Abstract
Recent investigations identified heterozygous CFC1 mutations in subjects with heterotaxy syndrome, all of whom had congenital cardiac malformations, including malposition of the great arteries. We hypothesized that a subset of patients with similar types of congenital heart disease—namely, transposition of the great arteries and double-outlet right ventricle, in the absence of laterality defects—would also have CFC1 mutations. Our analysis of the CFC1 gene in patients with these cardiac disorders identified two disease-related mutations in 86 patients. The present study identifies the first autosomal single-gene defect for these cardiac malformations and indicates that some cases of transposition of the great arteries and double-outlet right ventricle can share a common genetic etiology with heterotaxy syndrome. In addition, these results demonstrate that the molecular pathway involving CFC1 plays a critical role in normal and abnormal cardiovascular development.
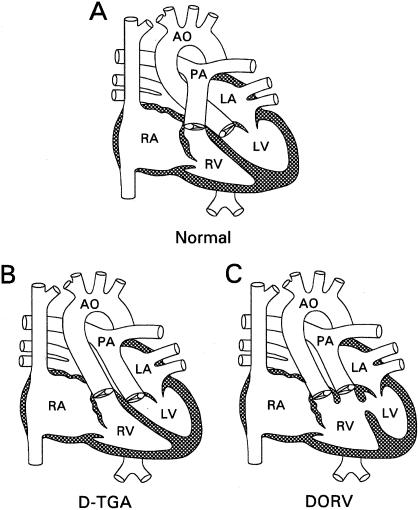
Congenital heart disease (CHD) is the most common major birth defect, occurring in 4–8/1,000 live births (Ferencz et al. 1985). Transposition of the great arteries (TGA) and double-outlet right ventricle (DORV) account for 5% and 2%, respectively, of all CHD (Perry et al. 1993). TGA and DORV are generally classified as conotruncal defects, or defects of the outflow tracts of the heart (for review, see Fyler 1992). The more common form of TGA (dextro-looped TGA [D-TGA]) consists of complete inversion of the great vessels, so that the aorta incorrectly arises from the right ventricle and the pulmonary artery incorrectly arises from the left ventricle (fig. 1). In the less common type of TGA (levo-looped TGA [L-TGA]), the ventricles are inverted, instead. DORV exhibits marked anatomic variability but is defined by both great vessels arising from the right ventricle (fig. 1). In some cases of DORV, the orientation of the great vessels is similar to that of D-TGA, even though both vessels arise from the right ventricle. Although D-TGA, L-TGA, and DORV are most frequently seen in isolation, they are also a characteristic finding in heterotaxy syndrome, which is defined by variable abdominal and thoracic laterality defects.
Figure 1.
Diagrams of the normal heart (A), D-TGA (B), and DORV (C). In the normal heart, the pulmonary artery arises from the right ventricle, and the aorta arises from the left ventricle. In D-TGA, the great arteries have been switched. In DORV, the aorta and pulmonary artery both arise from the right ventricle. Both D-TGA and DORV can display additional anatomic variations. RA = right atrium; LA = left atrium; LA = left ventricle; RV = right ventricle; AO = aorta; PA = pulmonary artery.
The etiology of CHD in general—and of TGA or DORV in particular—remains largely unknown. Environmental and genetic factors both have been implicated, but few single-gene defects have been identified. Most cases of TGA or DORV are sporadic, but there are rare reports of familial cases (Becker et al. 1996; Ferencz et al. 1997). Although a 22q11 deletion is frequently seen in patients with other types of conotruncal defects (e.g., interrupted aortic arch, truncus arteriosus, and tetralogy of Fallot), this chromosomal alteration is rarely found in patients with TGA or DORV (Takahashi et al. 1995; Goldmuntz et al. 1998). Unlike cases of TGA or DORV, familial cases of heterotaxy syndrome demonstrating X-linked (MIM 306955), autosomal dominant (MIM 605376), or autosomal recessive inheritance have been identified (Gebbia et al. 1997; Casey 1999; Kathiriya and Srivastava 2000). Molecular evaluation of several families with X-linked inheritance of heterotaxy syndrome identified ZIC3 (MIM 300265) as the disease-related gene in those subjects (Gebbia et al. 1997). A subsequent investigation identified a ZIC3 truncation mutation in a family with X-linked inheritance of complex CHD (Megarbane et al. 2000). In this family, two affected probands had isolated CHD, including D-TGA, in the absence of laterality defects, and one male relative with the identical mutation was found, on close inspection, to have no clinical phenotype consistent with decreased penetrance.
More recently, candidate genes for human CHD have been identified from gene-targeting studies in mice. For example, the EGF-CFC gene family encodes extracellular proteins that participate in early embryogenesis (Shen and Schier 2000). Cfc1 is one of the four known family members and has a symmetrical pattern of expression in the developing mouse embryo (Shen et al. 1997). Mice with homozygous mutations of Cfc1 develop visceral laterality defects and complex cardiac malformations reminiscent of human heterotaxy syndrome (Gaio et al. 1999; Yan et al. 1999). In particular, homozygous mutant mice frequently (i.e., in 82% of cases) have malposition of the great arteries, including TGA and DORV, as well as other cardiac malformations (Gaio et al. 1999).
Recent investigations of patients with heterotaxy syndrome identified three heterozygous CFC1 (MIM 605194) mutations (R112C, G174del1, and R189C) in four unrelated subjects, all of whom had congenital cardiac malformations (Bamford et al. 2000). The clinical phenotype of the four subjects varied substantially and included defects typical of both left-sided and right-sided isomerism. The cardiac phenotype also varied, and it included dextrocardia, TGA, a common atrioventricular canal, venous anomalies, and other unspecified anomalies. Two of the three mutations resulted in altered protein function, as demonstrated by cellular-localization and mutant-rescue assays. A fourth sequence alteration (R78W) was identified in an African American subgroup of the study population. Although this alteration was found in normal African American subjects, it demonstrated subtle functional abnormalities in the mutant rescue assay (Bamford et al. 2000).
Given the cardiac phenotype of the homozygous Cfc1 mutant mouse and the results of the studies of heterotaxy in humans, we hypothesized that a subset of patients with TGA or DORV in the absence of other thoracic or abdominal laterality defects would also have CFC1 mutations. Our investigations were designed to test whether, in certain cases, isolated TGA and DORV share a common genetic etiology with heterotaxy syndrome and thereby represent a spectrum of disease.
Patients with either TGA or DORV were recruited prospectively, from November 1991 to December 2000, by the Clinical Core of the Special Center of Research on the Genetics of Conotruncal Malformations, under a protocol approved by the internal review board of The Children’s Hospital of Philadelphia. A sample of whole blood was collected from the proband, and a lymphoblastoid cell line was prepared from a portion of this blood sample, through use of standard techniques. DNA was extracted from the whole blood and/or cell line, for further studies. All subjects were tested for a 22q11 deletion, as described elsewhere (Goldmuntz et al. 1998). Only those patients who did not have a 22q11 deletion or other known chromosomal anomalies were included in the present investigation. Parental samples were obtained when possible.
A total of 86 patients with either D-TGA (n=58), L-TGA (n=6), or DORV (n=22) who did not have evidence of heterotaxy syndrome were included in the present study. All subjects underwent complete physical examination, chest roentgenography (CXR), and echocardiography, at The Children’s Hospital of Philadelphia. Many subjects also underwent abdominal roentgenography (AXR; the so-called “babygram” of neonates). These clinical assessments independently identified normal situs of the heart, venous structures, stomach, and liver. Splenic anatomy was not evaluated. All cardiac medical records were reviewed by one of two pediatric cardiologists at The Children’s Hospital of Philadelphia, and patients with situs abnormalities were excluded from the present study. The medical records, original newborn CXR/AXR, and echocardiograms of all mutation carriers were reviewed again (by E.G.) to confirm normal chest and abdominal situs. A male preponderance (65%) was observed, consistent with previous epidemiologic reports (Ferencz et al. 1997). The ethnicities of the study cohort reflected those of the population served by the hospital and included white (72%), African American (10%), Hispanic (4%), Asian (2%), other (8%), and not available (4%).
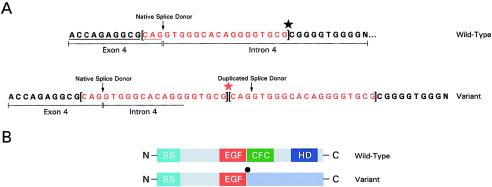
Subjects were tested for CFC1 mutations, by SSCP analysis and direct sequencing of aberrant bands, as described in detail elsewhere (Bamford et al. 2000). A total of three heterozygous sequence alterations were identified in three unrelated probands, as described in table 1. A novel, tandem duplication of the exon 4 splice-donor site (splice-site duplication) was identified in one patient with D-TGA (fig. 2). The 20 duplicated base pairs contain the entire spliceosome recognition motif that anneals with U1 RNA. Either recognition site would be predicted to interact with the spliceosome with equal affinity. Utilization of the duplicated donor site predicts a frameshift, after the EGF motif, that would eliminate the CFC domain and the carboxy terminus (fig. 2). This alteration was not identified in 200 normal chromosomes. Parental samples were not available for evaluation. We were unable to detect wild-type transcript in lymphoblast cell lines and thus could not further study the structure of the CFC1 transcript in this patient.
Table 1.
Molecular and Clinical Summary of CFC1 Alterations in TGA/DORV Study Cohort
| Sequence Alteration | Cardiac Lesion | Other Cardiac Features | Noncardiac Features |
| Splice-site duplication | D-TGA | Intact ventricular septum | Pyloric stenosis |
| G174del1 | DORV | Subpulmonic ventricular septal defect, aortic arch hypoplasia | None |
| R78W (polymorphism) | DORV | Pulmonary atresia, double inlet, single right ventricle | None |
Figure 2.
Splice-site duplication in a patient with TGA. A, Tandem 20-bp duplication (red) of the exon 4 splice-donor site found in a subject with TGA. The native and duplicated splice sites are indicated by an arrow (↓). The star marks where the duplication occurred. Both the wild-type and the mutant sequence are demonstrated. B, Wild-type and mutant gene structure demonstrates signal peptide sequence (SS; turquoise), conserved EGF-like domain (EGF; red), conserved-CFC domain (CFC; green), hydrophobic domain (HD; dark blue), and predicted altered sequence in mutant protein (light blue). The conserved CFC domain and hydrophobic region are not present in the predicted product.
A single-base-pair deletion (G174 del1) was identified in one subject with sporadic DORV (table 1). This mutation had previously been reported in two patients with heterotaxy syndrome (Bamford et al. 2000). The mother of this proband and the mother of one of the subjects with heterotaxy were found to carry the G174del1 alteration, though neither was reported to have a clinical phenotype on the basis of family history. However, neither mother was available for further clinical testing to identify subtle subclinical features. Nonetheless, this alteration was not identified in 200 normal chromosomes. Furthermore, the deletion is predicted to introduce a frameshift that disrupts the terminal membrane-associating domain and extends the open reading frame. Previous cellular-localization experiments and mutant-rescue assays indicate that the G174del1 alteration results in a functionally different protein product (Bamford et al. 2000). In particular, immunohistochemical studies revealed that wild-type protein is retained on the cell surface, whereas the G174del1 mutant protein is absent from the cell surface. Additional data indicate that the mutant protein accumulates to substantial levels in the cell (data not shown).
The missense alteration (R78W) was identified in one African American subject with DORV (table 1). Parental samples were not available for additional studies. Although this mutation initially was not found in 200 random controls of unknown ethnicity, further examination of an African American control population was undertaken, since, to date, all subjects identified as having this alteration have been of African American descent (Bamford et al. 2000; present study). The R78W sequence variation predicts the loss of a SmaI site, resulting in a higher migrating band on agarose-gel electrophoresis. Application of this assay to DNA from 154 African American control individuals demonstrated that 133 were homozygous for the wild-type allele, whereas 21 were heterozygous for the R78W alteration, giving a carrier frequency of 13.6%.
We conclude that the splice-site duplication and the G174del1 alteration represent disease-related mutations of CFC1 in these subjects. Neither mutation was identified in 200 normal control chromosomes, though the G174del1 mutation was identified in a presumably unaffected mother, which is consistent with incomplete penetrance. Similar cases of reduced penetrance have been reported in CHD and other complex congenital disorders (Nanni et al. 1999; Megarbane et al. 2000). Both mutations are predicted to eliminate domains essential for normal protein function and, thus, would result in a loss of function (Yeo and Whitman 2001). In addition, multiple experiments demonstrate that the function of the G174 del1 mutant protein is significantly altered (Bamford et al. 2000).
These mutations represent the first autosomal, single-gene defect identified for either TGA or DORV. Our results indicate that CFC1 mutations occur in ∼2% (2/86) of this population, though the present study may underestimate the true mutation frequency, given that the sensitivity of the mutation-detection method employed in this investigation is 80%–90%, depending on the gene (Muenke et al. 1994). Although the mutation frequency in this population is fairly low, this finding gives significant insight into the normal and abnormal mechanisms directing the orientation and development of the great vessels. In particular, these investigations identify the developmental pathway involving CFC1 as being critical for conotruncal formation. Investigations in model systems suggest that EGF-CFC proteins serve as cofactors for nodal signaling acting through the activin receptors (Shen and Schier 2000). This pathway may be distinct from other mechanisms, such as that of neural-crest cell migration, that are recognized to participate in conotruncal development. These findings also identify functionally critical regions of the encoded protein.
The potential biological significance of the R78W missense alteration remains unclear. On the one hand, it may confer a predisposition to develop situs and/or cardiovascular anomalies, given the functional alterations that this sequence change introduces (Bamford et al. 2000); on the other hand, unaffected control subjects of similar ethnicity carry the same change. In addition, the altered amino acid is not conserved among different species. However, analysis of a large number of African American subjects with heterotaxy syndrome, TGA, or DORV would be required in order to determine whether this change is observed with significantly increased frequency in the diseased—as compared with the unaffected—population.
The present study demonstrates that, in some cases, heterotaxy syndrome shares a common genetic etiology with malpositioned great arteries in the absence of laterality defects. Thus, in select cases, isolated TGA or DORV are part of the same disease spectrum that includes heterotaxy syndrome. Reports of familial heterotaxy syndrome support this finding as well. Several families have been described in which some members had heterotaxy syndrome whereas other members of the same family had isolated cardiovascular defects (Casey 1999). Such families presumably indicate that the phenotypic expression of a single genetic alteration can vary substantially. In addition, consistent—rather than random—types of congenital heart defects are seen in the context of heterotaxy syndrome and, most commonly, include D- or L-TGA, DORV, atrioventricular-canal defects, and systemic and pulmonic venous anomalies. Thus, some patients with isolated TGA or DORV may have a forme fruste of a laterality disorder confined to malpositioning of the great vessels, without overt noncardiac laterality defects. Finally, the report by Megarbane et al. (2000), of a ZIC3 mutation in a family with X-linked inheritance of complex CHD in the apparent absence of laterality defects, also demonstrates that heterotaxy syndrome and TGA can share a common genetic etiology. These findings suggest that other genetic etiologies for heterotaxy syndrome may also cause isolated TGA or DORV and may warrant further investigation.
Acknowledgments
This study was supported by National Institutes of Health–National Heart, Lung, and Blood Institute grant HL51533 (to E.G.) and by the National Institutes of Health–Division of Intramural Research, National Human Genome Research Institute (support to M.M.).
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for X-linked heterotaxy [MIM 306955], autosomal heterotaxy [MIM 605376], ZIC3 [MIM 300265], and CFC1 [MIM 605194])
References
- Bamford RN, Roessler E, Burdine RD, Saplakoglu U, dela Cruz J, Splitt M, Goodship JA, Towbin J, Bowers P, Ferrero GB, Marino B, Schier AF, Shen MM, Muenke M, Casey B (2000) Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet 26:365–369 [DOI] [PubMed] [Google Scholar]
- Becker TA, Van Amber R, Moller JH, Pierpont ME (1996) Occurrence of cardiac malformations in relatives of children with transposition of the great arteries. Am J Med Genet 66:28–32 [DOI] [PubMed] [Google Scholar]
- Casey BKK (1999) Genetics of human left-right axis malformations. In: Harvey RP (ed) Heart development. Academic Press, New York, pp 479–489 [Google Scholar]
- Ferencz C, Correa-Villasenor A, Loffredo CA, Wilson PD (1997) Malformations of the cardiac outflow tract. In: Ferencz C, Correa-Villasenor A, Loffredo CA, Wilson PD (eds) Genetic and environmental risk factors of major cardiovascular malformations: The Baltimore-Washington Infant Study 1981–1989. Vol 5 in: Anderson RH (ed) Perspectives in pediatric cardiology. Futura Publishing, Armonk, NY, pp 59–102 [Google Scholar]
- Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Neill CA, Perry LW, Hepner SI, Downing JW (1985) Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol 121:31–36 [DOI] [PubMed] [Google Scholar]
- Fyler DC (1992) Nadas' pediatric cardiology. Hanley & Belfus, Philadelphia [Google Scholar]
- Gaio U, Schweickert A, Fischer A, Garratt AN, Muller T, Ozcelik C, Lankes W, Strehle M, Britsch S, Blum M, Birchmeier C (1999) A role of the cryptic gene in the correct establishment of the left-right axis. Curr Biol 9:1339–1342 [DOI] [PubMed] [Google Scholar]
- Gebbia M, Ferrero GB, Pilia G, Bassi MT, Aylsworth A, Penman-Splitt M, Bird LM, Bamforth JS, Burn J, Schlessinger D, Nelson DL, Casey B (1997) X-linked situs abnormalities result from mutations in ZIC3. Nat Genet 17:305–308 [DOI] [PubMed] [Google Scholar]
- Goldmuntz E, Clark BJ, Mitchell LE, Jawad AF, Cuneo BF, Reed L, McDonald-McGinn D, Chien P, Feuer J, Zackai EH, Emanuel BS, Driscoll DA (1998) Frequency of 22q11 deletions in patients with conotruncal defects. J Am Coll Cardiol 32:492–498 [DOI] [PubMed] [Google Scholar]
- Kathiriya IS, Srivastava D (2000) Left-right asymmetry and cardiac looping: implications for cardiac development and congenital heart disease. Am J Med Genet 97:271–279 [DOI] [PubMed] [Google Scholar]
- Megarbane A, Salem N, Stephan E, Ashoush R, Lenoir D, Delague V, Kassab R, Loiselet J, Bouvagnet P (2000) X-linked transposition of the great arteries and incomplete penetrance among males with a nonsense mutation in ZIC3. Eur J Hum Genet 8:704–708 [DOI] [PubMed] [Google Scholar]
- Muenke M, Schell U, Hehr A, Robin NH, Losken HW, Schinzel A, Pulleyn LJ, Rutland P, Reardon W, Malcolm S, Winter RM (1994) A common mutation in the fibroblast growth factor receptor-1 gene in Pfeiffer syndrome. Nat Genet 8:269–274 [DOI] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, de Die-Smulders de C, Giannotti A, Imaizumi K, Jones KL, Del Campo M, Martin RA, Meinecke P, Pierpont MEM, Robin NH, Young ID, Roessler E, Muenke M (1999) The mutational spectrum of the Sonic Hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet 8:2479–2488 [DOI] [PubMed] [Google Scholar]
- Perry LW, Neill CA, Ferencz C, Rubin JD, Loffredo CA (1993) Infants with congenital heart disease: the cases. In: Ferencz C, Rubin JD, Loffredo CA, Magee CA (eds) Epidemiology of congenital heart disease: The Baltimore-Washington Infant Study 1981–1989. Vol 4 in: Anderson RH (ed) Perspectives in pediatric cardiology. Futura Publishing, Mount Kisco, NY, pp 33–62 [Google Scholar]
- Shen MM, Schier AF (2000) The EGF-CFC gene family in vertebrate development. Trends Genet 16:303–309 [DOI] [PubMed] [Google Scholar]
- Shen MM, Wang H, Leder P (1997) A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development 124:429–442 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kido S, Hoshino K, Ogawa K, Ohashi H, Fukushima Y (1995) Frequency of a 22q11 deletion in patients with conotruncal cardiac malformations: a prospective study. Eur J Pediatr 154:878–881 [DOI] [PubMed] [Google Scholar]
- Yan YT, Gritsman K, Ding J, Burdine RD, Corrales JD, Price SM, Talbot WS, Schier AF, Shen MM (1999) Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev 13:2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C, Whitman M (2001) Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell 7:949–957 [DOI] [PubMed] [Google Scholar]