Abstract
Satellite cells, the quintessential skeletal muscle stem cells, reside in a specialized local environment whose anatomy changes dynamically during tissue regeneration. The plasticity of this niche is attributable to regulation by the stem cells themselves and to a multitude of functionally diverse cell types. In particular, immune cells, fibrogenic cells, vessel-associated cells and committed and differentiated cells of the myogenic lineage have emerged as important constituents of the satellite cell niche. Here, we discuss the cellular dynamics during muscle regeneration and how disease can lead to perturbation of these mechanisms. To define the role of cellular components in the muscle stem cell niche is imperative for the development of cell-based therapies, as well as to better understand the pathobiology of degenerative conditions of the skeletal musculature.
Keywords: skeletal muscle satellite cells, muscle stem cell niche, accessory cell types, myogenic cell types, muscular dystrophy
See the Glossary for abbreviations used in this article.
Glossary.
- C/EBP
CCAAT/enhancer-binding protein
- DTX
diphtheria toxin
- ECM
extracellular matrix
- FAP
fibro-adipogenic progenitor
- FGF
fibroblast growth factor
- FSP
fibroblast-specific protein
- Fzd
Frizzled
- IGF
insulin-like growth factor
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MCP
macrophage inflammatory protein
- MIP
monocyte chemotactic protein
- Myf
myogenic factor
- MyHC
myosin heavy chain
- MyoD
myogenic differentiation
- Par
partitioning defective
- Pax
Paired box
- PDGFR
platelet-derived growth factor receptor
- Rbpj
recombining binding protein-J
- Sca
stem cell antigen
- Sdc
syndecan
- SMA
smooth muscle actin
Introduction
Homeostatic adult skeletal muscle has a relatively uniform architecture. Bundles of longitudinally aligned muscle fibres surrounded by sheets of extracellular matrix (ECM) are attached to tendons and bone through myotendinous junctions [1]. A dense network of blood vessels supplies the tissue with nutrients and oxygen. Quiescent muscle stem cells, or satellite cells, are found underneath the ECM sheet attached to the muscle fibre plasma membrane [2]. Adult muscle tissue also contains several types of interstitial and vessel-associated cells that show little to no mitotic activity under resting conditions [3,4,5]. After injury, these cells begin to proliferate and, in conjunction with infiltrating immune cells, disperse throughout the muscle tissue (Fig 1A,B; [3,4,5,6]). The cellular dynamics during muscle regeneration are highly complex and occur with distinct temporal and spatial kinetics. In the course of muscle regeneration, satellite cells become activated and some will eventually upregulate transcription factors that trigger the myogenic differentiation programme [7]. Once differentiated into myocytes, the cells will align and form new syncytial muscle fibres or fuse to existing fibres. On completion of this regenerative response, the tissue returns to its homeostatic state and the resident cell populations re-enter a resting state.
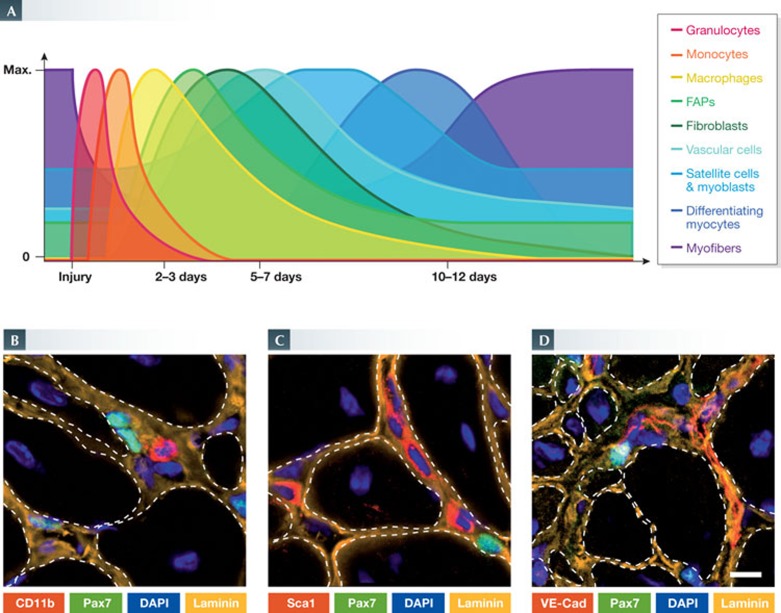
Figure 1.
Overview of tissue histology during mouse skeletal muscle regeneration. (A) A time course of histological changes in regenerating skeletal muscle. H&E staining of uninjured TA muscles and regenerating TA muscles at 5, 10 and 30 days after intramuscular cardiotoxin injection. Regenerating muscles are reduced to mostly mononuclear cells at day 5, but are able to re-establish multinucleated myofibres by day 10. Notably, the nuclei of uninjured myofibres are located at the periphery, whereas those of regenerating muscle fibres are centrally located. Scale bar, 50 μm. (B) Longitudinal view of whole tissue preparations of uninjured (left) and regenerating (right) skeletal muscle. Immunostaining for the extracellular matrix protein laminin (green) labels the basal lamina surrounding myofibres and capillaries. In regenerating conditions, the proliferation of satellite cells can be observed by the increase in the number of Pax7 (red) expressing cells (arrows). DAPI staining of nuclei (blue) reveals accessory cells in the satellite cell niche. Scale bar, 50 μm. H&E, haematoxylin and eosin; TA, tibialis anterior.
Despite there being many differentiating cells, the total number of satellite cells remains constant through multiple rounds of regeneration [8]. This equilibrium is due to the ability of satellite cells to self-renew, which provides progeny for differentiation while uncommitted mother cells are retained [9]. Satellite cell commitment to myogenic differentiation is mediated by the myogenic regulatory factors Myf5 and MyoD. Cre/lox reporter systems show that a subpopulation of about 10% of satellite cells has never expressed Myf5 [10]. Such satellite stem cells can self-renew through asymmetrical cell divisions that give rise to Myf5-positive satellite cells in response to the demand for committed myogenic progenitors. Asymmetric satellite cell division is controlled by the Par complex, which allows activation of p38α/β MAPK and upregulation of MyoD in the committed daughter cell [11]. Self-renewing satellite cells express higher levels of Pax7 than do cells that are primed for differentiation [12]. Moreover, a subpopulation of MyoD-expressing satellite cells can downregulate this factor to resist differentiation and re-enter quiescence [13]. These mechanisms of self-renewal allow the satellite cell pool to be maintained over multiple rounds of injury and repair, and are ultimately responsible for the outstanding regenerative capacity of muscle tissue.
Self-renewal and the three basic states of satellite cells—quiescence, proliferation and differentiation—are predominantly regulated by extrinsic factors in the local environment, the so-called stem cell niche [14]. These environmental cues include growth factors, cytokines, adhesion molecules and ECM contributed by the various cell types present in regenerating muscle tissue. In addition, satellite cells and their committed progeny actively participate in the remodelling of the niche during regenerative myogenesis. The diverse non-satellite cell types in muscle tissue can be categorized into cells with myogenic potential and into cells with accessory function for muscle regeneration [3,4,5]. Ablation studies have clearly shown an essential role of many accessory cell types during adult myogenesis [3,5,6]. By contrast, the physiological relevance of non-satellite cell types with myogenic potential is less clear [4]. Importantly, these cell types have unique characteristics that render them suitable for cell therapy approaches directed at treating skeletal muscle diseases.
In this article, we review the literature and present an overview of the cellular dynamics in the muscle stem cell niche in homeostasis, during regeneration and in disease. We introduce the niche under quiescent conditions, which we follow by a discussion of the cell types that constitute and modulate the niche during muscle regeneration. Subsequently, we elaborate on the deregulation of the niche under pathogenic conditions, and lastly we discuss potential importance of the niche for the recruitment of non-satellite cells with myogenic potential.
The quiescent niche
Under homeostatic conditions, adult muscle tissue has slow turnover [15]. Thus, after a proliferative phase during juvenile development, most satellite cells remain quiescent in the absence of an injury stimulus [7]. Importantly, the composition of the niche and the receptors that allow satellite cells to sense extrinsic signals are fundamentally different in quiescence than in the activated state [14,16]. In the quiescent niche, a few cell types are found in the proximity of satellite cells, for instance vessel-associated cells and muscle fibres (Fig 1B). This environment remains essentially static and imposes signals that promote the quiescent stem cell state.
Several studies have identified the Notch receptors as being critical for the maintenance of satellite cell quiescence [17,18]. Notch proteins are transmembrane receptors that are activated on exposure to ligands presented by juxtaposed cells [19]. The Notch pathway is highly pleiotropic and has important functions throughout development. In adult skeletal muscle, genetic loss of Rbpj, a downstream factor in the Notch pathway, leads to spontaneous satellite cell activation and premature differentiation. Since the satellite cell niche contains few heterogeneous cell types under quiescent conditions, regulatory Notch signals are most likely to be presented by the myofibres.
Quiescent satellite cells express high levels of integrin α7 and β1, as well as dystroglycan [20,21]. These receptors could transduce signals from the laminin-rich ECM that covers the satellite cells on their host muscle fibres [21,22]. Under homeostatic conditions, satellite cells also express M-cadherin and the glycoprotein CD34, which are involved in adhesion to myofibres [23,24]. Moreover, quiescent satellite cells are decorated with the heparan sulphate proteoglycans Sdc-3 and Sdc-4, which serve as co-receptors for integrins and sequester soluble growth factors and ECM in the immediate cellular microenvironment [25,26]. Interestingly, Sdc-3 also binds to Notch in satellite cells, and this interaction is required for self-renewal and reversible quiescence [27].
In contrast to the quiescent state, during muscle regeneration the composition of the niche is in a flux that is regulated by a spectrum of cell types (Fig 2). Satellite cell activation is coupled to the upregulation of specific receptors that integrate these niche signals to trigger the appropriate cellular responses. In the subsequent sections we discuss the different cell types involved in regulation of the niche during muscle repair.
Figure 2.
Schematic representation of the various cell types involved in muscle regeneration. Within the complexity of regenerating muscles, satellite cells are subject to a distinct environment determined by the spatial and temporal presence of cytokines, growth factors and other cell types.
Immune cells are critical effectors of the satellite cell niche
Acute sterile muscle injuries trigger a precisely orchestrated inflammatory process aimed at the removal of damaged cells, coordination of the regenerative response and, ultimately, restoration of tissue homeostasis (Fig 3A; Table 1). The onset, development and resolution of inflammation involve diverse interactions between leukocytes and local cell types, including satellite cells (Fig 3B). In resting conditions, adult skeletal muscle contains different types of resident leukocyte. The most abundant are mast cells and macrophages. These resident cell types, in conjunction with ‘patrolling’ circulatory monocytes, act as sensors for distress and secrete a number of chemoattractive molecules following muscle injury [28,29]. Particularly, damage-activated mast cells almost instantly begin to secrete TNF-α, histamine and tryptase and then initiate the de novo synthesis of other cytokines, such as interleukin (IL)-6 [30]. At low physiological concentrations, TNF-α, tryptase and IL-6 promote activation and proliferation of satellite cells [31,32,33]. Moreover, inhibition of mast cell activity leads to reduced leukocyte extravasation and impairs muscle repair [34]. Thus, immune cells contribute substantially to the satellite cell niche in the earliest stages of muscle regeneration.
Figure 3.
Participation of non-myogenic cell types in muscle regeneration. (A) The relative presence of immune, fibrotic, vascular and myogenic cell types after muscle injury. (B–D) Immunofluorescence micrographs of tissue sections from regenerating mouse muscles. In their niche, Pax7-positive satellite cells (green) are in close proximity to various non-myogenic cell types (red): (B) CD11b-positive leukocytes; (C) Sca1-positive interstitial cells; and (D) VE-Cad-positive endothelial cells. ECM is shown in orange and nuclei are labelled with DAPI (blue). Scale bar, 10 μm.
Table 1. Cell types in the muscle satellite niche.
| Cell type | Markers | Presence (days after injury) | Effect on myogenic cells | References |
|---|---|---|---|---|
| Granulocyte | CD11b+, CD31+, Gr1+, CD43+ | 0–3 | Indirectly stimulate myogenesis through FAPs | [37,38] |
| Monocyte | CD11b+, CD31+, Ly6C(±) | 0–3 | Promote satellite cell activation and proliferation (Ly6C+) or differentiation (Ly6C−) | [28,41,42] |
| M1 macrophage | CD11b+, CD31+, CD68+, iNOS+ | Peak at 2–3, return to baseline after 7–9 | Promote myoblast proliferation, repress myogenic differentiation | [43,44,45,46,49,50,51,52] |
| M2 macrophage | CD11b+, CD31+, CD68+, CD206+, CD163+ | Peak at 3–4, return to baseline after 8–10 | Regulate entry into myogenic differentiation, promote myotube formation | [43,44,47,48,49,50,51,52,59] |
| FAP | CD34+, Pdgfrα+, Sca1+(high) | Peak at 3–4, return to baseline after 7–9 | Promote myogenic differentiation | [37,61,62,63,65] |
| Fibroblast | Tcf4+, SMAα+, vimentin+, desmin+, FSP1+ | Peak at 5–7, persist throughout regeneration | Promote myoblast proliferation, enhance self-renewal | [72] |
| Vascular cell | VE-Cad+, SMAα+, CD31+ | Peak at 5–7, return to baseline after ∼28 | Promote myoblast proliferation, enhance self-renewal | [73,74,75,76,77,78] |
| Satellite cell and myoblast | Pax7+, MyoD+*, α7β1-int+, Sdc4+, VCAM-1+ | Peak at 5–7, return to baseline after ∼28 | Enhance self-renewal of satellite cells | [10,56,57,82] |
| Differentiating myocyte | Myogenin+, α7β1-int+, Sdc4+, desmin+ | Appear around day 4–5 and peak by day 10 | — | [124,125] |
| Newly formed myofibre | MyHC+ | Return to baseline numbers after ∼28 (with most myofibres remaining centrally nucleated) | Enhance self-renewal | [10,17,18,85] |
*Myoblasts only.
The initial burst of cytokines and chemokines produced by resident leukocytes, which include TNF-α and MIP-2, along with cellular and extracellular contents released by the damaged tissue, lead to the rapid attraction of circulating granulocytes [35,36]. These consist mainly of neutrophils and, to a lesser extent, eosinophils [37]. Neutrophils promote the proinflammatory environment that is necessary for the clearance of cellular debris. Under certain conditions, this cell type has been suspected to transiently aggravate tissue damage [38]. Neutrophils also secrete the chemokines MIP-1α, MCP-1 and others that favour the recruitment of monocytes [39,40]. Beyond the first day after injury, monocytes gradually become the predominant leukocytes in the exudate. Globally, monocytes are divided in two categories: the classic monocytes (Ly6C+) that are predominantly present during the first few days after injury and the non-classical monocytes (Ly6C−) that slowly replace Ly6C+ cells as regeneration progresses [41]. Although the origin of this switch in monocyte subpopulations is still debated, distinct functions for both cell types have been established [41,42]. Indeed, Ly6C+ monocytes promote the recruitment of other monocytes by secreting proinflammatory cytokines, such as TNF-α and IL-1β, whereas Ly6C− monocytes express high levels of anti-inflammatory molecules and growth factors [41]. Importantly, the switch of monocyte subtypes not only influences the general course of inflammation but also is important in the satellite cell niche. The proinflammatory environment established by Ly6C+ monocytes promotes the proliferation of myogenic cells and reduces their differentiation and fusion capacity. On the other hand, the anti-inflammatory signals from Ly6C− monocytes have opposite effects and stimulate differentiation [41]. Therefore, the emergence of Ly6C+ monocytes before Ly6C− monocytes is important to ensure appropriate proliferation of myogenic cells and to prevent their premature differentiation.
Once monocytes have invaded the tissue, they begin to differentiate into macrophages. Macrophages can be divided into several subtypes. Analogous to monocytes, this classification of macrophages during muscle regeneration can be simplified into an initial wave of proinflammatory, or M1, macrophages that is followed by a second wave of anti-inflammatory, or M2, macrophages. These macrophage subsets, however, are not mutually exclusive, and, at a given time point, distinct subtypes can be found in the same regenerating area [43]. Depletion models of different types of acute sterile injury have shown that suppression of M1 macrophages leads to persistence of necrotic cells, impaired myoblast proliferation, increased fibrosis and fat accumulation [44,45,46]. By contrast, inhibition of the transition from M1 to M2 macrophages in mice negative for IL-10 or the transcription factor C/EBPβ resulted in reduced myogenin expression and fibre growth [47,48]. Therefore, M1 and M2 macrophages stimulate, respectively, the early and the late phases of myogenesis. These results are supported by the observation that in injured human muscle, M1 macrophages are found close to proliferating myogenic cells and M2 macrophages interact with differentiating myocytes [43]. The proximity is important for macrophages to mediate myogenic effects and is favoured by attractive reciprocal chemotactic signals [49]. Indeed, direct physical contact, for instance through VCAM1–VLA4, allows macrophages to inhibit apoptosis and promote survival of myogenic cells [50]. Treatment of myogenic cells with macrophage-conditioned medium revealed that the effects of M1 and M2 macrophages are also mediated by paracrine signalling [43,51]. IL-1β, IL-6 and TNF-α secreted by M1 macrophages are particularly important to induce proliferative effects on myogenic cells, whereas IL-4 and IGF-1 released by M2 macrophages promote their differentiation [43,52,53]. Moreover, macrophages also secrete different ECM proteins according to the stage of macrophage differentiation. For example, M2 macrophages secrete a more mature form of fibronectin and a higher amount of ColVI than do M1 macrophages [54,55]. The ECM proteins secreted by M2 macrophages are important components of the muscle stem cell niche and promote self-renewal of satellite cells [56,57].
The regulatory function of immune cells for myogenesis is sensitive to perturbation and efficient muscle repair depends on their precise coordination. For instance, M2 macrophages can secrete anti-inflammatory molecules and pro-resolving mediators [58]. The anti-inflammatory properties of these molecules allow M2 macrophages to efficiently decrease the oxidative activity and the cytolytic muscle damage caused by neutrophils and M1 macrophages [58,59]. Thus, even slight imbalances in immune cell populations due to sustained and successive inflammatory signals in diseased muscles can disrupt the cellular dynamics in the niche and provide inappropriate environmental cues to satellite cells (see below). Nonetheless, if appropriately synchronized and controlled, immune cells serve as key effectors in the muscle stem cell niche to guide satellite cells through the regeneration process.
Fibrogenic cells remodel the niche during regeneration
During muscle regeneration the extracellular environment in the stem cell niche is dynamically rearranged [60]. The functions of various ECM components being deposited in regenerating tissue are only beginning to be elucidated. Structurally, this transitional fibrillar ECM serves to preserve the gross integrity of the tissue until degenerated fibres have been cleared and innervated young muscle fibres have been formed in the correct anatomical position. The main source of these ECM proteins during muscle regeneration is fibrogenic mesenchymal stromal cells, such as fibroblasts and FAPs.
FAPs are mesenchymal stem cells resident in skeletal muscle that have the ability to differentiate into fibroblasts, adipocytes and possibly into bone and cartilage cells, although not into satellite cells or muscle fibres [61,62,63]. This cell type is marked by the expression of the mesenchymal stem cell surface markers, CD34, Sca-1 and PDGFRα, and by the absence of the haematopoietic markers CD45 and CD31 and of the satellite cell marker integrin α7 [3]. Under quiescent conditions FAPs localize close to blood vessels [64]. On muscle injury, these cells are activated, expand and take over the interstitium, where they have a promyogenic function (Fig 3C; [63]). The number of FAPs increases rapidly and peaks 3–4 days after injury, then returns to baseline levels after 7–9 days (Fig 3A).
FAPs are major contributors to the deposition of several extracellular proteins—for instance, certain collagen isoforms that are abundant in the supportive transitional ECM during muscle regeneration [65]. This cell type also secretes high levels of IL-6, which promotes the differentiation of myogenic cells [63]. Vice versa, myotubes seem to inhibit the differentiation of FAPs into adipocytes [62]. Regulation of cellular crosstalk also involves eosinophils that are recruited during the early stages of regeneration [37]. Eosinophils release the cytokines IL-4 and IL-13, which induce the proliferation of FAPs and simultaneously block their adipogenic differentiation. In mice deficient for IL-4 and IL-13, adipocytes accumulated after muscle injury and reparative myogenesis was impeded. Remarkably, FAPs seem to remove necrotic debris from regenerating muscles more efficiently than macrophages [37]. The physiological contribution of these two cell populations to debris clearance, however, remains to be investigated, since their abundance and temporal regulation in regenerating muscle probably differs.
Fibroblasts are elongated cells with extended cell processes and a fusiform or spindle-like shape that are identifiable by high expression levels of the intermediate-filament-associated proteins vimentin, desmin, FSP1 and α-SMA [66,67]. This cell type is of a non-vascular, non-epithelial and non-inflammatory nature. Fibroblasts are heterogeneous, with expression profiles that differ depending on the tissue source [68]. A major function of this cell type is the deposition of fibrillar ECM, such as collagen and fibronectin, and basement membrane constituents [68,69,70]. Moreover, fibroblasts can actively remodel the ECM by secretion of matrix metalloproteinases [71].
Under homeostatic conditions in adult skeletal muscle, fibroblasts reside in the interstitium between myofibres [72]. After muscle injury, they quickly start to proliferate and become highly abundant. Tissue fibroblast content is greatest at about 5 days after muscle damage and coincides with the peak of satellite cell proliferation (Fig 3A). DTX-driven ablation of a subpopulation of fibroblasts from regenerating muscles, achieved with a tamoxifen-inducible Cre allele driven by the Tcf4 promoter, resulted in premature satellite cell differentiation and impaired regeneration [72]. Similar to FAPs, Tcf4-positive fibroblasts express PDGFRα, and it remains to be determined whether the Tcf4-Cre allele is also expressed in the former cell type [61,62,63,72]. Within the muscle fibroblast population, the Tcf4-Cre allele is only active in about 40% of cells. Despite this low percentage of fibroblasts that were depleted by DTX with this Cre driver, a notable phenotype was observed [72]. These results emphasize the critical role of ECM-producing cell types in the stem cell niche during regenerative myogenesis. A possible interpretation of this study is that fibroblasts allow for transient expansion of satellite cells during muscle regeneration while preventing their differentiation. Interestingly, such a role for fibroblasts during muscle regeneration would be opposed to the effects of FAPs, which seem to have prodifferentiation effects [63].
DTX-mediated ablation of satellite cells with a tamoxifen-inducible Pax7-Cre driver revealed that a proper satellite cell response to an injury stimulus is reciprocally required for a normal fibroblast response [72]. The number of fibroblasts in satellite cell-depleted muscles was reduced by ∼50% at the peak of regeneration, 5 days after injury, when the first centrally nucleated fibres are normally formed. This effect on fibroblasts might be due to the absence of a trophic signal from proliferating satellite cells or from the young muscle fibres that cannot be established in satellite cell-depleted muscle. Taken together, the complex role of mesenchymal fibrogenic cells in the muscle stem cell niche only begins to be understood and future studies will be required to explore their interplay with myogenic cells in more detail.
Endothelial and periendothelial cells in the niche
Skeletal muscle is laced with a dense microvasculature, and most quiescent satellite cells are found in close proximity to these vessels [73]. During muscle injury, the number of capillaries in the tissue initially increases and then returns to baseline about 4 weeks after injury (Fig 3D; [74,75]). In co-culture experiments, endothelial cells promote the proliferation of satellite cell-derived myoblasts. Reciprocally, differentiating myogenic cells are proangiogenic and increase the formation of capillary-like structures [73]. Endothelial cells secrete a variety of mitogenic and/or anti-apoptotic factors, such as VEGF, that influence muscle cells [76]. Intriguingly, differentiating myogenic cells also secrete VEGF and their proangiogenic function mainly depends on this factor [77]. This finding suggests an intricate feed-forward mechanism through which VEGF in the stem cell niche co-regulates both myogenesis and angiogenesis.
In contrast to the predominantly promitotic effects of endothelial cells on myogenic progenitors, cells in the periendothelial position, such as smooth muscle cells and fibrogenic cell types, are crucial for re-entry into quiescence on completion of regeneration [76]. Satellite cells transitioning into quiescence increase expression of the Ang1 receptor Tie-2. Forced expression of Ang1 in mouse muscles increases the number of quiescent cells and inhibition of Tie-2 prevents cell-cycle exit on completion of regeneration [78]. Importantly, periendothelial cells seem to be the major source of Ang1 during muscle regeneration. In summary, vessel cells in the stem cell niche coordinate both the acute satellite cell response and the late stages of muscle regeneration when the tissue returns to homeostasis.
Regulation of the niche by cells of the muscle lineage
In many tissues, the committed or differentiated progeny of stem cells become components of the niche where they provide regulatory signals [79]. This feature is the same in the skeletal muscle lineage and, as discussed below, involves cell–cell interactions, as well as the secretion of growth factors and regulatory ECM.
Notch signals originating from differentiating myogenic cells allow for self-renewal of muscle progenitors while suppressing activation of the commitment factor MyoD during development [18,80,81]. This Notch-dependent developmental mechanism is preserved in adult satellite cells. Experiments using the Myf5-Cre-YFP reporter system revealed that during the regenerative response of mature skeletal muscle, committed proliferating YFP+ satellite cells of the Myf5-dependent lineage provide Notch signals to the self-renewing YFP− satellite stem cells [10]. Satellite stem cells contain high levels of Notch-3, while their committed Myf5/MyoD-positive progeny express the Notch ligand Delta-1. In asymmetric divisions, Delta-1 localizes to the cell interface with the YFP− cell and probably activates self-renewal signals by binding to Notch-3 [10]. Thus, next to its established role in maintaining satellite cell quiescence (see above), Notch also plays a role in activated cells.
When compared with quiescent cells, activated satellite cells and their differentiated progeny express high levels of ECM components and various molecules involved in the remodelling of extracellular space [56,82]. An intriguing role of Notch signalling during myogenic development is the regulation of ECM synthesis by myogenic progenitors [83]. Genetic loss of Rbpj from myogenic cells, whose terminal differentiation is blocked due to knockout of MyoD, leads to an inability to acquire the satellite cell position underneath the basal lamina surrounding the developing muscle fibres. Importantly, the expression of several ECM components is strongly disrupted in such cells, suggesting that they actively contribute to the formation of the basal lamina. Thus, the regulation of ECM synthesis could be another Notch-related developmental process that plays a role in adult myogenesis.
The pool of Myf5-independent satellite stem cells in muscle tissue is critically controlled by Wnt7a, a lipophilic factor that is released into the stem cell niche by newly formed fibres [10,84,85]. Expansion of the satellite stem cell population is a mechanism that is essential for maintenance of the myogenic progenitor pool after muscle injury. Consequently, knockout of Wnt7a severely reduces overall satellite cell number after regeneration. The effect of Wnt7a on satellite stem cells depends on the Fzd7–Sdc4 co-receptor complex, the function of which is modulated by fibronectin that is secreted into the niche microenvironment by committed Myf5-positive satellite cells [56]. Binding of Wnt7a and fibronectin to the Fzd7–Sdc4 co-receptor complex allows for downstream GTPase signalling and the induction of symmetric expansion of satellite stem cells. Similar to the loss of Wnt7a, loss of fibronectin from regenerating muscle severely reduces the overall pool of satellite cells. Thus, the release of fibronectin into the stem cell niche represents a feedback mechanism originating from committed satellite cell progeny that, in concert with Wnt7a, modulates the self-renewing stem cell pool. Other cell types in muscle express high levels of fibronectin and, therefore, might also contribute to fine-tuning of the Wnt7a response [68,69,70].
Another ECM molecule critical to the satellite cell niche is ColVI [57]. Mutations in ColVI are the underlying cause of Bethlem myopathy and Ullrich congenital muscular dystrophy [86]. ColVI knockout mice show deficiency in muscle regeneration and mild myopathy [57,87]. Satellite cells express high levels of ColVI and secrete this factor to autoregulate the softness of their niche. In elegant grafting experiments, wild-type satellite cells ameliorated the regenerative phenotype of ColVI-deficient muscle tissue, which demonstrates a cell-autonomous requirement for this factor. Interestingly, in this mouse model, the ColVI content in the satellite cell niche can also be restored by transplantation of wild-type fibroblasts.
In summary, important regulatory functions of the stem cell niche are controlled by committed satellite cell progeny or by differentiated fibres. Specifically, satellite cells actively autoregulate their immediate microenvironment. The emerging mechanisms that integrate feedback and feed-forward signals within the myogenic lineage are highly complex and further investigation will undoubtedly unravel important new concepts in basic stem cell biology.
The satellite cell niche in ageing and pathology
Chronic degenerative conditions of skeletal muscle can lead to permanent changes within the muscle stem cell niche. Evidence suggests that, under specific conditions, satellite cells can differentiate into brown fat, osteocytes and myofibroblasts [88,89,90]. Pathological deterioration of the niche or systemic changes can influence these fate decisions and disrupt normal cellular responses to injuries [91,92]. Ultimately, imbalance within the local satellite cell milieu attenuates the formation of new myofibres and leads to the eventual loss of muscle function. Therefore, understanding of pathological conditions of the muscle stem cell niche is important to treat muscle diseases.
Although ageing should not be considered a pathological state, the deterioration in muscle regeneration through this process is directly correlated with changes to local and circulating factors that influence satellite cell function [93]. With use of heterochronic parabiosis to connect the circulatory systems of young and old mice, serum from young mice reduced age-related tissue fibrosis and restored satellite cell function in old mice. Systemically, serum levels of TGF-β1 are increased in elderly humans and mice [94]. This profibrotic factor not only stimulates the expansion of tissue-resident fibroblasts but also inhibits the myogenic differentiation of satellite cells, which diminishes the regenerative capacity of ‘old’ muscle. Additionally, altered levels of osteopontin secreted by CD11b+ macrophages that are found in the serum of old mice are associated with reduced proliferative and differentiation capacities of satellite cells [95]. This finding suggests that immune responses to injuries can shift with age. Accordingly, subtle changes to the systemic milieu can affect the cellular response of support cells within the muscle stem cell niche and affect the efficiency of myogenic regeneration. Combined with localized FGF-2 secreted by the myofibres that disrupt the ability of aged muscle stem cells to return to quiescence in old mice, the aged niche creates an unfavourable environment for the proper function of muscle stem cells [96]. The full extent of these gradual changes is not yet known and requires further investigation, along with whether the ‘young’ stem cell niche can be therapeutically restored.
In degenerative muscle diseases, localized muscle pathologies can transform the normal wound-healing programme into a positive feedback loop that prevents the proper function of satellite cells. Notably, the attenuation of muscle repair in most forms of muscular dystrophy is correlated with a build-up of fibrotic scarring, adipose tissue and immune infiltrations [97]. The increased susceptibility of dystrophic muscle fibres to damage leads to cycles of degeneration and regeneration. In most cases, necrotic and regenerating areas occur concurrently throughout dystrophic muscles. Unlike the beneficial effects of transient ECM protein upregulation during normal regeneration, increased inflammation and persistent expression of ECM proteins reduce the differentiation of myoblasts into myofibres [98,99]. Moreover, the altered elasticity of fibrotic muscle tissue is likely to have a negative influence on the self-renewal of satellite cells [57,100]. The lack of efficient myofibre formation in diseased muscle reduces the inhibitory feedback on the fibrogenic and adipogenic differentiation of FAPs [62]. This feedback mechanism persists until anti-myogenic signals accumulate exponentially and muscle regeneration is essentially halted.
Restoration of a functional muscle stem cell niche is an important aspect of treating degenerative muscle diseases. Experimental regulation of supportive cell types during regeneration can optimize the myogenic efficiency of satellite cells [37,63,72]. Therefore, it is conceivable that these cells can be effectively manipulated in disease conditions to combat the loss of muscle stem cell function over time. In agreement with this idea, anti-fibrotic and anti-inflammatory therapies have reduced the progression of Duchenne muscular dystrophy in the short term [101,102]. Furthermore, transplantation of corrective supportive cells can recondition the niche to restore satellite cell function [57,95]. The intricacies of the regenerating environment and altered systemic milieu in specific diseases, however, have made research into this area challenging, and there are many aspects regarding the structural and temporal regulation of the muscle stem cell niche that remain unknown. Thus, future research into the regulation of the stem cell niche in regeneration and disease holds great potential for the therapeutic enhancement or restoration of muscle regeneration.
Non-satellite cells with myogenic potential
Various cell types other than satellite cells are able to fuse into differentiating muscle fibres and, in certain cases, can also acquire Pax7 expression and become satellite cells [4]. These properties are attractive for the development of cell-based therapies for muscle diseases. Examples of these cell types include side population cells, PW1-positive interstitial cells, pericytes, mesoangioblasts, CD133-positive circulating cells and integrin β4-positive interstitial cells [103,104,105,106,107,108]. The existence of these cell types raises questions of whether a non-satellite cell progenitor could compensate for the role of satellite cells during physiological myogenesis and whether an exogenous stem cell type could feed into the myogenic lineage to generate new satellite cells and replenish the pool of cells available for regenerative myogenesis. Moreover, the niche signals that are required for myogenic conversion of these cell types remain largely unknown. Several groups have started to address this interesting subject by using ablation methods that are coupled to the expression of Pax7 [72,109,110]. These studies have used either a tamoxifen-inducible Pax7-Cre with a stop-flox DTX allele or the human DTX receptor inserted into the Pax7 locus, so that the respective cells can be selectively ablated by exposure to DTX. Targeted elimination of satellite cells with DTX in these mouse models resulted in a severely impaired regenerative response of muscle tissue. Importantly, in one study, regeneration was induced in muscles that had been depleted of satellite cells 2 weeks earlier, and virtually no fibre formation was observed up to 5 weeks after injury [110]. These results indicate that no other cell type can compensate for the loss of satellite cells by direct differentiation into myofibres, and that no other cell type can replenish the satellite cell pool in the intermediate term after injury. As discussed above, however, signals originating from satellite cells and their committed and differentiated progeny are critical for the function and the recruitment of many different cell types in the niche, for instance fibroblasts, endothelial cells and FAPs [62,72,77]. Therefore, DTX-induced loss of satellite cells and the absence of newly formed muscle fibres on injury could lead to impaired recruitment of myogenic non-satellite cell types due to the absence of a trophic signal. In support of this idea, in vitro, most myogenic non-satellite cell types require co-culture and co-differentiation with myoblasts to substantially contribute to the formation of myotubes. In spite of their unclear physiological role in directly contributing to adult myogenesis and to the stem cell niche, non-satellite cells with myogenic potential seem to have tremendous therapeutic value that enables, for instance, systemic delivery or extensive ex vivo expansion [111,112,113,114,115,116,117,118,119,120,121,122]. Thus, their use for cell therapy might allow bypassing of several problems associated with the isolation and expansion of conventional myogenic cells for transplantation [123]. Importantly, an improved understanding of the niche signals required to recruit these cells to myogenesis will help to advance such therapies.
Conclusion and outlook
On injury, the stem cell niche in muscle transitions from a relatively steady state involving few cell types into an enormously complex environment with spatiotemporally regulated cascades of direct and indirect cellular interactions (Fig 4, Table 1). The sum of these interactions, combined with intrinsic stem cell programming, controls the regenerative dynamics in the tissue and ultimately allows for the re-establishment of muscle structure and function. The study of muscle regeneration has taken us away from a view that is centred on intrinsic satellite cell regulation towards an understanding that integrates the immense relevance of the niche. With the mouse as a versatile model to study the biology of skeletal muscle, it is becoming increasingly apparent how elaborately fine-tuned is the role of the different cell types involved in muscle regeneration, and how detrimental are the consequences of disease-related imbalances in these dynamics.
Figure 4.
Schematic of extrinsic signals in the muscle stem cell niche. Paracrine signals (thin arrows) regulate the recruitment, proliferation rate and differentiation (bold arrows) of each cell type.
An integrative understanding of the cellular complexity in the niche will allow for the development of therapeutic strategies targeted to normalize or adapt the global behaviour of specific cell populations rather than single signalling pathways. The field has taken great steps forward due to the development of several important genetic tools allowing the manipulation and observation of specific cell populations in muscle tissue. The further refinement of these tools and the identification of mutually exclusive cellular markers will be crucial to answering many of the outstanding questions (Sidebar A) and to a future holistic understanding of the dynamics of muscle regeneration.
Sidebar A | In need of answers.
How does the niche instruct fate decisions of satellite cells?
What are the main circulating signals that influence the satellite cell niche in systemic conditions, such as ageing, cancer cachexia and diabetes? What changes in the niche do these factors trigger?
Is there a specialized ECM microenvironment that instructs the maintenance of satellite cells in quiescence?
What are the niche signals that recruit non-satellite cell types with myogenic potential?
What are the critical components required to create a functional artificial niche for the expansion of uncommitted satellite cells ex vivo?
Is it possible to develop an experimental system that allows the observation of cellular dynamics in a completely undisturbed niche?
Are there differences in the composition of the satellite cell niche between mice and humans?
C Florian Bentzinger, Yu Xin Wang, Nicolas A Dumont & Michael A Rudnicki
Acknowledgments
C.F.B. is supported by a grant from the Swiss National Science Foundation. Y.X.W. is supported by fellowships from QEII-GSST and the Canadian Institutes of Health Research. N.A.D. is supported by a fellowship of the Canadian Institutes of Health Research. M.A.R. is supported by the Canadian Institutes for Health Research, the National Institutes of Health, the Canadian Stem Cell Network, Ontario Ministry of Economic Development and Innovation, and the Canada Research Chair Program.
References
- Lieber RL (2010) Skeletal Muscle Structure, Function, and Plasticity. Baltimore, MD, USA: Wolters Kluwer/Lippincott Williams & Wilkins [Google Scholar]
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RN, Zhang RH, Rossi FM (2013) Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? Febs J 280: 4100–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannerec A, Marazzi G, Sassoon D (2012) Stem cells in the hood: the skeletal muscle niche. Trends Mol Med 18: 599–606 [DOI] [PubMed] [Google Scholar]
- Mounier R, Chretien F, Chazaud B (2011) Blood vessels and the satellite cell niche. Curr Top Dev Biol 96: 121–138 [DOI] [PubMed] [Google Scholar]
- Pillon NJ, Bilan PJ, Fink LN, Klip A (2013) Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 304: E453–E465 [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol [Epub 1 Feb] ; DOI: 10.1101/cshperspect.a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Rudnicki MA (2011) Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol 13: 127–133 [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122: 289–301 [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy A, Cadwallader AB, Fedorov Y, Tyner K, Tanaka KK, Olwin BB (2012) Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell 11: 541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S (2012) A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148: 112–125 [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, von Maltzahn J, Rudnicki MA (2010) Extrinsic regulation of satellite cell specification. Stem Cell Res Ther 1: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS (1997) Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther 8: 1429–1438 [DOI] [PubMed] [Google Scholar]
- Cheung TH, Rando TA (2013) Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14: 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA (2012) Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30: 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S (2012) A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30: 243–252 [DOI] [PubMed] [Google Scholar]
- Koch U, Lehal R, Radtke F (2013) Stem cells living with a Notch. Development 140: 689–704 [DOI] [PubMed] [Google Scholar]
- Blanco-Bose WE, Yao CC, Kramer RH, Blau HM (2001) Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res 265: 212–220 [DOI] [PubMed] [Google Scholar]
- Cohn RD et al. (2002) Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110: 639–648 [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K (1997) Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet 17: 318–323 [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A (1994) Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199: 326–337 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB (2001) Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 239: 79–94 [DOI] [PubMed] [Google Scholar]
- Xian X, Gopal S, Couchman JR (2010) Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res 339: 31–46 [DOI] [PubMed] [Google Scholar]
- Pisconti A, Cornelison DD, Olguin HC, Antwine TL, Olwin BB (2010) Syndecan-3 and Notch cooperate in regulating adult myogenesis. J Cell Biol 190: 427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C et al. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670 [DOI] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12: 1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J (2001) Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol 10: 312–320 [DOI] [PubMed] [Google Scholar]
- Chen SE, Jin B, Li YP (2007) TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol 292: C1660–C1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne E, Tremblay MH, Cote CH (2011) Mast cell tryptase stimulates myoblast proliferation; a mechanism relying on protease-activated receptor-2 and cyclooxygenase-2. BMC Musculoskelet Disord 12: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P (2008) Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44 [DOI] [PubMed] [Google Scholar]
- Dumont N, Lepage K, Cote CH, Frenette J (2007) Mast cells can modulate leukocyte accumulation and skeletal muscle function following hindlimb unloading. J Appl Physiol 103: 97–104 [DOI] [PubMed] [Google Scholar]
- Wang Y, Thorlacius H (2005) Mast cell-derived tumour necrosis factor-alpha mediates macrophage inflammatory protein-2-induced recruitment of neutrophils in mice. Br J Pharmacol 145: 1062–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigitte M et al. (2010) Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum 62: 268–279 [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A (2013) Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153: 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont N, Bouchard P, Frenette J (2008) Neutrophil-induced skeletal muscle damage: a calculated and controlled response following hindlimb unloading and reloading. Am J Physiol Regul Integr Comp Physiol 295: R1831–R1838 [DOI] [PubMed] [Google Scholar]
- Kasama T, Strieter RM, Standiford TJ, Burdick MD, Kunkel SL (1993) Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J Exp Med 178: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA (2000) The neutrophil as a cellular source of chemokines. Immunol Rev 177: 195–203 [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M et al. (2013) Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31: 384–396 [DOI] [PubMed] [Google Scholar]
- Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L (2011) Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J 25: 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa M et al. (2008) Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res 314: 3232–3244 [DOI] [PubMed] [Google Scholar]
- Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP (2006) Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol 290: R1488–1495 [DOI] [PubMed] [Google Scholar]
- Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG (2012) IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 189: 3669–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C (2009) A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA 106: 17475–17480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK (2003) Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnet C, Lafuste P, Arnold L, Brigitte M, Poron F, Authier FJ, Chretien F, Gherardi RK, Chazaud B (2006) Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci 119: 2497–2507 [DOI] [PubMed] [Google Scholar]
- Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L (2002) Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci 23: 189–194 [DOI] [PubMed] [Google Scholar]
- Dumont N, Frenette J (2010) Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am J Pathol 176: 2228–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Jansen KM, Mills ST, Pavlath GK (2003) IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113: 483–494 [DOI] [PubMed] [Google Scholar]
- Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S (2001) Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol 53: 386–392 [DOI] [PubMed] [Google Scholar]
- Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, Rauterberg J, Lorkowski S (2008) Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol 180: 5707–5719 [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA (2013) Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12: 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urciuolo A et al. (2013) Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 4: 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O, Lindbom L (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10: 427–439 [DOI] [PubMed] [Google Scholar]
- Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG (2009) Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 18: 482–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ (2003) Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics 14: 261–271 [DOI] [PubMed] [Google Scholar]
- Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ (2012) Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res 27: 1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12: 143–152 [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretheeban T, Lemos DR, Paylor B, Zhang RH, Rossi FM (2012) Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A et al. (2011) Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124: 3654–3664 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6: 392–401 [DOI] [PubMed] [Google Scholar]
- Tarin D, Croft CB (1969) Ultrastructural features of wound healing in mouse skin. J Anat 105: 189–190 [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 99: 12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann HP, Muller GA (1991) Characterization of human renal fibroblasts in health and disease: II. In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am J Kidney Dis 17: 684–686 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363 [DOI] [PubMed] [Google Scholar]
- Lindner D, Zietsch C, Becher PM, Schulze K, Schultheiss HP, Tschope C, Westermann D (2012) Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem Res Int 2012: 875742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G (2011) Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C et al. (2007) Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque E, Pena J, Martin P, Jimena I, Vaamonde R (1995) Capillary supply during development of individual regenerating muscle fibers. Anat Histol Embryol 24: 87–89 [DOI] [PubMed] [Google Scholar]
- Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK (2007) Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661 [DOI] [PubMed] [Google Scholar]
- Abou-Khalil R, Mounier R, Chazaud B (2010) Regulation of myogenic stem cell behavior by vessel cells: the “menage a trois” of satellite cells, periendothelial cells and endothelial cells. Cell Cycle 9: 892–896 [DOI] [PubMed] [Google Scholar]
- Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE (2009) Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296: C1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Khalil R et al. (2009) Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 5: 298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E (2012) A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol 13: 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabe de Angelis M, McIntyre J 2nd, Gossler A (1997) Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386: 717–721 [DOI] [PubMed] [Google Scholar]
- Vasyutina E, Lenhard DC, Wende H, Erdmann B, Epstein JA, Birchmeier C (2007) RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc Natl Acad Sci USA 104: 4443–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, Buckingham M (2010) An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res 4: 77–91 [DOI] [PubMed] [Google Scholar]
- Brohl D, Vasyutina E, Czajkowski MT, Griger J, Rassek C, Rahn HP, Purfurst B, Wende H, Birchmeier C (2012) Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev Cell 23: 469–481 [DOI] [PubMed] [Google Scholar]
- von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA (2012) Wnt signaling in myogenesis. Trends Cell Biol 22: 602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA (2009) Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemann CG (2011) The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol 7: 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM (1998) Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet 7: 2135–2140 [DOI] [PubMed] [Google Scholar]
- Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P (2010) Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol Biol Cell 21: 1111–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H et al. (2013) MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab 17: 210–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A, Komaki M, Rudnicki M (2001) Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245–253 [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810 [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA (2013) Treating muscular dystrophy by stimulating intrinsic repair. Regen Med 8: 237–240 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764 [DOI] [PubMed] [Google Scholar]
- Carlson ME et al. (2009) Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell 8: 676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal P, Pishesha N, Wijaya D, Conboy IM (2012) Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany NY) 4: 553–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS (2012) The aged niche disrupts muscle stem cell quiescence. Nature 490: 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P (2011) Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JD et al. (2002) A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 11: 263–272 [DOI] [PubMed] [Google Scholar]
- Serrano AL, Muñoz-Cánoves P (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316: 3050–3058 [DOI] [PubMed] [Google Scholar]
- Gilbert PM et al. (2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lu H (2010) Targeting fibrosis in Duchenne muscular dystrophy. J Neuropathol Exp Neurol 69: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur AY, Kuntzer T, Pike M, Swan A (2008) Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev CD003725. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC (1999) Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401: 390–394 [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA (2010) Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol 12: 257–266 [DOI] [PubMed] [Google Scholar]
- Dellavalle A et al. (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267 [DOI] [PubMed] [Google Scholar]
- Torrente Y et al. (2004) Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest 114: 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M et al. (2003) Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301: 487–492 [DOI] [PubMed] [Google Scholar]
- Liadaki K, Casar JC, Wessen M, Luth ES, Jun S, Gussoni E, Kunkel LM (2012) beta4 integrin marks interstitial myogenic progenitor cells in adult murine skeletal muscle. J Histochem Cytochem 60: 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R et al. (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656 [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach E et al. (2006) Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve 34: 44–52 [DOI] [PubMed] [Google Scholar]
- Asakura A, Rudnicki MA (2002) Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30: 1339–1345 [DOI] [PubMed] [Google Scholar]
- Tedesco FS et al. (2011) Stem cell-mediated transfer of a human artificial chromosome ameliorates muscular dystrophy. Sci Transl Med 3: 96ra78. [DOI] [PubMed] [Google Scholar]
- Dellavalle A et al. (2011) Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2: 499. [DOI] [PubMed] [Google Scholar]
- Berry SE, Liu J, Chaney EJ, Kaufman SJ (2007) Multipotential mesoangioblast stem cell therapy in the mdx/utrn-/- mouse model for Duchenne muscular dystrophy. Regen Med 2: 275–288 [DOI] [PubMed] [Google Scholar]
- Sampaolesi M et al. (2006) Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444: 574–579 [DOI] [PubMed] [Google Scholar]
- Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M (2007) Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol Chapter 2: Unit 2B 1. [DOI] [PubMed] [Google Scholar]
- Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, Torrente Y, Butler-Browne GS, Mouly V (2009) In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther 17: 1771–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Ishikawa M, Kamei N, Nakasa T, Adachi N, Deie M, Asahara T, Ochi M (2009) Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood-derived CD133-positive cells. Stem Cells 27: 949–960 [DOI] [PubMed] [Google Scholar]
- Zheng B et al. (2007) Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol 25: 1025–1034 [DOI] [PubMed] [Google Scholar]
- Vauchez K et al. (2009) Aldehyde dehydrogenase activity identifies a population of human skeletal muscle cells with high myogenic capacities. Mol Ther 17: 1948–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Rudnicki MA (2013) The emerging biology of muscle stem cells: implications for cell-based therapies. Bioessays 35: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DR, Criswell DS, Carson JA, Booth FW (1997) Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol (1985) 83: 1270–1275 [DOI] [PubMed] [Google Scholar]
- Jin Y, Murakami N, Saito Y, Goto Y, Koishi K, Nonaka I (2000) Expression of MyoD and myogenin in dystrophic mice, mdx and dy, during regeneration. Acta Neuropathol 99: 619–627 [DOI] [PubMed] [Google Scholar]