Abstract
Non healing chronic wounds are difficult to treat in patients with diabetes and can result in severe medical problems for these patients and for society. Negative-pressure wound therapy (NPWT) has been adopted to treat intractable chronic wounds and has been reported to be effective. However, the mechanisms underlying the effects of this treatment have not been elucidated. To assess the vasculogenic effect of NPWT, we evaluated the systemic mobilization of endothelial progenitor cells (EPCs) during NPWT. Twenty-two of 29 consecutive patients who presented at the clinic of Seoul National Universty Hospital between December 2009 and November 2010 who underwent NPWT for diabetic foot infections or skin ulcers were included in this study. Peripheral blood samples were taken before NPWT (pre-NPWT) and 7–14 days after the initiation of NPWT (during-NPWT). Fluorescence-activated cell sorting (FACS) analysis showed that the number of cells in EPC-enriched fractions increased after NPWT, and the numbers of EPC colony forming units (CFUs) significantly increased during NPWT. We believe that NPWT is useful for treating patients with diabetic foot infections and skin ulcers, especially when these conditions are accompanied by peripheral arterial insufficiency. The systemic mobilization of EPCs during NPWT may be a mechanism for healing intractable wounds in diabetic patients with foot infections or skin defects via the formation of increased granulation tissue with numerous small blood vessels.
Keywords: diabetic foot, endothelial progenitor cell, negative-pressure wound therapy
Introduction
Non-healing chronic diabetic wounds are difficult to treat. They are associated with high economic burden and increased length and frequency of hospitalization. Furthermore, diabetes is a significant risk factor for foot amputation.1 Adequate surgical debridement, effective antibiotic therapy, correction of metabolic abnormalities and proper wound management are essential for healing the chronic wounds of diabetic patients.2, 3 Despite painstaking management, chronic wounds in the feet of diabetic patients frequently fail to heal completely. Therefore, many trials using bioengineered tissue or skin substitutes, growth factors, electronic stimulation or advanced moist wound therapy have been performed.4 Negative-pressure wound therapy (NPWT) is an adjuvant therapy that uses negative pressure to evacuate infected fluid from open wounds through a sealed dressing and a tube that is connected to suction.5, 6 NPWT has been adopted to treat many acute and chronic wounds, and several randomized controlled studies have shown efficacy in treating diabetic foot infections and pressure ulcers and in aiding skin grafts.6, 7
The following mechanisms of NPWT have been suggested to enhance wound healing: (1) the removal of excessive extracellular fluid and tissue edema, which leads to increased blood flow;8, 9 (2) stabilization of the wound environment; (3) macrodeformation, that is, foam shrinkage that induces the contraction of the wound and facilitates wound closure;10 and (4) microdeformation at the foam–wound interface, which induces cellular proliferation and angiogenesis.6, 11 However, we focused on the effect of increased blood flow during NPWT.
The suggestion that postnatal neovascularization is mediated by putative endothelial progenitors was first made in 1997,12 and subsequent studies have led to a new field of cardiovascular science, which, in turn, has provided new insights into other fields of medicine. ‘Endothelial progenitor cells (EPCs)' are now considered to be mobilized from the bone marrow during acute ischemia and to contribute to the neovascularization of ischemic tissues,13, 14 and circulating EPC number is now used as a marker for endothelial dysfunction or repair in various diseases.13, 15, 16 Researchers working on the musculoskeletal system have also become interested in the roles of EPCs during healing of diabetic foot ulcers, and bone marrow-derived EPCs have been proposed to participate in angiogenesis.17, 18, 19, 20
The purpose of the present study was to determine the level of circulating EPCs in diabetic patients with chronic wounds and to assess the effect of NPWT on the number of circulating EPCs and wound healing.
Materials and methods
Study population and surgical procedures
This study was approved by our Institutional Review Board, and informed consent was obtained from patients and/or their parents or guardians. Twenty-two of the 29 consecutive patients (2009.12–2010.11) who underwent NPWT for diabetic foot infections or skin ulcers were included in this study. Seven patients were excluded because of the following reasons: unstable comorbidities, refusal to participate or missing samples. The control group was composed of 16 patients who did not have diabetes but who had undergone an orthopedic operation for a non-traumatic disease. Some data for the control group were also included from previous publications.21 The average age of the patients in the study group was 66.7 years (range 28–83 years), whereas that of patients in the control group was 17.1 years (range 12–52 years).
A common aspect of the procedure was that NWPT was performed on the foot or skin ulcer of a diabetic patient after debridement or partial foot amputation; however, the details of the adopted surgical procedures differed between patients. Medical-grade polyurethane foam (CuraVAC, Daewoong Pharma, Seoul, Korea) was applied, with adhesive drapes for sealing and connector tubing that were connected to a wall-mounted suction device (Figure 1). A negative pressure of 120 mm Hg was maintained continuously throughout the procedure.
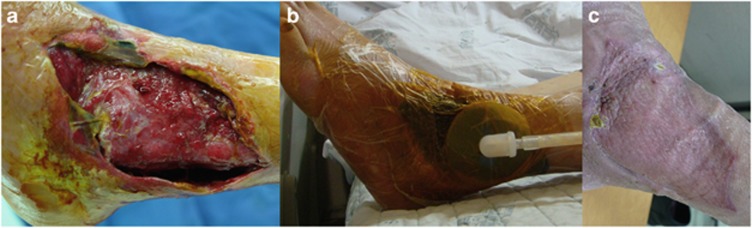
Figure 1.
An illustrative case of NPWT for an intractable wound in a diabetic patient. (a) A large-sized skin defect with infection developed in a diabetic patient. (b) Medical-grade polyurethane foam (CuraVAC, Daewoong Pharma) was applied, with adhesive drapes for sealing and connector tubing connected to a wall-mounted suction device. A negative pressure of 120 mm Hg was maintained continuously. (c) The large-sized skin defect healed without amputation after 20 days of NPWT and a split-thickness skin graft.
Acquisition of peripheral blood from patients, mononuclear cell isolation and the culture of adherent cell colonies
Peripheral blood samples were taken at two time points: before operation (pre-NPWT) and 7–14 days after the start of NPWT (during-WPWT). Peripheral blood mononuclear cells (PB MNCs) were isolated from whole blood and cultured as reported previously.15 Briefly, MNCs were isolated from 3–5 ml of human peripheral blood using density gradient centrifugation over Ficoll (Ficoll-Paque PLUS, Amersham Biosciences, Buckinghamshire, UK) for 20 min at 2580 r.p.m. and washed three times in phosphate-buffered saline. The isolated MNCs were then resuspended in EGM-2 (catalog number CC-3162; Clonetics, San Diego, CA, USA), which consisted of endothelial basal medium, 5% fetal bovine plasma, human epidermal growth factor, VEGF (vascular endothelial growth factor), hFGF (human fibroblast growth factor)-B, insulin-like growth factor-1 and ascorbic acid. Mononuclear cells (3 × 106 cells per well) were seeded on 10 μg ml−1 fibronectin-coated 24-well plates (Sigma Aldrich, St Louis, MO, USA) and incubated in a 5% CO2 incubator at 37 °C. MNCs that were isolated at different times (that is, pre-NPWT and during-NPWT) from each patient were cultured on at least three plates. The plates were observed daily, and the first media changes were performed 6 days after plating. Thereafter, the media were changed every 3 days, and each cluster or colony was followed up every day. The colony forming units (CFUs) were defined as a central core of rounded cells that were surrounded by elongated, spindle-shaped cells, and the numbers of CFUs per plate were counted 14 days after plating.
To evaluate the EPC characteristics of the CFUs, plated cell colonies were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-conjugated, acetylated-low-density protein (DiI-Ac-LDL, Molecular Probes, Carlsbad, CA, USA) (2.5 μg Ml−1) for 2 h at 37 °C.14 The cells were then fixed in 1% paraformaldehyde and stained with fluorescein isothiocyanate (FITC)-labeled lectin that was obtained from Ulex Europaeus (Sigma Aldrich). The cells were counterstained with 4,6-diamidino-2-phenylindole to identify nuclei that were not stained with DiI-Ac-LDL and FITC-lectin. After staining, the cells were observed using an inverted fluorescent microscope (Nikon Eclipse E300, Nikon Inc., Melville, NY, USA).
Proportion of EPCs among PB MNCs
Isolated PB MNCs (3 × 105 cells) were washed with cold phosphate-buffered saline that was supplemented with 5 mM EDTA and 0.1% bovine plasma albumin. CD34, VEGFR2 and CD133 are cell surface markers that are most widely used to define EPCs.22 Therefore, cells were incubated for 30 min at 4 °C with 5 μl of a phycoerythrin-conjugated mouse anti-human VEGFR2 (R&D Systems, Minneapolis, MN, USA) and 5 μl of an allophycocyanin-conjugated mouse anti-human CD133 (Miltenyi Biotec, Auburn, CA, USA) antibodies. Another 3 × 105 PB MNCs were reacted with 10 μl of FITC-conjugated mouse anti-human CD34 (BD Bioscience, San Jose, CA, USA) and 5 μl of allophycocyanin-conjugated mouse anti-human alkaline phosphatase (ALP, R&D Systems) antibodies. To avoid nonspecific staining, the cells were stained with phycoerythrin-, FITC- or allophycocyanin-conjugated human immunoglobulin G1 as negative controls. The cells were fixed with 200 μl 1% paraformaldehyde (Sigma-Aldrich) in phosphate-buffered saline for 10 min and analyzed using a FACStar flow cytometer (Becton Dickinson, Palo Alto, CA, USA) for at least 10 000 events. After appropriate gating, two-dimensional, side scatter-fluorescence dot-plot analysis was performed, and the specific surface antigen-positive cell fractions were determined as previously described20 and quantified by deducting the values of the isotype controls. Isolated PB MNCs from each patient were tested in duplicate.
Assessment of C-reactive protein and EPC-mobilizing cytokines in the peripheral blood
Plasma from peripheral blood was obtained before each operation (pre-NPWT) and 7–14 days after the initiation of NPWT (during-NPWT). We assessed the level of C-reactive protein in the peripheral blood to evaluate whether the acute inflammatory process was active after NPWT application, and quantitative immunoassays of SDF-1A and VEGF were performed using appropriate enzyme-linked immunosorbent assay kits (ELISA, R&D Systems). Plasma from each patient was tested in triplicate at each time point. Diurnal variation was not considered in measurements of plasma cytokine levels.
Statistical analysis
All data are expressed as the means±s.d.'s. The Wilcoxon-signed rank test for paired data was used to compare values at different time points, and the Mann–Whitney test was used to compare the values from the study and control groups. Probability values of <0.05 were interpreted to denote statistical significance, and the SPSS software (IBM SPSS 19.0; IBM, New York, NY, USA) was used for the statistical analysis.
Results
The proportion of PB MNCs was lower in diabetic patients with a non-healing chronic wound and was higher after NPWT
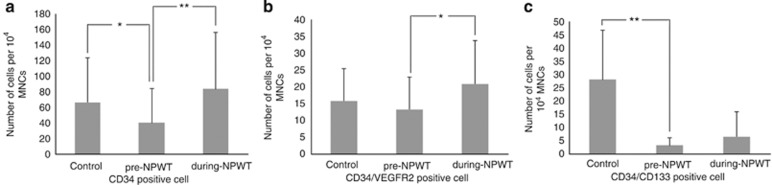
Two-dimensional, side scatter-fluorescence dot-plot analysis of freshly isolated PB MNCs showed that the number of EPC-enriched cell fractions (that is, CD34+ cells, CD34+/CD133+cells) was lower in diabetic patients with a non-healing chronic wound than in the control group, and this value uniformly increased after the initiation of NPWT (Figure 2).
Figure 2.
Two-dimensional, side scatter-fluorescence dot-plot analysis of freshly isolated PB MNCs. The numbers of CD34+ cells (a), CD34+/VEGFR+ cells (b) and CD34+/CD133+ cells (c) were lower in diabetic patients with a non-healing chronic wound than in the control group, and the numbers uniformly increased after the initiation of NPWT. Data are presented as the mean±s.d., *P<0.05, and **P<0.01. The Mann–Whitney test was used for comparison to the control group, and the Wilcoxon-signed rank test was used for paired data.
When quantified by deducting the corresponding values for the isotype controls, the baseline (pre-NPWT) mean number of CD34+ cells was 40.3±44.0 cells per 1 × 104 MNCs in the study group and 66.5±56.8 cells in the control group (P=0.032). The number of CD34+ cells in the study group increased significantly to 83.5±72.4 cells per 1 × 104 MNCs (a 2.1-fold increase) during the NPWT period (P=0.001), and the mean number of CD34+/VEGFR2+ cells per 1 × 104 MNCs increased from 13.3±11.4 cells in the pre-NPWT period to 20.8±12.9 cells during NPWT (a 1.5-fold increase; P=0.031). The mean number of CD34+/CD133+ cells per 1 × 104 MNCs was also lower in the study group (3.33±2.9 cells) than in the control group (28.1±18.6 cells) (P=0.00). This value increased to 6.5±9.4 cells during NPWT; however, this difference was not statistically significant (P=0.145).
Numbers of EPC colonies increased after NPWT
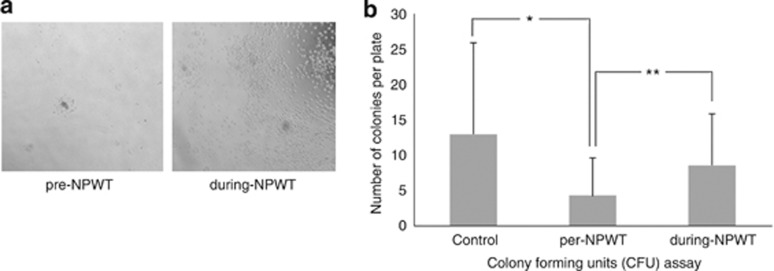
The CFUs were confirmed to exhibit EPC characteristics based on morphologic characteristics, as visualized using a light microscope and staining with DiI-Ac-LDL and FITC-lectin.
The number of CFUs at the baseline was variable between individuals, as was reported previously,21 and were scarce in diabetic foot infection patients (Study group 4.2±5.3 versus the Control group 13.0±13.3, P=0.016). For the most part, the CFU numbers increased from 4.2±5.3 (range, 0–15.8) per plate in the pre-NPWT period to 8.6±7.3 (range, 0.7–23) per plate during NPWT (P=0.006) (Figure 3).
Figure 3.
Colony forming unit assay to evaluate the functional capacity of circulating EPCs. (a) Illustrative photomicrograph of colony formation. (b) The numbers of CFUs per plate increased significantly during NPWT (during NPWT). Data are presented as the mean±s.d., *P<0.05 and **P<0.01. The Mann–Whitney test was used for comparison with the control group, and the Wilcoxon-signed rank test was used to compare paired data.
Plasma levels of EPC-mobilizing cytokines decreased during NPWT
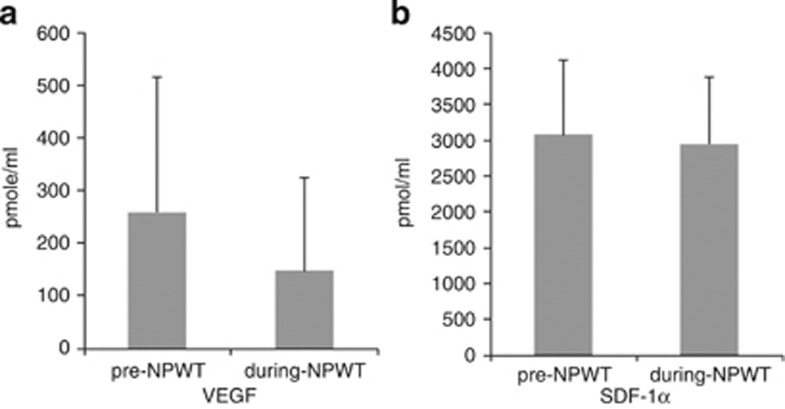
Our analysis of EPC-mobilizing cytokines showed decreases in plasma VEGF levels during NPWT, whereas the levels of C-reactive protein and SDF-1α remained essentially unchanged. The baseline serum level of C-reactive protein was 9.06±9.17 mg dl−1 in the pre-NPWT period, and this value decreased, but not significantly, to 5.75±6.11 mg dl−1 during NPWT (P=0.236). The baseline plasma level of VEGF was 259.8±256.5 pmol ml−1 during the pre-NPWT period, and this value decreased to 148.3±177.1 pmol ml−1 during NPWT (P=0.005). However, the mean plasma level of SDF-1α remained unchanged from 3071.4±1044.0 pmol ml−1 during the pre-NPWT period to 2947.0±938.9 pmol ml−1 during NPWT (P=0.959) (Figure 4).
Figure 4.
Plasma levels of EPC-mobilizing cytokines. (a) The plasma level of VEGF decreased significantly after NPWT. (b) The plasma level of SDF-1α was maintained regardless of NPWT. Data are presented as the mean±s.d., and *P<0.05 using the Wilcoxon-signed rank test.
Discussion
This study shows that the proportion of EPCs in the PB MNC population increases during NPWT. We hypothesized that EPC-mediated neovascularization might have a role as a bridging mechanism between the HIF/VEGF pathway and increased blood supply during NPWT and that postnatal vasculogenesis by bone marrow-derived EPCs could have a role in neovascularization at an injured site.
Despite a growing number of reports on EPC biology, the precise identification of EPCs remains problematic. Some investigators have used flow cytometry to estimate EPC counts,20, 23, 24 whereas others utilize CFUs.13, 15 The most widely used cell surface markers for EPC identification are CD34, VEGFR2 and CD133. CD34+ cell fractions have been reported to be enriched for EPCs and hematopoietic stem cells and to have a role in postnatal vasculogenesis.12, 25 In particular, CD34+/VEGFR2+ and/or CD34+/CD133+ cells are considered to represent an immature cell population with endothelial progenitor capacity. CFUs are defined as a central core of rounded cells that are surrounded by elongated and spindle-shaped cells, and CFU numbers are considered to represent the functional properties of EPCs better than EPC numbers.12, 25 Thus, we utilized both methods to evaluate EPC mobilization during NPWT.
In the present study, CD34+ and CD34+/VEGFR2+ cell fractions increased significantly during NPWT, and an increase in functional EPCs was confirmed using CFU assays. As we had shown in a previous article,20, 21 even after hazardous surgical procedures such as fractures and bone lengthening, an active inflammation process because of the index operation did not persist for more than 7 days after the procedure, and this finding was confirmed by an unchanged level in the C-reactive protein in the blood during NPWT. These findings suggest that NPWT-induced stimuli provoked systemic mobilizations of vascular-forming precursor cells—that is, EPCs. Mobilized CD34+ cells are thought to secret various cytokines and growth factors that induce intrinsic angiogenesis,18, 19, 26 and the proposed effects of EPCs might explain the mechanism of neovascularization during NPWT. However, further research is required to clarify this issue.
Changes in wound biochemistry and mechanical deformation alter the local environment through the process of mechanotransduction (that is, the conversion of a mechanical stimulus into chemical activity). In vitro, stretching increases human fibroblast growth and migration, and in the animal wound closure model, NPWT increased collagen organization and increased the expression of VEGF and fibroblast growth factor 2. The EPC mobilization that was induced during NPWT and observed during this study appears to have been caused by negative-pressure strain.
Plasma levels of EPC-mobilizing cytokines were decreased during NPWT. Previously reported stimuli for EPC mobilization include various pathophysiological conditions, growth factors and cytokines, and drugs. Furthermore, surgical intervention and musculoskeletal trauma are also known to promote transient EPC mobilization that cannot be differentiated from an inflammatory response induced by injury. Of the various EPC-mobilizing stimuli, ischemia is considered to be one of the strongest and to be mediated by EPC-mobilizing cytokines such as VEGF and SDF-1α. However, in the present study, the systemic level of VEGF was found to be decreased during NPWT, whereas the level of SDF-1α was maintained. Assessment of mobilizing and angiogenic factors from local tissue may help in clarifying the mechanism of EPC mobilization during NPWT, which was not possible in this study because of limits in interpreting the phenomenon. Although we could not measure the levels of VEGF and SDF-1α directly in the tissue where NPWT had been applied, other scientists have reported that the application of NPWT increased the level of VEGF in the local tissue.27 The relationship between the systemic and local levels of cytokines, diurnal variation and the effect of the mode of negative pressure should be controlled for further evaluation.
In this study, we did not show that patients who tended to exhibit rapid wound regeneration also possessed more EPC CFUs after NPWT. Further research is required to clarify whether and how mobilized EPCs contribute to neovascularization. It should also be noted that a limitation of this study was that only early phases of NPWT were analyzed; therefore, we could not confirm the effect of prolonged NPWT on EPC mobilization.
Conclusions
This study demonstrates the systemic mobilization of EPCs during NPWT, which could be the mechanism underlying increased vascular supply and granulation tissue formation. Thus, the vasculogenic potential of NPWT has a role in treating diabetic patients with foot infections and skin ulcers, especially when accompanied by peripheral arterial insufficiency.
Acknowledgments
This work was supported by the Seoul National University Hospital Research Fund (SNUH-03-2012-0430) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Education, Science and Technology (2010-0021475).
The authors declare no conflict of interest.
References
- Lavery LA, Ashry HR, vanHoutum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion at diabetes-related amputations in minorities. Diabetes care. 1996;19:48–52. doi: 10.2337/diacare.19.1.48. [DOI] [PubMed] [Google Scholar]
- Bader MS. Diabetic foot infection. Am Fam Phys. 2008;78:71–79. [PubMed] [Google Scholar]
- Bader M, Brooks A. Medical management of diabetic foot infections. Postgrad Med. 2012;124:102. doi: 10.3810/pgm.2012.03.2541. [DOI] [PubMed] [Google Scholar]
- Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers. Diabetes care. 2008;31:631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- Nain PS, Uppal SK, Garg R, Bajaj K, Garg S. Role of negative pressure wound therapy in healing of diabetic foot ulcers. J Surg Tech Case Rep. 2011;3:17–22. doi: 10.4103/2006-8808.78466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schintler M. Negative pressure therapy: theory and practice. Diabetes Metab Res Rev. 2012;28:72–77. doi: 10.1002/dmrr.2243. [DOI] [PubMed] [Google Scholar]
- Kirby M. Negative pressure wound therapy. Br J Diabet Vasc Dis. 2007;7:230–234. [Google Scholar]
- Rejzek A, Weyer F. Der Einsatz des VAC-Systems in der Therapie von Ulcus cruris und diabetischer Gangrän. Acta Chir Austriaca. 1998;150:12–13. [Google Scholar]
- Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117:121S. doi: 10.1097/01.prs.0000225450.12593.12. [DOI] [PubMed] [Google Scholar]
- Torbrand C, Ugander M, Engblom H, Arheden H, Ingemansson R, Malmsjö M. Wound contraction and macro-deformation during negative pressure therapy of sternotomy wounds. J Cardiothorac Surg. 2010;5:75. doi: 10.1186/1749-8090-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086. doi: 10.1097/01.prs.0000135330.51408.97. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke. 2008;39:1441–1447. doi: 10.1161/STROKEAHA.107.499236. [DOI] [PubMed] [Google Scholar]
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kuroda R, Mifune Y, Kawamoto A, Shoji T, Miwa M, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43:434–439. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Mifune Y, Kawamoto A, Kuroda R, Shoji T, Iwasaki H, et al. Fracture induced mobilization and incorporation of bone marrow-derived endothelial progenitor cells for bone healing. J Cell Physiol. 2008;215:234–242. doi: 10.1002/jcp.21309. [DOI] [PubMed] [Google Scholar]
- Lee DY, Cho TJ, Kim JA, Lee HR, Yoo WJ, Chung CY, et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008;42:932–941. doi: 10.1016/j.bone.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Lee DY, Cho TJ, Lee HR, Park MS, Yoo WJ, Chung CY, et al. Distraction osteogenesis induces endothelial progenitor cell mobilization without inflammatory response in man. Bone. 2010;46:673–679. doi: 10.1016/j.bone.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Mifune Y, Matsumoto T, Kawamoto A, Kuroda R, Shoji T, Iwasaki H, et al. Local delivery of granulocyte colony stimulating factor-mobilized CD34-positive progenitor cells using bioscaffold for modality of unhealing bone fracture. Stem Cells. 2008;26:1395–1405. doi: 10.1634/stemcells.2007-0820. [DOI] [PubMed] [Google Scholar]
- Labler L, Rancan M, Mica L, Harter L, Mihic-Probst D, Keel M. Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma. 2009;66:749–757. doi: 10.1097/TA.0b013e318171971a. [DOI] [PubMed] [Google Scholar]