Abstract
Although intensive efforts have been undertaken to elucidate the genetic background of immunoglobulin A nephropathy (IgAN), genetic factors associated with the pathogenesis of this disease are still not well understood. We designed a case-control association study that was based on linkage disequilibrium among single-nucleotide polymorphisms (SNPs) in the selectin gene cluster on chromosome 1q24-25, and we found two SNPs in the E-selectin gene (SELE8 and SELE13) and six SNPs in the L-selectin gene (SELL1, SELL4, SELL5, SELL6, SELL10, and SELL11) that were significantly associated with IgAN in Japanese patients. All eight SNPs were in almost complete linkage disequilibrium. SELE8 and SELL10 caused amino acid substitutions from His to Tyr and from Pro to Ser (χ2=9.02, P=.0026, odds ratio = 2.73 [95% confidence interval {CI} 1.38–5.38] for His-to-Tyr substitutions; χ2=17.4, P=.000031, odds ratio = 3.61 [95% CI 1.91–6.83] for Pro-to-Ser substitutions), and SELL1 could affect promoter activity of the L-selectin gene (χ2=19.5, P=.000010, odds ratio = 3.77 [95% CI 2.02–7.05]). The TGT haplotype at these three loci was associated significantly with IgAN (χ2=18.67, P=.000016, odds ratio = 1.88 [95% CI 1.41–2.51]). Our results suggest that these eight SNPs in selectin genes may be useful for screening populations susceptible to the IgAN phenotype that involves interstitial infiltration.
Immunoglobulin A nephropathy (IgAN [MIM 161950]) is the most common form of glomerulonephritis, a principal cause of end-stage renal disease worldwide (Maisonneuve et al. 2000). Surveys of patients with primary glomerulonephritis that were conducted in 1985 and 1993 by the Research Group on Progressive Renal Diseases in Japan revealed a high prevalence of IgAN and a relatively poor prognosis for patients (Koyama et al. 1997). The rate of survival for the 502 patients with IgAN who were monitored, with renal-related death taken as the end point, was 61% at 20 years from the time renal abnormalities were first detected. The strong evidence from human studies of a role for genetic factors in the development and progression of IgAN has been provided by descriptive reports of familial aggregations of IgAN and by analyses of affected sib pairs and parent-child pairs from multiple ethnic groups (Hsu et al. 2000).
Accumulation of leukocytes within the glomerulus and interstitium of the kidney is considered to be a key pathogenetic mechanism in various types of glomerulonephritis (Adler and Brady 1999). The three major groups of adhesion molecules involved in these interactions are the selectins, the integrins, and certain proteins belonging to the immunoglobulin supergene family. To date, three selectins have been characterized: E-selectin is expressed predominantly in cytokine-activated endothelium, L-selectin in circulating leukocytes, and P-selectin in activated endothelial cells and platelets. Chaudhury et al. (1996) reported a marked increase in E-selectin and P-selectin expression on the extraglomerular vascular endothelium, with prominent interstitial infiltrates, in some biopsy samples from patients with IgAN; and a large number of L-selectin–positive cells were present within glomerular and interstitial infiltrates in affected kidneys. Lai et al. (1994) demonstrated an apparent increase in serum levels of E-selectin that was associated with increased histopathologic grade, when patients with IgAN were stratified according to the severity of their glomerular and interstitial lesions. Moreover, increased expression of E-selectin has been observed on renal interstitium that contains lymphocytes and macrophages, along with an increase in circulating soluble L-selectin (Kennel-De March et al. 1999).
Single-nucleotide polymorphisms (SNPs) in the human genome have great potential for application to association studies of complex diseases (Kruglyak 1999). Because SNPs are the most frequent type of genetic variation, we have been screening SNPs on a genomewide scale to clarify various complex diseases, including IgAN. For the work reported here, we designed a case-control association study, using SNPs found in the selectin genes (GenBank), and estimated haplotypes that might serve to identify regions containing loci responsible for IgAN phenotypes (Takeoka et al. 2001).
Peripheral blood samples were obtained from 346 patients (196 female and 150 male, mean age 44 years) who were diagnosed with IgAN on the basis of clinical manifestations as well as renal-biopsy findings at several surgical centers in Japan (Department of Medicine, Kidney Center, Tokyo Women’s Medical University; Department of Urology, Iwate Medical University; Department of Urology, Sanai Hospital; Department of Urology, Iwate Prefectural Ofunato Hospital; and Department of Medicine, Niigata University School of Medicine). The mean value of serum creatinine at the time of renal biopsy was 1.07 mg/dl, ranging from 0.3 to 2.5 mg/dl. We analyzed blood from 408 control subjects randomly selected from population-based volunteers. DNA was prepared from each sample, according to standard protocols.
SNPs in the three selectin genes were screened according to methods described elsewhere (Ohnishi et al. 2000; Yamada et al. 2000). Information about each SNP we discovered can be obtained at the Japanese SNPs (JSNP) Web site.
We amplified multiple genomic fragments, using 20 ng of genomic DNA for each PCR, as described elsewhere (Ohnishi et al. 2001). Sequences of the primers used in the present study are available at JSNP. Each PCR reaction was performed in a 20-μl solution containing 50 pmol of each primer, 10 units of Ex-Taq DNA polymerase (TaKaRa Shuzo), and 0.55 μg of TaqStart (Clontech Laboratories) in the GeneAmp PCR system 9700 (Applied Biosystems). Initial denaturation was at 94°C for 2 min, followed by 37 cycles of amplification at 94°C for 15 s and annealing at 60°C for 45 s, with final extension for 2 min at 72°C.
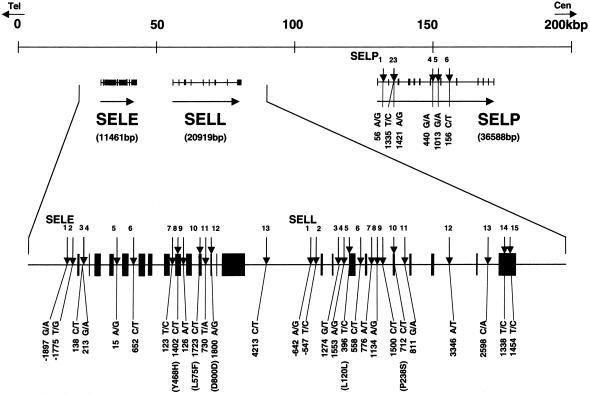
We genotyped a total of 34 SNPs present in three selectin genes—13 for E-selectin (SELE 1–13), 15 for L-selectin (SELL 1–15), and 6 for P-selectin (SELP 1–6) (fig. 1)—by means of the Invader assay that combines a structure-specific cleavage enzyme with a universal fluorescent resonance energy transfer system (Mein et al. 2000).
Figure 1.
High-density map and association with IgAN of SNPs in the selectin gene cluster at chromosome 1q24-25. SNPs (downward arrows) are numbered for E-selectin (SELE1–13), L-selectin (SELL1–15), and P-selectin (SELP1–6), in the telomeric to centromeric direction.
Comparison of allelic and genotypic frequencies in patients who have IgAN versus frequencies in control subjects disclosed significant associations between IgAN and eight SNPs present in two of the selectins (table 1 and table 2table 1 and table 2). Among the disease-associated SNPs, two were in the E-selectin gene (SELE8 in exon 9 and SELE13 in the 3′ flanking region). The other six were in the L-selectin gene (SELL1 in the 5′ flanking [promoter] region, SELL4 in intron 2, SELL5 in exon 3, SELL6 in intron 3, SELL10 in exon 5, and SELL11 in intron 5). These eight SNPs all lie within a 24-kb span of genomic DNA in the region harboring the three-gene cluster of selectins on chromosome 1q24-25 (fig. 1).
Table 1.
Statistically Significant Differences in Eight SNPs among Patients with IgAN versus Control Subjects
|
No. [%] of Subjects with |
||||||||
| Zygosity | SELE8 | SELE13 | SELL1 | SELL4 | SELL5 | SELL6 | SELL10 | SELL11 |
| IgAN: | ||||||||
| Major homozygotes | 187 [57.89%] | 189 [57.27%] | 185 [54.41%] | 157 [45.38%] | 166 [48.26%] | 133 [46.18%] | 156 [53.24%] | 161 [57.50%] |
| Heterozygotes | 109 [33.75%] | 115 [34.85%] | 114 [33.53%] | 138 [39.88%] | 131 [38.08%] | 106 [36.81%] | 101 [34.47%] | 95 [33.93%] |
| Minor homozygotes | 27 [8.36%] |
26 [7.88%] |
41 [12.06%] |
51 [14.74%] |
47 [13.66%] |
49 [17.01%] |
36 [12.29%] |
24 [8.57%] |
| Total | 323 [100.00%] | 330 [100.00%] | 340 [100.00%] | 346 [100.00%] | 344 [100.00%] | 288 [100.00%] | 293 [100.00%] | 280 [100.00%] |
| Major allele | 483 [74.77%] | 493 [74.70%] | 484 [71.18%] | 452 [65.32%] | 463 [67.30%] | 372 [64.58%] | 413 [70.48%] | 417 [74.46%] |
| Minor allele | 163 [25.23%] |
167 [25.30%] |
196 [28.82%] |
240 [34.68%] |
225 [32.70%] |
204 [35.42%] |
173 [29.52%] |
143 [25.54%] |
| Total | 646 [100.00%] | 660 [100.00%] | 680 [100.00%] | 692 [100.00%] | 688 [100.00%] | 576 [100.00%] | 586 [100.00%] | 560 [100.00%] |
| Control | ||||||||
| Major homozygotes | 269 [66.92%] | 274 [67.16%] | 246 [61.65%] | 218 [53.96%] | 227 [56.05%] | 218 [58.76%] | 235 [62.67%] | 262 [67.88%] |
| Heterozygotes | 120 [29.85%] | 121 [29.66%] | 139 [33.53%] | 159 [39.36%] | 154 [38.02%] | 132 [35.58%] | 126 [33.60%] | 112 [29.02%] |
| Minor homozygotes | 13 [3.23%] |
13 [3.19%] |
14 [3.51%] |
27 [6.68%] |
24 [5.93%] |
21 [5.66%] |
14 [3.73%] |
12 [3.11%] |
| Total | 402 [100.00%] | 408 [100.00%] | 399 [100.00%] | 404 [100.00%] | 405 [100.00%] | 371 [100.00%] | 375 [100.00%] | 386 [100.00%] |
| Major allele | 658 [81.84%] | 669 [81.99%] | 631 [79.07%] | 595 [73.64%] | 608 [75.06%] | 568 [76.55%] | 596 [79.47%] | 636 [82.38%] |
| Minor allele | 146 [18.16%] |
147 [18.01%] |
167 [20.93%] |
213 [26.36%] |
202 [24.94%] |
174 [23.45%] |
154 [20.53%] |
136 [17.62%] |
| Total | 804 [100.00%] | 816 [100.00%] | 798 [100.00%] | 808 [100.00%] | 810 [100.00%] | 742 [100.00%] | 750 [100.00%] | 772 [100.00%] |
Table 2.
Statistically Significant Differences in Eight SNPs among Patients with IgAN versus Control Subjects[Note]
|
Statistical Significance in Subjects with |
||||||||
| Stratified Comparison | SELE8 | SELE13 | SELL1 | SELL4 | SELL5 | SELL6 | SELL10 | SELL11 |
| χ2: | ||||||||
| Allele frequency | 10.686095 | 11.572843 | 12.354986 | 12.242864 | 11.007155 | 22.701719 | 14.379202 | 12.292278 |
| Genotype frequency | 11.704698 | 11.980555 | 19.773932 | 14.392873 | 13.899272 | 24.560263 | 18.609493 | 12.969646 |
| Major homozygotes/others | 6.244945 | 7.624198 | 3.961227 | 5.494191 | 4.530285 | 10.306812 | 6.018957 | 7.538820 |
| Minor homozygotes/others | 9.025162 | 8.026010 | 19.481165 | 12.982878 | 12.975893 | 22.013996 | 17.377607 | 9.470623 |
| P: | ||||||||
| Allele frequency | .001079 | .000669 | .000440 | .000467 | .000908 | .000002 | .000149 | .000455 |
| Genotype frequency | .002873 | .002503 | .000051 | .000749 | .000959 | .000005 | .000091 | .001526 |
| Major homozygotes/others | .012455 | .005759 | .046560 | .019080 | .033300 | .001325 | .014153 | .006038 |
| Minor homozygotes/others | .002663 | .004611 | .000010 | .000314 | .000316 | .000003 | .000031 | .002088 |
Note.— The χ2 test was used to evaluate possible associations between different genotypes, allele frequencies, or patients.
Homozygosity for minor alleles was significantly more common among patients with IgAN than among control subjects. The most significant association of a single SNP with IgAN involved SELL6 (χ2=22.0, P=.000003, odds ratio 3.42 [95% CI 2.00–5.85]). Among the eight SNPs for which we found positive associations, two alter amino acid sequences: SELE8 substitutes tyrosine for histidine at codon 468 in the short consensus-repeat domain of E-selectin (χ2=9.02, P=.0026, odds ratio = 2.73 [95% CI 1.38–5.38]), and SELL10 substitutes serine for proline at codon 238 in a short consensus-repeat domain of L-selectin (χ2=17.4, P=.000031, odds ratio = 3.61 [95% CI 1.91–6.83]). A third SNP in the promoter region, SELL1, could affect promoter activity of the L-selectin gene (χ2=19.5, P=.000010, odds ratio = 3.77 [95% CI 2.02–7.05]).
Analysis of linkage disequilibrium (LD) coefficients among these eight SNPs indicated quasi-complete LD (table 3). When we estimated haplotypes for three SNPs (SELE8, SELL1, and SELL10) that may affect the quality and quantity of the gene products, the TGT haplotype revealed a stronger association with the IgAN phenotype (χ2=18.67, P=.000016, odds ratio of 1.88 [95% CI 1.41–2.51]) than did the genotype at either locus singly (table 4).
Table 3.
LD Coefficients between Eight SNPs[Note]
| SELE8 | SELE13 | SELL1 | SELL4 | SELL5 | SELL6 | SELL10 | |
| SELL11 | .95284 | .95969 | .85654 | .73947 | .78009 | .80668 | .97308 |
| SELL10 | .85928 | .88022 | .93324 | .82487 | .87217 | .89132 | |
| SELL6 | .77048 | .78813 | .87736 | .89847 | .94944 | ||
| SELL5 | .76206 | .78182 | .88943 | .95107 | |||
| SELL4 | .72427 | .74805 | .85863 | ||||
| SELL1 | .84004 | .86222 | |||||
| SELE13 | .95929 |
Note.— LD coefficients were calculated and expressed as the D′=D/Dmax, as described elsewhere (Devlin and Risch 1995)
Table 4.
Estimated Haplotype Frequencies of SNPs SELE8, SELL1, and SELL10[Note]
| Haplotype | IgAN | Control | Total |
| CAC | 374.55 [68.10%] | 568.9 [81.97%] | 943.45 [75.84%] |
| CAT | 6.05 [1.10%] | 2.01 [.29%] | 8.06 [.65%] |
| CGC | 9.9 [1.80%] | 2.02 [.29%] | 11.92 [.96%] |
| CGT | 20.9 [3.80%] | 17.07 [2.46%] | 37.97 [3.05%] |
| TAC | 3.3 [.60%] | 3.09 [.45%] | 6.39 [.51%] |
| TAT | 3.3 [.60%] | .03 [.00%] | 3.33 [.27%] |
| TGC | 0 [.00%] | .98 [.14%] | .98 [.08%] |
| TGTa | 132 [24.00%] |
99.9 [14.39%] |
231.9 [18.64%] |
| Total | 550 [100.00%] | 694 [100.00%] | 1,244 [100.00%] |
Note.— Haplotype frequencies for pairs of alleles were estimated by the Estimating Haplotype-Frequencies software program, as described elsewhere (Terwilliger and Ott 1994).
χ2 (TGT/others) = 18.67, P=.000016.
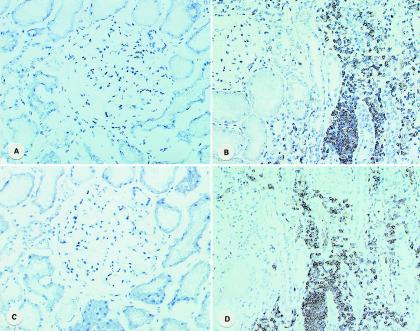
To investigate a potential role of E-selectin and/or L-selectin in the pathogenesis of IgAN, we performed immunohistological staining of these molecules in biopsy specimens (fig. 2). Strong staining of both E-selectin and L-selectin was observed in interstitial infiltrates of renal tissue from a patient with IgAN, whereas staining of both selectins was very weak in the renal tissues without infiltration.
Figure 2.
Immunohistochemical localization of E-selectin and L-selectin in the renal tissues of patients with IgAN. Control tissues were normal portions obtained from patients who underwent nephrectomy because of renal tumor. The monoclonal antibodies used in this study were anti-human E-selectin (clone 1.2B6) and anti-human L-selectin (clone FMC46) (DAKO Japan). A, Control E-selectin. B, IgAN E-selectin. C, Control L-selectin. D, IgAN L-selectin. Original magnification ×200.
In the present study, we have demonstrated significant association of eight SNPs in selectin genes with IgAN, as well as accumulation of two selectin proteins in renal tissues. Our findings suggest that one or more of the three specific SNPs could affect the quality or quantity of the gene products and could play a significant role in the etiology of IgAN.
Although a genomewide analysis that used familial cases has been reported (Gharavi et al. 2000), most patients with IgAN have no familial history of the disease. IgAN is a complex disease whose etiology involves immunological, environmental, and genetic factors (Hsu et al. 2000). Since it was hard for us to locate patients with familial histories of IgAN, we chose to perform a case-control association study, using SNPs at the three-gene selectin complex on chromosome 1q24-25.
One study suggested that LD in the human genome was likely to extend only a few kilobases (Dunning et al. 2000), but other investigators have suspected that it could extend much farther, to distances >100 kb in some cases (Abecasis et al. 2001). In fact, we now know that distances encompassing LD can vary according to the chromosomal regions and/or the populations examined (Reich et al. 2001). We found that LD at the selectin locus extended over ∼24 kb. This result implies that ∼125,000 SNPs will be required for whole-genome scanning in the Japanese population.
Selectins are cell-cell adhesion molecules involved in the leukocyte–endothelial cell interaction that is required for extravasation at sites of tissue injury. They have a single C-type lectin domain at their extracellular amino-termini, followed by an epidermal growth factor (EGF)-like domain, short consensus repeat domains, a transmembrane domain, and a short cytoplasmic tail. In the E-selectin gene, substitutions from Ser to Arg at codon 149 in the EGF-like domain and from Leu to Phe at codon 575 in the transmembrane domain have been associated with severe atherosclerosis in German patients (Wenzel et al. 1996). However, in our Japanese population sample, we found no substitutions at codon 149 and no significant association between IgAN and the SNP (SELE10) at codon 575 of E-selectin. Instead, in patients with IgAN we found a significant increase in tyrosine alleles at codon 468 in E-selectin, and in serine alleles at codon 238 in L-selectin, both of them in short consensus-repeat domains. Certain alleles of these two SNPs are likely to increase susceptibility to the IgAN phenotype with interstitial infiltration. The accumulation of these two isoforms of selectin that we observed in IgAN kidneys may provide a useful clue for development of novel treatments for this form of glomerulonephritis.
Acknowledgments
The authors are grateful to Wataru Obara, Fumihiro Akiyama, Akihiro Sekine, Susumu Saito, and technicians at the SNP Research Center, The Institute of Physical and Chemical Research (RIKEN). This work was supported, in part, by a Research for the Future Program Grant of the Japan Society for the Promotion of Science (to Y.N. and S.M.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for selectin genes [accession numbers AL021940.1, NT000026.1, and AL022146.1])
- JSNP, http://snp.ims.u-tokyo.ac.jp/ (for SNPs and primers)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for IgAN [MIM 161950])
References
- Abecasis GR, Noguchi E, Heinzmann A, Traherne JA, Bhattacharyya S, Leaves NI, Anderson GG, Zhang Y, Lench NJ, Carey A, Cardon LR, Moffatt MF, Cookson WOC (2001) Extent and distribution of linkage disequilibrium in three genomic regions. Am J Hum Genet 68:191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S, Brady HR (1999) Cell adhesion molecules and the glomerulonephritis. Am J Med 107:371–386 [DOI] [PubMed] [Google Scholar]
- Chaudhury PR, Wu B, King G, Campbell M, Macleod AM, Haites NE, Simpson JG, Power DA (1996) Adhesion molecule interactions in human glomerulonephritis: importance of the tubulointerstitium. Kidney Int 49:127–134 [DOI] [PubMed] [Google Scholar]
- Devlin B, Risch N (1995) A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics 29:311–322 [DOI] [PubMed] [Google Scholar]
- Dunning AM, Durocher F, Healey CS, Teare MD, McBride SE, Carlomagno F, Xu C-F, Dawson E, Rhodes S, Ueda S, Lai E, Luben RN, Van Rensburg EJ, Mannermaa A, Kataja V, Rennart G, Dunham I, Purvis I, Easton D, Ponder BAJ (2000) The extent of linkage disequilibrium in four populations with distinct demographic histories. Am J Hum Genet 67:1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, Amoroso A, Viola BF, Battini G, Caridi G, Canova C, Farhi A, Subramanian V, Nelson-Williams C, Woodford S, Julian BA, Wyatt RJ, Lifton RP (2000) IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet 26:354–357 [DOI] [PubMed] [Google Scholar]
- Hsu SIH, Ramirez SB, Winn MP, Bonventre JV, Owen WF (2000) Evidence for genetic factors in the development and progression of IgA nephropathy. Kidney Int 57:1818–1835 [DOI] [PubMed] [Google Scholar]
- Kennel-De March A, Bene MC, Renoult E, Kessler M, Faure GC, Kolopp-Sarda MN (1999) Enhanced expression of L-selectin on peripheral blood lymphocytes from patients with IgA nephropathy. Clin Exp Immunol 115:542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, Igarashi M, Kobayashi M, and Members and Coworkers of the Research Group on Progressive Renal Diseases (1997) Natural history and risk factors for immunoglobulin A nephropathy in Japan. Am J Kidney Dis 29:526–532 [DOI] [PubMed] [Google Scholar]
- Kruglyak L (1999) Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat Genet 22:139–144 [DOI] [PubMed] [Google Scholar]
- Lai KN, Wong KC, Li PKT, Chan CHS, Lui SF, Chui YL, Haskard DO (1994) Circulating leukocyte-endothelial adhesion molecules in IgA nephropathy. Nephron 68:294–300 [DOI] [PubMed] [Google Scholar]
- Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney APS, Briggs D, McCredie M, Boyle P (2000) Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis 35:157–165 [DOI] [PubMed] [Google Scholar]
- Mein CA, Barratt BJ, Dunn MG, Siegmund T, Smith AN, Esposito L, Nutland S, Stevens HE, Wilson AJ, Phillips MS, Jarvis N, Law S, de Arruda M, Todd JA (2000) Evaluation of single nucleotide polymorphism typing with invader on PCR amplicons and its automation. Genome Res 10:330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y, Tanaka T, Ozaki K, Yamada R, Nakamura Y (2001) A high-throughput SNP typing system for genome-wide association studies. J Hum Genet 46:471–477 [DOI] [PubMed] [Google Scholar]
- Ohnishi Y, Tanaka T, Yamada R, Suematsu K, Minami M, Fujii K, Hoki N, Kodama K, Nagata S, Hayashi T, Kinoshita N, Sato H, Sato H, Kuzuya T, Takeda H, Hori M, Nakamura Y (2000) Identification of 187 single nucleotide polymorphisms (SNPs) among 41 candidate genes for ischemic heart disease in the Japanese population. Hum Genet 106:288–292 [DOI] [PubMed] [Google Scholar]
- Reich DE, Cargill M, Bolk S, Ireland J, Sabeti PC, Richter DJ, Lavery T, Kouyoumjian R, Farhadian SF, Ward R, Lander ES (2001) Linkage disequilibrium in the human genome. Nature 411:199–204 [DOI] [PubMed] [Google Scholar]
- Takeoka S, Unoki M, Onouchi Y, Doi S, Fujiwara H, Miyatake A, Fujita K, Inoue I, Nakamura Y, Tamari M (2001) Amino-acid substitutions in the IKAP gene product significantly increase risk for bronchial asthma in children. J Hum Genet 46:57–63 [DOI] [PubMed] [Google Scholar]
- Terwilliger J, Ott J (1994) Handbook of human genetic linkage. Johns Hopkins University Press, Baltimore, pp 199–210 [Google Scholar]
- Wenzel K, Ernst M, Rohde K, Baumann G, Speer A (1996) DNA polymorphisms in adhesion molecule genes: a new risk factor for early atherosclerosis. Hum Genet 97:15–20 [DOI] [PubMed] [Google Scholar]
- Yamada R, Tanaka T, Ohnishi Y, Suematsu K, Minami M, Seki T, Yukioka Maeda A, Murata N, Saiki O, Teshima R, Kudo O, Ishikawa K, Ueyoshi A, Tateishi H, Inaba M, Goto H, Nishizawa Y, Tohma S, Ochi T, Yamamoto K, Nakamura Y (2000) Identification of 142 single nucleotide polymorphisms in 41 candidate genes for rheumatoid arthritis in the Japanese population. Hum Genet 106:293–297 [DOI] [PubMed] [Google Scholar]