned anxiety-related behavior. Expression of the receptor in the mouse forebrain during development is sufficient to restore normal anxiety-related behavior to knock-out mice, demonstrating a role for serotonin in the developmental programming of anxiety circuits. However, the precise developmental period as well as the signaling pathways and neural substrates involved in this phenomenon are unknown. Here, we show that pharmacological blockade of the receptor from postnatal day 13 (P13)–P34 is sufficient to reproduce the knock-out phenotype in adulthood, thus defining a role for serotonin in the maturation and refinement of anxiety circuits during a limited postnatal period. Furthermore, we identify increases in the phosphorylation of α-Ca2+/calmodulin-dependent protein kinase II (αCaMKII) at threonine 286 in the hippocampus of young Htr1aKO mice under anxiety-provoking conditions. Increases in αCaMKII phosphorylation were most pronounced in the CA1 region of the hippocampus and were localized to the extrasynaptic compartment, consistent with a tissue-specific effect of the receptor. No changes in αCaMKII phosphorylation were found in adult knock-out mice, suggesting a transient role of αCaMKII as a downstream target of the receptor. Finally, the anxiety phenotype was abolished when knock-out mice were crossed to mice in which αCaMKII phosphorylation was compromised by the heterozygous mutation of threonine 286 into alanine. These findings suggest that modulation of αCaMKII function by serotonin during a restricted postnatal period contributes to the developmental programming of anxiety-related behavior.
Keywords: CaMKII, anxiety, 5-HT1A receptor, postnatal development, CaMKII T286A, autophosphorylation
Introduction
Mice lacking serotonin receptor 1A [Htr1a knock-out (Htr1aKO)] display increased behavioral inhibition and avoidance in tests of innate anxiety-related behavior such as in the open field, elevated plus maze, elevated zero maze, and novelty-suppressed feeding tests (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998; Gross et al., 2002). In addition, Htr1aKO mice exhibit increased conditioning to ambiguous, but not nonambiguous cues, a hallmark of human anxiety (Klemenhagen et al., 2006; Tsetsenis et al., 2007). Inhibition of dentate gyrus granule cells in the hippocampus is able to reverse the ambiguous-cue conditioning phenotype of Htr1aKO mice, suggesting that defects in hippocampal processing contribute to the anxiety-related phenotype in these animals (Tsetsenis et al., 2007). Conditional expression of the receptor in mouse forebrain restores normal anxiety-related behavior to the knock-out and expression of the receptor during development, but not in adulthood, was sufficient to rescue the knock-out phenotype (Gross et al., 2002). These findings suggest that serotonin plays a critical role in the maturation and/or refinement of forebrain circuits critical for normal anxiety-related behavior in adulthood. Consistent with this hypothesis, changes in dendritic arborization (J. P. Hornung, personal communication) and neural excitability (Sibille et al., 2000; Monckton et al., 2002) have been documented in the CA1 region of Htr1aKO mice.
In vitro studies have shown that activation of Htr1a is coupled via Gαi to inhibition of adenylyl cyclase, activation of K+ and Ca2+ channels, and mobilization of intracellular Ca2+ (Raymond et al., 1999). However, little is known about the signaling pathways critical for receptor function in vivo. Coupling to G-protein-coupled inward-rectifying K+ (GIRK) channels has been shown to be essential for the hyperpolarizing effects of Htr1a agonists in hippocampal neurons (Luscher et al., 1997). Activation of Htr1a in cultured cortical neurons decreased autophosphorylation of α-Ca2+/calmodulin-dependent protein kinase II (αCaMKII) and decreased AMPA and NMDA receptor function via inhibition of cAMP (Cai et al., 2002). However, whether these signaling pathways are relevant to the anxiety-related phenotype of Htr1aKO mice is not known.
Here, we used a pharmacological approach to show that Htr1a activity is required from P13 to P34 for normal anxiety-related behavior in adulthood. Blocking the receptor for a similar length of time in adulthood, however, did not alter anxiety behavior. We also show that phosphorylation of αCaMKII is increased in the hippocampus of Htr1aKO mice under anxiety-provoking conditions and that this activation is correlated with anxiety-related behavioral measures. Finally, we show that a mutation that reduces αCaMKII phosphorylation blocks the behavioral effects seen in Htr1aKO mice, suggesting a role for αCaMKII in mediating the developmental programming effects of serotonin on anxiety.
Materials and Methods
Mouse strains and husbandry
Mice were maintained on a 129S6/SvEvTac;C57BL/6;CBA mixed background (Gross et al., 2002), except for those used in the Camk2aT286A–Htr1aKO interaction experiment, which were congenic C57BL/6 (N7). All testing groups derived from heterozygous breeding except mice used for the developmental N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY100635) treatment immunoblotting experiment, the adult WAY100635 treatment experiment, and some of the mice used for the developmental WAY100635 treatment behavioral experiment (n = 12). Camk2aT286A mice were generously provided by K. P. Giese (UCL, London, UK). Fathers were always removed before parturition and mothers and offspring were left undisturbed for the first week. Tail biopsy for genotyping was performed at postnatal day 7 (P7) as described (Giese et al., 1998); after weaning, at P21 mice were group housed (three to five per cage) on a 12 h light/dark cycle (lights off at 7:00 P.M.) in a temperature-controlled environment (21°C). Food and water were provided ad libitum. Male mice were used for all experiments.
Osmotic pump implantation
Osmotic minipumps (0.20 μl/h; model 1002; Alzet) were implanted subcutaneously to deliver WAY100635 (Sigma-Aldrich) or saline (0.9% NaCl) continuously for 21 d. Pumps were filled with 7.1 mg/ml WAY100635 to deliver a dose of 0.15 mg/h per kg of body weight. Pumps were soaked overnight in Ringer's solution (Eurospital) at 37°C to assure steady-state pumping after implantation. Before surgery, all pups were removed from the mother, placed in a beaker containing home cage bedding and kept on a heating pad. Mice were anesthetized with halothane (Merial Animal Health), pumps were subcutaneously implanted in the dorsal thoracic area, and wounds were closed with a 9 mm stainless-steel clip (Stoelting). After 21 d of treatment, mice were anesthetized, pumps removed, and the wounds closed with a clip.
8-OH-DPAT-induced hypothermia
Animals were singly housed the day of the test. 8-Hydroxy-N-(di-n-propyl)-aminotetralin (8-OH-DPAT; Sigma-Aldrich) was dissolved in 0.9% saline and injected subcutaneously at a concentration of 0.3 mg/kg body weight at t = 40 min from the beginning of the test. Body temperature was recorded in 10 min intervals with a digital rectal thermometer (Bioseb).
Behavioral testing
Procedures were performed according to European Molecular Biology Laboratory and Italian guidelines for ethical animal treatment following authorized protocols. Mice were transferred to the phenotyping facility 1 week before testing and were habituated to the testing room 1 h before testing, unless otherwise indicated. Mice were tested at 8–10 weeks of age, unless otherwise indicated; subsequent behavioral tests were separated by 1 week intervals to reduce intertest interactions and were performed in the order listed below. After testing, mice were held in a holding cage until all animals in a cage had been tested.
Open field.
Mice were placed into a gray plastic arena (50 × 50 cm) for 1 h. Locomotion data were collected by a video tracking system (TSE Systems). Animals were initially placed along one side of the arena, and the center region was defined as the central 26 × 26 cm area and the corners as the areas 10 cm from adjacent walls.
Novelty-suppressed feeding
Mice were food deprived for 24 h before testing; testing was performed between 4:00 and 8:00 P.M. Mice were habituated to the room for 10 min before being placed for 10 min into the open field arena (see above) with a food pellet fixed in the middle. The latency to visit and begin chewing food was recorded.
Elevated plus maze.
Mice were habituated to the room for 10 min before being placed into the central platform (5 × 5 cm) of a gray plastic maze facing toward an open arm (open arm, 30 × 5 cm, surrounded by a 0.25 cm high border; closed arms, 30 × 5 cm, surrounded by 15 cm high walls). The entire apparatus was elevated 45 cm above the floor. Locomotion data were collected for 5 min by a video tracking system (TSE Systems).
Protein analysis
Tissue homogenates for immunochemical analysis of proteins were obtained as follows.
Whole hippocampal lysates.
To obtain samples under conditions relevant to anxiety-related behavior, mice were placed into the open field for 20 min with no habituation. Locomotion data were collected by a video tracking system as previously described and data were used subsequently for correlation analysis; for baseline measurements, one mouse was removed from the home cage and immediately killed by cervical dislocation; the hippocampus was quickly dissected (within 2 min after dislocation) and frozen in liquid nitrogen. To avoid effects of testing order, no more than one mouse per day was taken from a cage. Individual hippocampal samples were dounce-homogenized in SDS lysis buffer (SDS 1%, glycerol 10%, and 50 mm Tris-HCl, pH 6.8) at 95°C and centrifuged at 10,000 × g for 10 min.
Hippocampal microdissection.
Mice were killed after 20 min exposure to an open field (see above) and whole brains were quickly dissected and frozen in powdered dry ice. Hippocampal subfields enriched in CA1, CA3, and dentate gyrus (DG) regions were microdissected from dorsal hippocampus at −20°C under a magnifying lens using a cold tissue corer (Fine Science Tools; diameter, 2.0 mm); tissue lysates from each fraction were then processed as described above.
Synaptosomal and extrasynaptic lysates.
Crude synaptosomal fractionation of hippocampus was performed as described previously (Nagy and Delgado-Escueta, 1984). Mice were killed after a 20 min exposure to the open field (see above) and the hippocampus was rapidly dissected, pooled, and dounce-homogenized in ice-cold lysate buffer (0.32 m sucrose, 1 mm EDTA, 1 mg/ml BSA, and 5 mm HEPES, pH 7) containing fresh protease and phosphatase inhibitors (1 mm PMSF, 10 mm NaF, 1 mm NaPP, 80 mm β-glycerophosphate, and Complete Mini protease inhibitor cocktail, EDTA-free; Roche Diagnostics) using a 15 ml Teflon pestle dounce (Wheaton) at 660 rpm. The homogenate was centrifuged at 3000 × g for 10 min and the supernatant (S1) was centrifuged again at 14,000 × g for 12 min. The new supernatant (S2) was kept for extrasynaptic preparation; to isolate synaptosomes, the pellet fraction (P2) was resuspended in 45% (v/v) Percoll (Pharmacia) in Krebs–Ringer solution [containing (in mm) 140 NaCl, 5 KCl, 10 HEPES, 1 EDTA, and 5 glucose, pH 7.4] and centrifuged shortly at 14,000 × g. The top layer, containing synaptosomes, was removed, washed with Krebs–Ringer solution and isolated by centrifugation. Synaptosomes were lysed in SDS buffer as described above. Extrasynaptic proteins were isolated by overnight precipitation of S2 in acetone at −20°C; after centrifugation (18,000 × g), the pellet was resuspended in SDS lysis buffer as described above.
Western blotting
Protein concentration was determined with the bicinchoninic acid protein assay (Pierce). Equal amounts of protein were separated on 12% polyacrylamide gels by SDS-PAGE and transferred to Immobilon polyvinylidene difluoride membranes (Millipore) using standard protocols (Towbin et al., 1992). Immunoblots were probed with primary antibodies including anti-αCaMKII (Santa Cruz Biotechnology; 1:1000) and anti-phospho-CaMKII-T286 (Promega; 1:1000); signals were visualized with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) and an enhanced chemiluminescent (ECL) system (GE Healthcare). Blots were washed with washing buffer (Tris-buffered saline, Tween 20–0.1%) and reprobed with anti-actin antibody (Millipore; 1:8000) to normalize for protein content. Signals from the ECL films (GE Healthcare) were quantified by densitometry using NIH ImageJ software.
Statistical analysis
Behavioral data were analyzed by ANOVA followed by post hoc comparisons using Fisher's test in cases of significance (p < 0.05). For quantitative immunochemical analyses, optical density (OD) values within each blot were first standardized to facilitate interblot comparison and the z scores obtained with 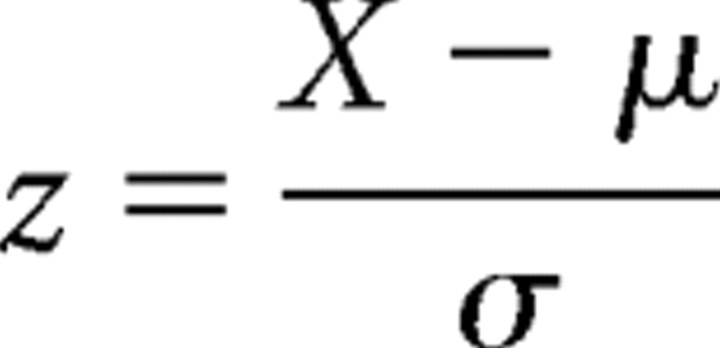 were analyzed by ANOVA followed by post hoc comparisons using Fisher's test in cases of significance (p < 0.05; x, relative optical density value; μ, mean of replicate values within the blot; σ, SD of replicate values within the blot). OD data within extrasynaptic as well as synaptosomal pools were analyzed by the Kolmogorov–Smirnov nonparametric test to allow comparisons with a variance-free wild-type measurement. For correlation analysis, OD data within each blot were normalized as percentage over the mean of immunoreactivity, and Pearson's coefficient was calculated. All analyses were performed using either Excel (Microsoft) or StatView 5.0 (SAS Institute).
were analyzed by ANOVA followed by post hoc comparisons using Fisher's test in cases of significance (p < 0.05; x, relative optical density value; μ, mean of replicate values within the blot; σ, SD of replicate values within the blot). OD data within extrasynaptic as well as synaptosomal pools were analyzed by the Kolmogorov–Smirnov nonparametric test to allow comparisons with a variance-free wild-type measurement. For correlation analysis, OD data within each blot were normalized as percentage over the mean of immunoreactivity, and Pearson's coefficient was calculated. All analyses were performed using either Excel (Microsoft) or StatView 5.0 (SAS Institute).
Results
The conditional genetic strategy we used previously to establish a developmental role for Htr1a (Gross et al., 2002) did not allow rapid regulation of protein expression and, thus, could not be used to determine the precise developmental time window for the anxiety-moderating effects of the receptor. Determining the precise susceptibility period is important because the mouse forebrain goes through several major developmental milestones during the preweaning period. Hippocampal dendrite formation and pruning occurs primarily between P10 and P42 (Pokorny and Yamamoto, 1981), and functional coupling of Htr1a to inhibitory GIRK channels does not occur until P14 (Béïque et al., 2004). These observations suggest that the receptor may be involved in modulating developmental events in the late postnatal period.
To determine whether Htr1a function during the late postnatal period is necessary for normal anxiety-related behavior in adulthood, we treated wild-type mice chronically with either saline or the selective Htr1a antagonist WAY100635 from P13 to P34 using subcutaneous osmotic minipumps and measured anxiety-related behavior in adulthood. Persistent blockade of Htr1a function during WAY100635 treatment was confirmed by measuring the hypothermic response to an acute treatment with the Htr1a agonist 8-OH-DPAT at various times after minipump implantation. Hypothermic responses to 8-OH-DPAT were completely blocked 1 d after implantation, remained absent through the treatment period, and were restored 1 d after minipump explantation (supplemental Figs. 1, 2, available at www.jneurosci.org as supplemental material). Normal hypothermic responses to 8-OH-DPAT were also observed in adult mice tested 2 months after minipump removal (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). These findings confirm that WAY100635 treatment rapidly and persistently blocks Htr1a function when delivered via osmotic minipump, but does not have long-term effects on receptor activity after minipump removal.
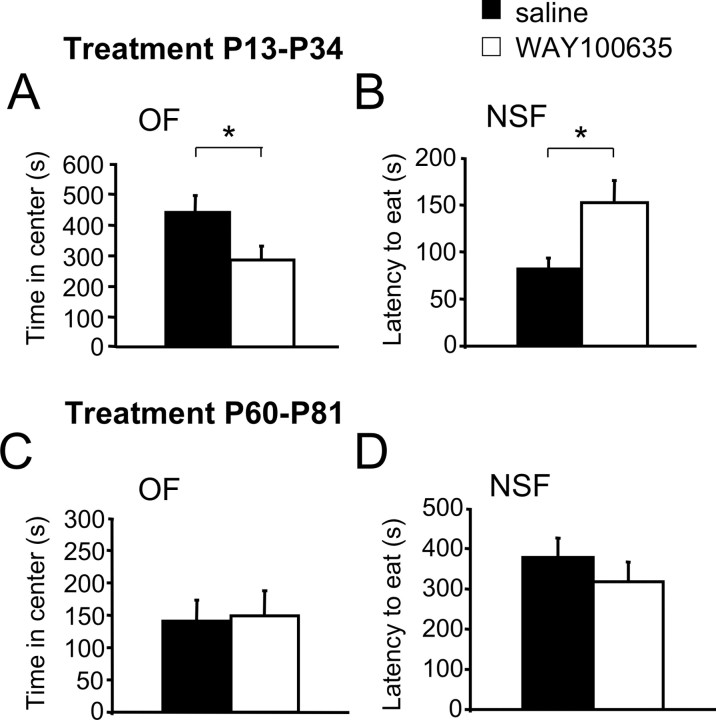
Assessment of anxiety-related behavior in saline and WAY100635-treated mice was performed in the open field, elevated plus maze, and novelty-suppressed feeding tests when mice reached adulthood (14–16 weeks). Mice treated with WAY100635 showed significantly reduced time in the center, percentage of path in the center, and total path in the open field, and increased latency to feed in the novelty-suppressed feeding tests when compared with saline-treated control mice (Fig. 1A,B, supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material). No significant effect of the antagonist was seen in the elevated plus maze (data not shown). These behavioral changes match the increase in behavioral inhibition to novelty seen in Htr1aKO mice (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). Consistent with a selective effect of WAY100635 on Htr1a, similar treatment of Htr1aKO mice with WAY100635 from P13–P34 failed to alter anxiety-related measures in the open field and novelty-suppressed feeding tests (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). These findings show that blockade of Htr1a during postnatal weeks 3 to 5 is sufficient to reproduce the increased anxiety-related behavior seen in Htr1aKO mice.
Figure 1.
Increased anxiety-related behavior in adult mice treated during development, but not adulthood, with WAY100635. A, B, Adult wild-type mice treated with the selective Htr1a antagonist WAY100635 from P13 to P34 (WAY100635) showed increased anxiety-related behavior when compared with saline-treated littermates (saline) in the open field (OF; saline, n = 29; WAY100635, n = 27; A) and novelty-suppressed feeding test (NSF; saline, n = 19; WAY100635, n = 18; B). C, D, Adult mice treated with the selective Htr1a antagonist WAY100635 from P60 to P81 did not display altered anxiety-related behavior when compared with saline-treated littermates in the open field (saline, n = 23; WAY100635, n = 24; C) or novelty-suppressed feeding test (saline, n = 14; WAY100635, n = 14; D). Error bars indicate mean ± SEM. *p < 0.05.
Our conditional genetic rescue experiments showed that blocking expression of Htr1a in adulthood was not sufficient to recapitulate the Htr1aKO phenotype (Gross et al., 2002). To test whether blockade of Htr1a during a similar 3 week period in adulthood could alter anxiety-related behavior later in adulthood, we implanted mice with minipumps containing saline or WAY100635 from P60–P81 and measured anxiety-related behavior 3 weeks later (week 14). Consistent with our previous results, blockade of Htr1a during adulthood did not affect anxiety-related behavior in either the open field or novelty-suppressed feeding tests when tested 3 weeks later (Fig. 1C,D, supplemental Figs. 3C,D, 4B, available at www.jneurosci.org as supplemental material). These findings point to a restricted susceptibility period during the late postnatal period when blockade of Htr1a can program long-term anxiety behavior.
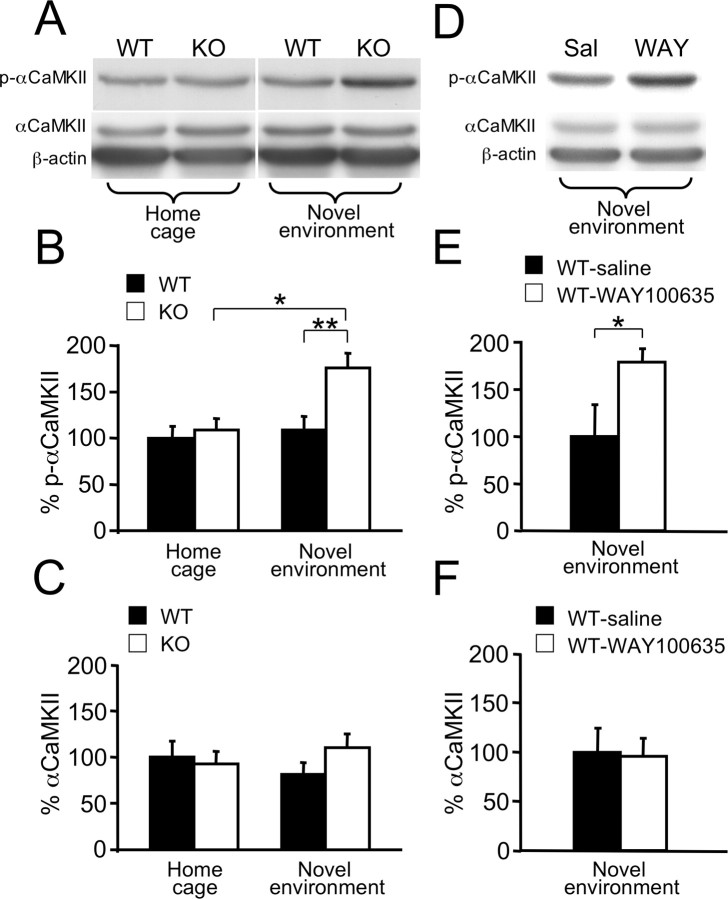
Next, we examined Htr1aKO mice for changes in the phosphorylation of αCaMKII during the susceptible developmental period. We chose to focus on the hippocampus for several reasons: (1) high levels of expression of Htr1a in hippocampal CA1 pyramidal neurons (Chalmers and Watson, 1991; Kia et al., 1996; Gross et al., 2002), (2) Htr1aKO mice exhibit increased dendritic arborization (J. P. Hornung, personal communication) and neural excitability in the hippocampal CA1 region (Sibille et al., 2000; Monckton et al., 2002), (3) lesions of the hippocampus are associated with decreased innate anxiety-related behavior (Kjelstrup et al., 2002; Bannerman et al., 2004), and (4) inhibition of dentate gyrus granule cells suppresses conditioned responses to ambiguous aversive cues and reverses the Htr1aKO phenotype (Tsetsenis et al., 2007). Quantitative immunoblotting of P21 hippocampal extracts with antibodies recognizing αCaMKII phosphorylated at threonine 286 (T286) (p-αCaMKII) revealed no significant difference between Htr1aKO mice and wild-type littermates under baseline conditions (home cage) (Fig. 2A,B). However, levels of p-αCaMKII are known to be sensitive to stress (Suenaga et al., 2004) and, thus, it was possible that an effect of the Htr1aknock-out might be revealed under conditions of stress such as those experienced during anxiety testing. When identical immunoblotting experiments were performed on hippocampal extracts from mice after 20 min exposure to the open field (novel environment), a significant increase in p-αCaMKII was observed in knock-out mice when compared with littermate controls (Fig. 2A,B). Similar increases in p-αCaMKII were observed in wild-type mice treated since P13 with WAY100635 when compared with saline-treated littermates (Fig. 2D,E). No differences in levels of total αCaMKII were observed in either Htr1aKO (Fig. 2C) or WAY100635-treated mice (Fig. 2F). These findings demonstrate that Htr1a signaling during the third postnatal week is important to maintain normal levels of αCaMKII phosphorylation under stressful situations.
Figure 2.
Increased αCaMKII T286 phosphorylation in the hippocampus of Htr1aKO mice or mice treated with WAY100635. A, Representative bands from Western blot analysis of p-αCaMKII, total αCaMKII, and actin immunoreactivity in total hippocampal extracts of P21 wild-type (WT) and Htr1aKO (KO) mice either exposed (novel environment) or not exposed (home cage) to a novel environment for 20 min before being killed. B, Quantification of immunoreactivity revealed a significant increase in p-αCaMKII of KO mice compared with WT in the novel environment, but not home cage condition (home cage: WT, n = 14; KO, n = 16; novel environment: WT, n = 10; KO, n = 9). C, Level of total αCaMKII immunoreactivity was similar in WT and KO mice (home cage: WT, n = 10; KO, n = 13; novel environment: WT, n = 11; KO, n = 9). D, Representative bands from Western blot analysis of p-αCaMKII, total αCaMKII, and actin immunoreactivity in total hippocampal extracts of P21 wild-type mice during chronic treatment with the selective Htr1a antagonist WAY100635 (WAY; 0.15 mg/kg/h) or with saline (Sal) and after novel environment exposure. E, F, Quantification of immunoreactivity revealed a significant increase in p-αCaMKII (E) but not total αCaMKII (F) for mice treated with WAY100635 (WT-WAY100635; n = 6) when compared with the saline control (WT-saline; n = 5). Data are expressed as a percentage of optical density (intensity units per square millimeter) versus WT, home cage (B, C), or WT-saline (E, F). Error bars indicate mean ± SEM. Intensity of bands was normalized versus actin. *p < 0.05; **p < 0.01.
If the changes in p-αCaMKII seen in Htr1aKO mice directly contribute to the anxiety phenotype, then these changes should persist into adulthood. However, immunoblotting of hippocampal extracts from adult mice under either baseline conditions (home cage) or after 20 min exposure to the open field (novel environment) did not reveal significant differences in p-αCaMKII between Htr1aKO and wild-type littermates (Fig. 3, supplemental Fig. 6, available at www.jneurosci.org as supplemental material). Thus, deletion of Htr1a is associated with a transient increase in αCaMKII phosphorylation that is limited to a circumscribed developmental time period.
Figure 3.
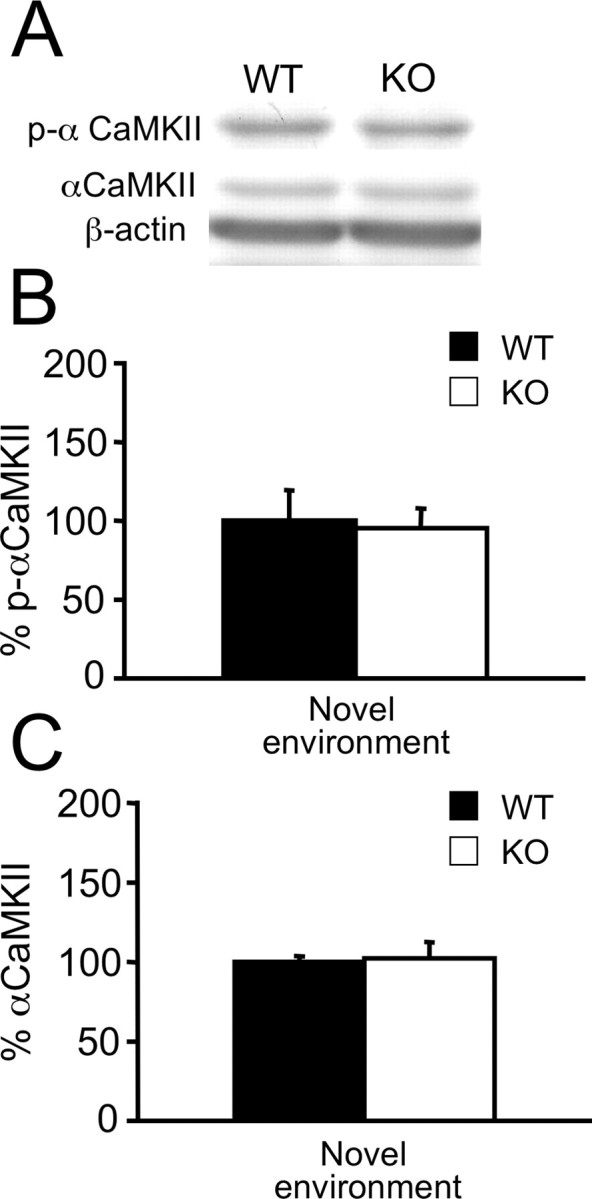
Unaltered level of αCaMKII T286 phosphorylation in the hippocampus of adult Htr1aKO mice. A, Representative bands from Western blot analysis of p-αCaMKII, total αCaMKII, and actin immunoreactivity in total hippocampal extracts of adult wild-type (WT) or Htr1aKO (KO) mice after novel environment exposure. B, C, Quantification of p-αCaMKII (B) and total αCaMKII immunoreactivity (C) were similar in WT and KO mice. Data are expressed as a percentage of optical density (intensity units per square millimeter) versus WT; intensity of bands was normalized versus actin (WT, n = 10; KO, n = 9). Error bars indicate mean ± SEM.
Within the hippocampus, Htr1a expression is most pronounced in excitatory neurons of the CA1 region with lower levels of receptor protein detected in CA3 and DG (Chalmers and Watson, 1991; Kia et al., 1996; Gross et al., 2002). Thus, changes in Htr1a-dependent signaling should be greatest in this hippocampal region. To examine region-specific changes in p-αCaMKII, we isolated CA1, CA3, and DG tissue from hippocampus of knock-out and wild-type littermates by micropunching frozen brain tissue. Immunoblotting revealed a significant increase in p-αCaMKII restricted to the CA1 region (Fig. 4A). These findings are consistent with a tissue-specific action of the receptor on αCaMKII signaling.
Figure 4.
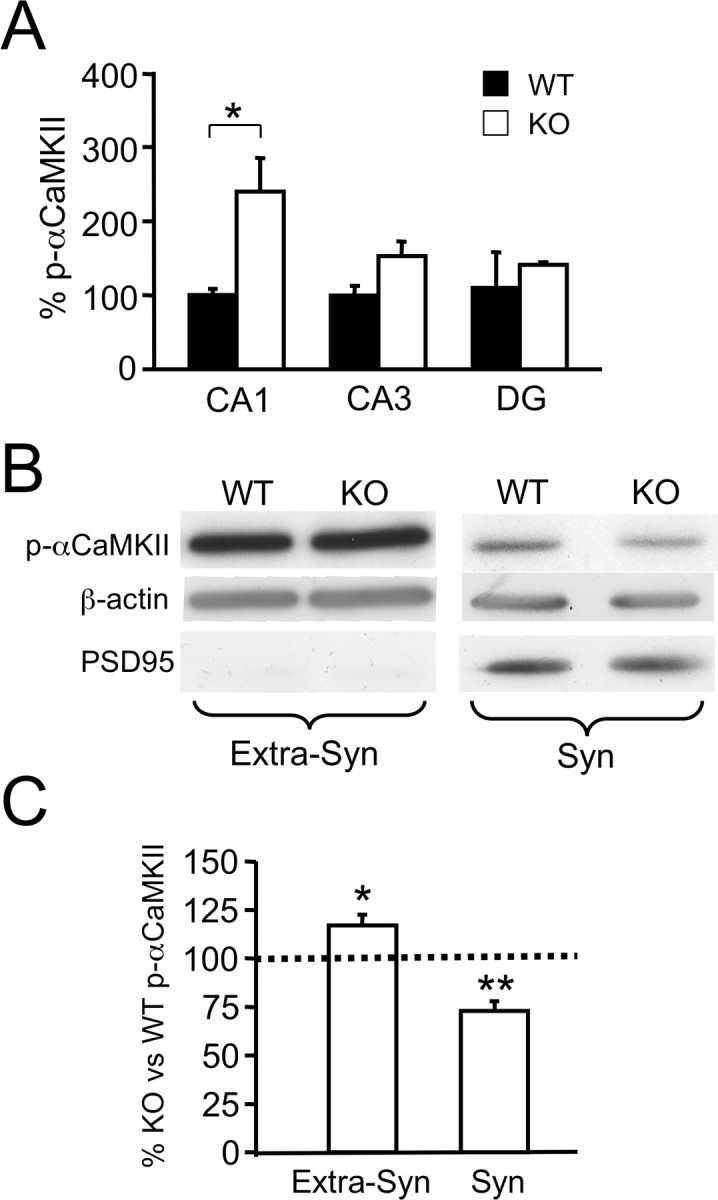
Changes in αCaMKII T286 phosphorylation in the hippocampus of Htr1aKO mice at a subregional and subcellular level. A, Quantification of p-αCaMKII immunoreactivity from Western blot analysis of hippocampal fractions enriched in CA1, CA3, or DG region from P21 wild-type (WT) or Htr1aKO (KO) mice after 20 min exposure to a novel environment. Level of p-αCaMKII in hippocampal CA1 but not CA3- or DG-enriched fractions was significantly increased in KO compared with WT mice (CA1: WT, n = 4; KO, n = 3; CA3: WT, n = 4; KO, n = 3; DG, WT, n = 4; KO, n = 2). Data are expressed as percentage optical density (intensity units per square millimeter) versus WT, CA1. B, Representative bands from Western blot analysis of p-αCaMKII, actin, and postsynaptic density-95 (PSD95) immunoreactivity in synaptosomal (Syn) and nonsynaptosomal (Extra-Syn) cytosolic fractions from pooled hippocampi of P21 WT and KO mice. C, Quantification of immunoreactivity revealed a significant increase in nonsynaptosomal p-αCaMKII as well as a decrease in synaptosomal p-αCaMKII in KO mice compared with WT (Syn, n = 6 pools; Extra-Syn, n = 4 pools). Mice were exposed for 20 min to a novel environment before being killed. Data are expressed as the percentage of optical density (intensity units per square millimeter) relative to WT (mean ± SEM); the intensity of bands was normalized versus actin; PSD95 immunoreactivity was used to assess the purity of the nonsynaptosomal fraction. A Kolmogorov–Smirnov (nonparametric) test was used to assess statistical significance in the pooled-samples experiment (C). Error bars indicate mean ± SEM. *p < 0.05; **p < 0.01.
Immunohistochemical studies have shown that Htr1a is localized to the somatodendritic compartment of excitatory neurons in the hippocampus with no particular enrichment at synapses (Chalmers and Watson, 1991; Kia et al., 1996; Riad et al., 2000). αCaMKII, however, is localized both extrasynaptically as well as at both presynaptic and postsynaptic sites (Kennedy et al., 1983; Ouimet et al., 1984; Benfenati et al., 1996; Ouyang et al., 1997). A local effect of Htr1a on αCaMKII phosphorylation might, therefore, be expected to occur throughout the somato-dendritic compartment rather than specifically at synaptic sites. Distinguishing these effects could have important implications for the functional consequences of altered αCaMKII activity because these two kinase populations have distinct roles in neuronal homeostasis (Miller et al., 2002). To test this hypothesis, we examined αCaMKII phosphorylation in fractionated hippocampal extracts from knock-out and wild-type littermate mice. Crude synaptosomal and extrasynaptic fractions were isolated from pooled total hippocampal extracts. Immunoblotting showed a significant increase in p-αCaMKII in the extrasynaptic fraction, consistent with a local effect of Htr1a on this pathway (Fig. 4B,C). Surprisingly, however, immunoblotting also revealed a significant decrease in αCaMKII phosphorylation in the synaptosomal fraction of knock-out mice (Fig. 4B,C). This change is most likely the result of either a local effect of Htr1a on different signaling pathways in extrasynaptic and synaptic compartments or an indirect effect of somatodendritic Htr1a receptor signaling on synaptic activity or function.
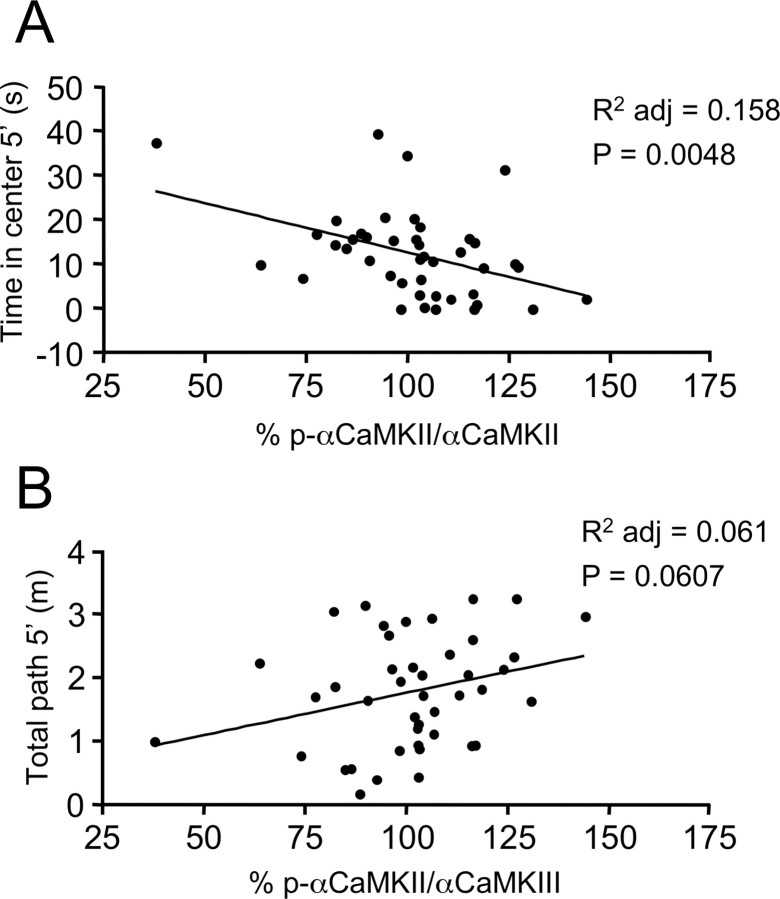
Our findings indicate that changes in αCaMKII phosphorylation occur in the extrasynaptic compartment of CA1 hippocampal neurons as a result of the absence of Htr1a-dependent signaling during the third week of life. These data suggest that changes in αCaMKII activity may play a role in the developmental programming of anxiety-related behavior by Htr1a. If this were the case, then we would expect natural variations in the level of p-αCaMKII to be significantly correlated with anxiety-related, but not nonanxiety-related behavioral measures in our mice. To test this possibility, we performed a Pearson's correlation between fractional p-αCaMKII and behavioral measures obtained during the 20 min exposure to the open field immediately before being killed at P21. At this age, rodents show clear avoidance behavior when exposed to a novel environment, and anxiety-related behavioral measures taken at this age correlate significantly with anxiety-related measures obtained in adult animals (A. M. Depino, unpublished observations). A significant negative correlation was found between the fraction of p-αCaMKII and the time in the center of the open field (Fig. 5A). No significant correlation was found between fractional p-αCaMKII and the total path, although there was a trend for a correlation in the opposite direction (Fig. 5B). These findings are consistent with a role for p-αCaMKII in the behavioral phenotype of the Htr1aKO mice.
Figure 5.
Hippocampal αCaMKII T286 phosphorylation correlates with anxiety-related measures in the open field. A, B, Anxiety parameters, as measured in the open field paradigm, were correlated with individual relative hippocampal levels of p-αCaMKII (% p-αCaMKII/αCaMKII) immunoreactivity, analyzed at the end of the behavioral test (20 min) in P21 mice. The level of p-αCaMKII/αCaMKII negatively correlated with time in the center (R2 = 0.18; R2 adj = 0.16; p = 0.0048; n = 43) (A), but did not significantly correlate with total path (B) during the first 5 min in the open field (R2 = 0.08; R2 adj = 0.06; p = 0.060; n = 43). To permit statistical comparison between different experimental groups, individual levels of p-αCaMKII/αCaMKII are presented as percentage values over intrablot means of immunoreactivity.
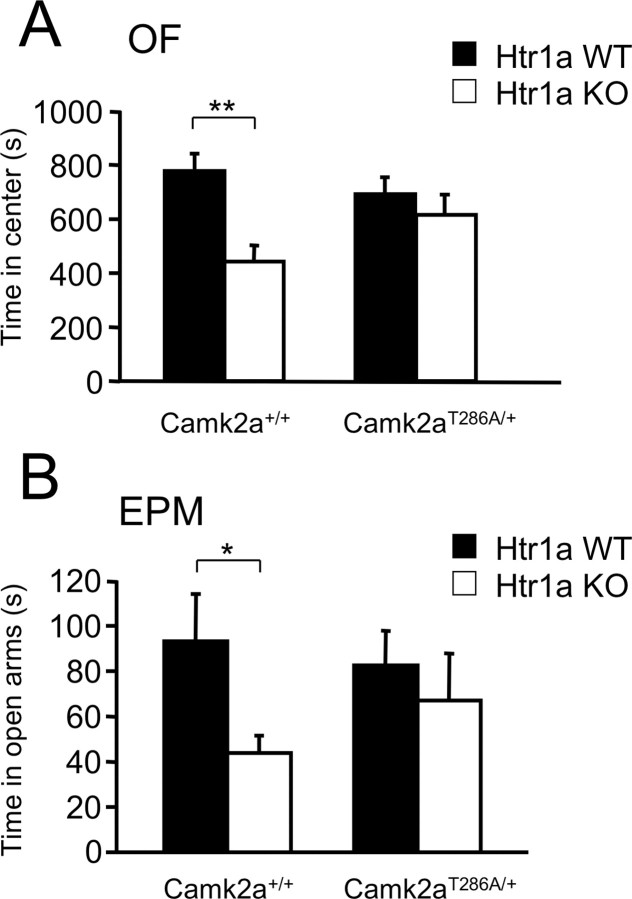
However, to directly test whether phosphorylation of αCaMKII might play an essential role in the behavioral effects of serotonin signaling during development, we used a genetic approach to modify αCaMKII phosphorylation at threonine 286. The mutation of threonine 286 into alanine (T286A) has been used in mice (Camk2aT286A) (Giese et al., 1998) to show that phosphorylation at this site is essential for normal synaptic plasticity (Giese et al., 1998; Hardingham et al., 2003; Cooke et al., 2006). Homozygous Camk2aT286A mice show no NMDA receptor-dependent long-term synaptic potentiation (LTP) at hippocampal CA1 synapses and have severely impaired spatial learning (Cho et al., 1998; Giese et al., 1998; Need and Giese, 2003; Cooke et al., 2006). Heterozygous Camk2aT286A mice, however, show a 50% reduction in phosphorylation at T286, normal experience-dependent plasticity (Glazewski et al., 2000; Taha et al., 2002), and reduced CA1 LTP (Ohno et al., 2001). We reasoned that the heterozygous Camk2aT286A mutation might completely or partially interfere with the increased phosphorylation seen in Htr1aKO mice without altering the learning capacity of the animals. Such a functional interaction should be revealed as a genetic interaction between the Htr1aKO and Camk2aT286A mutations. To test this possibility, we first confirmed that heterozygous Camk2aT286A mice show a specific and significant decrease in levels of p-αCaMKII in hippocampal extracts of P21 mice. As expected, heterozygous Camk2aT286A mice showed a twofold reduction in T286 phosphorylation when compared with wild-type littermates, and Htr1aKO mice heterozygous for the Camk2aT286A allele showed levels of p-αCaMKII indistinguishable from wild-type littermates (supplemental Fig. 7, available at www.jneurosci.org as supplemental material). No change in total levels of αCaMKII protein were observed (supplemental Fig. 7, available at www.jneurosci.org as supplemental material). Next, we examined whether the anxiety-related phenotype of Htr1aKO mice could be modulated by the heterozygous Camk2aT286A mutation. Strikingly, the significant decrease in the time in the center, percentage of path in the center, and the total path in the open field, and time in open arms of the elevated plus maze associated with the Hr1aKO mutation in a wild-type background was absent in littermates heterozygous for Camk2aT286A (Fig. 6, supplemental Fig. 8, available at www.jneurosci.org as supplemental material). This genetic interaction between Camk2aT286A and Htr1aKO suggests that phosphorylation at T286 is critical for the expression of the Htr1aKO phenotype and supports a causal role of increased p-αCaMKII in the anxiety phenotype of the knock-out mice.
Figure 6.
Heterozygous Camk2aT286A mutation moderates Htr1aKO phenotype. A, B, Htr1aKO mice (Htr1a KO) show decreased time in the center of the open field (OF; A) as well as decreased time in the open arms of the elevated plus maze (EPM; B) on a wild-type (Camk2a+/+) but not on a heterozygous Camk2aT286A (Camk2aT286A/+) genetic background (Camk2a+/+: Htr1a WT, n = 9; Htr1a KO, n = 13; Camk2aT286A/+: Htr1a WT, n = 14; Htr1a KO, n = 9). Error bars indicate mean ± SEM. *p < 0.05; **p < 0.01.
Discussion
Our finding that continuous pharmacological blockade of Htr1a during the third, fourth, and fifth postnatal weeks is sufficient to recapitulate the behavioral phenotype of Htr1a knock-out mice (Fig. 1A,B) points to this period as a critical time for serotonin signaling in the maturation and/or refinement of brain circuits controlling anxiety behavior. These data extend previous experiments showing that conditional expression of Htr1a in mouse forebrain during development is sufficient to restore normal anxiety-related behavior to the knock-out (Gross et al., 2002). Moreover, pharmacological blockade of Htr1a during the third, fourth, and fifth postnatal weeks is also able to reproduce the enhanced conditioning responses to ambiguous cues seen in knock-out mice (Tsetsenis et al., 2007). Together, these experiments argue that activation of Htr1a by endogenous serotonin serves a moderating role in the development of cognitive circuits that influence the processing of anxiety-related cues in adulthood.
The third, fourth, and fifth postnatal weeks are a period of dramatic synaptogenesis in mouse forebrain, with the majority of excitatory and inhibitory synapses that enervate CA1 pyramidal neurons appearing during this period (Pokorny and Yamamoto, 1981; Bahr and Wolff, 1985). Htr1a is highly expressed in CA1 pyramidal neurons (Chalmers and Watson, 1991; Kia et al., 1996), and Htr1aKO mice exhibit increased neuronal excitability (Sibille et al., 2000; Monckton et al., 2002) as well as increased arborization and synapse number in the proximal apical dendritic region of these neurons (J. P. Hornung, personal communication). Although it remains to be shown that the anxiety phenotype of the knock-out is directly linked to structural changes in CA1, alterations in hippocampal function are likely to underlie at least part of the anxiety-related phenotype of these mice because their enhanced response to ambiguous conditioned cues can be reversed by the pharmacological inhibition of hippocampal dentate gyrus granule cells (Tsetsenis et al., 2007).
At the same time, our observation that blockade of Htr1a during a prolonged period of adult life was not sufficient to reproduce the knock-out phenotype (Fig. 1C,D) is consistent with conditional genetic studies showing that suppression of receptor expression in adulthood is not able to mimic the knock-out phenotype (Gross et al., 2002). Two phenomena may explain why receptor blockade in adulthood is ineffective. First, electrophysiological studies have shown that forebrain Htr1a receptors are not tonically activated in adult animals (Haddjeri et al., 2004), presumably because endogenous serotonin levels are not sufficient to activate the receptor, at least under baseline conditions. Second, if deficits in hippocampal neuronal structure or wiring are critical for the developmental programming effects of serotonin, then the time of maximal synaptic growth may represent a particularly sensitive period for such modulation. Similar sensitive periods have been described for synaptogenesis in visual and auditory circuits during the same developmental period (Hensch, 2004).
Our finding of increased αCaMKII phosphorylation at T286 in Htr1aknock-out mice (Fig. 2) is consistent with studies demonstrating decreased phosphorylation at this site after pharmacological activation of the receptor in cortical slices and cultures (Cai et al., 2002). Several factors may have contributed to the fact that increased phosphorylation in the knock-out only occurs under conditions of behavioral challenge. First, extracellular serotonin is increased after exposure to stress (Kirby et al., 1997; Parsons et al., 2001) and, although modest, this may contribute to enhance activation of the receptor after exposure to a novel environment. Second, exposure to a novel environment may increase the activity of signaling pathways that promote αCaMKII phosphorylation and, thus, unmask an inhibitory action of the receptor. Furthermore, modulation of αCaMKII by Htr1a was shown to depend on cAMP inhibition via Gαi signaling and subsequent protein phosphatase activation (Cai et al., 2002). Behavioral challenge can increase cAMP levels in hippocampus (Mitchell et al., 1992; Meaney et al., 2000) and such activation may be necessary to reveal inhibitory effects of Htr1a. Finally, our finding that increased phosphorylation of αCaMKII in knock-out mice is transient and not observed in adulthood (Fig. 3) is consistent with the inability of receptor blockade in adulthood to alter anxiety-related behavior (Fig. 1C,D).
Htr1a protein is found throughout the plasma membrane of the somato-dendritic compartment of hippocampal pyramidal neurons (Riad et al., 2000). A primarily extrasynaptic rather than synaptic expression of the receptor reflects nonsynaptic release of serotonin in projection areas (Tork, 1990) and is consistent with our observation that increased αCaMKII phosphorylation is restricted to the extrasynaptic compartment (Fig. 4B,C). αCaMKII is abundant in both extrasynaptic and synaptic compartments (Kennedy et al., 1983; Ouimet et al., 1984; Benfenati et al., 1996) and both neural activity and Ca2+ entry are associated with CaMKII holoenzyme (containing both αCaMKII and βCaMKII) self-association and translocation to synapses (Shen and Meyer, 1999; Hudmon et al., 2005). T286 phosphorylation of αCaMKII, however, reduces self-association and translocation (Hudmon et al., 2005), and this may explain the lower levels of synaptic αCaMKII phosphorylation seen in Htr1aknock-out mice. Although the function of extrasynaptic αCaMKII is not well understood, the CaMKII holoenzyme is known to associate with the actin cytoskeleton (Shen et al., 1998). The cytoskeletal modulating effects of αCaMKII have been shown to play a role in dendritogenesis and synaptogenesis (Wu and Cline, 1998; Fink and Meyer, 2002; Fink et al., 2003; Jourdain et al., 2003; Gaudilliere et al., 2004), and we speculate that Htr1a may modulate hippocampal dendritic structure by regulating αCaMKII activity.
Although our findings of a behavioral interaction between the Htr1aKO and Camk2aT286A mutations coupled with our biochemical studies suggest that these molecules act in a common biochemical pathway, other more indirect explanations for these data are conceivable. It is possible, for example, that changes in αCaMKII phosphorylation are a secondary consequence of changes in neuronal activity caused by the absence of the receptor. Alternatively, although increased αCaMKII phosphorylation appears to be restricted to the CA1 region where Htr1a expression is high, we cannot rule out indirect effects of the Htr1aKO and Camk2aT286A mutations in other brain areas on the behavioral consequences of Htr1a signaling. Tissue-specific manipulations of Htr1a and/or αCaMKII would be required to address these possibilities. Furthermore, the failure of heterozygous Camk2aT286A mice to show altered anxiety-related behavior in the absence of the Htr1aKO mutation (Fig. 6) suggests that either altered αCaMKII phosphorylation is necessary but not sufficient to moderate anxiety behavior, or the life-long alterations in αCaMKII function associated with this mutation (Giese et al., 1998) (supplemental Fig. 7, available at www.jneurosci.org as supplemental material) mask its behavioral effects.
In conclusion, we have documented phasic increases in αCaMKII phosphorylation in the CA1 region of the hippocampus in Htr1a knock-out mice during a limited developmental period. Pharmacological blockade of the receptor during this period is sufficient to recapitulate the anxiety phenotype of knock-out mice and a mutation of αCaMKII that decreases phosphorylation is able to reverse the knock-out phenotype. These data place the αCaMKII-signaling pathway downstream of serotonin and suggest that it is likely to play a critical role in the maturation and/or refinement of brain circuits controlling anxiety responses.
Footnotes
This work was supported by the European Molecular Biology Laboratory PhD Program (L.L.I.) and National Institutes of Health Grant MH64948 (C.G.). We thank Karl-Peter Giese for generously providing the Camk2aT286A mice, Pietro Pilo Boyl for helping to establish the synaptosome preparation, Enrica Audero for advice in immunochemistry and critical reading of this manuscript, Valeria Carola for help with statistical analysis, Amaicha Mara Depino for communicating results prior to publication, and Simone Santanelli and Francesca Zonfrillo for mouse husbandry.
References
- Bahr S, Wolff JR. Postnatal development of axosomatic synapses in the rat visual cortex: morphogenesis and quantitative evaluation. J Comp Neurol. 1985;233:405–420. doi: 10.1002/cne.902330309. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus: memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F, Onofri F, Czernik AJ, Valtorta F. Biochemical and functional characterization of the synaptic vesicle-associated form of CA2+/calmodulin-dependent protein kinase II. Brain Res Mol Brain Res. 1996;40:297–309. doi: 10.1016/0169-328x(96)00053-8. [DOI] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain—a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in αCaMKIIT286A and CREBαδ- mice. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Wu J, Plattner F, Errington M, Rowan M, Peters M, Hirano A, Bradshaw KD, Anwyl R, Bliss TV, Giese KP. Autophosphorylation of αCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J Physiol (Lond) 2006;574:805–818. doi: 10.1113/jphysiol.2006.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the α calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Giese KP, Silva A, Fox K. The role of α-CaMKII autophosphorylation in neocortical experience-dependent plasticity. Nat Neurosci. 2000;3:911–918. doi: 10.1038/78820. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology. 2004;29:1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Glazewski S, Pakhotin P, Mizuno K, Chapman PF, Giese KP, Fox K. Neocortical long-term potentiation and experience-dependent synaptic plasticity require α-calcium/calmodulin-dependent protein kinase II autophosphorylation. J Neurosci. 2003;23:4428–4436. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB, Bennett MK, Erondu NE. Biochemical and immunochemical evidence that the “major postsynaptic density protein” is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci USA. 1983;80:7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Betito K, Rowe W, Boksa P, Meaney MJ. Serotonergic regulation of type II corticosteroid receptor binding in hippocampal cell cultures: evidence for the importance of serotonin-induced changes in cAMP levels. Neuroscience. 1992;48:631–639. doi: 10.1016/0306-4522(92)90407-s. [DOI] [PubMed] [Google Scholar]
- Monckton JE, Gross CT, Hen R. Electrophysiological correlates of anxiety in serotonin 1A receptor knock-out mice. Soc Neurosci Abstr. 2002;28:550–1. [Google Scholar]
- Nagy A, Delgado-Escueta AV. Rapid preparation of synaptosomes from mammalian brain using nontoxic isoosmotic gradient material (Percoll) J Neurochem. 1984;43:1114–1123. doi: 10.1111/j.1471-4159.1984.tb12851.x. [DOI] [PubMed] [Google Scholar]
- Need AC, Giese KP. Handling and environmental enrichment do not rescue learning and memory impairments in αCamKII(T286A) mutant mice. Genes Brain Behav. 2003;2:132–139. doi: 10.1034/j.1601-183x.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ. Inducible, pharmacogenetic approaches to the study of learning and memory. Nat Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, McGuinness TL, Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci USA. 1984;81:5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Kantor D, Harris KM, Schuman EM, Kennedy MB. Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci. 1997;17:5416–5427. doi: 10.1523/JNEUROSCI.17-14-05416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Kerr TM, Tecott LH. 5-HT(1A) receptor mutant mice exhibit enhanced tonic, stress-induced and fluoxetine-induced serotonergic neurotransmission. J Neurochem. 2001;77:607–617. doi: 10.1046/j.1471-4159.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull. 1981;7:113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knock-out: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br J Pharmacol. 1999;127:1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIβ functions as an F-actin targeting module that localizes CaMKIIα/β heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the serotonin(1A) receptor in mice results in downregulation of major GABAA receptor α subunits, reduction of GABAA receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga T, Morinobu S, Kawano K, Sawada T, Yamawaki S. Influence of immobilization stress on the levels of CaMKII and phospho-CaMKII in the rat hippocampus. Int J Neuropsychopharmacol. 2004;7:299–309. doi: 10.1017/S1461145704004304. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of αCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci. 1990;600:9–35. doi: 10.1111/j.1749-6632.1990.tb16870.x. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology. 1992;24:145–149. [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]