Abstract
Protein tyrosine phosphatase 1B (PTP1B) inhibits insulin signaling and, when overexpressed, plays a role in insulin resistance (Ahmad et al. 1997). We identified, in the 3′ untranslated region of the PTP1B gene, a 1484insG variation that, in two different populations, is associated with several features of insulin resistance: among male individuals, higher values of the insulin resistance HOMAIR index (P=.006), serum triglycerides (P=.0002), and total/HDL cholesterol ratio (P=.025) and, among female individuals, higher blood pressure (P=.01). Similar data were also obtained in a family-based association study by use of sib pairs discordant for genotype (Gu et al. 2000). Subjects carrying the 1484insG variant showed also PTP1B mRNA overexpression in skeletal muscle (6,166 ± 1,879 copies/40 ng RNA vs. 2,983 ± 1,620; P<.01). Finally, PTP1B mRNA stability was significantly higher (P<.01) in human embryo kidney 293 cells transfected with 1484insG PTP1B, as compared with those transfected with wild-type PTP1B. Our data indicate that the 1484insG allele causes PTP1B overexpression and plays a role in insulin resistance. Therefore, individuals carrying the 1484insG variant might particularly benefit from PTP1B inhibitors, a promising new tool for treatment of insulin resistance (Kennedy and Ramachandran 2000).
The insulin resistance/metabolic syndrome—characterized by the variable coexistence of hyperinsulinemia, dislipidemia, obesity, and hypertension—is influenced by both environmental and genetic background, the latter being mostly unknown (Trischitta et al. 1997; Virkamaki et al. 1999). Protein tyrosine phosphatase 1B (PTP1B) is a major regulator of insulin sensitivity and body fat (Ahmad et al. 1995; Kenner et al. 1996; Ahmad et al. 1997; Elchebly et al. 1999; Goldstein et al. 2000; Klaman et al. 2000). In fact, PTP1B directly interacts with and dephosphorylates the activated insulin receptor (Seely et al. 1996; Bandyopaddhyay et al. 1997), thus inhibiting insulin signaling and action. In addition, type 2 diabetes and obesity have been linked to markers on human chromosome 20q13.1 (Lembertas et al. 1997; Lee et al. 1999; Klupa et al. 2000), which harbors PTP1B. Also, the mouse PTP1B region (i.e., the distal arm of chromosome 2, syntenic with human chromosome 20) is likely to harbor a gene for obesity (Lembertas et al. 1997). These data indicate that PTP1B is a candidate gene for insulin resistance/metabolic syndrome.
We searched for polymorphisms in both the regulatory and coding regions of the human PTP1B gene (Forsell et al. 2000). Table 1 shows the primer sets used for the screening by PCR and SSCP. Because of an alternative splicing in intron 9, two different 3′ UTRs are transcribed for PTP1B (Forsell et al. 2000). Both 3′ UTRs were screened. Samples carrying different electrophoretic patterns were automatically sequenced after cloning (at least five clones) in pCR II TOPO vector (Invitrogen).
Table 1.
Primers and PCR Conditions
| Exon | Primers | AnnealingTemperature(°C) | Amplimer Size(bp) |
| PR1 (−594/−431) | Forward: 5′-TGCAGCACCCAAGTGGAAATTC-3′ | 60 | 229 |
| Reverse: 5′-TCATGCACCTCCTTTCCTGCAG-3′ | |||
| PR2 (−510/−215) | Forward: 5′-TGATACTCCTGAGTTTCAT-3′ | 58 | 338 |
| Reverse: 5′-AGCACCGCGAGATATCTAATAGC-3′ | |||
| PR3 (−261/−55) | Forward: 5′-GTCGCCTAGGCAACAGGCGCG-3′ | 60 | 262 |
| Reverse: 5′-TAGCCGCTGCTCTTCTTCATG-3′ | |||
| PR4 (−68/+108) | Forward: 5′-GCAGGCGTGATGCGTAGTTC-3′ | 60 | 218 |
| Reverse: 5′-GACTTGTCGATCTGCTCGAA-3 | |||
| Exon 1 | Forward: 5′-CTGGCAGGCGTGATGCGTAG- 3′ | 68 | 300 |
| Reverse: 5′-GATGTTCAAGCGGCCTAAGCG-3′ | |||
| Exon 2 | Forward: 5′-GTCTTCCTCAGTGTCTGACGGTC-3′ | 68 | 192 |
| Reverse: 5′-GCCTGCAGCAAAGGAAGAGC-3′ | |||
| Exon 3 | Forward: 5′-TGTACACATTCAGCTTTTCC-3′ | 58 | 226 |
| Reverse: 5′-TGAGGTGTGGATAACAGC-3′ | |||
| Exon 4 | Forward: 5′-TCGTTAGCTGACTGCAGAAGG-3′ | 60 | 244 |
| Reverse: 5′-ATGGTAACTATATGGAGTGG-3′ | |||
| Exon 5 | Forward: 5′-TCATGAAGCTTGTGGGATGTGC-3′ | 60 | 316 |
| Reverse: 5′-TGACATTAGGTAATATCACC-3′ | |||
| Exon 6 | Forward: 5′-GAAGGTGACTCTGTGTGTAC-3′ | 64 | 349 |
| Reverse: 5′-TCACAGCAGAGCAGGTAGAGGAGC-3′ | |||
| Exon 7 | Forward: 5′-TGAGAATTGGACCTGGC-3′ | 62 | 283 |
| Reverse: 5′-TACAACTGACAGCCTCCTTC-3′ | |||
| Exon 8 | Forward: 5′-TGACAAACCAGCCGAAGTGAAC-3′ | 62 | 354 |
| Reverse: 5′-TCAGTACCAGCGTGTGTTTC-3′ | |||
| Exon 9 | Forward: 5′-GAGTACCCATCTCTGCCCTCTG-3′ | 62 | 324 |
| Reverse: 5′-CAGATGCACCACAGAACTGAATCC-3′ | |||
| Exon 10a | Forward: 5′-CATGAGGCGACAGCACTGC-3′ | 62 | 350 |
| Reverse: 5′-CTTCCATTCCCAGTACTACCTGA-3′ | |||
| 3′ UTR A | Forward: 5′-AGGACGGTTGTAAGCAGTTGTT-3′ | 62 | 329 |
| Reverse: 5′-GGAACCACAGCCAGTTTATGAT-3′ | |||
| 3′ UTR B | Forward: 5′-TCTCTGCTTACTAATGTGCCCC-3′ | 60 | 351 |
| Reverse: 5′-TCAAGAGTGTCGACTTGGA-3′ | |||
| 3′ UTR C | Forward: 5′-TCTGGACATGATTTAGGGAAGC-3′ | 62 | 320 |
| Reverse: 5′-TGCCGTGTTTTTCATGTTAAAA-3′ | |||
| 3′ UTR D | Forward: 5′-AAAGGGAACTGAAGACCTCCAC-3′ | 64 | 314 |
| Reverse: 5′-GGAGGTTAAACCAGTACGTCCA-3′ | |||
| 3′ UTR E | Forward: 5′-ATTCTGAGCTGGCTTGTTGTTT-3′ | 62 | 234 |
| Reverse: 5′-GGTTTATTCCATGGCCATTGTA-3′ | |||
| Intron 9 A | Forward: 5′-CTGGTCAACATGTGCGTGG-3′ | 62 | 267 |
| Reverse: 5′-CTTGGGACCAGAGGGCTC-3′ | |||
| Intron 9 B | Forward: 5′-TTAAGGATCGATGCACTGGG-3′ | 62 | 324 |
| Reverse: 5′-TTGGGATTCCTTCCCTGGG-3′ | |||
| Intron 9 C | Forward: 5′-CCTTAGGTGATGTAATCAGCC-3′ | 62 | 350 |
| Reverse: 5′-AGGCCTCGAGGACACCC-3′ | |||
| Intron 9 D | Forward: 5′-CCTGTGACAGCCATCTTGC-3′ | 62 | 327 |
| Reverse: 5′-CATCTGATGTACTCAGATGCC-3′ | |||
| Intron 9 E | Forward: 5′-ACTAGCCTCAGAGCTCTGG-3′ | 58 | 348 |
| Reverse: 5′-GTGGAGGTGGAGTGGAGG-3′ |
Proximal region of the 3′ UTR.
Several single-nucleotide polymorphisms (SNPs) were identified in 100 unrelated subjects from the Gargano area (on the east coast of central Italy) (table 2). Because of a low allele frequency (AF) (i.e., <2%) and/or the nature of sequence variations (i.e., either silent or intronic), only the SNP localized in the 3′ UTR (1484insG SNP, according to the published sequence [GenBank accession number M33689]) was considered for association studies with insulin resistance/metabolic syndrome. For this purpose, RFLP analysis was used, because a SacII restriction site was created by 1484insG.
Table 2.
SNPs in the PTP1B Gene
| Designation | Location | Type | RestrictionEnzyme | AF(%) |
| −105delA | 5′ UTR | Noncoding | … | 1 |
| A669G | Exon 6 | Synonymous | … | 3 |
| C981T | Exon 8 | Synonymous | … | 7 |
| C1207T | Exon 9 | Missensea | … | .9 |
| delT | Intron 3 | … | 1.1 | |
| A/C | Intron 5 | 50 | ||
| 1484insG | 3′ UTR | Noncoding | SacII | 7.7 |
| 1737insG | 3′ UTR | Noncoding | … | 1.9 |
| T2251C | 3′ UTR | Noncoding | … | 1 |
| A2261G | 3′ UTR | Noncoding | … | 1 |
Leu379Phe.
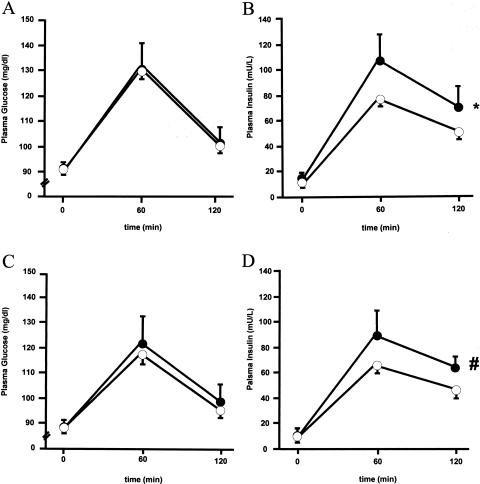
To minimize the inclusion of genetic determinants of β-cell failure, rather than insulin resistance, and to avoid the confounding effect of hyperglycemia on insulin-resistance–related abnormalities, we analyzed only nondiabetic subjects. First, 477 normoglycemic (fasting plasma glucose <126 mg/dl), unrelated white subjects from the Gargano area were studied. Also, 335 normoglycemic individuals from Sicily were analyzed, to replicate the association study. In all subjects, the following parameters were assessed: BMI; waist and hip circumference; blood pressure; and fasting glucose, insulin, and lipid profiles. Plasma glucose (mmol/l), serum insulin (pmol/l), and lipid profile (total serum cholesterol, HDL cholesterol, and serum triglycerides) were measured by use of commercially available enzymatic kits, as described elsewhere (De Cosmo et al. 1997). The insulin-resistance index HOMAIR (homeostasis model assessment) was calculated as fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/l)/22.5 (Bonora et al. 2000). In individuals from Sicily, glucose and insulin levels were also measured before, 60 min after, and 120 min after a 75-g oral-glucose load was given. Values are given as mean ± SEM. Mean values of unrelated individuals from the two genotype groups were compared by Student t-test or Mann-Whitney U test, as appropriate. Informed consent was obtained from participants before they entered the study, which was approved by the local research ethics committee. Among the 477 unrelated individuals from the Gargano region (165 male subjects, aged 37.9 ± 0.9 years, and 312 female subjects, aged 38.2 ± 0.6 years; 1484insG AF 7.7%, in Hardy-Weinberg equilibrium), male subjects carrying the 1484insG allele (n=15) showed, compared with wild-type individuals, higher BMI values (29.2 ± 1.1 kg/m2 vs. 25.7 ± 0.3; P=.001), fasting plasma insulin (10.0 ± 1.1 mU/L vs. 7.8 ± 0.3; P=.048), serum triglycerides (168 ± 39 mg/dl vs. 118 ± 6; P=.034), total/HDL cholesterol ratio (5.2 ± 0.4 vs. 4.4 ± 0.1; P=.035) and the insulin-resistance HOMAIR index (Bonora et al. 2000) (2.4 ± 0.3 vs. 1.7 ± 0.1; P=.025). Female subjects carrying the 1484insG allele (n=56, including 3 homozygous subjects) showed higher values of systolic (114 ± 1.6 mm Hg vs. 110 ± 0.7; P=.024) and diastolic (75 ± 0.9 mm Hg vs. 73 ± 0.5; P=.038) blood pressure but not of other insulin-resistance–related parameters. As mentioned above, 335 nondiabetic Sicilians (170 male subjects, aged 38.5 ± 0.9 years, and 165 female subjects, aged 35.2 ± 0.9 years; 1484insG AF 5.2%) were studied, to replicate the data in a population that, although geographically close to the first population, is known to be of different ethnicity (Piazza et al. 1988). This would minimize the risk of false-positive results due to “population stratification” (Altshuler et al. 1998; “Freely Associating” 1999). Male subjects carrying the 1484insG allele (n=18, including 1 homozygous subject) showed higher levels of fasting plasma insulin (14.6 ± 3.1 μU/L vs. 10.6 ± 0.6; P=.041) and serum triglycerides (171 ± 19 mg/dl vs. 118±5; P=.001) as compared to individuals carrying the wild-type genotype. No difference between the two groups was observed in BMI, HOMAIR index, or total/HDL cholesterol ratio. Also, no difference in any of the above-mentioned variables was observed between female subjects carrying the 1484insG allele (n=20) and those not carrying that allele. In this second population, glucose and insulin levels during oral-glucose–tolerance test (OGTT) were also available. Although glucose levels were similar between the two genotype groups (fig. 1A and 1C), insulin levels during OGTT were higher both in male subjects (P=.005, by two-way analysis of variance [ANOVA]) and, to a lesser extent (P=.024), in female subjects carrying the 1484insG allele, as compared with wild-type individuals (fig. 1B and 1D) (i.e., compensatory hyperinsulinemia, a typical feature of insulin resistance in normoglycemic individuals). Then the two populations were pooled and were examined together (table 3). Male subjects carrying the 1484insG allele (n=33 [10%]) had higher values of insulin resistance HOMAIR index (P=.006), total/HDL cholesterol ratio (P=.025), and serum triglycerides (P=.0002) and had a 3.5-fold (95% CI 1.64–7.47) higher (P=.001) risk to show a cluster of insulin-resistance–related metabolic abnormalities (i.e., values in two or three of the three above-mentioned parameters were in the highest quartile of the entire cohort). Female subjects carrying the 1484insG allele (n=76 [16%]) had higher systolic (P=.03) and diastolic (P=.007) blood pressure, the latter parameter remaining significantly (P=.01) different also when adjusted (by analysis of covariance) for the slightly different observed age (table 3). The proportion of individuals carrying the 1484insG allele was higher (P=.02, by χ2 test) in female subjects (16%) than in male subjects (10%) (table 3). This may be the consequence of the apparently stronger association between the 1484insG allele and insulin resistance observed in male subjects than is observed in female subjects. One could, in fact, speculate that male 1484insG carriers are more likely to have been removed from the cohort of normoglycemic subjects we recruited, because of a more rapid progression to type 2 diabetes and/or early mortality for cardiovascular disease—both events being possible outcomes of insulin resistance/metabolic syndrome. To further minimize the risk of population-stratification bias, sib pairs concordant for sex and discordant for genotype from the Gargano region were also studied. Of 181 sib pairs concordant for sex, 13 were discordant for the PTP1B genotype. The differences in continuous variables between the siblings were estimated by use of a permutation test for paired replicates, as described elsewhere (Gu et al. 2000). The permutation test does not make any assumptions about the normality, the homogeneity of the variance, or the precise form of the underlying distribution. In the permutation test for 13 pairs, there are 213 equally likely outcomes for each variable, under the assumption of no difference between the paired siblings. Because of computational limitations, the two-tailed P values were estimated by use of a very large (107) random sample from all possible permutations. If the observed sum of differences (OSD) entered the 5% region of rejection, the differences between pairs was considered significant. The differences in phenotypic values were computed as the value in the sibling with the 1484insG variant minus the value in the sibling with the wild-type genotype. Sibs carrying the 1484insG allele showed higher BMI, total/HDL cholesterol ratio, triglycerides, and diastolic blood pressure (table 4). All together, these data show that the 1484insG variant of the PTP1B gene 3′ UTR associates with several features of insulin resistance/metabolic syndrome. This association seems to be stronger among male subjects than among female subjects. This is not surprising, because a sex-specific effect of PTP1B (Klaman et al. 2000) and other insulin-resistance genes (Bruning et al. 2000) has been reported in animal models. In several instances, the 3′ UTRs may regulate gene expression through the modulation of mRNA stability (Day and Tuite 1998; Xia et al. 1998; Frittitta et al. 2001). Accordingly, PTP1B mRNA levels were measured in skeletal-muscle specimens by competitive PCR, as described elsewhere (Frittitta et al. 2000). For this purpose, a competitor was created. A PTP1B cDNA portion containing nt 662–1251, according to the published sequence (GenBank accession number M33689), was amplified from the pAD.CMVPTP1B plasmid. An internal EarI fragment (nt 931–1073) was removed and the deleted cDNA, cloned in pCR II TOPO vector (Invitrogen), was used as the competitor. Constant amounts of PTP1B reverse-transcription first-strand products were coamplified with increasing copy-number amounts of competitor, and the equivalence point was determined after PCR and electrophoretic analysis.
Figure 1.
Glucose (A and C) and insulin (B and D) plasma levels before (time 0), 60 min after, and 120 min after a 75-g oral-glucose load was given to 170 male subjects (A and B) and 165 female subjects (C and D) from Sicily. Blackened circles indicate subjects carrying the 1484insG. Unblackened circles indicate subjects not carrying the 1484insG. Data are mean ± SEM. An asterisk (*) denotes a P value <.01, by two-way ANOVA, versus subjects not carrying 1484insG. A pound sign (#) denotes a P value <.05, by two-way ANOVA, versus subjects not carrying 1484insG.
Table 3.
Clinical Features of Subjects from the Two Different Populations Pooled Together[Note]
|
Mean ± SEM Values for Subjects |
||||
| Male |
Female |
|||
| Feature | Wild Type(n=302 [90%]) | 1484insG(n=33 [10%]) | Wild Type(n=401 [84%]) | 1484insG(n=76 [16%]) |
| Age (years) | 38.2 ± .7 | 38.1 ± 1.8 | 36.7 ± .6 | 39.8 ± 1.3a |
| BMI (kg/m2) | 28.2 ± .4 | 29.4 ± 1.3 | 26.5 ± .3 | 25.7 ± .7 |
| Fasting plasma glucose (mg/dl) | 91.6 ± .6 | 93.4 ± 1.3 | 88.7 ± .5 | 88.9 ± .9 |
| Fasting plasma insulin (mU/l) | 9.2 ± .3 | 12.5 ± 1.8b | 8.9 ± .3 | 8.1 ± .6 |
| HOMAIR | 2.1 ± .1 | 3.0 ± .5b | 2.0 ± .1 | 1.8 ± .1 |
| Fasting serum cholesterol:fasting serum HDL cholesterol ratio | 4.6 ± .1 | 5.2 ± .3a | 3.7 ± .1 | 3.7 ± .1 |
| Fasting serum triglycerides (mg/dl) | 118.0 ± 4.0 | 169.8 ± 20.3c | 83.7 ± 2.6 | 87.2 ± 5.1 |
| Systolic blood pressure (mm Hg) | 119.1 ± .7 | 117.6 ± 1.9 | 111.1 ± .6 | 114.2 ± 1.5a |
| Diastolic blood pressure (mm Hg) | 78.7 ± .5 | 76.7 ± 1.1 | 73.0 ± .4 | 75.7 ± .8b |
Note.— All differences—except fasting serum cholesterol/fasting serum HDL cholesterol, in male subjects, and systolic blood pressure, in female subjects—remained significant after correction for multiple comparisons.
P<.05 versus wild type.
P<.01 versus wild type.
P<.001 versus wild type.
Table 4.
Clinical Features of 13 Sib Pairs Discordant for the PTP1B Genotype
|
Mean ± SEM Valuesfor Sibs |
||||
| Feature | Wild Type | 1484insG | OSD | Pa |
| Age (years) | 35.7 ± 2.6 | 36.1 ± 2.9 | 22.0 | NS |
| BMI (kg/m2) | 23.7 ± .88 | 26.3 ± 1.14 | 34.2 | .02 |
| Fasting plasma glucose (mg/dl) | 88.2 ± 1.16 | 88.2 ± 1.9 | .50 | NS |
| Fasting plasma insulin (mU/L) | 7.5 ± 1.0 | 7.8 ± .7 | 4.45 | NS |
| HOMAIR | 1.63 ± .2 | 1.72 ± .2 | 1.07 | NS |
| Fasting serum cholesterol:fasting serum HDL cholesterol ratio | 3.5 ± .3 | 4.35 ± .4 | 11.3 | .02 |
| Fasting serum triglycerides (mg/dl) | 92.4 ± 14.5 | 116.6 ± 16.6 | 315.0 | .03 |
| Systolic blood pressure (mm Hg) | 109.6 ± 4.2 | 113.4 ± 4.5 | 50.0 | NS |
| Diastolic blood pressure (mm Hg) | 73.3 ± 2.5 | 77.1 ± 2.8 | 50.0 | .05 |
NS = not significant.
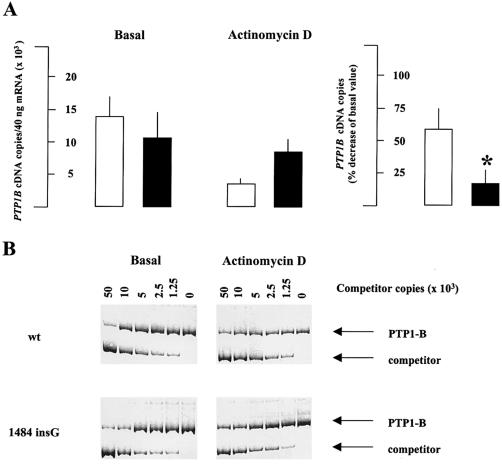
To assess the reproducibility of competitive PCR, samples were analyzed in triplicate, with a mean coefficient of variation of 15%. PTP1B mRNA levels were higher in five muscle samples from 1484insG carriers than in 11 age- and sex-matched wild-type individuals (6,166 ± 1,879 copies/40 ng RNA vs. 2983 ± 1620; P<.01).
To investigate whether the 1484insG variation may be responsible for changes in PTP1B mRNA stability, human embryo kidney 293 cells were transiently transfected (Chen and Okayama 1987) with either 1484insG or wild-type cDNA. Specific PTP1B mRNA level (by competitive PCR) before and after 40 h of 5 μg/ml actinomycin D pre-exposure (i.e., inhibition of transcription) was then measured. The decrease of mRNA level after transcription inhibition was significantly (P<.01) lower for 1484insG PTP1B transfected cells as compared to wild-type PTP1B transfected cells (fig. 2). This indicates that the G insertion at position 1484 stabilizes PTP1B mRNA. The 3′ UTR sequence has an essential role for the regulation of mRNA stability, and variants in this region have been associated with insulin resistance (Xia et al. 1998; Maegawa et al. 1999). The 3′ UTR may regulate mRNA stability through the binding with specific proteins, which occurs mostly but not exclusively at AU-rich regions (Conne et al. 2000; Day and Tuite 1998). The 3′ UTR of the PTP1B gene does not contain adenylate/uridylate–rich elements. Therefore, the 1484insG variation is likely to play a role in PTP1B mRNA stability through the modulation of protein binding to not-yet-identified 3′ UTR elements.
Figure 2.
A, left, specific mRNA content (by competitive PCR before and after transcription inhibition for 40 h with 5 μg/ml actinomycin D) in human embryo kidney 293 cells transfected with either wild-type (white columns) or 1484insG (black columns) cDNA. A, right, data from the left panel are recalculated as % decrease after treatment with actinomycin D. Data are mean ± SEM of the results of three independent experiments. B, Representative competitive PCR for both wild-type (wt) and 1484insG transfected cells.
In conclusion, the 1484insG variation increases PTP1B mRNA stability and associates with several features of insulin resistance/metabolic syndrome. This association has been validated (Altshuler et al. 1998; “Freely Associating” 1999) by replication of data in unrelated individuals of different ethnicity and in a family-based study.
Screening for the 1484insG variation may, therefore, identify those subjects in whom PTP1B overexpression can be recognized as a molecular cause of insulin resistance. These individuals might particularly benefit from PTP1B inhibitors, a promising new tool for treatment of insulin resistance (Kennedy and Ramachandran 2000).
Acknowledgments
The pAD.CMVPTP1B plasmid was kindly provided by Dr. B. J. Goldstein (Jefferson University, Philadelphia). This research was supported by Téléthon Italia grant E1239 and by the Italian Ministry of Health (Ricerca Finalizzata e Finalizzata Strategica 1999 and Ricerca Corrente 2000).
References
- Ahmad F, Li PM, Meyerovitch J, Goldstein BJ (1995) Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B in negative regulation of the insulin action pathway. J Biol Chem 270:20503–20508 [DOI] [PubMed] [Google Scholar]
- Ahmad F, Azevedo JL, Cortright R Jr, Dohm JL, Goldstein BJ (1997) Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J Clin Invest 100:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler D, Kruglyak L, Lander E (1998) Genetic polymorphisms and disease. N Engl J Med 338:1626 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D, Kusari A, Kenner AK, Liu F, Chernoff J, Gustafson TA, Kusari J (1997) Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine phosphorylated in the presence of insulin. J Biol Chem 272:1639–1645 [DOI] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 23:57–63 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillett J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conne B, Stutz A, Vassalli JD (2000) The 3′ untranslated region of messenger RNA: a molecular “hotspot” for pathology? Nat Med 6:637–641 [DOI] [PubMed] [Google Scholar]
- Day DA, Tuite MF (1998) Post-transcriptional gene regulatory mechanisms in eukaryotes: an overview. J Endocrinol 157:361–371 [DOI] [PubMed] [Google Scholar]
- De Cosmo S, Bacci S, Piras GP, Cignarelli M, Piacentino G, Margaglione M, Colaizzo D, Di Minno G, Giorgino R, Liuzzi A, Viberti GC (1997) High prevalence of risk factors for cardiovascular disease in parents of IDDM patients with albuminuria. Diabetologia 40:1191–1196 [DOI] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chang CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548 [DOI] [PubMed] [Google Scholar]
- Forsell PKAL, Boie Y, Montalibet J, Collins S, Kennedy BP (2000) Genomic characterization of the human and mouse protein tyrosine phosphatase-1B genes. Gene 260:145–153 [DOI] [PubMed] [Google Scholar]
- Freely associating (1999) Nat Genet 22:1–2 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Sbraccia P, Costanzo BV, Tassi V, D’Adamo M, Spampinato D, Ercolino T, Purrello F, Tamburano G, Vigneri R, Trischitta V (2000) High insulin levels do not influence PC-1 gene expression and protein content in human muscle tissue and hepatoma cells. Diabetes Metab Res Rev 16:26–32 [DOI] [PubMed] [Google Scholar]
- Frittitta L, Erccolino T, Bozzali M, Argiolas A, Graci S, Santagati MG, Spampinato D, Di Paola R, Cisternino C, Tassi V, Vigneri R, Pizzuti A, Trischitta V (2001) A cluster of 3 single nucleotide polymorphisms in the 3′-untranslated region of human glycoprotein PC-1 gene stabilizes PC-1 mRNA and associates with increased PC-1 protein content and insulin resistance related abnormalities. Diabetes 50:1952–1955 [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M (2000) Tyrosine dephosphorylation and deactivation of insulin receptor substrate–1 by protein–tyrosine phosphatase 1B. J Biol Chem 275:4283–4289 [DOI] [PubMed] [Google Scholar]
- Gu HF, Almgren P, Lindholm E, Frittitta L, Pizzuti A, Trischitta V, Groop LC (2000) Association between the human glycoprotein PC-1 gene and elevated glucose and insulin levels in paired-sibling analysis. Diabetes 49:1601–1603 [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Ramachandran C (2000) Protein tyrosine phosphatase-1B in diabetes. Biochem Pharmacol 60:877–833 [DOI] [PubMed] [Google Scholar]
- Kenner KA, Anyanwu E, Olefsky JM, Usari J (1996) Protein tyrosine phosphatase 1B is a negative regulator of insulin and insulin-like growth factor-I stimulated signaling. J Biol Chem 271:19810–19816 [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JN, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB (2000) Increased energy expenditure decreased adiposity and tissue-specific insulin sensitivity in protein tyrosine phosphatase 1B- deficient mice. Mol Cell Biol 20:5479–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupa T, Malecki MT, Pezzolesi M, Ji L, Curtis S, Langefeld CD, Rich SS, Warren JH, Krolewski AS (2000) Further evidence for a susceptibility locus for type 2 diabetes on chromosome 20q131-q132. Diabetes 49:2212–2216 [DOI] [PubMed] [Google Scholar]
- Lee JH, Reed DR, Li WD, Xu W, Joo EJ, Kilker RL, Nanthakumar E, North M, Sakul H, Bell C, Price RA (1999) Genome scan for human obesity and linkage to markers in 20q13. Am J Hum Genet 64:196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembertas AV, Perusse L, Changon YC, Fisler JS, Warden CH, Purcell-Huynh DA, Dionne FT, Gaghon J, Nadeau A, Lusis AJ, Bouchard C (1997) Identification of an obesity quantitative trait locus on mouse chromosome 2 and evidence of linkage to body fat and insulin on the human homologous region 20q. J Clin Invest 100:1240–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa H, Shi K, Hidaka H, Iwai N, Nishio Y, Egawa K, Kojima H, Haneda M, Yasuda H, Nakamura Y, Kinoshita M, Kikkawa R, Kashiwagi A (1999) The 3′-untranslated region polymorphism of the gene for skeletal muscle-specific glycogen-targeting subunit of protein phosphatase 1 in type 2 diabetic Japanese population. Diabetes 48:1469–1472 [DOI] [PubMed] [Google Scholar]
- Piazza A, Cappello N, Olivetti E, Rendine SA (1988) Genetic history of Italy. Ann Hum Genet 52:203–213 [DOI] [PubMed] [Google Scholar]
- Seely BL, Staubs PA, Reichart DR, Bernhanu P, Milarski KL, Saltiel AR, Kusari J Olefsky JM (1996) Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 45:1379–1385 [DOI] [PubMed] [Google Scholar]
- Trischitta V, Frittitta L, Vigneri R (1997) Early molecular defects in human insulin resistance: studies in healthy subjects with low insulin sensitivity. Diabetes Metab Rev 13:147–162 [DOI] [PubMed] [Google Scholar]
- Virkamaki A, Ueki K, Kahn CR (1999) Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest 103:931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JB, Sherer SW, Cohen PT, Majer M, Xi T, Norman RA, Knowler WC, Bogardus C, Prochazka M (1998) A common variant in PPP1R3 associated with insulin resistance and type 2 diabetes. Diabetes 47:1519–1524 [DOI] [PubMed] [Google Scholar]