Abstract
Targeted genomic selection methodologies, or sequence capture, allow for DNA enrichment and large-scale resequencing and characterization of natural genetic variation in species with complex genomes, such as rapeseed canola (Brassica napus L., AACC, 2n=38). The main goal of this project was to combine sequence capture with next generation sequencing (NGS) to discover single nucleotide polymorphisms (SNPs) in specific areas of the B. napus genome historically associated (via quantitative trait loci –QTL– analysis) to traits of agronomical and nutritional importance. A 2.1 million feature sequence capture platform was designed to interrogate DNA sequence variation across 47 specific genomic regions, representing 51.2 Mb of the Brassica A and C genomes, in ten diverse rapeseed genotypes. All ten genotypes were sequenced using the 454 Life Sciences chemistry and to assess the effect of increased sequence depth, two genotypes were also sequenced using Illumina HiSeq chemistry. As a result, 589,367 potentially useful SNPs were identified. Analysis of sequence coverage indicated a four-fold increased representation of target regions, with 57% of the filtered SNPs falling within these regions. Sixty percent of discovered SNPs corresponded to transitions while 40% were transversions. Interestingly, fifty eight percent of the SNPs were found in genic regions while 42% were found in intergenic regions. Further, a high percentage of genic SNPs was found in exons (65% and 64% for the A and C genomes, respectively). Two different genotyping assays were used to validate the discovered SNPs. Validation rates ranged from 61.5% to 84% of tested SNPs, underpinning the effectiveness of this SNP discovery approach. Most importantly, the discovered SNPs were associated with agronomically important regions of the B. napus genome generating a novel data resource for research and breeding this crop species.
Introduction
Brassica napus L. var oleifera Delile (2n=4x=38, AACC) known as rape, oilseed rape or rapeseed belongs to one of the three cultivated allotetraploid Brassica crops that form U’s triangle [1]. Since the 1990´s, the cultivation of rapeseed has been primarily devoted to the production of edible oil, high value protein meals and more recently, biofuels [2,3]. Canadian scientists developed varieties which were declared suitable for human and animal consumption by the United States Food and Drug Administration in 1985 [4,5] after reducing the seed levels of two antinutritional factors, erucic acid (<2% of total oil) and glucosinolates (<30 mg in the meal). This transformation of rapeseed from an industrial (lubricants) into an edible oil [6,7] has been regarded as one of the major achievements of modern plant breeding [5]. In addition to improving the nutritional quality of rapeseed, major breeding efforts have been devoted to increasing seed yield (e.g. hybrid development) and identifying resistance genes for blackleg and sclerotinia, among other diseases [3,8].
Rapeseed researchers have developed a large number of genomic tools in order to accelerate the breeding process and to understand the genetic basis of complex traits in a polyploid genome [3,9]. Among these tools, the development and application of molecular markers in rapeseed research has prevailed and has grown immensely since their humble beginnings in the 1980s to the impressive high-throughput technologies of today [2,10,11]. Molecular markers like Restriction Fragment Length Polymorphism (RFLP), Randomly Amplified Polymorphic DNA (RAPD) and Simple Sequence Repeats (SSR) have at one time or another, all been developed with the ultimate goal of associating DNA sequence differences with desirable phenotypic variation [12]. Largely, this task was conducted by developing more than 34 molecular linkage maps (reviewed in 3) that were used as reference charts for quantitative trait loci (QTL) analysis [13-20]. Additionally, molecular markers have also been used to understand the structure of the Brassica A and C genomes [13,21-23], organize germplasm collections [24,25] and predict heterosis through the formation of heterotic groups for their subsequent combination in hybrid varieties [19,26].
The recent availability of sequence data for Brassica crops [2,9,27,28] is now steering researchers into developing newer, more efficient and high-throughput molecular markers. Among these, the most popular choice of marker is single nucleotide polymorphisms (SNPs), which were first embraced in human and animal genetics [29,30] and have subsequently made their way into plant genomics and genetics [30-32]. Hayward et al. [33] reviewed the latest technologies available for SNP discovery as well as the potential application that SNP markers may have in B. napus genetic and genomic research.
A logical step during the process of associating molecular markers with desirable phenotypes is to make use of all the genetic and genomic information available for a given individual and/or population through targeted discovery approaches. This can be achieved by enriching for chromosomal regions underlying QTLs of agronomical and nutritional interest via hybridization-based platforms, in combination with high-throughput next generation sequencing (NGS) technologies. This could provide rapeseed researchers with a unique opportunity to discover SNPs under QTL peaks and their corresponding confidence intervals. Given the vast number of QTL mapping studies for various agronomical and nutritional traits in rapeseed [2] and the recent advances made in genomics and bioinformatics [33], it is now possible to investigate sequence variation (among individuals) of specific QTL targeted genomic regions with the aim of discovering SNPs tightly linked to a desired phenotype. This can also be useful to further elucidate the genetic and molecular basis underlying a trait.
NGS offers a faster, more systematic and cost-effective way of assessing genetic variation [34,35]. However, a major challenge to broadly apply these NGS technologies continues to be the targeted DNA enrichment of large and complex eukaryotic genomes. For example, sequencing a thousand genes would require designing and synthesizing several thousands of PCR primers and performing several thousands of PCR reactions, a costly and lengthy process. Recently, the development of microarray-based or liquid-based genomic selection methods have proven extremely useful for the isolation of user-defined unique genomic sequences in one single enrichment step [36-38]. These methodologies are commonly referred to as “Sequence Capture” and have been broadly applied in human genetics [39-41]. In plant genetics, resequencing of targeted genomic areas has resulted in the discovery of SNP markers, the study of gene copy number variation and characterization of homoeologous coding and non-coding sequences in allopolyploid genomes [42-45]. One crucial advantage of using sequence capture protocols in complex polyploid genomes is that duplicated loci can be specifically targeted and subsequently resolved using their interlocus polymorphisms, avoiding the risk of illegitimately comparing duplicated copies in the genome [2,32].
In this study, we have combined the use of sequence capture with NGS technologies in order to i) target 47 genomic regions underlying traits of agronomical and nutritional interest previously identified via QTL analysis, ii) re-sequence 10 contrasting genotypes, iii) discover and validate SNP markers for the interrogated QTL regions, and iv) characterize the discovered SNPs (genic vs. intergenic; coding vs. intronic sequence; transition vs. transversion).
Materials and Methods
Brassica napus plant material
The ten genotypes used in this study were grown under greenhouse conditions in a 16h/day photoperiod regime. These varieties or breeding lines were chosen in order to exploit available sequence, genotypic and phenotypic information (Table S1). Genotypes were classified as either low or high in both erucic acid and glucosinolates (hereinafter referred to as 00 and ++, respectively). Briefly, Tapidor (00 and late flowering) and Ningyou7 (++ and early flowering) are two divergent winter cultivars and the parents of a doubled haploid (DH) population that has been used to map several QTL for seed oil and erucic acid content [46]. Express (00 elite variety) and V8 (++ synthetic line) are the parents of another DH population that has and continues to be extensively tested for a number of useful agronomical and nutritional traits including seed yield, seedling vigour, heterosis, oil content and nitrogen use efficiency [47,48]. DH12075 (00, early flowering and blackleg resistant) and PSA12 (seedling vigor) are spring type parents of the Canadian reference mapping population [13]. Extensive genomic and EST sequence data are available from DH12075. YN-429 (early flowering) is a yellow seed line that is also the parent of a DH population with DH12075. This DH population has been genotyped and is presently being phenotyped for agronomical and quality traits (Isobel Parkin, personal communication). Rainbow (00) is an Australian spring type that has been bred for robust blackleg resistance. CGNA1 (00, late flowering and susceptible to sclerotinia) and CGNA 2 (++, early flowering and susceptible to blackleg disease) are winter type genotypes that have been bred under the agro-climatic conditions of south central Chile.
NGS library preparation
Nuclei DNA was extracted from flower buds and leaf tissue following standard lab protocols [49]. The DNA was checked for quality (A260/A280 ratio=1.98-2.0) and quantified using a Nanodrop spectrophotometer (10-15 ug per sample). All ten genotypes were used to prepare 454 Life Sciences NGS libraries. In addition, two lines (DH12075 and Express) underwent Illumina library preparation in order to compare the results from the two approaches. Briefly, 454 GS-FLX Titanium sequencing libraries were constructed using the 454 Life Sciences (454, Branford, CT) GSFLX Titanium Kit as described in the user guide. The entire genomic DNA product from this library preparation (e.g. SST library) was used as template in a Pre-Hybridization Linker Mediated PCR (LMPCR) reaction to ensure that the majority of the molecules contained adapters on both sides of the inserts. LMPCR consisted of 5 reactions each containing 5µl 10X Platinum High Fidelity Polymerase Buffer (Invitrogen, CA), 2.5 µl MgSO4, 1 µl 25nM dNTPs, 1 µl of 25 µM Primer A (5’-ACCATCTCATCCCTGCGTGTC), 1 µl of 25 µM Primer B (5'-CCTATCCCCTGTGTGCCTTG), 0.4 µl Platinum High Fidelity Polymerase (Invitrogen, CA). DNA in equal amounts was apportioned for each of the five reactions, with water to 50 µl. The reactions were then subjected to 94°C for 4 minutes followed by 8 cycles of: 94°C for 30 seconds, 1 minute at 58°C and 1.5 minutes at 68°C. The last step was an extension at 72°C for 5 minutes. The reactions were then kept at 4°C until further processing. The amplified material was recovered with a Qiagen Qiaquick column according to the manufacturer’s instructions (Qiagen, CA), except the DNA was eluted in 50 µl water instead of the EB buffer. The DNAs were quantified using the NanoDrop-1000 (Wilmington, DE) and the library was evaluated electrophoretically with an Agilent Bioanalyzer 2100 (Agilent, CA) using a DNA 7500 chip. The library fragment sizes were found to be 500 to 700 bp, which are well within the expected range.
Illumina Paired End libraries (Illumina Inc., CA) were constructed using Illumina’s PE Kit, with modifications. The agarose gel excision was performed at 250-300 base pairs (bp) to produce libraries with an approximate insert size of 130-180 bp. DNA was gel purified using a Qiaquick column (Qiagen, CA) and eluted in 30µl of water. The entire recovery product was used as template in the pre-hybridization library amplification via the Illumina sequencing adapters (i.e. LMPCR). Pre-hybridization LMPCR consisted of one reaction containing 50 µl Phusion High Fidelity PCR Master Mix (New England BioLabs, MA), 2µM of primers Illumina PE 1.0: (5'-AATGATACGGCGACCACCGAGATCTACACTCTT TCCCTACACGACGCTCTTCCGATC*T) and 2.0: (5'-CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGCTGAACCGCTCTTCCGATC*T) where the asterisk denotes a phosphorothioate bond, 30µl DNA, and water up to 100µl. PCR cycling conditions were as follows: 98°C for 30 seconds, followed by 8 cycles of 98°C for 10 seconds, 65°C for 30 seconds, and 72°C for 30 seconds. The last step was an extension at 72°C for 5 minutes. The reaction was then kept at 4°C until further processing. The amplified material was cleaned again with a Qiagen Qiaquick column according to the manufacturer´s instructions, except the DNAs were eluted in 50 µl water. The DNAs were quantified using a NanoDrop-1000 (Thermo Scientific, DE) and the library was evaluated electrophoretically with an Agilent Bioanalyzer 2100 using a DNA1000 chip (Agilent, CA). The mean library fragment size was found to be 328 bp. Fragment sizes are well in range with the expected ones.
Selection of B. napus genome sequence information to be captured
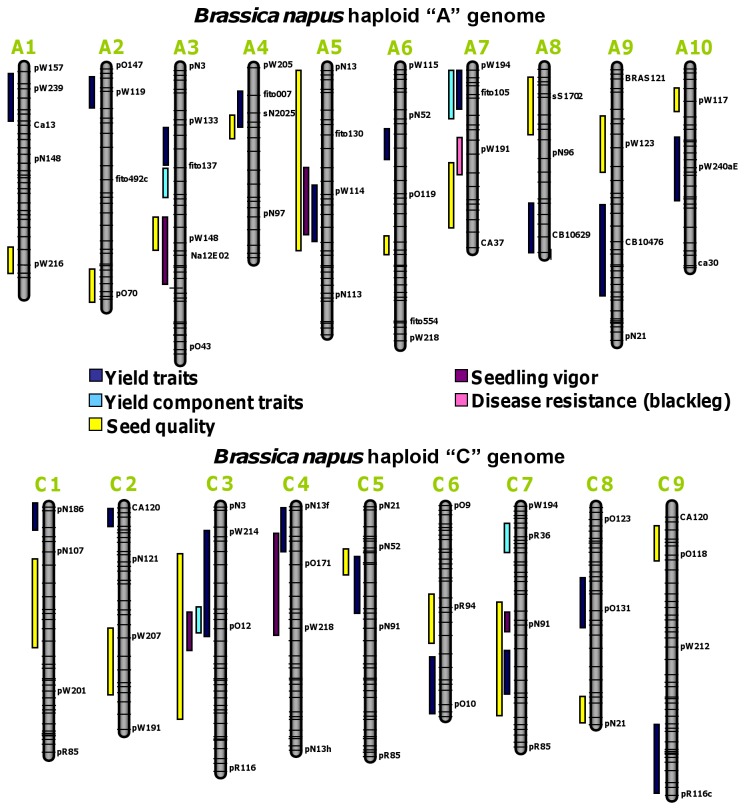
A total of 47 genomic regions from the A and C genomes of B. napus L. that were previously found to be associated with yield and yield component traits, seedling vigor, seed quality and disease resistance traits (Figure 1) were selected to develop probes for the sequence capture platform. This was conducted by selecting sequenced molecular markers that were located under QTL peaks and identifying their corresponding intervals in the 10 A genome haploid chromosomes of B. napus (Table S2). Since the genome sequence for B. rapa (equivalent to the A genome progenitor of B. napus) was available, sequences were extracted from the A genome according to the coordinates/regions identified to be associated with traits of interest. Predicted genes from those regions were BLASTed against available B. oleracea (or C genome) contigs to identify the orthologous regions. The regions from the C genome that had the most compelling synteny with the A genome segments were selected. This information was compiled into 883 FASTA sequences (Capture Design Reference) from sequenced scaffolds of B. rapa [51] and preliminary contigs from B. oleracea (Parkin and Sharpe, unpublished data), totaling approximately 51.2 Mb (38.8 Mb and 12.4 Mb corresponding to the A and C Brassica genomes, respectively).
Figure 1. Selected genomic regions underlying traits of agronomical and nutritional interest in B. napus.
Map integration was conducted according to common molecular markers and parental lines used in three different mapping studies [15, 22, 50]. QTL locations were inferred from relative map positions previously described [13,15,17-19, 22] always using common sets of molecular markers and genetic stocks.
Sequence capture design
The first step during the design of the sequence capture array was the development of a probe database set (70 to 100-mer oligos to be placed on the sequence capture microarray device) for the 51.2 Mb of selected genomic sequence. The second step involved the selection of probes using proprietary Roche NimbleGen software algorithms. As a result, a set of probes was selected to provide a 93.4% to 98.3% coverage of the targeted genomic sequence.
Sequence capture hybridization
Sequence capture array hybridization for all genotypes was conducted by Roche NimbleGen (Madison, WI) following their proprietary protocol. The 2.1M features array used to hybridize the ten genotypes had a coverage representation of 93.4-98.3% of the target areas of interest. The captured or enriched DNA was assessed using quantitative PCR. Five genotypes (DH12075, Ningyou_7, PSA12, Tapidor and YN-429) were captured using a solid array platform and analyzed using a 7500 Bioanalyzer chip. The remaining five genotypes (Rainbow, Express, V8, CGNA1 and CGNA2) were captured using a liquid platform and analyzed using a 1000 Bioanalyzer chip. Captured DNA elutions after hybridization and enrichment assessment were lyophilized and sent for NGS.
NGS of enriched DNA: 454 Life Sciences and Illumina chemistries
454 Life Sciences chemistry NGS was conducted for all ten captured genotype DNAs (Table 1). In addition, captured DNA samples from DH12075 and Express underwent NGS using the Illumina HiSeq. Standard protocols described by the manufacturers (454 Life Sciences, a Roche company, CT and Illumina, Inc., CA) were used for sequencing the captured DNA at Roche Nimblegen (Madison, WI). Sequence data files were deposited in the Short Read Archive of NCBI under the BioProject ID: PRJNA212782. Sequence reads were mapped to a reference dataset as described below.
Table 1. Summary of Next Generation Sequencing (NGS) results.
| Lines | Chemistry | Sequenced Reads | Total Num. of Bases | Avg. Length* | SD Length | Avg. Q-score | SD Q-score |
|---|---|---|---|---|---|---|---|
| DH12075 | 454 | 1,289,496 | 450,850,003 | 350 | 131.6 | 33.1 | 8.4 |
| PSA12 | 454 | 826,680 | 289,078,010 | 350 | 101.1 | 32.6 | 8.5 |
| Express | 454 | 827,074 | 313,062,567 | 379 | 105.4 | 31.4 | 8.4 |
| V8 | 454 | 711,244 | 261,475,787 | 368 | 121.5 | 33.7 | 8.2 |
| Tapidor | 454 | 778,116 | 257,650,369 | 331 | 95.1 | 31.6 | 8.5 |
| Ningyou-7 | 454 | 803,553 | 288,413,071 | 359 | 112.8 | 33.2 | 7.9 |
| Rainbow | 454 | 742,283 | 248,197,873 | 334 | 129.9 | 33.7 | 8.1 |
| YN-429 | 454 | 735,005 | 254,920,458 | 347 | 101.3 | 32.4 | 8.5 |
| CGNA1 | 454 | 742,361 | 267963689 | 364 | 118.7 | 33.1 | 8.4 |
| CGNA2 | 454 | 717,016 | 270,568,629 | 374 | 117.6 | 33.3 | 8.3 |
| DH12075 | Illumina | 167,215,495 | 8,225,138,523 | 49 | - | 40 | 2.3 |
| Express | Illumina | 184,559,482 | 10,908,314,681 | 59 | - | 42 | 2.1 |
Abbreviations: Num.=Number; Avg. = Average; SD = Standard deviation; Q-score = Quality.
* Length in base pairs (bp).
Reference sequence datasets
Three reference sequence datasets were developed to fully interrogate the capture design process. The capture design reference (883 sequences) was previously described. A second dataset containing the capture design sequences as well as their orthologous sequences was generated to increase the resolution of homoeologous sequences during the read mapping. To identify the orthologue of each sequence used in the capture design, sequences were individually aligned to the pseudomolecules of the complementary genome using NUCmer (MUMmer 3.0 package, [52]). Resulting delta files from the NUCmer alignments were passed through a Perl script to filter out the majority of the repetitive hits. The resulting files were plotted using mummerplot (MUMmer 3.0 package, [52]) and investigated manually before the complementary region was determined. The third dataset contains de novo assembles of the Brassica A and C genome pseudomolecules (representing 283.8 Mb of B. rapa and 488.6 Mb of B. oleracea, respectively) and was used to determine sequence coverage in the target and non-target regions of the genome.
Captured DNA read mapping and SNP discovery
The sequence reads (for all ten genotypes) were aligned to the each of the three reference data sets using the CLC Bio Genomics Server software version 4.5.1 (Katrinebjerg, Denmark; www.clcbio.com). Default parameters for the mapping algorithm were used except the mapping identity parameter was increased to 98% in order to resolve homoeologous sequence reads.
The mapped reads were then interrogated for sequence variation using the CLC Bio probabilistic variant calling tool with a minimum depth of 3 reads for the 454 data, and 8 reads for the Illumina data. Custom Perl scripts were used to compile information from SAM files and unfiltered SNP calling files generated by CLC Bio. The SNPs were filtered according to the following criteria: i) removal of SNPs with multiple variations within the 10 lines, ii) SNPs containing a high proportion of heterozygous calls or bias, and iii) SNPs without flanking sequence suitable for KASPar or Illumina Infinium design. For KASPar primer design, a SNP requires 100 bp of flanking sequence on both sides of the SNP. For Illumina Infinium assay design, a SNP requires 60 bp of flanking sequence that is free from additional SNPs on at least one side.
SNP characterization: filtered and unfiltered SNPs were characterized as genic or intergenic using the available annotation of the A and C diploid Brassica genomes. Coordinates for genes and the corresponding coding sequences (CDS) were extracted from GFF annotation files using a Perl script and SNP positions were then compared to the list of gene coordinates. For each of these sets the breakdown of transitions and transversions was determined using standard UNIX utilities to extract and count the SNP types. Coverage of the target and non-target regions of the sequence capture was compared using BEDTools [53] and custom Perl scripts.
SNP Validation
KASPar chemistry validation assay: DNA was extracted from two segregating populations and a diversity panel using the CTAB procedure scaled for 1.7 ml extractions [54]. DNA was quantified using the Quant-iT PicoGreen dsDNA reagent (Invitrogen Inc., CA) and adjusted to 5 ng/µl. The KASPar assay version 4.0 (LGC Genomics, UK) was used to validate a set of 100 SNPs discovered in this study. The reactions were conducted according to the manufacturer´s instructions in an 8 µl final volume reaction. The assays were carried out in a B. napus diversity panel composed of 25 highly inbred lines (Rod Snowdon, personal communication) and in two segregating populations (DH12075 x PSA12 and V8 x Express) of 30 and 29 individuals, respectively (Table S3). SNPs randomly distributed in the 47 target genomic areas were chosen for KASPar validation (Table S4). Nineteen and 37 KASPar oligonucleotide sets were designed to specifically detect polymorphisms in the DH12075 x PSA12 and V8 x Express populations, respectively. In addition, 44 KASPar oligonucleotide sets were designed to detect polymorphisms in both populations at the same time. All the primer sets were designed using PrimerPicker (KBioscience, 2009) with default parameters.
60K Illumina array validation: SNPs were assayed against a selection of B. napus lines using the Brassica 60K iSelect 24x1HD Custom Genotyping Beadchips and samples were prepared and assayed as per the Infinium HD Assay Ultra Protocol (Infinium HD Ultra User Guide 11328087_RevB, Illumina, Inc. San Diego, CA). The Brassica 60K beadchips were imaged using an Illumina HiScan system, and the SNP alleles were called using the Genotyping Module v1.9.4, within the GenomeStudio software suite v2011.1 (Illumina, Inc. San Diego, CA).
Mapping of a subset of validated SNP markers: linkage analysis and map construction were conducted separately for each population using JoinMap® v4.0. Linked loci were grouped using a LOD threshold of 5-8 and a maximum recombination fraction of 0.4. Grouped marker loci, including the newly mapped SNP markers, were arranged into a scoring matrix using JoinMap® v4.0. The data set was inspected for the presence of spurious double crossovers (identified using JoinMap® v4.0 data tool kit) generated by missing data and taken into account in the final linkage group construction. After the original scores were rechecked, a final linkage map was constructed for each population. Map distances in centiMorgans (cM) were calculated using the Kosambi mapping function.
Results
Brassica napus genomic DNA sequence enrichment using 2.1M NimbleGen custom sequence capture arrays
To investigate meaningful DNA sequence variation, genomic regions of interest were selected for sequence capture that had shown consistent QTL locations in B. napus genome across a variety of different genotypes, years and experimental locations. Examples of consistent QTL findings for important agronomical and nutritional traits (e.g. yield, yield components, seed quality, seedling vigor, disease resistance) could be found in several studies [13-19]. As a result, a total of 47 genomic regions comprising a total of 51.2 Mb (38.8 Mb and 12.4 Mb corresponding to the A and C Brassica genomes, respectively) were selected using sequenced molecular markers to approximate QTL locations in available genome sequence (Figure 1). Selected genomic sequences (51.2 Mb) were used to design solid and liquid sequence capture platforms, which allowed us to interrogate large portions of the B. napus genome per unit array and also to replicate internal controls, ensuring good coverage and depth for the sequencing of the captured genomic regions.
Captured B. napus genomic DNA sequencing, mapping and organization
A summary of the obtained NGS results for each of the 10 B. napus genotypes can be found in Table 1. Briefly, the average number of reads obtained for the 454 Life Sciences and Illumina HiSeq chemistries were 817,283 and 175,887,488, respectively. The average sequence read length was 382 bp using the 454 sequencer and 54 bp for the data obtained using the Illumina HiSeq sequencer (Table 1). Sequence quality parameters (Q-scores) were high for both NGS methods, with a 33 Q-score average for the 454 data and a 40 Q-score average for the Illumina data (Table 1).
Sequenced reads were mapped to two reference sets, the capture design reference (883 A and C Brassica genome reference sequences) and the A and C genome pseudomolecules (Parkin and Sharpe, unpublished data) [51], using the CLC Bio Genomics Server read mapping algorithm (Table 2). On average, 29% (236,966) of the 454 reads for each line uniquely matched the capture design reference, resulting in the calling of 831,767 putative SNPs for the ten genotypes. When the same 454 reads were mapped to the A and C genome pseudomolecules, an average of 479,421 reads (58%) uniquely matched the reference, increasing the number of putative SNP calls for the 10 genotypes to 1,549,530. For the two genotypes surveyed, on average 51% (88,438,604) of the Illumina HiSeq sequence reads uniquely matched the capture design reference while an average of 103,535,628 (59%) reads uniquely matched the A and C pseudomolecules increasing the number of putative SNP calls from 1,479,400 to 2,675,732.
Table 2. Result summary of sequenced reads mapped against the A and C Brassica genomes.
|
|
454 Chemistry Data
|
||||||
|---|---|---|---|---|---|---|---|
|
|
Capture design reference (883 Sequences)
|
A & C Genome Pseudomolecules (19 Sequences)
|
|||||
| Lines | SR | RM | RMp | SNPs | RM | RMp | SNPs |
| DH12075 | 1,289,496 | 414,569 | 32 | 159,442 | 775,554 | 60 | 263,195 |
| PSA12 | 826,680 | 241,867 | 29 | 78,683 | 497,595 | 60 | 138,398 |
| Express | 827,074 | 226,406 | 27 | 81,595 | 475,214 | 57 | 167,462 |
| V8 | 711,244 | 190,312 | 27 | 65,744 | 411,708 | 58 | 136,009 |
| Tapidor | 778,116 | 230,934 | 30 | 75,238 | 453,606 | 58 | 140,756 |
| Ningyou-7 | 803,553 | 240,828 | 30 | 89,273 | 480,899 | 60 | 150,945 |
| Rainbow | 742,283 | 207,465 | 28 | 67,546 | 432,873 | 58 | 132,747 |
| YN-429 | 735,005 | 219,859 | 30 | 76,381 | 427,669 | 58 | 134,101 |
| CGNA1 | 742,361 | 201,604 | 27 | 69,718 | 426,791 | 57 | 144,337 |
| CGNA2 | 717,016 | 195,811 | 27 | 68,147 | 412,298 | 58 | 141,580 |
| Illumina Chemistry Data | |||||||
| Lines | SR | RM | RMp | SNPs | RM | RMp | SNPs |
| DH12075 | 167,215,494 | 92,287,337 | 55 | 745,049 | 102,396,687 | 61 | 1,068,906 |
| Express | 184,559,482 | 84,589,870 | 46 | 834,391 | 104,674,569 | 57 | 1,606,826 |
Abbreviations: SR=sequenced reads, RM=reads matching the reference genome, RMp=reads mapped to reference genome in %, SNP=single nucleotide polymorphism.
SNP discovery among ten contrasting B. napus genotypes
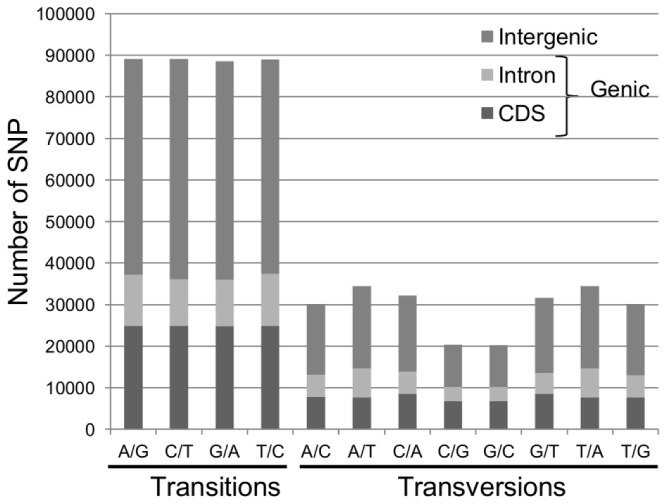
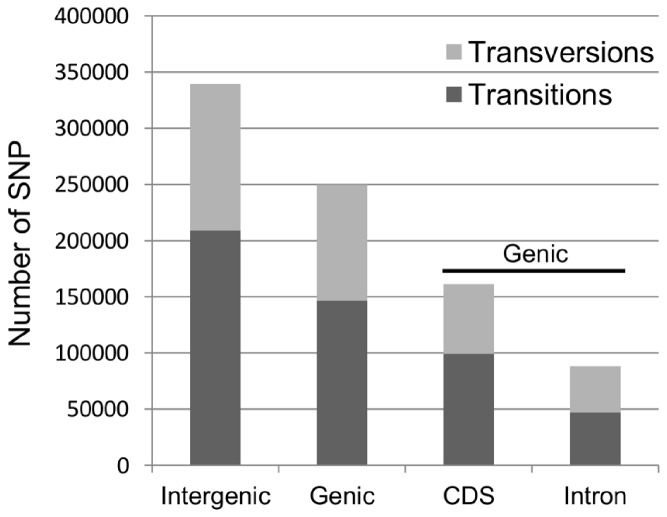
A total of 589,367 filtered SNPs were identified from 2,740,205 unfiltered SNPs (Table 3). The discovered SNPs were classified according to type (transition vs. transversion) and whether they were found in genic or non-genic sequences. A significant number of the discovered SNPs (249,623, 42.4%) were located in a gene region and of these, 64.6% (161,189) were found within annotated coding sequences (CDS). The remaining 57.6% (339,744) were found to be intergenic. Genic and intergenic SNPs were further broken down into transitions and transversions. Transitions represent approximately 60.4% of all post-filtered SNPs (355,714), with 41.2% (146,644) of these being located in genic regions. The breakdown of each SNP type (e.g. A-T, C-A, etc.) by genomic location is illustrated in Figure 2. Of the 146,644 genic transitions, 67.9% were found in CDS, whereas 59.8% of the 102,979 genic transversions were found in CDS (Figure 3). Figures S1 and Figure S2 show these breakdowns based on a SNP set filtered only for multiple variant SNPs (minimally filtered set).
Table 3. Summary of SNP filtering criteria and discovery pipeline results.
| Filtering Criteria | Number of SNPs Excluded | Number of SNPs Remaining |
|---|---|---|
| None | 0 | 2,740,205 |
| Multiple Variants hits | 33,917 | 2,706,288 |
| Heterozygous and Bias* | 2,111,439 | 594,849 |
| Flanking Sequence** | 5,482 | 589,367 |
| Candidate SNPs | 589,367 |
* Removal of SNPs containing only heterozygous and bias SNP calls. It also removes SNPs with a percentage of heterozygous calls over a threshold (0.2).
** Removal of SNPs not meeting KASPar or Illumina Infinium flanking sequence requirements.
Figure 2. Filtered SNP types and their classification according to genomic regions.
Transition and transversion SNP types were classified to show the proportion of SNPs annotated to each of three genomic regions (Intergenic, Intron, and CDS). CDS: coding sequence.
Figure 3. Filtered SNP counts characterization summary.
Total SNP counts were classified by genomic location (Intergenic vs. Genic) and further separated into transitions and transversions. Genic SNPs are also described in terms of their location within the gene (CDS vs. intron). CDS: coding sequence.
To assess the difference in sequencing platforms, the number of filtered SNPs unique to each sequencing type was determined for DH12075 and Express. Of the 589,367 filtered SNPs, 487,537 have a call in DH12075 or Express. Of these, 95,827 were unique to the Illumina sequencing data while 4,070 were unique to the 454 Life Sciences sequencing data. The flanking sequence for the filtered SNPs was categorized based on the downstream assay type, as KASPar, Illumina Infinium, or both. Our analysis showed that 587,261 SNPs are valid for KASPar assays, with 297,802 of those also having potential value for Illumina Infinium assay design. There were 289,459 SNPs that could only be used for KASPar design while 2,106 SNPs were only useful for Illumina Infinium design. This difference is largely due to the requirement that there are no flanking SNPs on at least one side of the target SNP for Infinium design.
Analysis of sequence capture coverage
In order to analyze the efficacy of the sequence capture process, the depth of coverage was surveyed for both target and non-target regions. The original capture design was based on incomplete diploid Brassica genome sequence with a bias to the A genome (75.7% of the capture sequence). However, the strong sequence identity between the A and C genomes (on average 94%) would not allow B. napus homoeologous sequences to be differentiated during the capture process [55]. Thus, to accurately assess the true target region, it was necessary to identify the orthologous sequence from the complementary genome for each of the 883 sequences used in the design process. As described in the methods each sequence was aligned to the pseudomolecules of the complementary genome using NucMER, which resulted in 1,074 orthologous sequences (or 131.1 Mb) being identified, 39 for the 35 A genome capture regions and 1035 for the 848 C genome capture regions.
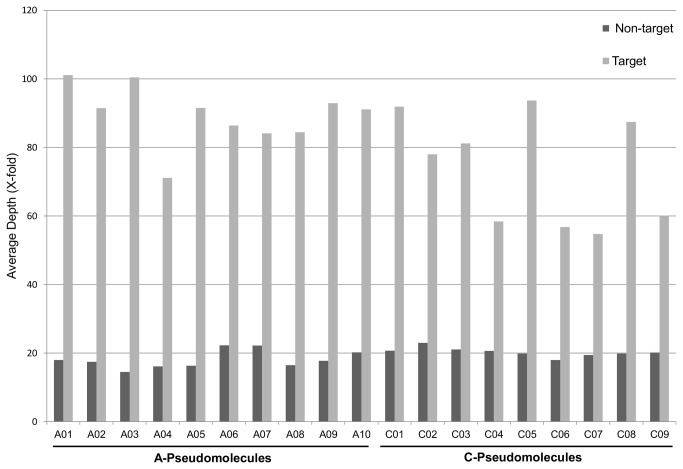
Sequence reads from each of the ten B. napus genotypes were then mapped using CLC Bio Genomics Server to three different reference sets: the original 883 capture sequences, the complemented capture regions (883 capture sequences + 1,074 orthologous sequences), and the A and C genome pseudomolecules. Sequence read mapping is presented across all ten lines for both sequencing platforms for these three reference sequence sets in Table 4. A percentage increase of reads mapped over the previous set (RMpi) is also included to illustrate better resolution of homoeologous sequences as well as the benefit of mapping to complete genome sequences. A coverage map was generated for each of the B. napus lines providing an average depth of coverage for target and non-target regions (Figure 4). The average depth of coverage for non-target regions was 19.6X while the average depth for the target regions was almost four-fold higher (77X). Additionally, the set of filtered SNPs were analyzed to determine their origin, 333,379 SNPs were found in the target regions, representing 56.6% of all filtered SNPs.
Table 4. Summary of sequence read mapping performance using multiple reference sequence sets.
|
|
454 Chemistry Data
|
||||||
|---|---|---|---|---|---|---|---|
|
|
Capture Design Reference (883 Sequences)
|
Capture Design with Orthologues (883 + 1074 Sequences)
|
A & C Genome Pseudomolecules (19 Sequences)
|
||||
| Lines | SR | RM | RMpi | RM | RMpi | RM | RMpi |
| DH12075 | 1,289,496 | 414,569 | 0 | 457,448 | 10.3 | 775,554 | 69.5 |
| PSA12 | 826,680 | 241,867 | 0 | 279,959 | 15.7 | 497,595 | 77.7 |
| Express | 827,074 | 226,406 | 0 | 271,137 | 19.8 | 475,214 | 75.3 |
| V8 | 711,244 | 190,312 | 0 | 230,314 | 21 | 411,708 | 78.8 |
| Tapidor | 778,116 | 230,934 | 0 | 266,100 | 15.2 | 453,606 | 70.5 |
| Ningyou | 803,553 | 240,828 | 0 | 277,338 | 15.2 | 480,899 | 73.4 |
| Rainbow | 742,283 | 207,465 | 0 | 248,757 | 19.9 | 432,873 | 74 |
| YN-429 | 735,005 | 219,859 | 0 | 247,101 | 12.4 | 427,669 | 73.1 |
| CGNA1 | 742,361 | 201,604 | 0 | 241,700 | 19.9 | 426,791 | 76.6 |
| CGNA2 | 717,016 | 195,811 | 0 | 233,568 | 19.3 | 412,298 | 76.5 |
| Illumina Chemistry Data | |||||||
| Lines | SR | RM | RMpi | RM | RMpi | RM | RMpi |
| DH12075 | 167,215,494 | 92,287,337 | 0 | 74,985,743 | -18.7 | 102,396,687 | 36.6 |
| Express | 184,559,482 | 84,589,870 | 0 | 78,787,995 | -6.9 | 104,674,569 | 32.9 |
Abbreviations: SR = sequenced reads, RM = reads matching the reference genome, RMpi = percentage increase in reads mapped over previous reference.
Figure 4. Summary of DNA sequence coverage.
The average depth of coverage in captured and non-captured regions across all 19 A and C Brassica pseudomolecules is illustrated. Captured regions are those from the original sequence capture selection combined with the orthologous sequence from the complementary genome.
Discovered SNP validation assays
The robustness of the SNP discovery pipeline was assessed by validating a set of 100 SNPs in two reference mapping populations (DH12075 x PSA12; Express x V8) and a B. napus diversity panel using the KASPar SNP assay (Table 5). Nineteen sets of KASPar oligonucleotides were designed to test for SNPs identified between the parental lines of the spring type mapping population (DH12075 x PSA12), 37 were designed to test for SNPs identified between the parental lines of the winter type mapping population (Express x V8) and 44 evaluated SNPs were common to both populations. Table 5 summarizes the results obtained using the singleplex KASPar SNP validation assay for these 100 SNPs. The assay for SNP robustness among the set of 25 diverse B. napus lines showed that 71% of SNPs (71/100) were informative and could discriminate among the different alleles tested. Higher rates of positively confirmed polymorphisms were observed when SNPs were designed for specific parental sets, 84% for DH12075 x PSA12 and 78% for Express x V8. When KASPar oligonucleotides were designed to test for polymorphisms in both mapping populations, the percentage of positively confirmed polymorphisms dropped to 55% probably due to the cumulative effect of failures in both populations (Table 5).
Table 5. Singleplex KASPar SNP validation assay of a set of 100 discovered SNP markers.
|
|
Parental lines specific SNP*
|
|||
|---|---|---|---|---|
| Diversity Set | DH12075 x PSA12 | V8 x Express | Both populations | |
| Individuals tested | 25 | 30 | 29 | - |
| Total SNP tested** | 100 | 19 | 37 | 44 |
| Amplification type | ||||
| No amplification | 12 | 0 | 4 | 8 |
| Monomorphic | 9 | 1 | 5 | 6 |
| Multiple Loci | 8 | 2 | 0 | 6 |
| Polymorphic*** | 71 | 16 | 28 | 24 |
| % PA | 88 | 100 | 89 | 82 |
| % PS | 71 | 84 | 78 | 55 |
Abbreviations: PA = Positive Amplification; PS = Polymorphic SNPs.
* Represents markers specifically designed from SNPs that showed polymorphism for a specific set of reference mapping parental lines. For instance, polymorphisms between the spring type parents, (DH12075 and PSA12); polymorphisms between the winter type parents, (V8 and Express); and polymorphisms detected for both sets of parental lines at the same SNP locus.
** Out of the 100 SNPs tested 73 SNPs were A genome-specific and 27 were C genome-specific.
*** Polymorphic SNPs also include two dominant (presence/absence of the tested allele) marker types.
Additionally, 4,333 of the filtered SNPs were matched to SNPs used on a Brassica 60K Illumina Infinium array (Clarke et al, unpublished data). During array validation, tests using almost 100 B. napus lines indicated that 363 of the 4,333 SNPs failed, while 2,664 (61.5%) of the remaining SNPs were annotated as high quality, based on good cluster separation (AA θ 0 – 0.2 and BB θ 0.8 - 1) and a low proportion of heterozygous calls (AB Frequency <= 0.15) (data not shown). These preliminary results coupled with the KASPar assays serve as a confirmation of the filtering criteria used in the SNP discovery pipeline, suggesting sequence capture coupled with NGS is a promising tool for specific SNP marker development in polyploid genomes.
In order to confirm the chromosomal location for the discovered SNPs and associate them with the target QTL regions, we mapped a subset of the 100 validated SNP markers in both mapping populations. We were able to map 45 out of 48 SNP markers (93.8%) to the target QTL regions in the DH12075 x PSA12 mapping population. Similarly, 46 out of 50 SNP markers (92%) mapped to the target QTL regions in the Express x V8 mapping population (Figure S3).
Discussion
SNP discovery and molecular marker development in B. napus has increased remarkably during the past years since the first published work on genome-wide SNP identification by Trick and coauthors [56]. Such an increment has been mainly the result of the implementation of NGS technologies, microarrays and bioinformatics [2,57,58]. A major challenge to broadly apply NGS technologies, however, continues to be the targeted DNA enrichment of large and complex eukaryotic genomes, such as rapeseed canola (AACC, 2n=38). Sequence capture has addressed this limitation allowing for the isolation of user-defined genomic sequences in one simple step [36-38]. In this context, given the vast history of QTL mapping for various agronomical and nutritional traits in B. napus [13-19,48] the opportunity arose to investigate sequence variation of specific QTL targeted genomic regions [3,32]. We utilized sequence capture to target and re-sequence meta-QTL regions [2] for key complex traits including yield, yield components, seedling vigor, seed quality and disease (Table S2) in ten founder B. napus genotypes (Table 1) [13,47,48]. In total, 47 genomic regions were targeted comprising 51.2 Mb or approximately 4.3% of the estimated 1.2 Gbp B. napus genome [59]. However, since the capture process is unlikely to be able to differentiate the closely related sequences of the A and C genomes, a more accurate estimate of the genome surveyed would be 10.9% (883 + 1,074 orthologous sequences, 131.1 Mb).
Captured DNAs for the ten founder genotypes were sequenced using 454 sequencing technology. Two different reference sets were used to map the sequenced reads obtained, the capture design reference and the A and C genome pseudomolecules. This proved to be crucial allowing us to discern more efficiently between A and C homoeologues and resulted in a higher percentage (RMp) of sequence reads mapped (Table 2). The use of two different reference sets illustrated the benefit of a more complete genome reference as well as differences in sequencing platforms. Mapping of the sequenced reads obtained using Illumina HiSeq technology (2 genotypes) showed a higher RMp for the capture design reference set compared to 454. However, due to the lower specificity of the shorter reads a more subtle increase was observed on the RMp values when the Illumina sequenced reads were uniquely mapped to the A and C genome pseudomolecules, since the short reads have more opportunity for multiple equivalent matches (Table 2, Table 4). Nonetheless, the number of putative SNP calls increased for both sequencing platforms when the larger reference genome set was utilized (Table 2). In addition, the use of more complete reference sequences in the SNP identification process should increase the likelihood of identifying single loci in the amphidiploid genome. In previous attempts to use NGS to discover SNPs in B. napus in the absence of reference genomic sequence, the close homology between the A and C genomes often precluded the discrimination of homoeologous loci. In Trick et al [56], 87.5-91.2% of the identified genic SNPs were so called hemi-SNPs, where assays designed to such SNPs would amplify two loci, limiting their downstream application.
SNP discovery among the ten contrasting B. napus genotypes was performed following a stringent filtering procedure in order to remove SNPs exhibiting multiple variants at a single position, those which could result from co-assembly of homoeologous sequences and those that could represent polymorphisms that distinguish the B. napus species from the parental diploid species used as reference. In addition, SNPs that did not have flanking sequence suitable for either KASPar or Illumina Infinium validation were removed. As a result, 589,367 SNPs were identified from a total of 2,740,205 unfiltered SNPs (Table 3). The polymorphism rate was compared across all 10 genotypes using the equivalent 454 data sets. On average, one filtered SNP was detected every 421.3 bp in the capture regions, which is quite similar to the rate of one every 446 bp observed by Bus et al [60]. Outside of the capture regions, the rate dropped to an average of one SNP every 697.8 bp, indicating the efficiency of the capture process. Out of the filtered SNPs, 46% (268,944) mapped to the A genome pseudomolecules and 54% (320,423) mapped to the C genome pseudomolecules. Previously any bias in observed polymorphism in B. napus has been towards the A genome, which has been suggested to result from the introgression of B. rapa alleles during breeding [6,8]. However, in the current study the use of one resynthesized line (PSA12) and two B. napus lines with resynthesis in their recent pedigree (V8 and Ningyou) may have improved the levels of polymorphism found in the C genome.
Recently, Li and coworkers [61] explored the relative contributions of genic and nongenic SNPs to phenotypic variation for five quantitative traits in maize. Even though genic or nongenic trait associated SNPs (TASs) were found to contribute to nearly half of the phenotypic variation explained by all TASs, non-genic SNPs were significantly overrepresented among TASs [61]. This highlights the importance of discovering SNPs not only in genic but also in non-genic regions for genome wide association studies (GWAS) in crop species with complex genomes, like maize or rapeseed canola. This study provides a large and unique source of nongenic (339,744) and intronic (88,434) SNPs (Figure 3) that could not be discovered by EST-based or RNA-seq methods previously utilized [62-64]. Undoubtedly, these SNPs are not only the starting point to explore sequence polymorphisms under targeted QTL areas of the genome, but also they can be used together with other SNP sets to perform GWAS in rapeseed canola. This resource will provide a further tool to help enhance our understanding of the genetic architecture of quantitative traits in this species.
Discovered SNPs were also classified into transitions and transversions (Figures 2 and 3). As seen in plants and other organisms the percentage of transitions (60.4%) was higher than that of transversions (39.6%) [60,65-68]. The number of the four different types of transitions was found to be balanced, whereas the number of the eight different types of transversions varied (Figure 2). A similar result was recently reported for B. napus using RAD sequencing [60]. The ratio of total transitions/transversions was found to be 1.52 (355,714/233,653). This ratio is in agreement with previous reports in Brassica where transitions/transversions ranged from 1.03 to 1.65 [60,66]. The lowest ratio of transitions/transversions (1.14; 47,065/41,369) was found in intronic sequences and the highest in CDS or exonic sequences (1.62; 99,579/61,610). The same trend between exonic and intronic regions was also observed in B. rapa [66]. Since transversion substitutions are more likely to produce missense and nonsense mutations, a high ratio of transitions/transversions in exonic sequences could be indicative of the presence of purifying selection on amino acid substitutions as seen in B. rapa [66].
This study combined sequence capture and NGS technologies to discover SNPs in specific areas (target) of the B. napus genome (QTLs). The efficiency of target sequence enrichment was evaluated by comparing the mapping of the obtained sequenced reads to multiple reference sets (Table 4). As observed in other studies [36,42], approximately 30% of the sequence reads mapped to the capture regions for the ten genotypes re-sequenced using the 454 sequencing platform. However, due to the strong homology between the A and C genomes the reference set including the complemented capture regions is perhaps a more realistic representation of the target regions, which increased the efficacy of matching for the 454 reads to approximately 35% (Table 4). Beside those regions which are still unrepresented in the reference, the requirement for unique matches in a complex polyploid genome will necessarily limit the ability to map all sequenced reads. Comparing the reads mapped to the target regions to those mapped to the A and C genome pseudomolecules indicated that on average 57.4% of the reads which could be mapped were found in the target regions. In addition, the depth of coverage which effectively dictates the ability to accurately call SNPs was surveyed for both target and non-target regions across the A and C Brassica pseudomolecules (Figure 4). The four-fold increase in depth of reads mapped to the target regions ensured that almost 60% of the annotated SNPs were found in these regions.
Validation of discovered SNPs is a crucial step to estimate the percentage that could be converted into robust and informative molecular markers [69]. Filtered SNPs were classified based on their available flanking sequence, and thus, the possibility of being utilized in different downstream assays as KASPar (289,459), Illumina Infinium (2,106), or both (297,802). We validated a set of 100 SNPs in two reference mapping populations (DH12075 x PSA12; Express x V8) and a B. napus diversity panel using the KASPar SNP assay (Table 5). Validation rates varied depending on whether the KASPar oligonucleotides were specifically designed to test for SNPs identified between the parental lines of the two mapping populations. Importantly, 71 of the 100 SNPs were also informative when tested using a B. napus diversity set composed of 25 lines (Table 5). Since the singleplex KASPar SNP validation assay relies on consistent template sequences, amplification failure could result from non-specific oligonucleotide annealing and not be related to the nature of the tested SNP. Therefore, corrected validation rates, not considering the 12 evaluated SNPs showing no amplification, were as follows: 81% (71/88) for the diversity set, 84 % (16/19) for DH12075 x PSA12, 85% (28/33) for Express x V8, and 67% (24/36) for both mapping populations. These rates demonstrate the robustness of our SNP discovery pipeline protocol. In addition, an independent assay evaluated 4,333 SNPs in 96 B. napus lines using a Brassica 60K Illumina Infinium array (Clarke et al, unpublished data), resulting in 61.5% validation rate. To further corroborate that the discovered SNPs correspond to polymorphisms in the target QTL regions, we mapped a subset of the 100 KASPar SNP markers in both mapping populations (Figure S3). As a result, more than 90% of the tested SNPs were confirmed to reside in the target QTL regions or its vicinity, with only two SNPs (one on each mapping population) failing to be mapped. This was probably due to the small mapping population size used to perform the linkage analysis.
A previous study on B napus genome-wide SNP discovery used a combination of NGS and RAD sequencing and surveyed about 1% of the B. napus genome, detecting more than 20,000 SNPs among 8 inbred lines, with 84% (26/31) of the SNPs being confirmed by re-sequencing; however, none were tested in SNP assays [60]. Significantly, this approach utilized an improved reference sequence set, by combining a 95K Brassica unigene dataset [70] with the B. rapa reference sequence [27]. Our work expands on previous studies by: (i) targeting specific QTL regions representing 10.9% of the B. napus genome; (ii) using a more representative genomic reference, (iii) detecting more than 500 thousand SNPs among 10 contrasting genotypes, including genic (42%, 249,623) and intergenic (58%, 339,744) SNPs with an average SNP assay validation rate of over 80%. The development of high densities of robust molecular markers (e.g. SNPs) will be very powerful towards predicting the best individuals in plant breeding schemes through the use of genomic selection [71]. Illumination of the genome sequences and species-wide genetic diversity of B. napus crops (spring and winter types) will help to elucidate the genetic background of complex traits and broaden gene pools as a basis for more successful breeding.
Supporting Information
Minimally filtered SNP types and their classification according to genomic regions. Transition and transversion SNP types were classified to show the proportion of SNPs annotated to each of three genomic regions (Intergenic, Intron, and CDS). In the minimally filtered SNP set, only positions with more than one variant call were removed.
(TIFF)
Minimally filtered SNP counts characterization summary. Total SNP counts were classified by genomic location (Intergenic vs. Genic) and further separated into transitions and transversions. Genic SNPs are also described in terms of their location within the gene (CDS vs. intron). In the minimally filtered SNP set, only positions with more than one variant call were removed. CDS: coding sequence.
(TIFF)
Linkage mapping of polymorphic SNP markers discovered using sequence capture in B. napus. Mapped SNP markers are illustrated in red, each horizontal bar represents a molecular marker. Linkage analysis and map position was conducted separately for each population using JoinMap® v4.0. Linked loci were grouped using a LOD threshold of 5-8 and a maximum recombination fraction of 0.4. Grouped SNP marker loci were arranged into a scoring matrix using MSExcel. Distances were assigned in centiMorgans (cM) using the Kosambi mapping function. Scoring matrixes are available upon request.
(TIFF)
Genotype descriptions, fragment size estimation of captured DNA and final sample concentrations. (DOC).
(DOCX)
Brassica A Genome Sequence Capture Coordinates. (XLS).
(XLSX)
Genotypes tested in KasPar Assay. (XLS).
(XLSX)
List of 100 SNP and oligonucleotides used in KasPar validation assays. (XLS).
(XLSX)
Acknowledgments
The authors would like to thank Daniela Quezada and Fernando Westermeyer for their technical assistance and two anonymous reviewers for improving the quality of these manuscript. We acknowledge Fondecyt 1100732 and INIA for its support providing experimental fields and infrastructure.
Funding Statement
This research was funded by Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT), FONDECYT/Regular 1100732 and CONICYT REGIONAL/GORE LA ARAUCANIA/CGNA/R10C1001. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.U N (1935) Genome analysis in Brassica with special reference to the experimental formation of Brassica napus and its peculiar mode of fertilization. Jpn J Bot 7: 389-452. [Google Scholar]
- 2. Snowdon RJ, Iniguez-Luy FL (2012) Potential to improve oilseed rape and canola breeding in the genomics era. Plant Breed 131: 351-360. doi: 10.1111/j.1439-0523.2012.01976.x. [DOI] [Google Scholar]
- 3. Iniguez-Luy FL, Federico ML (2011) The genetics of Brassica napus L. In: Bancroft I, Schmidt R. Genetics and Genomics of the Brassicaceae. Springer, New York: pp.291-322. [Google Scholar]
- 4. Food and Drug Administration (1995) Code of Federal Regulations, Title 21, Food and Drugs, part 184, Direct Food Substances Affirmed as Generally Recognized as Safe, Sections; (1555). as of April 1, 1995, part 184 U.S. Government Printing Office, Washington, D.C.. [Google Scholar]
- 5. Shahidi F (1990) Rapessed and canola: global production and distribution. In: Sahidi F. Canola and rapeseed: production, chemistry and processing technology. Van Nostrand Reinhold, NY. pp 3-13. [Google Scholar]
- 6. Buzza GC (1995) Plant Breeding. In: Kimber DS, McGregor DI. Brassica oilseeds: production and utilization. CABI Publishing, Wallingford: pp. 153-175. [Google Scholar]
- 7. Hickling D (2001) Canola meal feed industry guide. third edition. Canola Council of Canada, Winnipeg, MB. [Google Scholar]
- 8. Snowdon RJ, Luhs W, Friedt W (2007) Oilseed rape. In: Kole C. Genome mapping and molecular breeding in plants Volume 2 Springer, Heidelberg: pp. 55-114. [Google Scholar]
- 9. Gali KK, Sharpe AG (2011) Molecular linkage maps: strategies, resources and achievements In: Edwards D, Batley J, Parkin I, Kole Ch. Genetics, genomics and breeding of oilseed Brassicas. CRC Press, New York: pp. 85-129. [Google Scholar]
- 10. Hayward A, Mason A, Dalton-Morgan J, Zander M, Edwards D et al. (2012) SNP discovery and applications in Brassica napus . Plant Biotechnol 39: 1-12. doi: 10.5010/JPB.2012.39.1.001. [DOI] [Google Scholar]
- 11. Edwards D, Gupta PK (2012) Sequence based DNA markers and genotyping for cereal genomics and breeding. In: Gupta PK, Varshney RK. Cereal Genomics II. Springer, New York: pp. 57-76. [Google Scholar]
- 12. Takeda S, Matsuoka M (2008) Genetic approaches to crop improvement: responding to environmental and population changes. Nat Rev Genet 9: 444-457. doi: 10.1038/nrg2342. PubMed: 18475268. [DOI] [PubMed] [Google Scholar]
- 13. Mayerhofer R, Wilde K, Mayerhofer M, Lydiate D, Bansal VK et al. (2005) Complexities of chromosome landing in a highly duplicated genome: Towards map based cloning of a gene controlling blackleg resistance in Brassica napus . Genetics 171: 1977-1988. doi: 10.1534/genetics.105.049098. PubMed: 16143600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Udall JA, Quijada PA, Osborn TC (2005) Detection of chromosomal rearrangements derived from homeologous recombination in four mapping populations of Brassica napus L. Genetics 169: 967- 979. doi: 10.1534/genetics.104.033209. PubMed: 15520255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Udall JA, Quijada PA, Lambert B, Osborn TC (2006) Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 2. Identification of alleles from unadapted germplasm. Theor Appl Genet 113: 597- 609 [DOI] [PubMed]
- 16. Quijada PA, Udall JA, Lambert B, Osborn TC (2006) Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 1. Identification of genomic regions from winter germplasm. Theor Appl Genet 113: 549-561 [DOI] [PubMed]
- 17. Radoev M, Becker HC, Ecke W (2008) Genetic analysis of heterosis for yield and yield components in rapeseed (Brassica napus L.) by quantitative trait locus mapping. Genetics 179: 1547-1558. doi: 10.1534/genetics.108.089680. PubMed: 18562665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kramer CC, Polewicz H, Osborn TC (2009) Evaluation of QTL alleles from exotic sources for hybrid seed yield in the original and different genetic backgrounds of spring-type Brassica napus L. Mol Breed 24: 419-431
- 19. Basunanda P, Spiller TH, Hasan M, Gehringer A, Schondelmaier J et al. (2007) Marker-assisted increase of genetic diversity in a double-low seed quality winter oilseed rape genetic background. Plant Breed 126: 581-587. doi: 10.1111/j.1439-0523.2007.01404.x. [DOI] [Google Scholar]
- 20. Raman R, Raman H, Kadkol GP, Coombes N, Taylor B et al. (2011) Genome-wide association analyses of loci for shatter resistance in Brassicas. In: Proceedings of the Australian Research Assembly on Brassicas, Wagga Wagga, NSW, Australia. pp 36-41. [Google Scholar]
- 21. Osborn TC, Lukens L (2003) The molecular genetic basis of flowering time variation in Brassica species. In: Nagata T, Tabata S. Biotechnology in agriculture and forestry. Brassica and Legumes: From gene structure to breeding. Springer-Verlag, New York: pp. 69-86. [Google Scholar]
- 22. Parkin IAP, Gulden SM, Sharpe AG, Lukens L, Trick M et al. (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana . Genetics 171: 765-781. doi: 10.1534/genetics.105.042093. PubMed: 16020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC (2007) Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotypic variation. Plant Cell 19: 3403-3417. doi: 10.1105/tpc.107.054346. PubMed: 18024568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bus A, Körber N, Snowdon RJ, Stich B (2011) Patterns of molecular variation in a species-wide germplasm set of Brassica napus . Theor Appl Genet 123: 1413-1423. doi: 10.1007/s00122-011-1676-7. PubMed: 21847624. [DOI] [PubMed] [Google Scholar]
- 25. Körber N, Wittkop B, Bus A, Friedt W, Snowdon RJ (2012) Seedling development in a Brassica napus diversity set and its relationship to agronomic performance. Theor Appl Genet 125: 1275-1287. doi: 10.1007/s00122-012-1912-9. PubMed: 22782254. [DOI] [PubMed] [Google Scholar]
- 26. Raiz A, Li G, Quresh Z, Swati MS, Quiros CF (2001) Genetic diversity of oilseed Brassica napus inbred lines based on sequence-related amplified polymorphism and its relation to hybrid performance. Plant Breed 120: 411-415. doi: 10.1046/j.1439-0523.2001.00636.x. [DOI] [Google Scholar]
- 27. The Brassicarapa Genome Sequencing Project Consortium (2011) The genome of the mesopolyploid crop species Brassicarapa. Nat Genet 43: 1035-1039 [Google Scholar]
- 28. Edwards D, Wang X (2012) Genome sequencing initiatives, In: Edwards D. Genetics, genomics and breeding of oilseed Brassicas. Science Publishers Inc., New Hampshire, USA: pp 152-157. [Google Scholar]
- 29. The International HapMap Consortium (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449: 851-861. doi: 10.1038/nature06258. PubMed: 17943122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ganal MW, Altmann T, Röder MS (2009) SNP identification in crop plants. Curr Opin Plant Biol 12: 211-217. doi: 10.1016/j.pbi.2008.12.009. PubMed: 19186095. [DOI] [PubMed] [Google Scholar]
- 31. Deschamps S, Campbell M (2009) Utilization of next-generation sequencing platforms in plant genomics and genetic variant discovery. Mol Breed 25: 553-570. [Google Scholar]
- 32. Kaur S, Francki MG, Forster JW (2012) Identification, characterization and interpretation of single-nucleotide sequence variation in allopolyploid crop species. Plant Biotechnol J 10: 125-138. PubMed: 21831136. [DOI] [PubMed] [Google Scholar]
- 33. Hayward A, Vighnesh G, Delay C, Samian MR, Jiang Y et al. (2012) Second-generation sequencing for gene discovery in the Brassicaceae. Plant Biotechnol J, 10: 750-759. doi: 10.1111/j.1467-7652.2012.00719.x. PubMed: 22765874. [DOI] [PubMed] [Google Scholar]
- 34. Margulies M, Egholm M, Altman WE, Attiya S, Bader JS et al. (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437: 376-380. PubMed: 16056220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L et al. (2008) The complete genome of an individual by massively parallel DNA sequencing. Nature 452: 872-876. doi: 10.1038/nature06884. PubMed: 18421352. [DOI] [PubMed] [Google Scholar]
- 36. Albert TJ, Molla MN, Muzny DM, Nazareth L, Wheeler D et al. (2007) Direct selection of human genomic loci by microarray hybridization. Nat Methods 4: 903-905. doi: 10.1038/nmeth1111. PubMed: 17934467. [DOI] [PubMed] [Google Scholar]
- 37. Olson M (2007) Enrichment of super-sized resequencing targets from the human genome. Nat Methods 4: 891-892. doi: 10.1038/nmeth1107-891. PubMed: 17971778. [DOI] [PubMed] [Google Scholar]
- 38. Okou DT, Steinberg KM, Middle C, Cutler CD, Albert TJ et al. (2007) Microarray-based genomic selection for high throughput resequencing. Nat Methods 4: 907-909. doi: 10.1038/nmeth1109. PubMed: 17934469. [DOI] [PubMed] [Google Scholar]
- 39. Stankiewicz P, Lupski JR (2010) Structural variation in the human genome and its role in disease. Annu Rev Med 61: 437-455. doi: 10.1146/annurev-med-100708-204735. PubMed: 20059347. [DOI] [PubMed] [Google Scholar]
- 40. Alkan C, Coe BP, Eichler EE (2011) Genome structural variation discovery and genotyping. Nat Rev Genet 12: 363-376. doi: 10.1038/nrg2958. PubMed: 21358748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mills RE, Walter K, Stewart C, Handsaker RE, Chen K et al. (2011) Mapping copy number variation by population scale genome sequencing. Nature 470: 59-65. doi: 10.1038/nature09708. PubMed: 21293372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fu Y, Springer NM, Gerhardt DJ, Ying K, Yeh CT et al. (2010) Repeat subtraction-mediated sequence capture from a complex genome. Plant J 62: 898-909. doi: 10.1111/j.1365-313X.2010.04196.x. PubMed: 20230488. [DOI] [PubMed] [Google Scholar]
- 43. Grover CE, Salmon A, Wendel JF (2012) Targeted sequence capture as a powerful tool for evolutionary analysis. Am J Bot 99: 312-319. doi: 10.3732/ajb.1100323. PubMed: 22268225. [DOI] [PubMed] [Google Scholar]
- 44. Henry RJ, Edwards M, Waters DLE, Krishnan SG, Bundock P (2012) Application of large-scale sequencing to marker discovery in plants. J Biosci 37: 829-841. doi: 10.1007/s12038-012-9253-z. PubMed: 23107919. [DOI] [PubMed] [Google Scholar]
- 45. Salmon A, Udall JA, Jeddeloh JA, Wendel J (2012). Targeted Capture of Homoeologous Coding and Noncoding Sequence in Polyploid Cotton. G3: 921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qiu D, Morgan C, Shi J, Long Y, Liu J et al. (2006) A comparative linkage map of oilseed rape and its use for QTL analysis of seed oil and erucic acid content. Theor Appl Genet 114: 67-80. doi: 10.1007/s00122-006-0411-2. PubMed: 17033785. [DOI] [PubMed] [Google Scholar]
- 47. Gehringer A, Snowdon R, Spiller T, Basunanda P, Friedt W (2007) New oilseed rape (Brassica napus L.) hybrids with high levels of heterosis for seed under nutrient-poor conditions. Breeding Sci 57: 315-320. doi: 10.1270/jsbbs.57.315. [DOI] [Google Scholar]
- 48. Basunanda P, Radoev M, Ecke W, Friedt W, Becker H, et al. (2010) Comparative mapping of quantitative trait loci involved in heterosis for seedling and yield traits in oilseed rape (Brassica napus L.). Theor Appl Genet 120: 271-281 [DOI] [PMC free article] [PubMed]
- 49. Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press. [Google Scholar]
- 50. Iniguez-Luy FL, Lukens L, Farnham MW, Amasino RM, Osborn TC (2009) Development of public immortal mapping populations, molecular markers and linkage maps for rapid cycling Brassica rapa and B. oleracea . Theor Appl Genet 120: 31-43. doi: 10.1007/s00122-009-1157-4. PubMed: 19784615. [DOI] [PubMed] [Google Scholar]
- 51. Wang J, Lydiate IA, Parkin IAP, Falentin C, Delourme R et al. (2011) Integration of linkage maps for the amphidiploid Brassica napus and comparative mapping with Arabidopsis and Brassica rapa . BMC Genomics 12: 101. doi: 10.1186/1471-2164-12-101. PubMed: 21306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M et al. (2004) Versatile and open software for comparing large genomes. Genome Biol 5: R12. doi: 10.1186/gb-2004-5-6-p12. PubMed: 14759262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26: 841-842. doi: 10.1093/bioinformatics/btq033. PubMed: 20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kidwell KK, Osborn TC (1992) Simple plant DNA isolation procedures. In: Beckman J, Osborn TC. Plant Genomes: Methods for Genetic and Physical Mapping. Kluwer Academic Publishers Group. AH; Dordrecht, The Netherlands: pp. 1-13. [Google Scholar]
- 55. Cho K, O’Neill CM, Kwon S-J, Yang T-J, Smooker AM (2010) Sequence-level comparative analysis of the Brassica napus genome around two stearoyl-ACP desaturase loci. Plant J 61: 591-599. doi: 10.1111/j.1365-313X.2009.04084.x. PubMed: 19929877. [DOI] [PubMed] [Google Scholar]
- 56. Trick M, Long Y, Meng JL, Bancroft I (2009) Single nucleotide polymorphism (SNP) discovery in the polyploid Brassica napus using Solexa transcriptome sequencing. Plant Biotechnol J 7: 334-346. doi: 10.1111/j.1467-7652.2008.00396.x. PubMed: 19207216. [DOI] [PubMed] [Google Scholar]
- 57. Glenn TC (2011) Field guide to next-generation DNA sequencers. Mol Ecol Resour 11: 759-769. doi: 10.1111/j.1755-0998.2011.03024.x. PubMed: 21592312. [DOI] [PubMed] [Google Scholar]
- 58. LaFrombiose T (2009) Single nucleotide polymorphism arrays: a decade of biological, computational and technological advances. Nucleic Acids Res 37: 4181-4193. doi: 10.1093/nar/gkp552. PubMed: 19570852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9: 208-218. doi: 10.1007/BF02672069. [DOI] [Google Scholar]
- 60. Bus A, Hecht J, Huettel B, Reinhardt R, Stich B (2012) High-throughput polymorphism detection and genotying in Brassica napus using nex-genetartion RAD sequencing. BMC Genomics 13: 281. doi: 10.1186/1471-2164-13-281. PubMed: 22726880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li X, Zhu C, Yeh C-T, Wu W, Takacs EM et al. (2012) Genic and nongenic contribution to natural variation of quantitative trait in maize. Genome Res 22: 2436–2444. doi: 10.1101/gr.140277.112. PubMed: 22701078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parkin IAP, Clarke AW, Sidebottton C, Zhang W, Robinson SJ et al. (2010) Towards unambiguous transcript mapping in the allotetraploid Brassica napus . Genome 53: 929-938. doi: 10.1139/G10-053. PubMed: 21076508. [DOI] [PubMed] [Google Scholar]
- 63. Bancroft I, Morgan C, Fraser F, Higgins J, Wells R et al. (2011) Dissecting the genome of the polyploid crop oilseed rape by transcriptome sequencing. Nat Biotechnol 29: 762-766. doi: 10.1038/nbt.1926. PubMed: 21804563. [DOI] [PubMed] [Google Scholar]
- 64. Hu Z, Huang S, Sun M, Wang H, Hua W (2012) Development and application of single nucleotide polymorphism markers in the polyploid Brassica napus by 454 sequencing of expressed sequence tags. Plant Breed 131: 293-299. doi: 10.1111/j.1439-0523.2011.01947.x. [DOI] [Google Scholar]
- 65. Barchi L, Lanteri S, Portis E, Acquadro A, Valè G et al. (2011) Identification of SNP and SSR markers in eggplant using RAD tag sequencing. BMC Genomics 12: 304. doi: 10.1186/1471-2164-12-304. PubMed: 21663628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Park S, Yu HJ, Mun JH, Lee SC (2010) Genome-wide discovery of DNA polymorphism in Brassica rapa . Mol Genet Genomics 283: 135-145. doi: 10.1007/s00438-009-0504-0. PubMed: 20024583. [DOI] [PubMed] [Google Scholar]
- 67. Collins DW, Jukes TH (1994) Rates of transition and transversion in coding sequence since the human-rodent divergence. Genomics 20: 386-396. doi: 10.1006/geno.1994.1192. PubMed: 8034311. [DOI] [PubMed] [Google Scholar]
- 68. Ebersberger I, Metzler D, Schwarz C, Pääbo S (2002) Genome-wide comparison of DNA between humans and chimpanzees. Am J Hum Genet 70: 1490-1497. doi: 10.1086/340787. PubMed: 11992255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mammadov J, Aggarwal R, Buyyarapu R, Kumpatla S (2012) SNP markers and their impact on plant breeding. Int J Plant Genomics Volume 2012: 728398. doi: 10.1155/2012/728398. PubMed: 23316221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Trick M, Cheung F, Drou N, Fraser F, Lobenhofer EK et al. (2009) A newly-developed community microarray resource for transcriptome profiling in Brassica species enables the confirmation of Brassica-specific expressed sequences. BMC Plant Biol 9: 50. doi: 10.1186/1471-2229-9-50. PubMed: 19426481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cowling WA, Cullis BR, Beeck CP, Nelson NM (2011) Towards genomic selection in oilseed Brassica. In: Edwards D. Genetics, Genomics and breeding of oilseed Brassicas. CRC Press, New York: pp. 219-229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Minimally filtered SNP types and their classification according to genomic regions. Transition and transversion SNP types were classified to show the proportion of SNPs annotated to each of three genomic regions (Intergenic, Intron, and CDS). In the minimally filtered SNP set, only positions with more than one variant call were removed.
(TIFF)
Minimally filtered SNP counts characterization summary. Total SNP counts were classified by genomic location (Intergenic vs. Genic) and further separated into transitions and transversions. Genic SNPs are also described in terms of their location within the gene (CDS vs. intron). In the minimally filtered SNP set, only positions with more than one variant call were removed. CDS: coding sequence.
(TIFF)
Linkage mapping of polymorphic SNP markers discovered using sequence capture in B. napus. Mapped SNP markers are illustrated in red, each horizontal bar represents a molecular marker. Linkage analysis and map position was conducted separately for each population using JoinMap® v4.0. Linked loci were grouped using a LOD threshold of 5-8 and a maximum recombination fraction of 0.4. Grouped SNP marker loci were arranged into a scoring matrix using MSExcel. Distances were assigned in centiMorgans (cM) using the Kosambi mapping function. Scoring matrixes are available upon request.
(TIFF)
Genotype descriptions, fragment size estimation of captured DNA and final sample concentrations. (DOC).
(DOCX)
Brassica A Genome Sequence Capture Coordinates. (XLS).
(XLSX)
Genotypes tested in KasPar Assay. (XLS).
(XLSX)
List of 100 SNP and oligonucleotides used in KasPar validation assays. (XLS).
(XLSX)