Abstract
Tumor cells develop resistance to chemotherapeutic drugs through multiple mechanisms. Overexpression of efflux transporters is an important source of drug resistance. Efflux transporters such as P-glycoprotein reduce intracellular drug accumulation and compromise drug efficacy. Various nanoparticle-based approaches have been investigated to overcome efflux-mediated resistance. These include the use of formulation excipients that inhibit transporter activity and co-delivery of the anticancer drug with a specific inhibitor of transporter function or expression. However, the effectiveness of nanoparticles can be diminished by poor transport in the tumor tissue. Hence, adjunct therapies that improve the intratumoral distribution of nanoparticles may be vital to the successful application of nanotechnology to overcome tumor drug resistance. This review discusses the mechanisms of tumor drug resistance and highlights the opportunities and challenges in the use of nanoparticles to improve the efficacy of anticancer drugs against resistant tumors.
Keywords: Nanoparticles, efflux transporters, excipients, endocytosis, efflux inhibitors, tumor penetration, interstitial fluid pressure, extracellular matrix, transport barriers
1. Introduction
Despite major advances in cancer diagnosis and therapy, development of drug resistance and tumor relapse are frequent occurrences [1, 2]. While tumor cells evade death through multiple mechanisms [3], overexpression of efflux transporters is an important source of drug resistance. Tumor cells either inherently express efflux transporters or upregulate their expression in response to chemotherapy. Efflux transporters are capable of actively clearing a wide variety of substrates out of the cells. This results in sub-optimal intracellular drug concentrations and lack of efficacy [4]. Several efforts have been directed at inhibiting efflux transporters in tumors. Many small molecule efflux inhibitors have been tested in combination with chemotherapeutics in the clinic. However, unfavorable pharmacokinetics and significant dose-limiting toxicities have hampered their progress [5–7]. Co-administration of the chemotherapeutic and efflux inhibitor in nanoparticles (NPs) may allow temporal co-localization of these molecules, limit their non-specific distribution, and hence their toxicities [8]. In addition, several studies have shown that some of the excipients used in the construction of NPs are capable of inhibiting efflux transporters [9]. Taken together, nanotechnology offers a promising approach for overcoming efflux pump-based drug resistance (Figure 1).
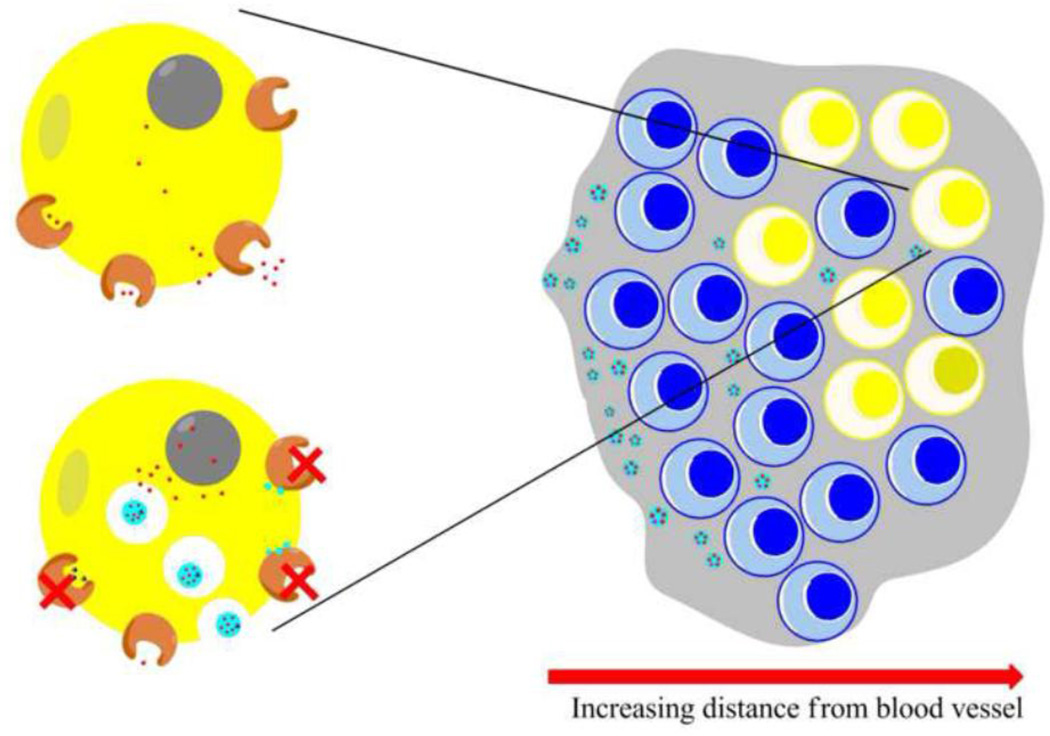
Figure 1. Opportunities and challenges in utilizing nanotechnology to overcome drug resistance.
Nanoparticles can inhibit drug efflux through (i) altered cellular distribution of the encapsulated drug (ii) use of specific excipients and (iii) co-delivery of agents that can inhibit efflux transporters. The drug resistant cells are often located farther away from blood vessels. High transport resistance within tumors limits NPs to regions adjacent to the blood vessels. It is critical that NPs reach areas distant from the blood vessels in order to effectively overcome drug resistance. Key: Blue cells - drug sensitive cells; yellow cells - resistant cells; grey area - tumor ECM; light blue circles - nanoparticle excipients; red dots - chemotherapeutic drug; blue triangles - efflux pump inhibitor
In order for NP-based therapies to be successful, however, it is essential that NPs are transported efficiently to the tumor cells. Tumors are characterized by inadequate blood supply and elevated interstitial fluid pressure (IFP) [10]. As a result, transport in the tumor is highly hindered. Several approaches have been proposed for improving intra-tumoral transport of macromolecules and drug carriers [10]. These include the use of anti-angiogenics [11] and modification of the tumor stroma [12].
In this review, we will focus on these two important issues: (1) the use of nanotechnology to overcome efflux activity in tumor cells and (2) inefficient transport of drugs and drug carriers within the tumor tissue. We will provide a mechanistic perspective of why nanotechnology holds such promise in overcoming drug resistance and why the use of adjunct therapies to improve transport may be critical to the success of nanotechnology-based anticancer therapies.
2. Mechanisms of tumor drug resistance
2.1 Efflux transporters
A majority of chemotherapeutics have intracellular targets. Thus, to kill the tumor cell, many anticancer drugs have to accumulate inside the cell at sufficient concentrations. A major hurdle to achieving adequate intracellular drug concentrations is the presence of efflux proteins on the tumor cell membrane [13, 14]. Drug efflux pumps belong to a family of transporters called the ATP-binding cassette (ABC) transporters. ABC transporters are one of the largest superfamily of proteins. The human genome encodes for 48 ABC proteins. These include 20 transporter proteins, further divided into 7 sub-families, ABC A–G [15]. Some of the important and well-studied transporters include ABCB 1 [P-glycoprotein (P-gp), multidrug resistance protein 1 (MDR1)], ABCC 1–3 [multi-drug resistance associated protein (MRP) 1–3], and ABCG 2 [breast cancer resistance protein (BCRP)]. P-gp was the first discovered efflux transporter [16, 17]. In 1983, Kartner and coworkers first demonstrated the correlation between increased expression of P-gp in tumor cells with the development of drug resistance [18]. This was followed by Chen and others, who described the sequence of the MDR1 cDNA and its homology to two bacterial transporters, thereby defining the first member of the ABC transporter family [19]. Ueda and coworkers demonstrated that expression of a full length cDNA for the human MDR1 gene confers drug resistance in tumor cells, confirming the role of MDR1 gene in drug resistance [20]. It was later discovered that some tumor cells that did not have upregulated P-gp levels could also actively efflux drugs. This led to the discovery of MRP 1 [21]. Since then, additional transporters have been identified and their roles in drug transport have been investigated [15]. Of these, P-gp is one of the most consistently over-expressed transporters in drug-resistant tumors [22].
Even under normal physiological conditions, efflux transporters are widely expressed in the body (reviewed in [23]). Some organs show a particularly high expression of these transporters. For example, P-gp, BCRP and MRP 2 are highly expressed on the apical sides of the lung, testis, placenta, and brain. On the other hand, MRP 1 is highly expressed on the basolateral side of these organs [3, 24–29]. These transporters create a formidable barrier that protects important organs from toxic xenobiotics. Consequently, these transporters play a key role in altering the absorption, distribution, metabolism, and excretion of drugs [30–32].
2.1.1 Structure and mechanism of P-gp
P-gp is a 170 kDa protein with broad substrate specificity [33]. Structurally, it comprises 2 transmembrane domains (TMDs) and 2 nucleotide binding domains (NBDs). The TMDs are hydrophobic domains consisting of 6 transmembrane segments, while NBDs are hydrophilic intracellular domains [34]. NBDs provide a docking site for the ATP molecules. While the exact mechanism by which P-gp interacts with its substrate is not fully understood, it is thought that binding of a substrate to the high-affinity binding site results in ATP hydrolysis, causing a conformational change that shifts the substrate to a lower affinity binding site and then into the extracellular space or outer leaflet of the membrane [35–37]. Whether P-gp extracts its substrate from the cytoplasm [38] or from within the membrane (‘vacuum cleaner’ hypothesis) is not clear, but evidence suggests that substrates diffuse from the lipid bilayer into the drug-binding pocket located in a hydrophobic environment [39, 40]. Studies from our laboratory suggest that drug released into the cytoplasm from NPs is susceptible to P-gp mediated efflux [41]. P-gp overexpression also confers resistance to drugs through mechanisms not directly related to transport. For example, overexpression of P-gp confers resistance to complement-mediated cytotoxicity due to delayed deposition of complement on the plasma membrane [42, 43]. Also, P-gp over-expressing cells are less sensitive to multiple forms of caspase-dependent cell death, including those mediated by Fas ligand [44] and serum withdrawal [45]. Some of the transport-independent effects of P-gp may be explained by the fact that over-expressed P-gp can constitute an important part of the plasma membrane. In Chinese hamster ovary (CHO) cells, P-gp alone accounted for about 20% of the total plasma membrane proteins [46]. This degree of overexpression could affect the activity of other membrane proteins.
2.1.2 Acquired and intrinsic resistance
In vitro studies have shown that the expression of efflux pumps increases in response to chemotherapy. These changes arise from copy number alterations of the gene or increased expression of these genes [47–50]. This change in efflux transporters in response to chemotherapy is evident in clinical studies as well [51]. Abolhoda et al. tested the effect of doxorubicin treatment on five patients with lung metastasis [52]. The authors found that after a 20-minute chemoperfusion, there was a 6–7 fold increase in MDR1 gene expression in these tumors. This phenomenon of upregulation of efflux transporters in response to drug treatment is termed as acquired resistance. Interestingly, Levchenko et al. reported the intercellular transfer of functional P-gp protein from P-gp positive cells to P-gp negative cells both in vitro and in vivo [53]. The transfer occurred between different cell types, and allowed the recipient drug-sensitive cells to survive toxic drug concentrations, leading to increased tumor resistance. This mechanism could explain how some sensitive cells acquire drug resistance.
Another striking feature of efflux transporters is the wide range of substrate specificity [33, 34]. Weakly basic and neutral compounds have been found to be the most vulnerable to these pumps [34, 54, 55]. However, some reports suggest that even acidic compounds can be subject to efflux [56, 57]. A rare common feature is that most compounds transported by these pumps are amphiphilic in nature [34]. The broad substrate specificity and upregulation in response to chemotherapy have serious consequences. Resistance arising from one drug can lead to cross-resistance to other chemotherapeutics that are substrates of the same transporter. Such a resistance is termed as multi-drug resistance (MDR). Because of this phenomenon, sequential chemotherapy or switching to a different drug class may not be useful once a patient develops resistance to one drug class.
A fraction of tumor cells intrinsically have a higher expression of efflux transporters even before exposure to chemotherapy [4]. This phenotype may be a manifestation of tumor microenvironmental conditions, tissue of origin, and/or rampant genetic mutations characteristic of cancer cells [3]. This phenomenon is termed as intrinsic resistance.
Acquired and intrinsic resistances may stem from mechanisms not involving efflux transporters as well. This is especially evident with drugs classified as “targeted therapies” [58]. These drugs target specific aberrant cellular pathways that are essential for the survival of cancer cells. For example, the epidermal growth factor receptor (EGFR) is upregulated in multiple cancers. Activation of EGFR results in the activation of multiple kinases that aid in tumor growth and survival. Hence, antagonists that block EGFR signaling are of considerable interest [59]. However, this enthusiasm has been dampened by the appearance of intrinsic and acquired resistances (reviewed in [60]). Upon continued exposure to EGFR antagonists, tumor cells resort to alternate pathways that enable survival and proliferation independent of EGFR activation [61]. Thus, in spite of EGFR inhibition, there is no effect on the tumor cell viability. On the other hand, some tumors do not rely on EGFR signaling at all. These tumors are intrinsically resistant to EGFR-targeted therapies.
Intracellular detoxification is another mechanism of drug resistance. Such mechanisms enable faster elimination of the drug from within the cell and hence reduce their intracellular concentration [62]. Glutathione conjugation is an example of the detoxification strategy employed by tumor cells [63, 64]. Mellish et al. showed that this mechanism can be upregulated in response to sustained exposure to chemotherapeutics [65]. The authors isolated a human ovarian carcinoma cell line from untreated patients. This cell line was exposed to increasing concentrations of cisplatin for 18 months. The resultant cell line was less susceptible to cell death induced by cisplatin and other platinum containing drugs. The authors found that the resistant cell line had higher levels of glutathione and correspondingly lower intracellular drug concentration [65].
Several anti-cancer agents induce DNA damage to bring about cell death. However, cancer cells can develop mechanisms to increase DNA repair and thereby develop resistance to these drugs. The mechanisms of DNA repair and drug resistance have been reviewed in detail elsewhere [66–68].
2.1.3 Efflux transporters and cancer stem cells
According to the consensus definition, cancer stem cells (CSCs) are minority cells that are capable of potentially unlimited self-renewal owing to assymetric cell division, and have the ability to produce multiple differentiated cell types that constitute solid tumors [69]. Although not fully established, the origins of CSCs could theoretically arise from oncogene activation in normal adult tissue stem cells or through the acquisition of stem cell-like properties via microenvironmentally triggered phenotypic changes in cancer cells. CSCs possess a number of intrinsic properties that contribute to therapy resistance and ultimately disease recurrence [70]. Similar to normal stem cells, CSCs have protective mechanisms against external insults from cytotoxic chemotherapy, which include alterations in the intrinsic apoptotic pathway, DNA repair, and most notably, overexpression of efflux transporters [71].
From the perspective of drug resistance, selective pressures within the tumor microenvironment can result in the generation of intrinsically resistant cells. In addition, standard therapeutic regimens, if ineffective in eradicating tumor cell burden, can result in a residual population of cells displaying acquired resistance. Both resistance mechanisms would ultimately result in the expansion of the CSC fraction within the tumor. These therapy-resistant cells, now considered the CSC population, are known to overexpress ABC transporters [72]. This principle is frequently exploited for their isolation. Rhodamine and Hoechst 33342 fluorescent dyes, substrates of both ABCG2 and ABCB1, are used in the analysis of the so-called side population of cells displaying low dye retention, via flow cytometric techniques. These side population cells display the ability to actively efflux ABC transporter substrates, as well as additional properties ascribed to CSCs, including tumor seeding at limiting dilution, a heightened anti-apoptotic state, relative proliferative quiescence, and resistance to conventional chemotherapy upon sorting these cells from the bulk population [73].
To demonstrate the role of CSCs in acquired resistance, numerous reports document a selective enrichment of the CSC fraction following conventional chemotherapy treatment in vitro. The ovarian cancer cell lines OVCA 433 and HEY, when treated with cisplatin and paclitaxel in vitro, result in cells with increased sphere forming efficiency, CSC marker gene expression, and tumor seeding efficiency in vivo [74]. Immortalized mammary epithelial (HMLE) cells induced to passage through epithelial to mesenchymal transition (EMT) have been shown to display the properties of CSCs. When these cells are spiked into non-CSC enriched HMLE cells and treated with paclitaxel in vitro, the CSCs selectively survive treatment, providing direct evidence for the role of CSCs in acquired drug resistance [75].
Another key reason for the therapeutic resistance of CSCs is their quiescent nature [76]. A number of chemotherapeutic agents are effective only against actively dividing cells. CSCs, like normal stem cells, divide infrequently and produce transient amplifying cells which populate the tumor. Hence, chemotherapy may be effective in eradicating the bulk of the tumor but may lack efficacy against the quiescent stem cell population [70].
2.1.4 Elevated levels of efflux transporters and poor prognosis
There is considerable evidence linking the presence of MDR cells with poor prognosis in cancer [77, 78]. Evidence for the role of P-gp in clinical tumor resistance was first provided by Trock and co-workers, who demonstrated P-gp expression in about 40% of breast cancer samples and its correlation with decreased treatment response [79]. Additional studies [3, 80, 81] further confirm this observation, and suggest that pretreatment P-gp expression is a strong predictor for clinical response to drug therapy [82]. Karaszi et al. examined the response to therapy of 93 acute leukemia patients [83]. These patients were treated with various therapies depending on the disease subtype. Based on a calcein efflux assay, these patients were then classified into MDR+ or MDR− groups. It was found that 72% of the MDR− patients responded to therapy, while only 31% of MDR+ patients did. Consequently, there was a three-fold difference in median patient survival. Similar results were reported by Leith et al., who found that elderly leukemic patients responded poorly to treatment in comparison to younger patients because of elevated P-gp expression [84].
2.2 Sequestration in acidic organelles
In addition to efflux pumps, sequestration in acidic organelles can reduce the bioavailability of anticancer drugs at their intracellular site of action [85]. Anthracyclines such as doxorubicin and daunorubicin accumulate in the nucleus (its site of action) in sensitive cells. In drug resistant cells, these weakly basic drugs are primarily distributed into acidic organelles such as late endosomes and lysosomes [86]. The elevated activity of the vacuolar H+-ATPase pump in drug resistant cells leads to highly acidified pH of these organelles [87, 88]. Basic drugs are expected to be highly ionized under these conditions. This results in their trapping within these organelles and loss of activity. The trapped drug is likely extruded out of the cell by exocytosis [89].
2.3 Resistance to transport of macromolecules and drug carriers
In addition to the tumor cells, the tumor extracellular matrix (ECM) is a source of resistance to chemotherapy. Transport of drug into and within the tumor is extremely inefficient [90, 91]. This leads to regions of high and low drug concentrations in the tumor [92]. The regions receiving lower drug concentrations often harbor the more aggressive and tumorigenic cells [93]. Thus, it is extremely important to achieve therapeutic drug concentrations in these under-supplied regions.
In order to address the issue of inefficient drug distribution, it is essential to understand the processes governing drug transport in the tumor. In the following sections, we provide a brief description of the physiological factors governing intratumoral drug transport.
2.3.1 Transport process in normal tissues
Exchange of fluid and nutrients (as well as drugs) between blood vessels and tissue is governed by several parameters, and this relationship is defined quantitatively by Starling’s law (Figure 2) [94, 95]. There are two components that drive the outward flow of soluble drug molecules into the tissue: the hydrostatic pressure head (arising from convection in the capillaries) and osmotic pressure head (arising from a difference in concentration of solutes). In normal tissues, this net pressure is directed towards the tissue and allows a convenient exchange of nutrients with the vascular compartment. The excess fluid draining into the tissues is cleared by the lymphatic system. As a result, a net negative pressure is maintained [12, 96]. Additionally, each cell in the body is only a few cell-diameters away from the nearest blood vessel. This restricts the distance a solute has to travel in the interstitium to encounter the farthest cell from the blood vessel [97]. Thus, the negative pressure difference and short interstitial distances allow efficient solute transport in normal tissues.
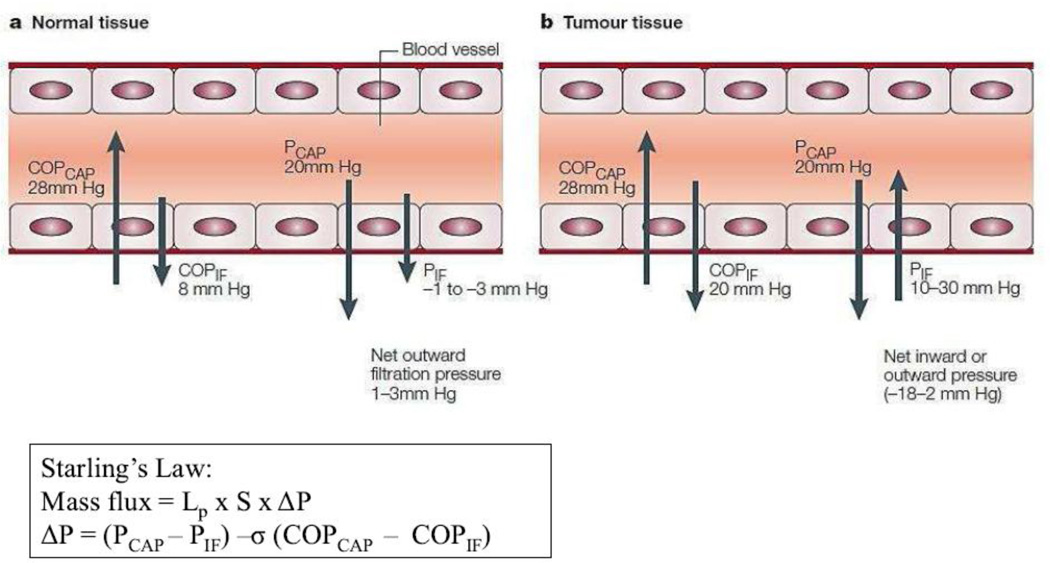
Figure 2. Forces that regulate transcapillary transport in tissues.
The figure shows the hydrostatic and colloid osmotic pressures in capillaries (PCAP and COPCAP, respectively) and the surrounding interstitium (PIF and COPIF, respectively) in normal tissues (a) and tumor tissues (b). It should be noted that values are approximate. (a) In normal human capillaries, the COPCAP is about 28mmHg, which tends to keep fluid in the capillaries. The forces that tend to move fluid out from the capillaries are the PCAP (about 20mmHg), the COPIF (about 8mmHg) and the PIF, which is normally negative (–1 to −3mmHg). So there is normally a net outward filtration pressure from the capillaries in tissues of 1–3mmHg. This outward pressure assures a flow of fluid out from the vessels and through the interstitium, and contributes to transport of molecules to and from cells. (b) In tumor tissues, the COPIF is increased to about 20 mm Hg and the PIF is increased to 10–30 mm Hg, resulting in some tumors in a net outward pressure of about 2 mm Hg (which is similar to that of normal tissues), but in other tumors in a net inward pressure of up to 18 mm Hg. Reprinted with permission from [12]. The equation describes Starling’s law. Mass flux across the capillaries is governed by the hydraulic conductivity (Lp), blood vessel surface area (S), and pressure difference (ΔP). σ indicates the reflection co-efficient.
2.3.2 Transport process in the tumor
Differences in tumor and normal tissue physiologies give rise to major obstacles to drug delivery to and within the tumor [98, 99]. The direction and magnitude of the driving force for drug transport is not constant throughout the tumor. This inconsistency in the driving force arises from the differences in the functionality of the lymphatic system. The advancing edge of the tumor exhibits normal lymphatic drainage. As a result, the fluid entering the tumor from the blood vessels is cleared normally [100]. This helps maintain a negative pressure gradient in this part of the tumor, similar to that in normal tissues [101]. However, the core of the tumor experiences no such driving force for drug transport. The lymphatic vessels are usually collapsed and show minimal hydraulic conductivity in the center of the tumor. As a result, excess fluid entering the tumor is not drained from this central core [102, 103]. Hence, the pressure differential in the core of the tumor is often in the opposite direction, i.e., from the tumor towards the blood vessels [12]. Additionally, the core of the tumor is characterized by poor vessel coverage [104–106]. Consequently, drug transport to the core of the tumor is reliant on the diffusion of the drug from well-perfused peripheral regions [107]. These disparities in normal and tumor physiologies arise from two major factors: angiogenesis and tumor microenvironment.
2.3.3 Angiogenesis
Tumor cells divide more rapidly than normal cells. As the tumor grows larger, it can no longer survive on the pre-existing blood vessels of the surrounding normal tissue. Tumors produce potent growth factors such as vascular endothelial growth factor (VEGF), which enable the sprouting of new blood vessels from existing ones. This process is known as angiogenesis [108, 109]. In normal tissues, this process is tightly regulated and involves a balance between pro- and anti-angiogenic factors, leading to well-formed blood vessels with a hierarchical architecture. In contrast, tumors are characterized by a pro-angiogenic environment. This results in poorly developed vascular anatomy (lack of pericyte coverage and leakiness) and architecture (dead ends and irregular flow patterns) [110–112].
The leakiness of tumor blood vessels leads to expulsion of vascular components including excess fluid into the tumor interstitium. The lack of lymphatic drainage restricts the removal of fluids and other vascular components from the tumor microenvironment. Presence of these vascular components in the limited ECM space results in elevated interstitial fluid pressure (IFP) [99, 101, 113–115] and prevents the entry of drugs into the tumor [116]. Elevated IFP has been shown to correlate with poor response to chemotherapy and immunotherapy [117]. A study by Curti and coworkers followed the response of 6 non-Hodgkin’s lymphoma patients to chemotherapy. The patients received a chemotherapy combination consisting of either ProMACE CytaBOM (cyclophosphamide, doxorubicin, etoposide cytozar, bleomycin, vincristine, methotrexate and prednisone) or EPOCH (etoposide, prednisolone, vincristine, doxorubicin and cyclophosphamide). Tumor IFPs were monitored before and after treatment. The authors found that the responders showed a lower pre-treatment IFP as compared to the non-responders. Additionally, the tumor IFPs of the responders decreased with treatment while that of the non-responders increased. This study also monitored the IFP of 10 melanoma patients treated with interleukin-1 and 2 based immunotherapy. The melanoma nodules that responded to immunotherapy showed a lower IFP than those that did not respond to immunotherapy. These results show that elevated IFP can significantly decrease therapeutic efficacy of multiple treatment modalities.
2.3.4 Reactive tumor microenvironment
Overexpression of cross-linking agents such as lysyl oxidase in tumors leads to a higher degree of polymerization of biopolymers like collagen and hyaluronic acid [118, 119]. Moreover, fibroblasts in the tumor microenvironment are in an activated state. Activated fibroblasts, or myofibroblasts, secrete copious amounts of ECM components. Additionally, myofibroblasts use specialized receptors on their cell surface to engage these biopolymers and increase the overall ECM rigidity [120, 121]. All these factors contribute to a reactive tumor microenvironment and an increased resistance to diffusional drug transport within the tumor ECM [122].
It should be noted, however, the rigidity of tumor ECM is likely heterogeneous. Overexpression of matrix metalloproteinases is a hallmark of many tumors and is associated with increased tumor invasiveness [123]. The expression of this enzyme brings about proteolysis of the collagen fibrils in the tumor [123], and should thus reduce matrix stiffness. However, this reduction in matrix stiffness may be spatially and temporally limited. Tumor cells present on the periphery are more likely to migrate [124]. Hence the stiffness of the bulk of the tumor may not be affected by the expression of the protease.
2.4 Acidic and hypoxic microenvironment
Limited solute distribution within the tumor ECM also means reduced transport of oxygen to the tumor cells. This leads to regions within the tumor that are hypoxic [125, 126]. Hypoxia can directly and indirectly affect the effectiveness of chemotherapy (reviewed in [127]). Many cytotoxics (eg.bleomycin) and photosensitizers (eg. porphyrin) rely on the production of free radicals for their activity [128, 129]. The activity of these drugs is compromised under hypoxia. Additionally, hypoxia reduces cell proliferation [130]. Since a number of anti-cancer drugs selectively kill rapidly dividing cells, these drugs are relatively ineffective in these regions.
Hypoxia leads to stabilization of an otherwise labile transcriptional factor called hypoxia inducible factor 1α (HIF1α) [131, 132]. This leads to the activation of several genes associated with the hypoxia responsive element (HRE). MDR1 gene is one of those genes regulated by HRE [133]. As discussed before, upregulation of MDR1 gene leads to resistance to drug therapy. It is thus conceivable that the hypoxic regions are rich in cells expressing the efflux transporters.
Tumor cells rely on glycolysis for energy production. This phenomenon is termed as the Warburg effect [134, 135]. Excess glycolysis, leads to the generation of lactic acid. Poor transport of nutrients in tumors is coupled with poor drainage of waste products as well. Thus, lactic acid is not cleared from the tumor efficiently, resulting in a drop in local pH. The acidic microenvironmental pH leads to ionization of weakly basic drugs such as doxorubicin in the tumor ECM. Since ionized drugs do not cross cell membranes efficiently, the acidic microenvironment limits intracellular drug accumulation and can, thus, lead to a loss in therapeutic efficacy [136].
3. Approaches to overcome tumor drug resistance
3.1 Inhibition and evasion of drug efflux
Upregulation of efflux transporters is correlated with poor prognosis in a number of cancers [77]. Consequently, a significant body of research has been directed towards overcoming drug resistance by inhibiting or circumventing these transport processes. The use of NPs has been central to many of these efforts [137]. NP-based therapies can be broadly categorized into three different approaches. In the first approach, efflux activity is inhibited through the use of specific formulation excipients. In the second approach, drug efflux is bypassed by altering the intracellular distribution of the drug. Co-delivery of specific inhibitors is another strategy to inhibit drug efflux.
3.1.1 Use of excipients that inhibit efflux transporters
NPs are multicomponent systems consisting of various excipients including polymers, lipids, and/or surfactants. While these materials have traditionally been considered inert, several studies have documented their ability to inhibit efflux activity.
3.1.1.1 Surfactants
Surfactants are amphiphilic molecules comprising both hydrophilic and hydrophobic groups. At concentrations above critical micellar concentration (CMC), surfactants self-assemble to form micelles. In aqueous solutions, the hydrophobic core of micelles can be used to solubilize lipophilic anti-cancer agents [138]. Surfactants are also used to stabilize the surface of polymeric or lipid NPs to form stable amphiphilic colloids in physiological fluids [139–141]. Thus, surfactants are arguably one of the most widely used excipients in nano drug delivery systems [142, 143].
The potential of surfactants to sensitize resistant cells to chemotherapeutics was first reported in drug-resistant CHO cells [144]. Since then, many groups have investigated the use of surfactants to inhibit efflux transporters [145]. Woodcock et al. showed that various surfactants were capable of overcoming drug resistance, with Cremophor® EL being the most potent [146]. Pre-treatment or concomitant treatment of MDR cells with Cremophor® EL significantly increased the cellular uptake and retention of daunorubicin. This effect resulted from enhanced membrane fluidity in the presence of the surfactant. Using fluorescence anisotropy, the authors observed a progressive decrease in membrane viscosity with an increase in surfactant concentration [147]. However, the use of Cremophor® EL has been associated with several toxicities including hypersensitivity and peripheral neuropathy [148]. This has significantly limited the use of this excipient in clinical practice.
Pluronics are another class of surfactants that are extensively used in NP formulations [149, 150]. Pluronics are A-B-A type of block co-polymers consisting of poly(ethylene oxide) and poly(propylene oxide) blocks. They have been shown to inhibit efflux transporters in different MDR-cell types [151]. In fact, multiple mechanisms have been attributed to their activity (Figure 3) (reviewed in [9]). In their seminal mechanistic studies, Batrakova et al. showed that pluronic-85 brought about a concentration-dependent depletion in intracellular ATP levels [152]. This energy depletion led to a decline in the activity of efflux transporters. Using confocal microscopy, the same group later showed colocalization of fluorescently labeled pluronic-85 with mitotracker red, a fluorescent label for mitochondria [153]. This provided additional evidence supporting the role of pluronics in interfering with mitochondrial processes and cellular energetics. Similar to the studies with Cremophor® EL, pluronic-85 also showed an increase in cell membrane fluidization [153]. It is possible that the changes in membrane permeability induced by surfactants are relevant not only to the cell membrane but also to intracellular organelle membranes. This may cause a loss in polarity of mitochondrial membranes and a depletion of cellular ATP.
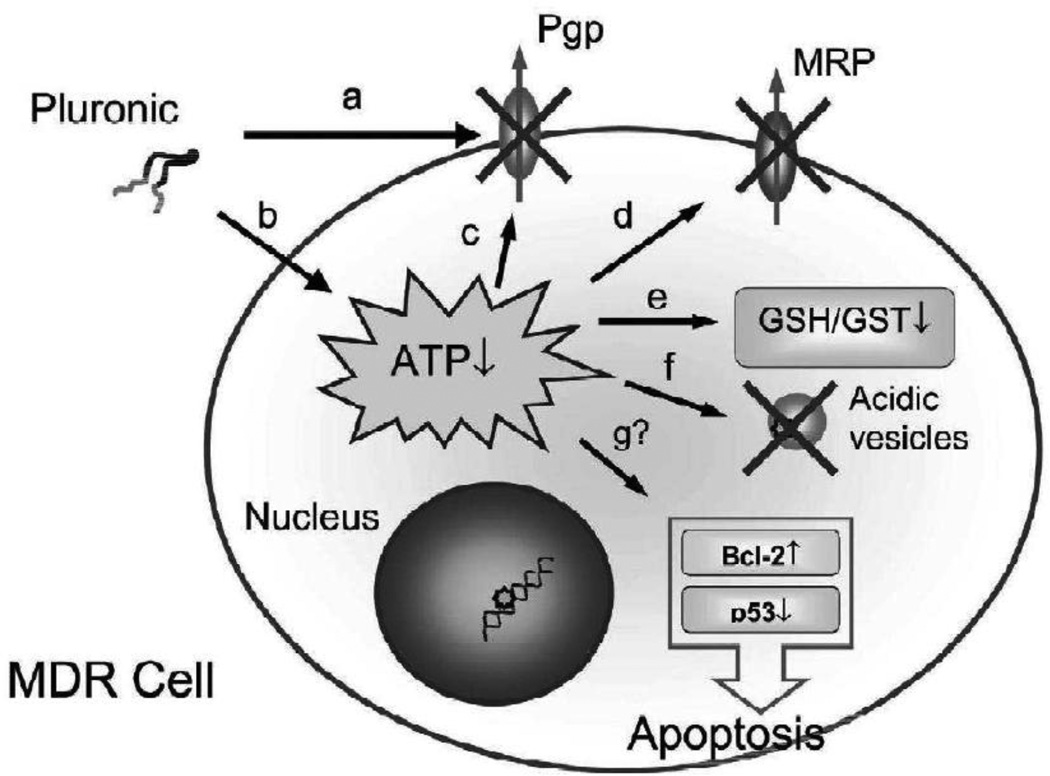
Figure 3. Schematic presenting multiple effects of Pluronic block copolymers displayed in MDR cell.
These effects include (a) decrease in membrane viscosity (‘fluidization’); (b) ATP depletion; (c, d) inhibition of drug efflux transport systems; (e) reduction in GSH/GST detoxification activity; and (f) drug release from acidic vesicles in the cell. Effects of Pluronic block copolymers on apoptosis (g) are not sufficiently studied at present. Reprinted with permission from [9].
Based on pre-clinical efficacy data, a pluronic formulation of doxorubicin, SP1049C, is in clinical trials [154]. SP1049C contains a mixture of two pluronics, L61 and F127. Results from a phase II clinical trial in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction were reported recently for this formulation [154]. These studies showed that the objective response rate in these patients was 47%. Previous clinical trials with doxorubicin have documented an objective response rate of ~20%. The improved response rate with SP1049C is highly promising and suggests that this formulation will likely have an impact on tumor drug resistance.
Other surfactants have shown comparable efficacy in preclinical studies [145]. For example, polyoxyl 15 hydroxystearate (solutol HS15) has shown potent activity in overcoming drug resistance. Coon et al. showed that treatment of drug resistant KB8-5–11 carcinoma epidermoid cells with solutol HS15 increased their sensitivity to doxorubicin [155]. Similarly, a recent study showed that paclitaxel encapsulated in lipid NPs stabilized with polyoxyethylene 20 stearyl ether (Brij® 78) had enhanced cellular uptake and efficacy. The authors confirmed that this action was due to ATP depletion caused by the surfactant [156, 157]. NPs stabilized with d-alpha tocopheryl polyethylene glycol 1000 succinate (Vitamin E TPGS) also showed a similar effect [158].
With a multitude of surfactants demonstrating efflux inhibition, some studies have attempted to identify structural features of the surfactant that are key to achieving maximal activity [159]. One such study focused on various esters of ethylene oxide and fatty acids. Two variables were evaluated: the type of fatty acid and the molar ratio of ethylene oxide to fatty acid. The unsaturated version of C18 fatty acid (oleic acid) resulted in better MDR modulation than the saturated C18 analog, stearic acid. In contrast to stearic acid, 12-hydroxy stearic acid did not show any effect on efflux transport [160]. Maximal efflux inhibition was found at a molar ratio of 20:1. This study shows that optimizing the ratio of the hydrophilic fraction (ethylene oxide) to hydrophobic fraction (fatty acid) is essential to maximizing the activity. In another study, Lo compared various surfactants ranging in HLB values from 4 to 40. A maximal inhibition of efflux transport was seen at HLB values between 10 and 17 [161]. Surfactants with different HLB values may vary in their ability to partition into the cell membrane, and this may explain the effect of HLB values on the MDR inhibitory activity of surfactants.
3.1.1.2 Polymers
Polymers lacking amphiphilic properties have also been shown to be useful in overcoming drug resistance. In particular, poly(alkyl cyanoacrylate) has been extensively studied for its ability to improve the intracellular transport of chemotherapeutics [162–164]. An interesting mechanism, distinct from the ones discussed before, was proposed by de Verdière et al. [165]. Doxorubicin, by itself, was ineffective against the drug-resistant P388-ADR leukemia cell line. However, NP-encapsulated drug showed a significantly higher toxicity. On further investigation, the authors found that NPs were not internalized effectively into cells, thus ruling out enhanced cellular uptake as a possible mechanism of improved efficacy. A degradation product of the polymer, poly(cyano acrylic acid), was found to form a complex with the cationic drug. This uncharged complex was transported into the cells much more efficiently than the charged drug molecule [165].
Another mechanism suggested by this research relates to saturation of efflux transport. NPs that rapidly release their entire payload near the cell membrane could achieve very high local drug concentration and thus saturate the efflux transporter. The authors showed that poly(isobutyl cyanoacrylate) NPs (showing rapid drug release) successfully overcame drug resistance through saturation of efflux activity. However poly(isohexyl cyanoacrylate) NPs (showing a slower drug release) were ineffective in saturating the efflux transporters. The proposed saturation mechanism is plausible in vitro where the concentration of the drug used was ~0.1–10 µg/mL [165]. However, such high local concentrations may not be achievable in vivo, potentially limiting the significance of this mechanism.
It was later shown that doxorubicin encapsulated in poly(isohexyl cyanoacrylate) NPs could successfully overcome tumor drug resistance in vivo [166]. In a chemo-resistant transgenic mouse model of hepatocellular adenocarcinoma, the authors found that free doxorubicin showed a modest cytotoxic effect. However, there was almost a 3-fold increase in the apoptotic index when doxorubicin was encapsulated in NPs [166]. This was likely due the formation of an uncharged complex between the drug and the degradation product of the polymer, leading to higher intracellular drug concentrations.
Another polymer with reported P-gp inhibitory potential is poly(ethylene glycol) (PEG) [167–169]. PEG is extensively used in NP formulations to provide a hydrophilic corona, to stabilize carriers in physiological fluids, and to evade macrophage uptake [170]. In a rat intestinal model, Shen et al. showed that various molecular weights of PEG were capable of inhibiting the P-gp-mediated efflux of rhodamine-123 [171]. However, PEG was not very potent in inhibiting P-gp. For example, PEG 20,000 decreased the secretory transport of rat intestinal membrane by ~65% at a concentration of 5% w/w. In contrast, pluronic-85 showed a 50% depletion in ATP levels at a concentration as low as 0.00067% w/w [9]. Additionally, it remains to be seen if such an inhibition is capable of reversing drug resistance in tumor cells.
3.1.2 Efflux bypass by altering sub-cellular localization of drug
3.1.2.1 Endocytosis Vs. diffusion
Most drug molecules enter cells by diffusion across the cell membrane [172]. This unprotected passage of drug through the cell membrane makes it vulnerable to the action of efflux transporters [172]. NPs are too large for diffusion-mediated transport. NP-encapsulated drug is taken up through endoctytic vesicles, which deposit the drug in the perinuclear regions, away from the cell membrane and closer to its site of action [173–176]. This can lead to a higher intracellular concentration of the drug and greater therapeutic activity (Figure 4).
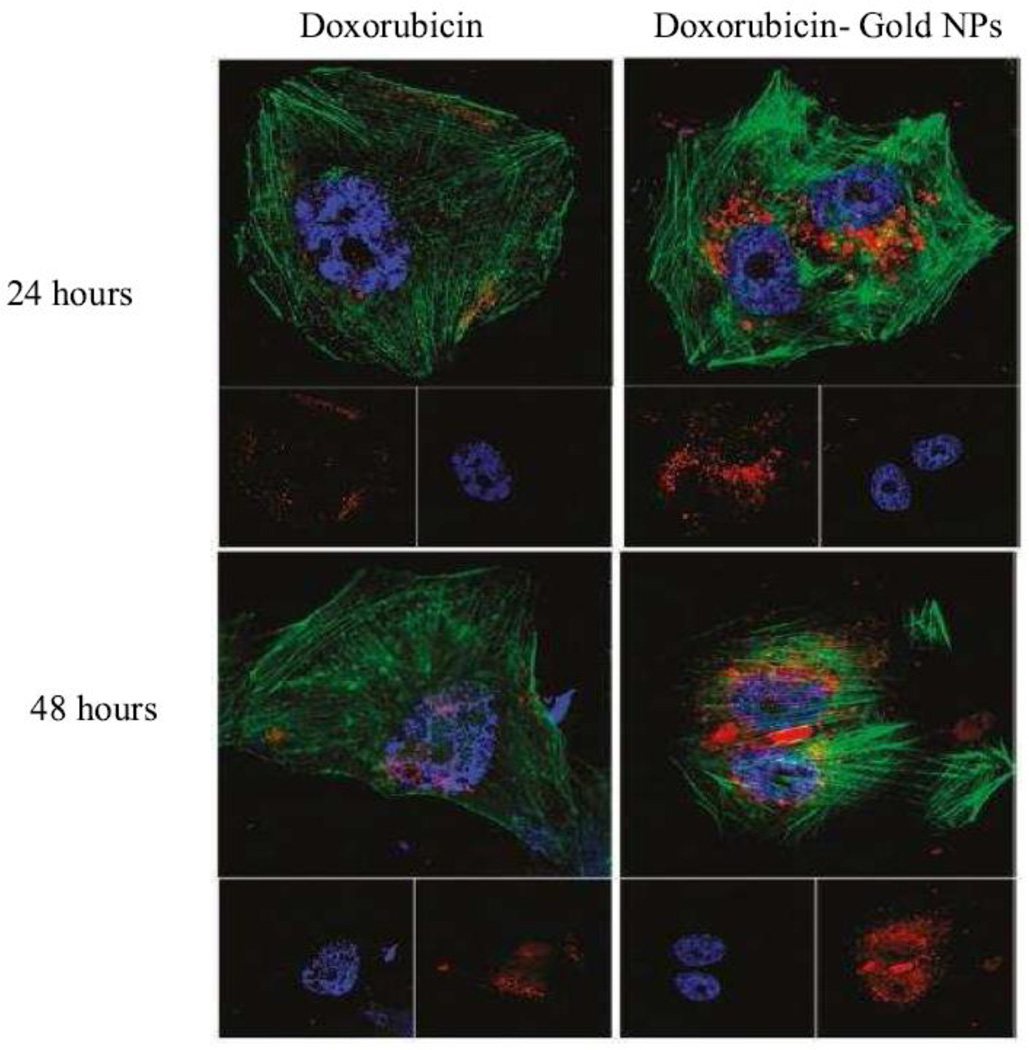
Figure 4. Different intracellular localization of NPs and free drug.
Confocal laser microscopic observation of MCF-7/ADR cells incubated with free doxorubicin (DOX) or DOX-tethered AuNPs for 24 and 48 h. The dose of doxorubicin or its equivalent was 5 µg mL1 in the cell culture. The cells were counterstained with DAPI (blue) for the cell nucleus and Alexa Fluor 488 phalloidin (green) for the cell membrane. Reprinted with permission from [187]. © 2011 American Chemical Society.
Though altered drug distribution was regarded as one of the mechanisms for NP-mediated MDR reversal, there was a lack of convincing evidence supporting this hypothesis [176–178]. Recently, a lipid-polymer NP system developed by Wong et al. offered some interesting insights [179]. The authors showed that the excipients used in the system did not have any effect on the efflux transport process [180]. Only encapsulation of the drug within this “hybrid” carrier resulted in a change in the sub-cellular distribution of the drug. This led to a reversal of drug resistance in a human cell line MDA435/LCC6/MDR1, and a mouse cell line, EMT6/AR1. This reversal was attributed to altered route of entry of the drug into the cells [179]. In another study, surfactant-polymer NPs loaded with doxorubicin were tested in NCI-ADR/RES cells [181]. The authors found that NP-encapsulated doxorubicin was significantly more cytotoxic than the free drug. Previous reports had shown that the polymer (alginate) used in these studies had no effect on drug efflux [162].While the surfactant used in the formulation (docusate sodium) may have P-gp inhibitory activity, the mechanism of efflux inhibition was not investigated. However, the intracellular localization of the drug was different when the drug was administered in the form of NPs.
Additional evidence for the role of altered intracellular distribution was provided by extensive work done in the field of polymer-drug conjugates. Polymer-drug conjugates, similar to NPs, are unable to enter the cell via diffusion. Omelyanenko et al. showed that the uptake of N-(2-hydroxypropyl) methacrylamide (HPMA) – adriamycin conjugate by endocytosis led to higher intracellular concentrations and higher potency in A2780/AD resistant ovarian cancer cell line [172, 182]. Confocal laser scanning microscopy confirmed that the increased potency was due to an altered route of entry into the cells for the drug-polymer conjugate.
3.1.2.2 Triggered intracellular drug release
An inherent limitation of NP systems is the leakage of drug while the carrier is in systemic circulation. As a result, a fraction of the drug is still subject to efflux. This decreases the targeted bioavailability and hence the effectiveness of the drug. An interesting approach to overcome this limitation is to trigger drug release in response to specific intracellular cues. Upon endocytosis, NPs are trafficked into early and late endosomes, which eventually fuse with lysosomes. This exposes NPs to a gradually decreasing pH environment. Several groups have utilized this low pH as a trigger to release drug from NPs [183–186]. These systems ideally show no or limited drug release at physiological pH.
A detailed investigation of such a system was reported by Wang and coworkers [187]. This group used gold NPs covalently conjugated to doxorubicin using a PEG spacer. Conjugation of doxorubicin to PEG was done via either a pH-sensitive hydrazone bond or a pH-insensitive carbamate bond. When conjugated to the NP surface, the close proximity of gold and doxorubicin quenched the fluorescence of the drug. This allowed for evaluation of the intracellular drug release. In comparison to that with the free drug, the intracellular concentrations achieved with NP-conjugated drug were higher in the drug resistant MCF-ADR cells but not in drug sensitive MCF-7 cells. However, drug conjugated via the pH-sensitive hydrazone bond but not via the carbamate bond was successfully released intracellularly. This resulted in a significant decrease in the IC50 values of doxorubicin encapsulated in the pH-sensitive formulation. In fact, the IC50 values of free drug and drug bound via the carbamate bond were identical. This report highlights two important properties a formulation should possess. First, it should be able to protect the drug from efflux pumps. Second, the formulation should be able to release the drug in the perinuclear regions, away from the efflux pumps and near the site of drug action [187].
A similar phenomenon has been shown by other groups using iron oxide nanoparticles [188], polymer micelles [189], and liposomes [190]. All these reports suggest the need to protect the drug from the environment until the drug reaches its target site of action.
3.1.2.3 Altering rate of drug release at the site of action
The rate of drug release has also been shown to play an important role in overcoming drug resistance. Gao et al. reported an elegant example of NP-engineering to improve drug delivery to resistant cancer cells [191]. This group synthesized doxorubicin loaded mesoporous silica NPs with varying pore sizes. With an increase in pore size, the rate of drug release from these particles increased. However, NPs showed drug release only under acidic conditions such as those found in late endosomes.
The authors found that free drug and NP-loaded drug were taken up to the same extent by sensitive MCF-7 cells. However, encapsulation in NPs resulted in a dramatic increase in the uptake of doxorubicin in resistant cells. On further investigation, the authors found that there was a significant difference in the intracellular drug concentrations and cytotoxicity achieved by the different NP-formulations in resistant cells. NPs that showed rapid drug release resulted in the highest intracellular drug concentrations and hence highest potency. Faster release, following uptake, led to a rapid increase in intracellular concentrations and greater cytotoxicity in vitro [191]. It must be noted, however, that even under acidic conditions, NPs released only 30–35% of their cargo over 30 hours. Hence, a major portion of the drug would likely remain bound to NPs and be potentially unused.
3.1.3 Simultaneous delivery of drugs and efflux inhibitors
In addition to the serendipitous use of active excipients, multiple pharmacologically active agents have been used intentionally for inhibiting efflux transporters. Initial studies were performed with 'first generation' inhibitors such as cyclosporine and verapamil, which were already in use for other indications [192]. Clinical trials with these agents failed to prove the role of P-gp in drug resistance [193]. A number of factors such as absence of confirmation of P-gp expression in the tumors and unexpected dose-limiting toxicities of P-gp inhibitors could have contributed to this failure [5]. In 2001, List et al. published the long-term results of treatment of acute myelogenous leukemia with daunomycin in combination with the P-gp inhibitor cyclosporine [194]. These results were the first to indicate the survival advantage of the combination treatment. Second generation inhibitors (e.g., PSC 833, VX-710) were developed solely for the purpose of overcoming drug resistance [195]. These agents were tested in clinical trials in various malignancies for which there was evidence of P-gp expression or were associated with a poorer therapeutic outcome [196]. One major limitation of these trials, however, was the reduction in anticancer drug doses that was required with concurrent administration of the inhibitor [6]. P-gp inhibitors increased the serum levels of the co-administered chemotherapeutic drug. Due to this pharmacokinetic interaction, the dose of the drug had to be reduced. A number of studies found that this reduction in dose led under-treatment of patients, which could have contributed to the failure of these combination treatments [6]. Pharmacokinetic interactions between the P-gp inhibitor and the drug could also result from inhibitors’ ability to inhibit other proteins involved in drug metabolism such as cytochrome P450 [7]. Third-generation inhibitors (tariquidar, zosuquidar, laniquidar, and ONT-093) have high potency and greater specificity for P-gp.
A primary concern even with the third generation inhibitors is that these agents may increase the side effects of chemotherapy by blocking physiological anticancer drug efflux from normal cells [197]. This is a relevant concern, because P-gp plays important roles in the physiological regulation of endogenous compounds and xenobiotics in the body [198]. It is therefore important to limit the exposure of normal cells and tissues to the efflux inhibitor and anticancer drug combination. Secondly, the differences in physico-chemical properties of the anticancer drug and efflux inhibitor may result in differences in the pharmacokinetics and tumor accumulation of the two agents. For optimal efficacy, both the drug and the inhibitor need to be temporally co-localized in the tumor cells.
Nano drug delivery platforms have the potential to overcome MDR by enabling simultaneous delivery of chemotherapeutics and efflux inhibitors. For example, administration of vincristine and verapamil in a single co-encapsulated poly(lactic-co-glycolic acid) (PLGA) NPs was more effective means of reversing drug resistance in vitro, than either single agent in multiple MDR cell lines [199, 200]. Similarly PLGA NPs loaded with both paclitaxel and tariquidar were effective in inducing cytotoxicity in drug resistant cell lines JC and NCI/ADR cells in vitro and in vivo (Figure 5) [8]. Polymer-lipid nanoparticle systems containing tristearin and steric acid as lipid components, with pluronic F68 polymer, was able to efficiently coencapsulate doxorubicin and elacridar and overcome MDR in a drug-resistant breast cancer cell line [201].
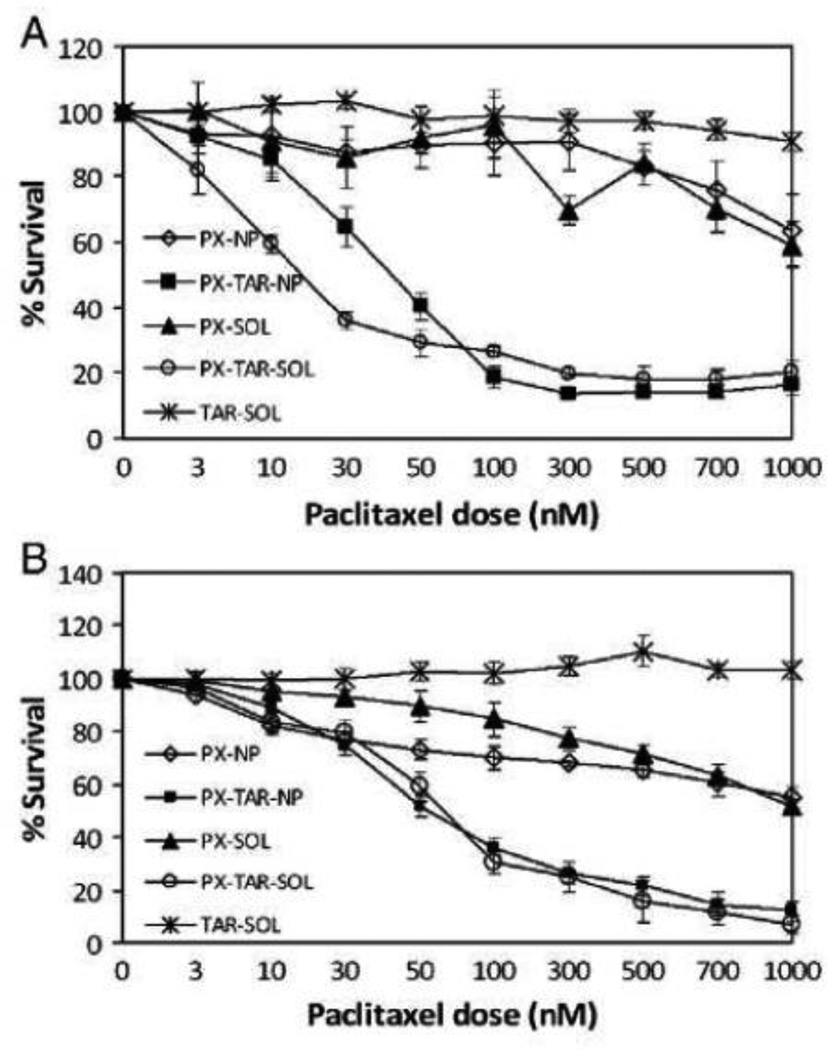
Figure 5. Enhanced cytotoxicity of dual agent nanoparticles in drug-resistant cell lines.
(A) JC and (B) NCI-ADR-RES. Cells were incubated with treatments for 24 h and the cell viability was determined by MTS assay. Legend: PX-NP — Nanoparticles containing paclitaxel; PXTAR-NP — Nanoparticles containing paclitaxel and tariquidar; PX-SOL — Paclitaxel insolution; PX-TAR-SOL — Paclitaxel and tariquidar in solution; and TAR-SOL— Tariquidar in solution. Data as mean±S.D.; n=10. Reprinted with permission from [8].
Another strategy involves the use of NPs containing both a chemotherapeutic agent and siRNA targeting the P-gp transcript. When using P-gp gene silencing to overcome drug resistance, the potential for kinetic differences in gene silencing and the availability of chemotherapeutic agents at the target site is a concern. Differences in size, biodistribution, and other physical characteristics of siRNA-transfection complexes and small molecule chemotherapeutics could give rise to differences in biodistribution. However, for optimum efficacy, the chemotherapeutic agent should be available at its target site when the gene is silenced. The use of mesoporous silica nanoparticles loaded with doxorubicin and siRNA targeting the P-gp transcript demonstrated synergistic inhibition of tumor growth than the single agent-loaded NPs in an orthotopic model of breast cancer [202]. In a similar study, poly(ethylene oxide)-modified poly(beta-amino ester) (PEO-PbAE) and PEO-modified poly(epsilon-caprolactone) (PEO-PCL) NPs were formulated to efficiently encapsulate MDR1 silencing siRNA and paclitaxel. Combination of MDR1 gene silencing and nanoparticle-mediated delivery significantly improved the cytotoxic activity of paclitaxel in SKOV3TR cells [203]. Active targeting of nanoparticles to cancer cells via biotin-functionalized PLGA NPs loaded with both P-gp-targeted siRNA and paclitaxel was able to overcome drug resistance in vitro as well as in vivo [204].
Encapsulation of efflux inhibitors in NPs can potentially limit the distribution of these agents and significantly limit their side effects. However, altered biodistribution of NP-encapsulated drug can have unintended consequences. As observed with Doxil, encapsulation of doxorubicin in liposomes was able to limit its cardiotoxicity. However, new side effects such as hand-foot syndrome and mucositis were observed because of certain physicochemical properties of the formulation [205]. Hence, it is possible that the side effects of efflux inhibitors may not be completely eliminated with the use of nano-encapsulation. However, if these newer side effects are milder than the existing ones, nanotechnology may still be an attractive alternative.
3.2 Improving transport
The vast body of evidence supporting the reversal of drug resistance by NPs offers a promising strategy to overcome an important problem in cancer therapy. In order to maximize this potential, it is critical that NPs (or at least the released therapeutic agent) reach every tumor cell. However, NPs are often limited to regions immediately adjacent to the blood vessels [90, 92]. Paradoxically, it is the regions away from the blood vessels that are rich in drug-resistant and aggressive cells [93]. The ability of NPs to overcome MDR will hence be realized only if they reach these poorly-perfused regions. Thus, any discussion of the use of nanotechnology to overcome drug resistance is incomplete without considering the problem of transport resistance in tumors. Several adjunct therapies have been proposed to enhance the transport of molecules in the tumor ECM. We provide here a summary of the progress made in this field and their possible implications for overcoming MDR using nanotechnology.
3.2.1 Inhibition of angiogenesis to improve drug delivery to tumors
Jain and co-workers proposed that the delivery of drugs to tumors is limited because of a faulty “delivery system” [111]. This “delivery system” referred to the blood vessels supplying the tumor. The leakiness of tumor blood vessels contributes to elevated IFP in tumors [116]. Consequently, it was hypothesized that repairing the tumor vasculature could reverse the elevated IFP. This would, in turn, lead to improved drug delivery and penetration. This process of inhibiting tumor vasculature and restoration of a normal phenotype has been termed as vascular normalization [111]. Such normalization includes various characteristics such as increased pericyte coverage, decreased vessel diameter, decreased blood volume, establishment of vessel hierarchy and enhanced tissue coverage by the blood vessels.
However, literature reports have been somewhat equivocal about the utility of this technique [206–209]. Some studies show that decreasing vascular permeability improves the delivery of drugs to the tumors [210]. Others suggest that increasing vascular permeability may improve drug delivery [211, 212]. Some of these conflicting results can be attributed to differences in tumor models used, and the inherent heterogeneity between tumors. Some reports suggest that lack of techniques to monitor and characterize the phenomenon of vascular normalization limits our understanding [111]. Nevertheless, a huge body of research has established that inhibiting angiogenesis is a highly effective but a temporary method to improve drug delivery and penetration into solid tumors [207, 213, 214]. In the following sections, we will summarize the pre-clinical and clinical studies that have investigated different strategies for inhibiting tumor vasculature.
3.2.1.1 VEGF inhibitors
Amongst several pro-angiogenic factors, VEGF is one of the most potent [109, 215]. It acts through tyrosine kinase receptors VEGFR1 and VEGFR2 [216, 217]. Initial efforts to inhibit VEGF resulted in the discovery of bevacizumab, a humanized monoclonal antibody that binds to VEGF and prevents its activity [218]. It is the first anti-angiogenic approved by the FDA for multiple indications including colorectal, lung, renal cancers, and glioblastoma [218, 219]. Other VEGF inhibitors include pazopanib, sorafenib, sunitinib, and vandetanib. Although inhibitors of the VEGF pathway have shown only modest efficacy as a monotherapy [220, 221], they hold tremendous promise in improving the delivery of co-administered chemotherapeutics [222].
The initial motive behind using VEGF inhibitors for monotherapy was to inhibit angiogenesis and ‘starve’ the tumor [223, 224]. The redundancy of angiogenic pathways has limited the clinical utility of this approach [225, 226]. Yet, certain transient morphological and functional changes to vasculature in response to VEGF inhibition (vascular normalization) leads to decreased IFP and improved drug delivery [227].
Tong et al. showed that DC101 (VEGFR2 blocker) could cause vessel normalization in mouse xenograft models of small cell lung cancer and glioblastoma [228]. This resulted in a significant decrease in vascular permeability and IFP. The decrease in IFP led to improved penetration of macromolecules like albumin and lectin in these tumors. Using immunostaining, the authors determined that there was no change in the lymphatic drainage from the tumor, suggesting that the decrease in tumor IFP was only due to the changes in the blood vessels [228].
3.2.1.2 Other targets for vascular normalization
Several other molecular targets have been explored for vessel normalization [12, 229]. The EGFR is upregulated in multiple cancers [230]. A consequence of EGFR activation is the increased secretion of VEGF. Thus, VEGF secretion can be decreased by inhibiting EGFR [231, 232]. In a recent report, Cerniglia and co-workers [233] showed that inhibiting the EGFR pathway could lead to vessel normalization. Treatment with erlotinib (an EGFR inhibitor) led to a decreased expression of VEGF, increased tumor perfusion and increased delivery of cisplatin. This resulted in enhanced therapeutic activity of cisplatin as compared to that with drug administration alone [233]. However, inhibiting the EGF pathway has resulted in a mixed response in clinical trials with no, moderate or good results [234–238].
Phosphoinositol-3-kinase (PI3K), like EGF, is another element upstream of VEGF. Qayum et al. showed that inhibiting PI3K leads to vessel normalization and improved therapeutic response to doxorubicin [239]. Similarly, selenium agents have been shown to have anti-angiogenic effects. They elicit their effects by down-regulating the expression of pro-angiogenic factors like cyclooxygenase-2 and nitric oxide synthase [240–242]. Bhattacharya et al. showed that treatment with methylselenocysteine led to an increased delivery of doxorubicin to human head and neck squamous carcinoma xenografts [243]. This effect was elicited through vessel maturation caused by methylselenocysteine [243].
The redundancy of angiogenic pathways can result in the development of resistance to therapies that rely on specific signaling pathways [217]. Escorcia and co-workers demonstrated that targeted radiation can be used to bring about vessel normalization [244]. The authors used a monoclonal antibody that identified specific epitopes on tumor neovasculature. This antibody was conjugated to actinium-225, which emits short range α particles. Pretreating tumors with targeted actinium 225 resulted in tumor vasculature normalization. This, in turn, led to an enhanced response to a combination treatment consisting of leucovorin and 5-fluorouracil [244].
3.2.1.3 Concentration and time dependency of vascular normalization
There has been considerable debate about the mechanism by which anti-angiogenic drugs improve the delivery of chemotherapeutics. Some reports suggest that inhibiting angiogenesis leads to decreased perfusion, while others have showed an increase. This has been complemented with data showing either decreased or increased drug delivery to the tumor [206].
The disparities in therapeutic response to anti-angiogenic therapies may be due to the concentration and time dependence of this technique. This dependence has been termed as the normalization window [111]. At sub-therapeutic concentrations of VEGF inhibitors, there may not be any effect on the blood vessels or on drug delivery. At very high concentrations, these therapies may completely destroy the vasculature. This will diminish drug delivery to the tumor [245]. Additionally, vessel normalization is highly transient. If the anti-angiogenic therapy is prolonged, the tumor vasculature could become inadequate for drug delivery [246]. Dickson et al. showed that the duration of vessel normalization depends on the physicochemical properties of the anti-angiogenic therapy as well as the type and location of the tumor [207]. The time dependence of vessel normalization also means that additional imaging techniques will be required to determine the normalization window for drug delivery. This somewhat limits the use anti-angiogenics. However, in a very interesting report, Rolny et al. presented a novel strategy to induce vascular normalization [247]. The authors showed that histidine-rich glycoprotein (HRG) could inhibit angiogenesis both directly and by converting tumor associated macrophages to an M1-like phenotype. The latter effect resulted in a longer vascular normalization window [248]. Such strategies with sustained responses hold significant promise in improving the delivery of chemotherapeutics [248].
Another source of disparity in measuring the activity of anti-angiogenics may stem from the current methods of characterization. Anti-angiogenic drugs can alter two parameters associated with chemotherapeutics: drug deposition and drug penetration. When one measures drug deposition in the tumor, concentrations are assessed as a whole. These concentrations may be localized in particular foci within the tumor and may not be representative of the therapeutic activity. Drug deposition may be a function of blood supply and may decrease with declining perfusion. However, the presentation of drug to resistant and hypoxic cells (function of drug penetration) is governed by intratumoral transport, which increases in response to a decrease in IFP [249]. Therapeutic response to the drug is a combined effect of both drug deposition and drug penetration. Thus, in order to comprehensively quantify the activity of anti-angiogenics, it is important to monitor both these parameters [249].
3.2.1.4 Suitability of anti-angiogenic therapy to improve NP transport
The use of anti-angiogenic drugs has been shown to have both positive and negative effects on the tumor delivery of nanomedicine. In a recent study, Vlahovic et al. found that pre-treatment with imatinib (a PDGFR-β inhibitor) led to enhanced accumulation of Doxil® in a mouse model of non-small cell lung cancer [250]. The same group later showed that treatment with pazopanib (inhibitor of VEGF and PDGF receptors) led to a decrease in the penetration of Doxil® in the same tumor model [251].
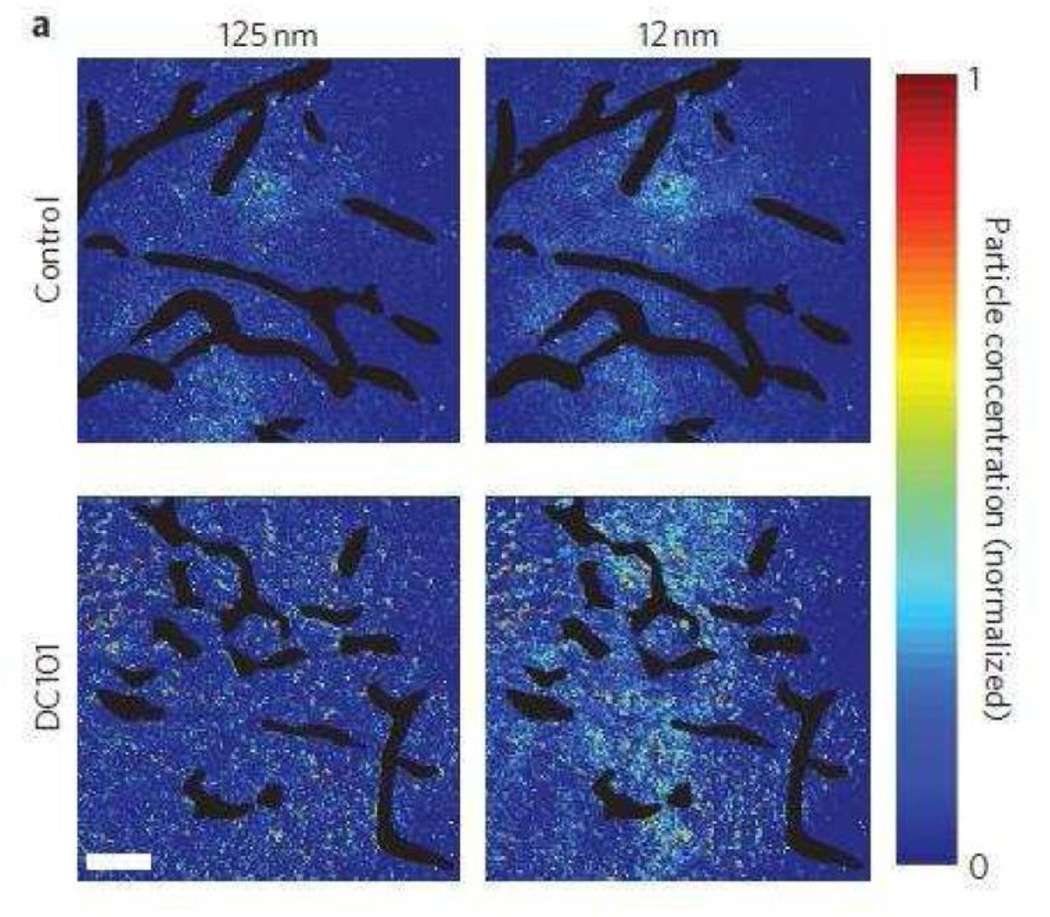
A recent study by Chauhan et al. offers some directions for the use of anti-angiogenics with nanotherapeutics [252]. The authors used DC101 as the VEGF blocker in combination with quantum dots of various sizes. In an orthotopic mouse mammary tumor model, the authors found that DC101 enhanced the penetration of NPs in a size-dependent manner. The advantage associated with the use of anti-angiogenic therapy was maximal for particles < 60 nm in size (Figure 6). The authors also found that there was a significant benefit of using DC101 in combination with Abraxane® (~10 nm diameter) but not with Doxil® (~100 nm diameter). The size dependence of this advantage is yet to be measured in different tumor models [252]. If the degree of normalization is variable amongst tumors, it is likely that this size dependence will also show a similar trend.
Figure 6. Effects of vascular normalization on nanoparticle delivery in tumors.
Nanoparticle penetration versus particle size in orthotopic 4T1 mammary tumors in response to normalizing therapy with DC101. Nanoparticle concentrations (denoted by pseudocolour) are relative to initial intravascular levels, with vessels shown in black. Normalization improves 12 nm particle penetration while not affecting 125 nm penetration. Scale bar, 100 mm. Reprinted with permission from [252].
3.2.2 Modifying tumor matrix to improve drug penetration
Tumor vessel normalization is an attractive strategy for enhancing the penetration of small molecules and macromolecules. Nonetheless, vascular normalization results in a reduction in the vascular pore size. To effectively utilize this technique for improving the delivery and penetration of NPs, the particle size of NPs has to be in the ~20 nm size range [253]. However, drug loading is severely compromised in such small particles, making these formulations impractical for in vivo use. Thus, alternative strategies to decrease IFP and to enhance intratumoral penetration of colloidal carriers are necessary. Altering the composition of the tumor extracellular matrix (ECM) provides another route to improve the tumor tissue distribution of NPs. The tumor ECM can be modified by either using enzymes that degrade specific ECM components or by modifying the tumor-associated cells that directly affect the behavior of ECM components.
3.2.2.1 Tissue digesting enzymes
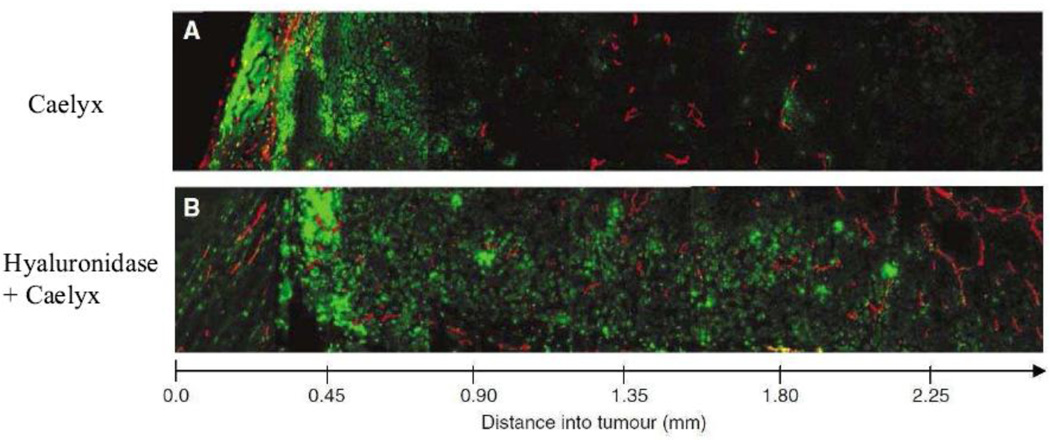
The tumor matrix is rich in collagen and hyaluronic acid [254], making them obvious targets for enhancing drug delivery. The effect of hyaluronic acid-digesting enzyme, hyaluronidase, was first reported by Maier et al. in patients with bladder cancer [255]. In their seminal studies, the authors found that co-administration of hyaluronidase with mitomycin-C significantly reduced the recurrence of bladder cancer in comparison to patients who received only the chemotherapeutic. Other groups later showed that the efficacy of chemotherapeutics could be enhanced by using hyaluronidase in multi-cellular spheroid models in vitro and in pre-clinical tumor models [256–259]. Brekken et al. were the first to show that intratumoral injection of hyaluronidase decreased IFP in orthotopic osteosarcomas in mice without affecting the arterial pressure [260]. The resultant increase in transvascular gradient may have led to the observed enhancement in drug delivery. The same group later confirmed that periodic fluctuations in IFP brought about by hyaluronidase administration increased the delivery of antibodies [261]. Subsequently, the effect of hyaluronidase administration on the uptake and distribution of CaelyxTM (pegylated liposomal doxorubicin hydrochloride) was measured [262]. Intratumoral administration of hyaluronidase led to an increase in tumor deposition of the formulation. More importantly, the distribution profile of the delivery system was altered. In the absence of the enzyme, liposomes were regionalized to the rim of the tumor. However co-administration of the enzyme led to increased penetration of the liposomes to the core of the tumor (Figure 7). Recently, Provenzano et al. reported a novel mechanism that may operate in improving drug delivery in response to hyaluronidase administration [263]. The authors found that K-ras driven pancreatic tumors in genetically modified mice were nearly avascular. Systemic administration of pegylated hyaluronidase led to the opening up of previously dysfunctional blood vessels. This led to enhanced delivery and efficacy of co-administered gemcitabine. This is the first study to show that enzymatic ablation of tumor ECM can restore blood supply, leading to increased drug delivery [263].
Figure 7. Distribution of liposomal doxorubicin in hyaluronidase treated osteosarcomas.
Distribution of liposomal doxorubicin in osteosarcoma xenografts treated with liposomal doxorubicin alone (16 mg kg1) (A) or liposomal doxorubicin combined with hyaluronidase (1500 U) (B). Representative images of doxorubicin (green) relative to capillaries (red) are presented from the rim to the center of the tumor sections. Reprinted with permission from [262].
The other ECM target for improving tumor penetration of chemotherapeutics is collagen. In an orthotopic osteosarcoma mouse model, Eikenes et al. showed that systemic administration of collagenase led to a rapid decline in tumor IFP, with only minor effects on the arterial blood pressure [264]. The resulting transcapillary gradient was maintained for nearly 24 hours. This led to enhanced delivery and penetration of a fluorescently-labeled antibody [264]. McKee et al. examined local injection of collagenase to improve the intratumoral distribution of an oncolytic virus [265]. Transfection of tumor cells with the virus was detected by expression of green fluorescent protein (GFP). Without collagenase co-administration, the virus was localized in regions that lacked collagen. Consequently, GFP expression was found only in limited regions within the tumor. Co-injection of collagenase significantly improved the distribution of the virus. This was confirmed by extensive GFP expression over the entire tumor. It is interesting to note that the virus had a hydrodynamic diameter of 150 nm, a size comparable to that of many NP formulations reported in the literature. Zheng and co-workers reported similar results [266]. The authors found that intratumoral administration of collagenase led to improved distribution of Doxil® in head and neck tumor xenografts. Interestingly, collagenase activity was observed only following local injections [266].
The above study by Zheng et al. highlights some key issues [266]. First, the toxicity associated with the use of these tissue-degrading enzymes is an important issue. Both collagen and hyaluronic acid are ubiquitously expressed in the body [263]. They form important components of the ECM and are essential for the function of vital organs. Intratumoral administration of these enzymes may prevent systemic toxicity. However, many tumors that require chemotherapeutic intervention may not be accessible by such local treatment. Second, the improvements in drug transport achieved with ECM degradation are transient. Collagen and hyaluronic acid are replenished within 8–24 hours [264, 267]. Since NP accumulation in the tumor is a relatively rapid event (few hours), transient decrease in ECM levels will help improve NP delivery to tumors [268]. However, NP penetration within a tumor is a relatively slower event [269, 270]. Thus, the transient nature of this technique may not improve the tissue transport of NPs. One possible strategy to overcome this problem is to immobilize the enzyme on the surface of NPs. This will ensure colocalization of NPs and the enzyme. Moreover, the passive targeting effect of NPs may limit the distribution and toxicity of the enzyme. An example of this approach was provided by Goodman et al.[271]. In that study, collagenase was physically adsorbed on the surface of polystyrene NPs. The adsorbed enzyme degraded collagen and improved the penetration of NPs in an in vitro multicellular spheroid model [271]. The study, however, did not examine the performance of this system in vivo. Rapid desorption of a physically adsorbed enzyme in the presence of plasma proteins may be a significant limitation of this approach. Covalent conjugation of the enzyme to the NP surface may overcome this limitation [272].
The choice of ECM component to be targeted for improving drug penetration is interesting. Collagenase administration has been unequivocally shown to increase drug penetration. However, there are conflicting reports regarding the utility of hyaluronidase for improving drug penetration [273]. In an interesting report, Netti et al. proposed that proteoglycans such as hyaluronic acid resemble “aqueous cages” [274]. These aqueous cages are passages through which the drug carrier can diffuse freely. Thus, eliminating these cages by degrading hyaluronic acid could hinder the transport of drug carriers. On the other hand, the solid collagen matrix may not offer a conducive environment for transport, and degrading collagen could, therefore, improve carrier distribution. The authors suggest that the amount of any ECM component does not dictate the magnitude of the effect it has on transport resistance. The structural assembly and organization of the component could play a more important role [274].
3.2.2.2 Modifying stromal cells
In addition to degrading the tumor stroma, an interesting alternative is to inhibit the secretion of ECM components by the stromal cells. An elegant example of this hypothesis was shown by Olive et al. [275]. Using a genetically engineered mouse model, the authors determined that pancreatic cancers had very little vascular coverage and that drug delivery to these tumors was severely impeded. The hedgehog signaling pathway was constitutively active in tumor-associated stromal cells. This led to the secretion of large amounts of ECM components. The authors showed that concomitant administration of a hedgehog pathway inhibitor (IPI 926) greatly increased the delivery and therapeutic efficacy of gemcitabine. A very recent report showed the safety of the IPI 926 in phase I clinical trials [276].
In another study, treatment with losartan was shown to decrease collagen I synthesis by fibroblasts in carcinomas [277]. Those effects were brought about through inhibition of the activity of transforming growth factor β (TGF β). The decrease in collagen content was sustained for a period of two weeks in a dose-dependent manner. Decreased collagen content was associated with an increase in the tumor penetration of liposomal doxorubicin and an increased therapeutic response [277].
4. Conclusions
Development of resistance to multiple drugs is a key obstacle to achieving successful treatment outcomes in many cancers. Tumor cells overexpress efflux transporters, which reduce intracellular drug accumulation and efficacy. NPs offer an attractive platform to overcome drug resistance. Many of the excipients used in fabricating NPs possess intrinsic efflux pump inhibitory activity. Intracellular distribution of NPs to specific loci in the cell, away from the activity of efflux pumps can also shield the encapsulated drug from transporters. An additional approach is to co-deliver specific inhibitors of transporter activity or function with the chemotherapeutic. However, poor intratumoral penetration of NPs limits their potential. Approaches that improve NP transport in tumors can significantly enhance their activity. Normalizing tumor vasculature has shown promising results with small molecules. Some NP formulations can also benefit from this approach. The field of ECM modification is relatively under-studied and holds tremendous potential for improving the therapeutic outcomes in hard-to-treat, avascular tumors.
Acknowledgements
The authors thank Drs. Yogesh Patil and Suresh Swaminathan for their useful input. Funding from NIH (grants CA116641 and CA093453).
Abbreviations
- NPs
Nanoparticles
- IFP
interstitial fluid pressure
- ABC
ATP-binding cassette
- P-gp
P-glycoprotein
- MDR
multidrug resistance
- MRP
multidrug resistance associated protein
- BCRP
breast cancer resistance protein
- TMDs
transmembrane domains
- NBDs
nucleotide binding domains
- ECM
extracellular matrix
- VEGF
vascular endothelial growth factor
- HIF
hypoxia inducible factor
- HRE
hypoxia responsive element
- CSCs
cancer stem cells
- CMC
critical micellar concentration
- PEG
poly(ethylene glycol)
- PLGA
poly(lactide-co-glycolide)
- EGF
epidermal growth factor
- TGF β
transforming growth factor β
- EMT
epithelial to mesenchymal transition
- HLB
hydrophilic lipophilic balance
- PI3K
phosphoinositol-3-kinase
- HRG
histidine rich glycoprotein
- EGFR
epidermal growth factor receptor
- VEGFR
vascular endothelial growth factor receptor
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 2.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 3.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Harris AL, Hochhauser D. Mechanisms of multidrug resistance in cancer treatment. Acta Oncol. 1992;31:205–213. doi: 10.3109/02841869209088904. [DOI] [PubMed] [Google Scholar]
- 5.Shabbits JA, Krishna R, Mayer LD. Molecular and pharmacological strategies to overcome multidrug resistance. Expert Rev Anticancer Ther. 2001;1:585–594. doi: 10.1586/14737140.1.4.585. [DOI] [PubMed] [Google Scholar]
- 6.Fracasso PM, Brady MF, Moore DH, Walker JL, Rose PG, Letvak L, Grogan TM, McGuire WP. Phase II study of paclitaxel and valspodar (PSC 833) in refractory ovarian carcinoma: a gynecologic oncology group study. J Clin Oncol. 2001;19:2975–2982. doi: 10.1200/JCO.2001.19.12.2975. [DOI] [PubMed] [Google Scholar]
- 7.Ma MK, McLeod HL, Westervelt P, Fracasso PM. Pharmacokinetic study of infusional valspodar. J Clin Pharmacol. 2002;42:412–418. [PubMed] [Google Scholar]
- 8.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136:21–29. doi: 10.1016/j.jconrel.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 12.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 14.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 15.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 16.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 17.Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973;323:466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- 18.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 19.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath T, Center MS. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988;48:3959–3963. [PubMed] [Google Scholar]
- 22.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 23.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Wright SR, Boag AH, Valdimarsson G, Hipfner DR, Campling BG, Cole SP, Deeley RG. Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res. 1998;4:2279–2289. [PubMed] [Google Scholar]
- 25.Scheffer GL, Pijnenborg AC, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, Vries EGde, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55:332–339. doi: 10.1136/jcp.55.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, Vandewalle A. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47:757–768. doi: 10.1177/002215549904700605. [DOI] [PubMed] [Google Scholar]
- 27.Chandra P, Brouwer KL. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res. 2004;21:719–735. doi: 10.1023/b:pham.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- 28.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 29.Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- 30.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, Deemter Lvan, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 31.Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol. 1997;8:161–170. doi: 10.1006/scbi.1997.0068. [DOI] [PubMed] [Google Scholar]
- 32.Roger E, Kalscheuer S, Kirtane A, Guru BR, Grill AE, Whittum-Hudson J, Panyam J. Folic Acid Functionalized Nanoparticles for Enhanced Oral Drug Delivery. Mol Pharm. 2012;9:2103–2110. doi: 10.1021/mp2005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 35.Sauna ZE, Smith MM, Muller M, Kerr KM, Ambudkar SV. The mechanism of action of multidrug-resistance-linked P-glycoprotein. J Bioenerg Biomembr. 2001;33:481–491. doi: 10.1023/a:1012875105006. [DOI] [PubMed] [Google Scholar]
- 36.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci U S A. 2000;97:2515–2520. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauna ZE, Ambudkar SV. Characterization of the catalytic cycle of ATP hydrolysis by human P-glycoprotein. The two ATP hydrolysis events in a single catalytic cycle are kinetically similar but affect different functional outcomes. J Biol Chem. 2001;276:11653–11661. doi: 10.1074/jbc.M011294200. [DOI] [PubMed] [Google Scholar]
- 38.Altenberg GA, Vanoye CG, Horton JK, Reuss L. Unidirectional Fluxes of Rhodamine 123 in Multidrug-Resistant Cells: Evidence Against Direct Drug Extrusion From the Plasma Membrane. PNAS. 1994;91:4654–4657. doi: 10.1073/pnas.91.11.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loo TW, Clarke DM. Do drug substrates enter the common drug-binding pocket of P-glycoprotein through “gates”? Biochem Biophys Res Commun. 2005;329:419–422. doi: 10.1016/j.bbrc.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 40.Lugo MR, Sharom FJ. Interaction of LDS-751 with P-Glycoprotein and Mapping of the Location of the R Drug Binding Site. Biochemistry. 2005;44:643–655. doi: 10.1021/bi0485326. [DOI] [PubMed] [Google Scholar]
- 41.Chavanpatil MD, Patil Y, Panyam J. Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoprotein-mediated drug efflux. Int J Pharm. 2006;320:150–156. doi: 10.1016/j.ijpharm.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 42.Weisburg JH, Curcio M, Caron PC, Raghu G, Mechetner EB, Roepe PD, Scheinberg DA. The multidrug resistance phenotype confers immunological resistance. J Exp Med. 1996;183:2699–2704. doi: 10.1084/jem.183.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisburg JH, Roepe PD, Dzekunov S, Scheinberg DA. Intracellular pH and multidrug resistance regulate complement-mediated cytotoxicity of nucleated human cells. J Biol Chem. 1999;274:10877–10888. doi: 10.1074/jbc.274.16.10877. [DOI] [PubMed] [Google Scholar]
- 44.Ruefli AA, Tainton KM, Darcy PK, Smyth MJ, Johnstone RW. P-glycoprotein inhibits caspase-8 activation but not formation of the death inducing signal complex (disc) following Fas ligation. Cell Death Differ. 2002;9:1266–1272. doi: 10.1038/sj.cdd.4401081. [DOI] [PubMed] [Google Scholar]
- 45.Robinson LJ, Roberts WK, Ling TT, Lamming D, Sternberg SS, Roepe PD. Human MDR 1 protein overexpression delays the apoptotic cascade in Chinese hamster ovary fibroblasts. Biochemistry. 1997;36:11169–11178. doi: 10.1021/bi9627830. [DOI] [PubMed] [Google Scholar]
- 46.Sognier MA, Zhang Y, Eberle RL, Sweet KM, Altenberg GA, Belli JA. Sequestration of doxorubicin in vesicles in a multidrug-resistant cell line (LZ-100) Biochem Pharmacol. 1994;48:391–401. doi: 10.1016/0006-2952(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 47.Wasenius VM, Jekunen A, Monni O, Joensuu H, Aebi S, Howell SB, Knuutila S. Comparative genomic hybridization analysis of chromosomal changes occurring during development of acquired resistance to cisplatin in human ovarian carcinoma cells. Genes Chromosomes Cancer. 1997;18:286–291. [PubMed] [Google Scholar]
- 48.Rao PH, Houldsworth J, Palanisamy N, Murty VV, Reuter VE, Motzer RJ, Bosl GJ, Chaganti RS. Chromosomal amplification is associated with cisplatin resistance of human male germ cell tumors. Cancer Res. 1998;58:4260–4263. [PubMed] [Google Scholar]
- 49.Rooney PH, Stevenson DA, Marsh S, Johnston PG, Haites NE, Cassidy J, McLeod HL. Comparative genomic hybridization analysis of chromosomal alterations induced by the development of resistance to thymidylate synthase inhibitors. Cancer Res. 1998;58:5042–5045. [PubMed] [Google Scholar]
- 50.Chin KV, Chauhan SS, Pastan I, Gottesman MM. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1990;1:361–365. [PubMed] [Google Scholar]
- 51.Grogan TM, Spier CM, Salmon SE, Matzner M, Rybski J, Weinstein RS, Scheper RJ, Dalton WS. P-glycoprotein expression in human plasma cell myeloma: correlation with prior chemotherapy. Blood. 1993;81:490–495. [PubMed] [Google Scholar]
- 52.Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. 1999;5:3352–3356. [PubMed] [Google Scholar]
- 53.Levchenko A, Mehta BM, Niu X, Kang G, Villafania L, Way D, Polycarpe D, Sadelain M, Larson SM. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci U S A. 2005;102:1933–1938. doi: 10.1073/pnas.0401851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seelig A, Blatter XL, Wohnsland F. Substrate recognition by P-glycoprotein and the multidrug resistance-associated protein MRP1: a comparison. Int J Clin Pharmacol Ther. 2000;38:111–121. doi: 10.5414/cpp38111. [DOI] [PubMed] [Google Scholar]
- 56.Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, Loscher W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007;52:333–346. doi: 10.1016/j.neuropharm.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 57.de Graaf D, Sharma RC, Mechetner EB, Schimke RT, Roninson IB. P-glycoprotein confers methotrexate resistance in 3T6 cells with deficient carrier-mediated methotrexate uptake. Proc Natl Acad Sci U S A. 1996;93:1238–1242. doi: 10.1073/pnas.93.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellis LM, Hicklin DJ. Resistance to Targeted Therapies: Refining Anticancer Therapy in the Era of Molecular Oncology. Clin Cancer Res. 2009;15:7471–7478. doi: 10.1158/1078-0432.CCR-09-1070. [DOI] [PubMed] [Google Scholar]
- 59.Ono M, Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res. 2006;12:7242–7251. doi: 10.1158/1078-0432.CCR-06-0646. [DOI] [PubMed] [Google Scholar]
- 60.Bianco R, Troiani T, Tortora G, Ciardiello F. Intrinsic and acquired resistance to EGFR inhibitors in human cancer therapy. Endocr Relat Cancer. 2005;12(Suppl 1):S159–S171. doi: 10.1677/erc.1.00999. [DOI] [PubMed] [Google Scholar]
- 61.Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, Helfrich BA, Doebele RC, Heasley LE. Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One. 5:e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 63.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 64.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mellish KJ, Kelland LR, Harrap KR. In vitro platinum drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br J Cancer. 1993;68:240–250. doi: 10.1038/bjc.1993.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem Sci. 1995;20:435–439. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
- 67.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 68.Kaina B, Christmann M. DNA repair in resistance to alkylating anticancer drugs. Int J Clin Pharmacol Ther. 2002;40:354–367. doi: 10.5414/cpp40354. [DOI] [PubMed] [Google Scholar]
- 69.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 70.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 71.Trumpp A, Wiestler OD. Mechanisms of Disease: cancer stem cells--targeting the evil twin. Nat Clin Pract Oncol. 2008;5:337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- 72.Pan CX, Zhu W, Cheng L. Implications of cancer stem cells in the treatment of cancer. Future Oncol. 2006;2:723–731. doi: 10.2217/14796694.2.6.723. [DOI] [PubMed] [Google Scholar]
- 73.Moserle L, Indraccolo S, Ghisi M, Frasson C, Fortunato E, Canevari S, Miotti S, Tosello V, Zamarchi R, Corradin A, Minuzzo S, Rossi E, Basso G, Amadori A. The side population of ovarian cancer cells is a primary target of IFN-alpha antitumor effects. Cancer Res. 2008;68:5658–5668. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- 74.Abubaker K, Latifi A, Luwor R, Nazaretian S, Zhu H, Quinn MA, Thompson EW, Findlay JK, Ahmed N. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden. Mol Cancer. 2013;12:24. doi: 10.1186/1476-4598-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baldini N, Scotlandi K, Barbanti-Brodano G, Manara MC, Maurici D, Bacci G, Bertoni F, Picci P, Sottili S, Campanacci M, et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med. 1995;333:1380–1385. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 78.Gregorcyk S, Kang Y, Brandt D, Kolm P, Singer G, Perry RR. p-Glycoprotein expression as a predictor of breast cancer recurrence. Ann Surg Oncol. 1996;3:8–14. doi: 10.1007/BF02409045. [DOI] [PubMed] [Google Scholar]
- 79.Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89:917–931. doi: 10.1093/jnci/89.13.917. [DOI] [PubMed] [Google Scholar]
- 80.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 81.Persidis A. Cancer multidrug resistance. Nat Biotechnol. 1999;17:94–95. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- 82.Singh Chintamani J, Mittal M, Saxena S, Bansal A, Bhatia A, Kulshreshtha P. Role of p-glycoprotein expression in predicting response to neoadjuvant chemotherapy in breast cancer-a prospective clinical study. World Journal of Surgical Oncology. 2005;3:61. doi: 10.1186/1477-7819-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karaszi E, Jakab K, Homolya L, Szakacs G, Hollo Z, Telek B, Kiss A, Rejto L, Nahajevszky S, Sarkadi B, Kappelmayer J. Calcein assay for multidrug resistance reliably predicts therapy response and survival rate in acute myeloid leukaemia. Br J Haematol. 2001;112:308–314. doi: 10.1046/j.1365-2141.2001.02554.x. [DOI] [PubMed] [Google Scholar]
- 84.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, Head DR, Appelbaum FR, Willman CL. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 85.Cleary I, Doherty G, Moran E, Clynes M. The multidrug-resistant human lung tumour cell line, DLKP-A10, expresses novel drug accumulation and sequestration systems. Biochem Pharmacol. 1997;53:1493–1502. doi: 10.1016/s0006-2952(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 86.Breuninger LM, Paul S, Gaughan K, Miki T, Chan A, Aaronson SA, Kruh GD. Expression of multidrug resistance-associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995;55:5342–5347. [PubMed] [Google Scholar]
- 87.Ma L, Center MS. The gene encoding vacuolar H(+)-ATPase subunit C is overexpressed in multidrug-resistant HL60 cells. Biochem Biophys Res Commun. 1992;182:675–681. doi: 10.1016/0006-291x(92)91785-o. [DOI] [PubMed] [Google Scholar]
- 88.Martinez-Zaguilan R, Raghunand N, Lynch RM, Bellamy W, Martinez GM, Rojas B, Smith D, Dalton WS, Gillies RJ. pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem Pharmacol. 1999;57:1037–1046. doi: 10.1016/s0006-2952(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 89.Raghunand N, Martinez-Zaguilan R, Wright SH, Gillies RJ. pH and drug resistance. II. Turnover of acidic vesicles and resistance to weakly basic chemotherapeutic drugs. Biochem Pharmacol. 1999;57:1047–1058. doi: 10.1016/s0006-2952(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 90.Jain RK. The next frontier of molecular medicine: delivery of therapeutics. Nat Med. 1998;4:655–657. doi: 10.1038/nm0698-655. [DOI] [PubMed] [Google Scholar]
- 91.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 92.Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11:8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 93.Milane L, Duan Z, Amiji M. Role of hypoxia and glycolysis in the development of multidrug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011;11:3. doi: 10.1186/1475-2867-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Starling EH. On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 96.Wiig H, Rubin K, Reed RK. New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand. 2003;47:111–121. doi: 10.1034/j.1399-6576.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 97.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 98.Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 99.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 100.Stanczyk M, Olszewski WL, Gewartowska M, Domaszewska-Szostek A. Lack of functioning lymphatics and accumulation of tissue fluid/lymph in interstitial "lakes" in colon cancer tissue. Lymphology. 43:158–167. [PubMed] [Google Scholar]
- 101.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- 102.Stanczyk M, Olszewski WL, Gewartowska M, Domaszewska-Szostek A. Lack of functioning lymphatics and accumulation of tissue fluid/lymph in interstitial “lakes” in colon cancer tissue. Lymphology. 2010;43:158–167. [PubMed] [Google Scholar]
- 103.Olszewski WL, Stanczyk M, Gewartowska M, Domaszewska-Szostek A, Durlik M. Lack of functioning intratumoral lymphatics in colon and pancreas cancer tissue. Lymphat Res Biol. 2012;10:112–117. doi: 10.1089/lrb.2012.0008. [DOI] [PubMed] [Google Scholar]
- 104.Gullino D, Masenti E, Trotti-Maina G. [Primary tumors of the pleura. II. Submesothelial tumors. (Anatomo-clinical considerations on 21 cases)] Arch Chir Torac Cardiovasc. 1968;25:83–115. [PubMed] [Google Scholar]
- 105.Hagendoorn J, Tong R, Fukumura D, Lin Q, Lobo J, Padera TP, Xu L, Kucherlapati R, Jain RK. Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 2006;66:3360–3364. doi: 10.1158/0008-5472.CAN-05-2655. [DOI] [PubMed] [Google Scholar]
- 106.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 108.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 109.Narang AS, Varia S. Role of tumor vascular architecture in drug delivery. Adv Drug Deliv Rev. 2011;63:640–658. doi: 10.1016/j.addr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 111.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 112.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 113.Young JS, Lumsden CE, Stalker AL. The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol Bacteriol. 1950;62:313–333. doi: 10.1002/path.1700620303. [DOI] [PubMed] [Google Scholar]
- 114.Roh HD, Boucher Y, Kalnicki S, Buchsbaum R, Bloomer WD, Jain RK. Interstitial hypertension in carcinoma of uterine cervix in patients: possible correlation with tumor oxygenation and radiation response. Cancer Res. 1991;51:6695–6698. [PubMed] [Google Scholar]
- 115.Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res. 1991;51:6691–6694. [PubMed] [Google Scholar]
- 116.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- 117.Curti BD, Urba WJ, Alvord WG, Janik JE, Smith JW, 2nd, Madara K, Longo DL. Interstitial pressure of subcutaneous nodules in melanoma and lymphoma patients: changes during treatment. Cancer Res. 1993;53:2204–2207. [PubMed] [Google Scholar]
- 118.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holback H, Yeo Y. Intratumoral drug delivery with nanoparticulate carriers. Pharm Res. 2011;28:1819–1830. doi: 10.1007/s11095-010-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 121.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Green CE, Liu T, Montel V, Hsiao G, Lester RD, Subramaniam S, Gonias SL, Klemke RL. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One. 2009;4:e6713. doi: 10.1371/journal.pone.0006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 126.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 127.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 128.Cunningham ML, Ringrose PS, Lokesh BR. Inhibition of the genotoxicity of bleomycin by superoxide dismutase. Mutat Res. 1984;135:199–202. doi: 10.1016/0165-1218(84)90122-8. [DOI] [PubMed] [Google Scholar]
- 129.Freitas I. Role of hypoxia in photodynamic therapy of tumors. Tumori. 1985;71:251–259. doi: 10.1177/030089168507100306. [DOI] [PubMed] [Google Scholar]
- 130.Amellem O, Pettersen EO. Cell inactivation and cell cycle inhibition as induced by extreme hypoxia: the possible role of cell cycle arrest as a protection against hypoxia-induced lethal damage. Cell Prolif. 1991;24:127–141. doi: 10.1111/j.1365-2184.1991.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 131.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 132.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 134.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 135.Racker E. Bioenergetics and the problem of tumor growth. Am Sci. 1972;60:56–63. [PubMed] [Google Scholar]
- 136.Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000;3:39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
- 137.Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34:592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 139.Chavanpatil MD, Khdair A, Panyam J. Surfactant-polymer nanoparticles: a novel platform for sustained and enhanced cellular delivery of water-soluble molecules. Pharm Res. 2007;24:803–810. doi: 10.1007/s11095-006-9203-2. [DOI] [PubMed] [Google Scholar]
- 140.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 141.Sadhukha T, Wiedmann TS, Panyam J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials. 2013;34:5163–5174. doi: 10.1016/j.biomaterials.2013.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawrence MJ. Surfactant systems: microemulsions and vesicles as vehicles for drug delivery. Eur J Drug Metab Pharmacokinet. 1994;19:257–269. doi: 10.1007/BF03188929. [DOI] [PubMed] [Google Scholar]
- 143.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 144.Riehm H, Biedler JL. Potentiation of drug effect by Tween 80 in Chinese hamster cells resistant to actinomycin D and daunomycin. Cancer Res. 1972;32:1195–1200. [PubMed] [Google Scholar]
- 145.Dong X, Mumper RJ. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine (Lond) 2010;5:597–615. doi: 10.2217/nnm.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Woodcock DM, Jefferson S, Linsenmeyer ME, Crowther PJ, Chojnowski GM, Williams B, Bertoncello I. Reversal of the multidrug resistance phenotype with cremophor EL, a common vehicle for water-insoluble vitamins and drugs. Cancer Res. 1990;50:4199–4203. [PubMed] [Google Scholar]
- 147.Woodcock DM, Linsenmeyer ME, Chojnowski G, Kriegler AB, Nink V, Webster LK, Sawyer WH. Reversal of multidrug resistance by surfactants. Br J Cancer. 1992;66:62–68. doi: 10.1038/bjc.1992.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 149.Sakai T, Alexandridis P. Single-step synthesis and stabilization of metal nanoparticles in aqueous pluronic block copolymer solutions at ambient temperature. Langmuir. 2004;20:8426–8430. doi: 10.1021/la049514s. [DOI] [PubMed] [Google Scholar]
- 150.Oh KS, Song JY, Cho SH, Lee BS, Kim SY, Kim K, Jeon H, Kwon IC, Yuk SH. Paclitaxel-loaded Pluronic nanoparticles formed by a temperature-induced phase transition for cancer therapy. J Control Release. 2010;148:344–350. doi: 10.1016/j.jconrel.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 151.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 152.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- 154.Valle JW, Armstrong A, Newman C, Alakhov V, Pietrzynski G, Brewer J, Campbell S, Corrie P, Rowinsky EK, Ranson M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Invest New Drugs. 2011;29:1029–1037. doi: 10.1007/s10637-010-9399-1. [DOI] [PubMed] [Google Scholar]
- 155.Coon JS, Knudson W, Clodfelter K, Lu B, Weinstein RS. Solutol HS 15, nontoxic polyoxyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance. Cancer Res. 1991;51:897–902. [PubMed] [Google Scholar]
- 156.Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release. 2006;112:312–319. doi: 10.1016/j.jconrel.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 157.Koziara JM, Lockman PR, Allen DD, Mumper RJ. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 2004;99:259–269. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 158.Dong X, Mattingly CA, Tseng MT, Cho MJ, Liu Y, Adams VR, Mumper RJ. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69:3918–3926. doi: 10.1158/0008-5472.CAN-08-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bogman K, Erne-Brand F, Alsenz J, Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J Pharm Sci. 2003;92:1250–1261. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- 160.Buckingham LE, Balasubramanian M, Emanuele RM, Clodfelter KE, Coon JS. Comparison of solutol HS 15, Cremophor EL and novel ethoxylated fatty acid surfactants as multidrug resistance modification agents. Int J Cancer. 1995;62:436–442. doi: 10.1002/ijc.2910620413. [DOI] [PubMed] [Google Scholar]
- 161.Lo YL. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J Control Release. 2003;90:37–48. doi: 10.1016/s0168-3659(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 162.Vauthier C, Dubernet C, Chauvierre C, Brigger I, Couvreur P. Drug delivery to resistant tumors: the potential of poly(alkyl cyanoacrylate) nanoparticles. J Control Release. 2003;93:151–160. doi: 10.1016/j.jconrel.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 163.Cuvier C, Roblot-Treupel L, Millot JM, Lizard G, Chevillard S, Manfait M, Couvreur P, Poupon MF. Doxorubicin-loaded nanospheres bypass tumor cell multidrug resistance. Biochem Pharmacol. 1992;44:509–517. doi: 10.1016/0006-2952(92)90443-m. [DOI] [PubMed] [Google Scholar]
- 164.Treupel L, Poupon MF, Couvreur P, Puisieux F. [Vectorisation of doxorubicin in nanospheres and reversion of pleiotropic resistance of tumor cells] C R Acad Sci III. 1991;313:171–174. [PubMed] [Google Scholar]
- 165.de Verdiere AC, Dubernet C, Nemati F, Soma E, Appel M, Ferte J, Bernard S, Puisieux F, Couvreur P. Reversion of multidrug resistance with polyalkylcyanoacrylate nanoparticles: towards a mechanism of action. Br J Cancer. 1997;76:198–205. doi: 10.1038/bjc.1997.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barraud L, Merle P, Soma E, Lefrancois L, Guerret S, Chevallier M, Dubernet C, Couvreur P, Trepo C, Vitvitski L. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J Hepatol. 2005;42:736–743. doi: 10.1016/j.jhep.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 167.Hugger ED, Audus KL, Borchardt RT. Effects of poly(ethylene glycol) on efflux transporter activity in Caco-2 cell monolayers. J Pharm Sci. 2002;91:1980–1990. doi: 10.1002/jps.10175. [DOI] [PubMed] [Google Scholar]
- 168.Wang J, Qu H, Jin L, Zeng W, Qin L, Zhang F, Wei X, Lu W, Zhang C, Liang W. Pegylated phosphotidylethanolamine inhibiting P-glycoprotein expression and enhancing retention of doxorubicin in MCF7/ADR cells. J Pharm Sci. 2011;100:2267–2277. doi: 10.1002/jps.22461. [DOI] [PubMed] [Google Scholar]
- 169.Johnson BM, Charman WN, Porter CJ. An in vitro examination of the impact of polyethylene glycol 400, Pluronic P85, and vitamin E d-alpha-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSci. 2002;4:E40. doi: 10.1208/ps040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 171.Shen Q, Lin Y, Handa T, Doi M, Sugie M, Wakayama K, Okada N, Fujita T, Yamamoto A. Modulation of intestinal P-glycoprotein function by polyethylene glycols and their derivatives by in vitro transport and in situ absorption studies. Int J Pharm. 2006;313:49–56. doi: 10.1016/j.ijpharm.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 172.Kopecek J, Kopeckova P, Minko T, Lu ZR, Peterson CM. Water soluble polymers in tumor targeted delivery. J Control Release. 2001;74:147–158. doi: 10.1016/s0168-3659(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 173.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 174.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 175.Murakami M, Cabral H, Matsumoto Y, Wu S, Kano MR, Yamori T, Nishiyama N, Kataoka K. Improving drug potency and efficacy by nanocarrier-mediated subcellular targeting. Sci Transl Med. 2011;3:64ra62. doi: 10.1126/scitranslmed.3001385. [DOI] [PubMed] [Google Scholar]
- 176.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thierry AR, Vige D, Coughlin SS, Belli JA, Dritschilo A, Rahman A. Modulation of doxorubicin resistance in multidrug-resistant cells by liposomes. FASEB J. 1993;7:572–579. doi: 10.1096/fasebj.7.6.8097173. [DOI] [PubMed] [Google Scholar]
- 178.Colin de Verdiere A, Dubernet C, Nemati F, Poupon MF, Puisieux F, Couvreur P. Uptake of doxorubicin from loaded nanoparticles in multidrug-resistant leukemic murine cells. Cancer Chemother Pharmacol. 1994;33:504–508. doi: 10.1007/BF00686509. [DOI] [PubMed] [Google Scholar]
- 179.Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J Pharmacol Exp Ther. 2006;317:1372–1381. doi: 10.1124/jpet.106.101154. [DOI] [PubMed] [Google Scholar]
- 180.Wong HL, Rauth AM, Bendayan R, Manias JL, Ramaswamy M, Liu Z, Erhan SZ, Wu XY. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 2006;23:1574–1585. doi: 10.1007/s11095-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 181.Chavanpatil MD, Khdair A, Gerard B, Bachmeier C, Miller DW, Shekhar MP, Panyam J. Surfactant-polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol Pharm. 2007;4:730–738. doi: 10.1021/mp070024d. [DOI] [PubMed] [Google Scholar]
- 182.Omelyanenko V, Kopeckova P, Gentry C, Kopecek J. Targetable HPMA copolymeradriamycin conjugates. Recognition, internalization, and subcellular fate. J Control Release. 1998;53:25–37. doi: 10.1016/s0168-3659(97)00235-6. [DOI] [PubMed] [Google Scholar]
- 183.Chan Y, Wong T, Byrne F, Kavallaris M, Bulmus V. Acid-labile core cross-linked micelles for pH-triggered release of antitumor drugs. Biomacromolecules. 2008;9:1826–1836. doi: 10.1021/bm800043n. [DOI] [PubMed] [Google Scholar]
- 184.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 185.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, Minko T, Discher DE. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. 2006;3:340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 186.Guo X, Szoka FC., Jr. Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG--diortho ester--lipid conjugate. Bioconjug Chem. 2001;12:291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- 187.Wang F, Wang YC, Dou S, Xiong MH, Sun TM, Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011;5:3679–3692. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]
- 188.Kievit FM, Wang FY, Fang C, Mok H, Wang K, Silber JR, Ellenbogen RG, Zhang M. Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J Control Release. 2011;152:76–83. doi: 10.1016/j.jconrel.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Y, Chen L, Ding Y, Yan W. Oxidized phospholipid based pH sensitive micelles for delivery of anthracyclines to resistant leukemia cells in vitro. Int J Pharm. 422:409–417. doi: 10.1016/j.ijpharm.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 190.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv Drug Deliv Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 191.Gao Y, Chen Y, Ji X, He X, Yin Q, Zhang Z, Shi J, Li Y. Controlled intracellular release of doxorubicin in multidrug-resistant cancer cells by tuning the shell-pore sizes of mesoporous silica nanoparticles. ACS Nano. 2011;5:9788–9798. doi: 10.1021/nn2033105. [DOI] [PubMed] [Google Scholar]
- 192.Ferry DR, Traunecker H, Kerr DJ. Clinical trials of P-glycoprotein reversal in solid tumours. Eur J Cancer. 1996;32A:1070–1081. doi: 10.1016/0959-8049(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 193.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 194.List AF, Kopecky KJ, Willman CL, Head DR, Persons DL, Slovak ML, Dorr R, Karanes C, Hynes HE, Doroshow JH, Shurafa M, Appelbaum FR. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98:3212–3220. doi: 10.1182/blood.v98.12.3212. [DOI] [PubMed] [Google Scholar]
- 195.Twentyman PR, Bleehen NM. Bleehen, Resistance modification by PSC-833, a novel non-immunosuppressive cyclosporin [corrected] Eur J Cancer. 1991;27:1639–1642. doi: 10.1016/0277-5379(91)90435-g. [DOI] [PubMed] [Google Scholar]
- 196.Oza AM. Clinical development of P glycoprotein modulators in oncology. Novartis Found Symp. 2002;243:103–115. discussion 115-108, 180-105. [PubMed] [Google Scholar]
- 197.Hubensack M, Muller C, Hocherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008;134:597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PubMed] [Google Scholar]
- 198.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 199.Song XR, Zheng Y, He G, Yang L, Luo YF, He ZY, Li SZ, Li JM, Yu S, Luo X, Hou SX, Wei YQ. Development of PLGA nanoparticles simultaneously loaded with vincristine and verapamil for treatment of hepatocellular carcinoma. J Pharm Sci. 2010;99:4874–4879. doi: 10.1002/jps.22200. [DOI] [PubMed] [Google Scholar]
- 200.Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ, Hou SX, Xiong SJ, Lei XJ, Wei YQ. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur J Pharm Sci. 2009;37:300–305. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 201.Wong HL, Bendayan R, Rauth AM, Wu XY. Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J Control Release. 2006;116:275–284. doi: 10.1016/j.jconrel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 202.Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin S, Wang X, Zhao Y, Ji Z, Zink JI, Nel AE. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yadav S, van Vlerken LE, Little SR, Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63:711–722. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- 204.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 206.Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J, Windhorst AD, Postmus PE, Verheul HM, Serne EH, Lammertsma AA, Smit EF. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 207.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF, Davidoff AM. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 208.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 209.Willett CG, Boucher Y, Tomaso Edi, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, de Bruijn EA, van Oosterom AT. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, Yuan F, Jain RK. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 212.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 213.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 215.McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(Suppl 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- 216.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 217.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 218.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 219.Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 220.Zakarija A, Soff G. Update on angiogenesis inhibitors. Curr Opin Oncol. 2005;17:578–583. doi: 10.1097/01.cco.0000183672.15133.ab. [DOI] [PubMed] [Google Scholar]
- 221.Quesada AR, Munoz-Chapuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 222.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 223.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 224.Gimbrone MA, Jr., Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 226.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 227.Le Serve AW, Hellmann K. Metastases and the normalization of tumour blood vessels by ICRF 159: a new type of drug action. Br Med J. 1972;1:597–601. doi: 10.1136/bmj.1.5800.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 229.Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 230.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 231.Maity A, Pore N, Lee J, Solomon D, O'Rourke DM. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3'-kinase and distinct from that induced by hypoxia. Cancer Res. 2000;60:5879–5886. [PubMed] [Google Scholar]
- 232.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, Xing X, Durduran T, Yodh AG, Evans SM, Koch CJ, Hahn SM, Quon H, Sehgal CM, Lee WM, Maity A. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One. 2009;4:e6539. doi: 10.1371/journal.pone.0006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 235.Krause M, Baumann M. Clinical biomarkers of kinase activity: examples from EGFR inhibition trials. Cancer Metastasis Rev. 2008;27:387–402. doi: 10.1007/s10555-008-9141-z. [DOI] [PubMed] [Google Scholar]
- 236.Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 237.Ciardiello F, Bianco R, Damiano V, Lorenzo SDe, Pepe S, Placido SDe, Fan Z, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–916. [PubMed] [Google Scholar]
- 238.Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr., Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 239.Qayum N, Im J, Stratford MR, Bernhard EJ, McKenna WG, Muschel RJ. Modulation of the tumor microvasculature by phosphoinositide-3 kinase inhibition increases doxorubicin delivery in vivo. Clin Cancer Res. 2012;18:161–169. doi: 10.1158/1078-0432.CCR-11-1413. [DOI] [PubMed] [Google Scholar]
- 240.Yin MB, Li ZR, Toth K, Cao S, Durrani FA, Hapke G, Bhattacharya A, Azrak RG, Frank C, Rustum YM. Rustum, Potentiation of irinotecan sensitivity by Se-methylselenocysteine in an in vivo tumor model is associated with downregulation of cyclooxygenase-2, inducible nitric oxide synthase, and hypoxia-inducible factor 1alpha expression resulting in reduced angiogenesis. Oncogene. 2006;25:2509–2519. doi: 10.1038/sj.onc.1209073. [DOI] [PubMed] [Google Scholar]
- 241.Fakih MG, Pendyala L, Smith PF, Creaven PJ, Reid ME, Badmaev V, Azrak RG, Prey JD, Lawrence D, Rustum YM. A phase I and pharmacokinetic study of fixed-dose selenomethionine and irinotecan in solid tumors. Clin Cancer Res. 2006;12:1237–1244. doi: 10.1158/1078-0432.CCR-05-2004. [DOI] [PubMed] [Google Scholar]
- 242.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 243.Bhattacharya A, Seshadri M, Oven SD, Toth K, Vaughan MM, Rustum YM. Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs. Clin Cancer Res. 2008;14:3926–3932. doi: 10.1158/1078-0432.CCR-08-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Escorcia FE, Henke E, McDevitt MR, Villa CH, Smith-Jones P, Blasberg RG, Benezra R, Scheinberg DA. Selective killing of tumor neovasculature paradoxically improves chemotherapy delivery to tumors. Cancer Res. 2010;70:9277–9286. doi: 10.1158/0008-5472.CAN-10-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, Tomaso Edi, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1,and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 247.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 248.Huang Y, Snuderl M, Jain RK. Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity. Cancer Cell. 2011;19:1–2. doi: 10.1016/j.ccr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 250.Vlahovic G, Ponce AM, Rabbani Z, Salahuddin FK, Zgonjanin L, Spasojevic I, Vujaskovic Z, Dewhirst MW. Treatment with imatinib improves drug delivery and efficacy in NSCLC xenografts. Br J Cancer. 2007;97:735–740. doi: 10.1038/sj.bjc.6603941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tailor TD, Hanna G, Yarmolenko PS, Dreher MR, Betof AS, Nixon AB, Spasojevic I, Dewhirst MW. Effect of pazopanib on tumor microenvironment and liposome delivery. Mol Cancer Ther. 2010;9:1798–1808. doi: 10.1158/1535-7163.MCT-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Burgess DJ. Microenvironment: A dense danger. Nat Rev Cancer. 2012;12:656–657. doi: 10.1038/nrc3375. [DOI] [PubMed] [Google Scholar]
- 255.Maier U, Baumgartner G. Metaphylactic effect of mitomycin C with and without hyaluronidase after transurethral resection of bladder cancer: randomized trial. J Urol. 1989;141:529–530. doi: 10.1016/s0022-5347(17)40881-0. [DOI] [PubMed] [Google Scholar]
- 256.Smith KJ, Skelton HG, Turiansky G, Wagner KF. Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of Kaposi's sarcoma. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR) J Am Acad Dermatol. 1997;36:239–242. doi: 10.1016/s0190-9622(97)70288-3. [DOI] [PubMed] [Google Scholar]
- 257.Schumer J, Klocker J, Tidstrand J, Rab B, Allmayer H. [Cisplatin, vindesine and hyaluronidase combined with simultaneous radiotherapy of advanced head and neck tumors] Onkologie. 1990;13:310–312. doi: 10.1159/000216782. [DOI] [PubMed] [Google Scholar]
- 258.Kohno N, Ohnuma T, Truog P. Effects of hyaluronidase on doxorubicin penetration into squamous carcinoma multicellular tumor spheroids and its cell lethality. J Cancer Res Clin Oncol. 1994;120:293–297. doi: 10.1007/BF01236386. [DOI] [PubMed] [Google Scholar]
- 259.Farr C, Menzel J, Seeberger J, Schweigle B. [Clinical pharmacology and possible applications of hyaluronidase with reference to Hylase “Dessau”] Wien Med Wochenschr. 1997;147:347–355. [PubMed] [Google Scholar]
- 260.Brekken C, de Lange Davies C. Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer Lett. 1998;131:65–70. doi: 10.1016/s0304-3835(98)00202-x. [DOI] [PubMed] [Google Scholar]
- 261.Brekken C, Hjelstuen MH, Bruland OS, de Lange Davies C. Hyaluronidase-induced periodic modulation of the interstitial fluid pressure increases selective antibody uptake in human osteosarcoma xenografts. Anticancer Res. 2000;20:3513–3519. [PubMed] [Google Scholar]
- 262.Eikenes L, Tari M, Tufto I, Bruland OS, de Lange Davies C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Eikenes L, Bruland OS, Brekken C, Davies Cde L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 265.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 266.Zheng X, Goins BA, Cameron IL, Santoyo C, Bao A, Frohlich VC, Fullerton GD. Ultrasound-guided intratumoral administration of collagenase-2 improved liposome drug accumulation in solid tumor xenografts. Cancer Chemother Pharmacol. 2011;67:173–182. doi: 10.1007/s00280-010-1305-1. [DOI] [PubMed] [Google Scholar]
- 267.Muckenschnabel I, Bernhardt G, Spruss T, Buschauer A. Pharmacokinetics and tissue distribution of bovine testicular hyaluronidase and vinblastine in mice: an attempt to optimize the mode of adjuvant hyaluronidase administration in cancer chemotherapy. Cancer Lett. 1998;131:71–84. doi: 10.1016/s0304-3835(98)00203-1. [DOI] [PubMed] [Google Scholar]
- 268.Patil YB, Toti US, Khdair A, Ma L, Panyam J. Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials. 2009;30:859–866. doi: 10.1016/j.biomaterials.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 271.Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- 272.Kuhn SJ, Finch SK, Hallahan DE, Giorgio TD. Proteolytic surface functionalization enhances in vitro magnetic nanoparticle mobility through extracellular matrix. Nano Lett. 2006;6:306–312. doi: 10.1021/nl052241g. [DOI] [PubMed] [Google Scholar]
- 273.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22:276–284. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 274.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 275.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jimeno A, Weiss GJ, Miller WH, Jr., Gettinger SN, Eigl B, Chang AL, Dunbar J, Devens S, Faia K, Skliris G, Kutok JL, Lewis KD, Tibes R, Sharfman WH, Ross RW, Rudin CM. Phase 1 Study of the Hedgehog Pathway Inhibitor IPI-926 in Adult Patients with Solid Tumors. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]