Abstract
Background
Verticillium wilt, caused by the fungal pathogen Verticillium dahliae, is the most severe disease in cotton (Gossypium spp.), causing great lint losses worldwide. Disease management could be achieved in the field if genetically improved, resistant plants were used. However, the interaction between V. dahliae and cotton is a complicated process, and its molecular mechanism remains obscure. To understand better the defense response to this pathogen as a means for obtaining more tolerant cultivars, we monitored the transcriptome profiles of roots from resistant plants of G. barbadense cv. Pima90-53 that were challenged with V. dahliae.
Results
In all, 46,192 high-quality expressed sequence tags (ESTs) were generated from a full-length cDNA library of G. barbadense. They were clustered and assembled into 23126 unigenes that comprised 2661 contigs and 20465 singletons. Those unigenes were assigned Gene Ontology terms and mapped to 289 KEGG pathways. A total of 3027 unigenes were found to be homologous to known defense-related genes in other plants. They were assigned to the functional classification of plant–pathogen interactions, including disease defenses and signal transduction. The branch of "SA→NPR1→TGA→PR-1→Disease resistance" was first discovered in the interaction of cotton–V. dahliae, indicating that this wilt process includes both biotrophic and necrotrophic stages. In all, 4936 genes coding for putative transcription factors (TF) were identified in our library. The most abundant TF family was the NAC group (527), followed by G2-like (440), MYB (372), BHLH (331), bZIP (271) ERF, C3H, and WRKY. We also analyzed the expression of genes involved in pathogen-associated molecular pattern (PAMP) recognition, the activation of effector-triggered immunity, TFs, and hormone biosynthesis, as well as genes that are pathogenesis-related, or have roles in signaling/regulatory functions and cell wall modification. Their differential expression patterns were compared among mock-/inoculated- and resistant/susceptible cotton. Our results suggest that the cotton defense response has significant transcriptional complexity and that large accumulations of defense-related transcripts may contribute to V. dahliae resistance in cotton. Therefore, these data provide a resource for cotton improvement through molecular breeding approaches.
Conclusions
This study generated a substantial amount of cotton transcript sequences that are related to defense responses against V. dahliae. These genomics resources and knowledge of important related genes contribute to our understanding of host–pathogen interactions and the defense mechanisms utilized by G. barbadense, a non-model plant system. These tools can be applied in establishing a modern breeding program that uses marker-assisted selections and oligonucleotide arrays to identify candidate genes that can be linked to valuable agronomic traits in cotton, including disease resistance.
Background
Cotton (Gossypium spp.) is widely cultivated because of its economically valuable fibers and oil seeds. During a plant’s life cycle, it is continuously threatened by Verticillium wilt, one of the most destructive diseases. The fungal pathogen, Verticillium dahliae, is extremely persistent in the soil and has a broad host range (Klosterman et al., [1]). It infects cotton roots and colonizes and occludes the xylem vessels, resulting in leaf curl, necrosis, defoliation, vascular tissue wilt, and discoloration (Additional file 1; Figure S1; Sink and Grey, [2]). A severe outbreak of this disease can reduce fiber quality and cause significant losses in yield. Between 2009 and 2010, 5.0 to 6.6 million acres, or more than 50% of the area in which cotton is grown in China, was affected (National Cotton Council of America–Disease Database).
No fungicides or other chemical means have proven effective for combating this disease. Although cultural practices such as appropriate seeding, irrigation, fertilization, and crop rotation can influence the development of this disease, none can efficiently control Verticillium wilt (Kamal, [3]). Genetic resistance is considered the most effective and sustainable management option, but has received little attention. Many crosses within certain lines of G. hirsutum can be readily made and multiple generations can be easily produced each year (Wang et al., [4]). However, a lack of genetics resources within such conventional breeding programs has meant that attempts to improve tolerance to Verticillium wilt have not been successful with this particular species, which accounts for >90% of the total acreage used for cotton production worldwide (Cai et al., [5]). However, plants of G. barbadense display high levels of wilt resistance, and could provide a good opportunity for genetically enhancing G. hirsutum. Despite this possibility, crosses between those species have historically resulted in abnormal separations, linkage drag, or hybrid dysgenesis. In addition, incorporating such materials from G. barbadense via molecular breeding techniques has been limited by a paucity of resistance genes and a lack of information about potential molecular markers. Therefore, the ability to explore functional genes or markers would be beneficial to researchers who are trying to introduce varieties with excellent wilt resistance.
Progress has been made in investigations of defense mechanisms by cotton against V. dahliae infection. For example, the cotton terpenoid pathway has been elucidated as an important contributor to the pathogen response by these plants (Tan et al., [6]; Luo et al., [7]; Xu et al., [8]). The phenylpropanoid pathway also has a critical protective role (Smit and Dubery, [9]; Pomar et al., [10]; Gayoso et al., [11]; Xu et al., [12]). Defense-responsive genes have been identified for the 14-3-3-like protein, PR10, anti-apoptosis (p35), HR-induced Hpa1Xoo, a thaumatin-like protein, major latex protein (MLP), and GbVe (Hill et al., [13]; Zhou et al., [14]; Wang et al., [15]; Chen and Dai, [16]; Munis et al., [17]; Tian et al., [18]; Zhang et al., [19]a). In addition, the transcriptional activator ethylene-responsive element binding factor gene (Qin et al., [20]), the oxygen transporter non-symbiotic hemoglobin gene (Qu et al., [21]), NDR1, and MAP kinase kinase (Gao et al., [22]) are associated with resistance to Verticillium. All of these findings indicate that various defense pathways are activated during the complicated response to V. dahliae by cotton. Nevertheless, although some defense-related genes have been identified from G. hirsutum, their molecular mechanisms remain obscure.
Several transcriptome analyses have been reported, such as from a drought-related cDNA library (Zhang et al., [23]) and an SSH library from cotton plants inoculated by V. dahliae (Zhang et al., [24]b). However, few studies of transcription have been described for G. barbadense. Although the D genome of cotton has recently been sequenced (Wang et al., [25]), its complexity means that publicly available data sets are of limited use for future research, including examinations of its entire transcriptome at specific developmental states or under certain stress conditions, as well as the elucidation of molecular mechanisms for the cotton defense response to V. dahliae. Such efforts would provide information on gene expression and regulation, and the amino acid content of proteins. Therefore, a thorough transcriptome analysis is essential for interpreting the functional elements of the genome and revealing the molecular constituents of cells and tissues. Extensive transcriptomic data would aid in our discovery of genes related to Verticillium wilt resistance, and would enable us to construct high density microarrays for further characterization of gene expression profiles during the cotton–V. dahliae interaction.
The technology used with full-length cDNA clones involves capturing mRNA via the 5’-end and stabilizing the full transcript when ligating into an appropriate vector and during reverse-transcription from the poly A tail (Carninci et al., [26]; Seki and Shinozaki, [27]). Therefore, full-length cDNA libraries can represent entire transcription units rather than partial gene sequences, making them extremely useful for transcriptome analysis and comparative genomics work (Seki et al., [28]). Such libraries also contain the transcriptional start site for most genes, and EST sequencing of the 5’-ends can reveal the untranslated region, methionine-encoding ATG codon, and translational start signal. Some researchers have used cDNA clones to construct microarrays for characterizing the binding of transcription factors (TFs) to promoter elements within the 5’-UTRs (Seki et al., [29]). Full-length cDNA clones also have a role in characterizing genetic structures in different species (Sakurai et al., [30]). Finally, as with other kinds of ESTs, results from the sequencing of such cDNA clones can be used for developing many types of genetic markers, including SSR (simple sequence repeat) and SNP (single nucleotide polymorphism) markers (Kofler et al., [31]; Galeano et al., [32]).
Because not enough genes/ESTs for G. barbadense are available in the public databases, and because the 'Pima90-53’ cultivar of this species shows significant advantages over G. hirsutum in both Verticillium wilt resistance and fiber quality, we decided to use full-length cDNA library construction and sequencing analysis to conduct an initial global analysis of the defense transcriptome dynamics in G. barbadense. Our study objectives were to create full-length cDNA libraries that would be useful for gene discovery in cotton, as well as for genome annotation and global investigations of the pathogen–plant interaction. We also proposed that the results would provide novel insights into the molecular mechanisms involved in defense processes by cotton. In doing so, we could obtain valuable resources for the development of molecular markers to study Upland cotton with recently published sequencing data for the D genome.
Methods
Fungal strain and inoculum preparation
The highly aggressive defoliating Verticillium dahliae fungal strain T5, isolated from an infected Upland cotton variety, was used for inoculation. To produce conidia, we took this strain from potato dextrose agar (PDA) plates and sub-cultured it onto Czapek’s medium (2 g of NaNO3, 1 g of K2HPO4, 1 g of MgSO4.7H2O, 1 g of KCl, 2 mg of FeSO4.7H2O, and 30 g L-1 sucrose) and incubated it at 25°C for 3 to 5 d. The resultant fungal cultures were filtered through sterile gauze to retain the mycelia. For root-dip inoculations, a conidial suspension (107 mL-1) was prepared.
Plant cultivation and inoculation
Seeds of cotton (Gossypium barbadense cv. Pima90–53) were surface-sterilized, then incubated on Petri dishes between sheets of moist filter paper for 48 h at 28°C. The new seedlings were transferred to tissue culture pots containing an MS medium (pH 6.0) supplemented with 10 g L-1 sucrose and 2 g L-1 Phytagel (Sangon Biotech Co., Ltd., Shanghai, China). They were then incubated for another 5 d at 28/25°C (day/night). Afterward, the seedlings were removed from the pots and inoculated via root-dipping for 30 s into either sterile water (for mock inoculation) or a conidial suspension of V. dahliae. They were transferred to fresh pots containing the same MS medium and incubated in a growth room at 25°C. The mock-inoculated and fungal-inoculated pots were placed in pairs, adjacent to each other, to minimize the effects of different microenvironments. Roots were harvested at 0, 1, 2, 4, 6, 8, 12, 24, 36, 48, 72, 96, and 120 hours post-inoculation (hpi). The treated tissues were quickly frozen in liquid nitrogen and stored at –80°C. As much mycelia as possible was removed from the roots.
Sections (approximately 0.5 mm) were cut from the surface-sterilized roots and stems of 10 seedlings per sampling time point. They were transferred to plates containing 25% PDA and incubated at 25°C for at least 2 d. They were then examined for the presence of mycelial growth from the vascular tissue; the percentage of sections showing such growth was recorded for each sample.
Extraction and purification of total RNA
Frozen tissues were ground mechanically to a fine powder in liquid nitrogen. Total RNA was isolated with TRIzol® reagent (Invitrogen), according to the manufacturer’s guidelines. Remaining traces of genomic DNA were removed by DNase after a purification step with the CleanUp protocol of the RNeasy Plant Mini kit (Qiagen). Pellets of total RNA were re-suspended in RNAse-free water and quantified spectrophotometrically. Quality was determined via denaturing agarose gels (1.5%) that contained formaldehyde and were stained with ethidium bromide. Equal amounts of total RNA from each sampling event were pooled. The mRNA was further isolated with the PolyATract mRNA Isolation System (Promega, Madison, WI, USA).
Library construction and EST sequencing
The cDNA was synthesized from 100 ng of mRNA with the Clontech Creator SMART cDNA synthesis system. It was recovered in autonomously replicating pSMART2IFD, using an in vivo fusion protocol provided by the manufacturer. Plasmid DNA was excised in Escherichia coli strain DH10B. Clones were picked randomly and transferred into 384-well plates. Single-pass sequencing from the 5’-end was carried out using a universal primer and BigDye Terminator, on an ABI 3730 automatic DNA sequencer (Sangon).
Pre-processing and assembly of ESTs
All sequences were processed to remove low-quality regions and adaptor sequences, using the LUCY programs (Chou and Holmes, [33]). This step was followed by SeqClean to shorten the Poly-A/T (http://compbio.dfci.harvard.edu/tgi/software). To remove possible contamination, we screened the resulting sequences against the National Center for Biotechnology Information (NCBI) UniVec database, E. coli genome sequences, and Gossypium on ribosomal RNA sequences. EST sequences longer than 100 bp after trimming were clustered and assembled into contigs and singletons (unisequences) via EST Clustering, which was designed based on the MegaBlast and CAP3 programs (Huang and Madan, [34]; Zhang et al., [35]).
Gene annotation
After clustering and assembly, the NCBI BLAST program version 2.2.6 (Altschul et al., [36]) was used to identify similarities between the ESTs and sequences deposited in public databases. We compared them against the GenBank non-redundant protein (Nr) and UniProt (TrEMBL and SwissProt) databases, using a cutoff E-value of 1e-5. The unigene sequences were also translated into proteins via ESTScan (Iseli et al., [37]). Translated protein sequences were then compared with information from pfam domain databases. Our Gene Ontology (GO) annotation (Harris et al., [38]) was performed with BLAST2GO (Conesa et al., [39]; Götz et al., [40]), based on sequence similarity in the UniProt databases and domains in the pfam database. WEGO (Ye et al., [41]) was used for GO functional classification of all unigenes and to plot the distribution of those same gene functions. The unigene sequences were also aligned to the Clusters of Orthologous Groups (COG) database to predict and classify functions. Pathway assignments were made based on information from the KEGG database (Kanehisa and Goto, [42]).
Quantitative real-time PCR
For selected genes, differential gene expression was verified by Q-PCR. The roots from triplicate samples (n=3 seedlings) were collected at each time point. Gene expression analysis was performed with three biological replicates and three technical replicates. First-strand cDNA was generated from 1 μg of total RNA isolated from the roots of both mock- and Verticillium-inoculated G. barbadense 'Pima90-53’ (resistant) and G. hirsutum 'Han208’ (susceptible), using the Superscript First-strand Synthesis System (Invitrogen). Primers for Q-PCR were designed according to the parameters of an optimum GC content of 50%, Tm>55 to 65°C, length 18 to 30 nucleotides, and an expected amplified fragment size of 80 to 200 bp. For qPCR, 20-μL samples were run in three technical replicates on a LightCycler® Real Time PCR System (Roche Germany), using 2 μL of first-strand cDNA and SYBR Green PCR Master Mix (Takara). Amplification conditions included the following: one cycle at 94°C for 15 s; then 40 cycles at 94°C for 10 s, 59°C for 10 s, and 72°C for 15 s. Afterward, all products were subjected to melt curve analysis. A negative control without a cDNA template was run with each analysis to evaluate the overall specificity. Both GhUBQ14 (GenBank: DW505546) and cotton actin (AF059484) were used as reference genes to normalize the total amount of cDNA in each reaction (Artico et al., [43]; Zhang et al., [24]b). A mean normalization from two reference genes was used to analyze the level of expression for each gene. Data from each biological replicate were given as the mean of three technical replicates. Each bar in the column chart represented the mean of three biological replicate experiments (n=3); vertical bars indicated standard errors. Relative fold-changes were calculated per the 2-△△Ct method, as described by Livak and Schmittgen [44]. All of the selected genes and primers are listed in Additional file 2: Table S1.
Transcription factor analysis
We used BLAST results for unique cotton sequences against Arabidopsis proteins to identify cotton sequences homologous to Arabidopsis TFs. The comprehensive Plant Transcription Factor Database was searched (PlantTFDB; http://planttfdb.cbi.pku.edu.cn) (Guo et al., [45]; Zhang et al., [46]a).
Analysis of microsatellite repeats
The EST-SSRs in unigene sequences were identified by the MISA program (http://pgrc.ipk-gatersleben.de/misa), a powerful pipeline for SSR detection. The minimum repeat number was six for dinucleotides and five for tri-, tetra-, penta-, or hexanucleotides.
Results
Establishment of experimental system
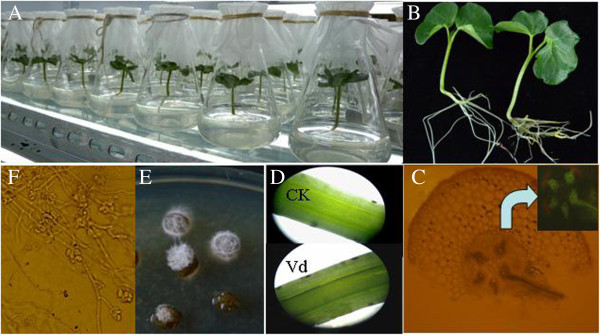
To minimize any environmental impacts, we grew the cotton seedlings in sterile culture pots and used a root dip-inoculation system for introducing the fungus. This system provided coordinated and reproducible infections under controlled conditions (Figure 1A and B). It enabled us to obtain symptoms characteristic of Verticillium wilt disease in the field. Initially, the pathogen attached to and grew on the root surface, then penetrated the roots at approximately 1 day post-infection (dpi) before entering the vascular system. It progressed through the plant to the hypocotyl vascular tissue (2 to 3 dpi; Figure 1C), where the infection was associated with he onset of vascular browning (Figure 1D). This ultimately led to wilting of the cotyledons and plant death. We monitored this movement of the pathogen by re-isolating V. dahliae from surface-sterilized sections of infected plants and observing the development of a GFP-tagged V. dahliae strain (Figure 1E and F).
Figure 1.
Infection of cotton seedlings with Verticillium dahliae. A: Aseptic growth in tissue culture flask. B: status at 3 dpi for roots treated with conidial suspension. C: Infection process, based on GFP-tagged V. dahliae strain. D: Vascular tissue of uninfected hypocotyl section (CK). Severe browning of vascular tissue in longitudinal section at 7 dpi. E: Mycelia growth in vascular tissue of surface-sterilized hypocotyl section prepared from cotton seedling at 2 dpi, then incubated for 3 d on 25% potato dextrose agar. F: Mycelium observed with optic microscope.
To obtain a global overview of the cotton transcriptome and gene activity at nucleotide resolution, we thoroughly mixed RNA that was extracted at 1, 2, 4, 6, 8, 12, 24, 36, 48, 72, 96, or 120 hpi. To minimize any systematic bias from transcriptome sampling, and to improve our accuracy in detecting low-abundance transcripts, we constructed and sequenced three cDNA libraries from the same pooled RNA sample.
Library construction
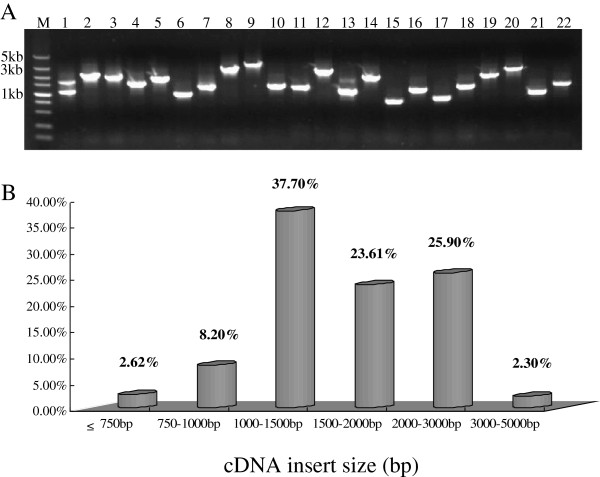
A high-quality full-length cDNA library was obtained. To analyze their average insert size and distribution, we randomly sampled cDNA clones from white plates. In all, 1000 clones were successfully amplified by PCR (Figure 2A). Inserts were 500 bp to 5 kb long (average 1.8 kb). As shown in Figure 1, 37.70%, 23.61%, and 25.90% of the clones had insert lengths of 1000 bp to 1.5 kb, 1.5 kb to 2.0 kb, and 2.0 kb to 3.0 kb, respectively. In addition, approximate 2.30% of the clones had inserts longer than 3.0 kb (Figure 2B). These results indicated that our full-length cDNA library contained a sufficient number of genes so that we could gain their complete sequence information.
Figure 2.
Detection and analysis of inserts for cDNA clones. PCR amplification of inserts for cDNA clones (A) and statistical analysis of insert fragment sizes in cDNA library (B). Lanes: M, DL5000 marker; 1-22, random clones from library.
EST sequencing and assembly
A total of 46,192 high-quality sequences (average length 818 bp) was generated after any short and low-quality sequences were removed. Among them, 23,126 unigenes were derived from cluster assembly and sequence alignments. They included 2661 contigs and 20,465 singletons. Each contig had 2 to 1537 ESTs, with an average length of 783 bp. The majority of contigs (78.0%) contained five or fewer ESTs, while only 5.0% had 26 or more. This demonstrated low redundancy for the library. The distribution of EST numbers in each unigene indicated that several highly abundant genes could be identified, with 99 unigenes being represented by over 25 ESTs (Figure 3).
Figure 3.
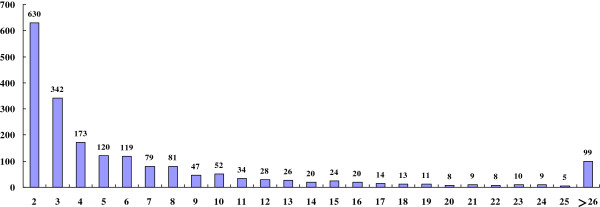
Distribution of 1981 contigs based on number of clustered ESTs.
Functional annotation of the cotton unigene dataset
A sequence similarity search was conducted against the NCBI, Nr, and SwissProt databases, using BLASTx and tBLASTn algorithms that specified E-values of less than 10-5. This analysis revealed that 22,446 unigenes had significant hits in the Nr database. However, of these, only 10,060 unigenes showed similarities to proteins of known function, 12,386 had similarities to predicted proteins of unknown function, and 680 unigenes had no significant similarity to any sequences contained in that database. This indicated that most of the unigenes could be assigned a known or putative function. Those without database hits were likely to include non-coding RNAs, genes whose sequences did not capture regions that contained conserved functional domains, or protein-coding genes that were novel in the database and/or cotton-specific.
GO enrichment analysis
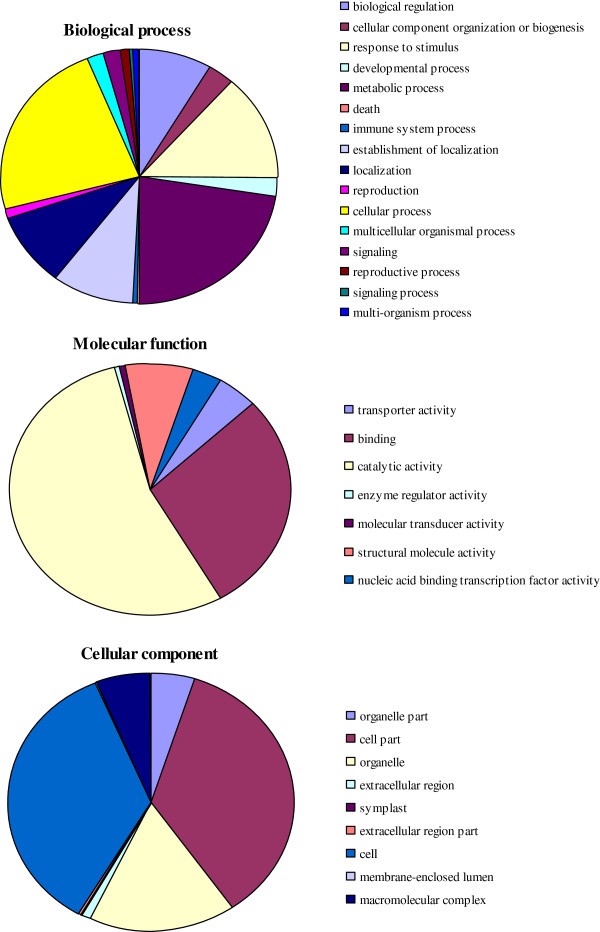
Based on our BLAST and SwissProt results, we further annotated the cotton unigenes with GO terms. In all, 20,397 (72.0%) could be assigned at least one term. Among them, 6,893 were placed in the category of biological processes, 8,383 in molecular functions, 11305 in cellular components, and 6184 in all three categories.
We also used a set of plant-specific GO slims, which are a list of high-level GO terms that provide a broad overview of the ontology content. Within our three categories, the most abundant slims were for cellular processes (biological), catalytic activity (molecular), and cell part (components) (Figure 4). Metabolic and cellular processes and responses to stimulus were among the most highly represented groups within the biological-process category. The most frequent family in this library was for PR protein, followed by dirigent-like protein, and glutathione S-transferase. A large number of expressed genes were also involved in plant defense mechanisms, cytoskeleton, signal transduction, cell wall/membrane/envelope biogenesis, and lipid transport and metabolism (Figure 5).
Figure 4.
Functional classification of unigenes from Verticillium dahliae-stressed cotton within categories of biological processes, molecular functions, and cellular components.
Figure 5.
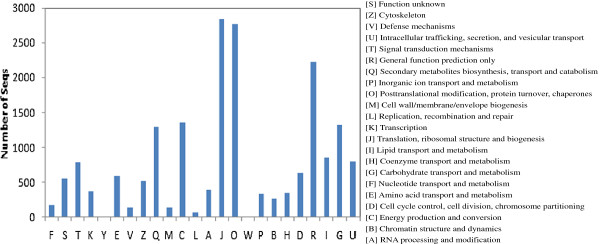
Classifications for Clusters of Orthologous Groups (COGs). Sequences with Nr hits were grouped into 22 COG classes.
Pathway classification of transcripts
Using Pathway Tools (Karp et al., [47], we predicted 289 active biochemical pathways for cotton resistance to Verticillium wilt from enzyme-coding unigenes in our EST collection. Enrichment was greatest for the metabolism category (8017), followed by the categories of diseases (3190), genetic information processing (GIP; 2354), organismal systems (OS; 1901), cellular processing (CP; 1077), and environmental information processing (EIP; 773). Among the subsets for metabolism (Table 1), the major contributors were carbohydrates (24.7%), xenobiotics biodegradation and metabolism (17.1%), energy (12.4%), and amino acid metabolism (7.4%). In the GIP category, translation (60.9%), and folding, sorting, and degradation (35.0%) were the most common when compared with replication and repair (4.1%). In the EIP category, the vast majority of unigenes (98.1%) was involved in signal transduction. Transport and catabolism (42.2%), cell growth and death (36.1%), and cell communication (13.6%) constituted the majority of unigenes in the CP category. The major constituent of the organismal category was the nervous system (21.7%), endocrine system (18.4%), environmental adaptation (13.9%), and immune system (13.8%). The most important metabolite biosynthesis pathways, e.g., plant–pathogen interactions, plant hormone signal transduction, calcium signaling, and phenylpropanoid biosynthesis were well covered by our EST collection.
Table 1.
Distribution of functions in the KEGG pathway
|
Functional |
|
Total |
Percent |
Percent |
|---|---|---|---|---|
|
category |
|
unigenes |
of |
of |
| unigenes | categories | |||
|
Metabolism (8017, 38.75%) |
Metabolism of other amino acids |
729 |
2.74% |
7/ |
| Xenobiotic biodegradation and metabolism |
1369 |
5.15% |
15/ |
|
| Energy metabolism |
997 |
3.75% |
8 |
|
| Carbohydrate metabolism |
1981 |
7.45% |
15/ |
|
| Terpenoid and polyketide metabolism |
597 |
2.25% |
10/ |
|
| Biosynthesis of other secondary metabolites |
442 |
1.66% |
14/ |
|
| Amino acid metabolism |
737 |
2.77% |
8 |
|
| Glycan biosynthesis and metabolism |
251 |
0.94% |
10 |
|
| Lipid metabolism |
598 |
2.25% |
12 |
|
| Nucleotide metabolism |
185 |
0.70% |
2 |
|
| Cofactor and vitamin metabolism |
131 |
0.49% |
11 |
|
|
GIP (2354, 6.68%) |
Translation |
1433 |
5.39% |
5 |
| Folding, sorting, and degradation |
825 |
3.10% |
7 |
|
| Replication and repair |
96 |
0.36% |
7 |
|
|
EIP (773, 2.91%) |
Signal transduction |
758 |
2.85% |
15 |
| Membrane transport |
14 |
0.05% |
2 |
|
| Signaling molecules and interactions |
1 |
0.00% |
1 |
|
|
CP (1077, 4.05%) |
Cell growth and death |
389 |
1.46% |
7 |
| Transport and catabolism |
454 |
1.71% |
5 |
|
| Cell motility |
87 |
0.33% |
1 |
|
| Cell communication |
147 |
0.55% |
4 |
|
| OS (1901, 7.05%) | Excretory system |
126 |
0.47% |
5 |
| Environmental adaptation |
264 |
0.99% |
4 |
|
| Endocrine system |
349 |
1.31% |
6 |
|
| Nervous system |
413 |
1.55% |
7 |
|
| Development |
87 |
0.33% |
3 |
|
| Immune system |
263 |
0.99% |
12 |
|
| Circulatory system |
100 |
0.38% |
2 |
|
| Digestive system |
183 |
0.69% |
8 |
|
| Sensory system | 116 | 0.44% | 3 |
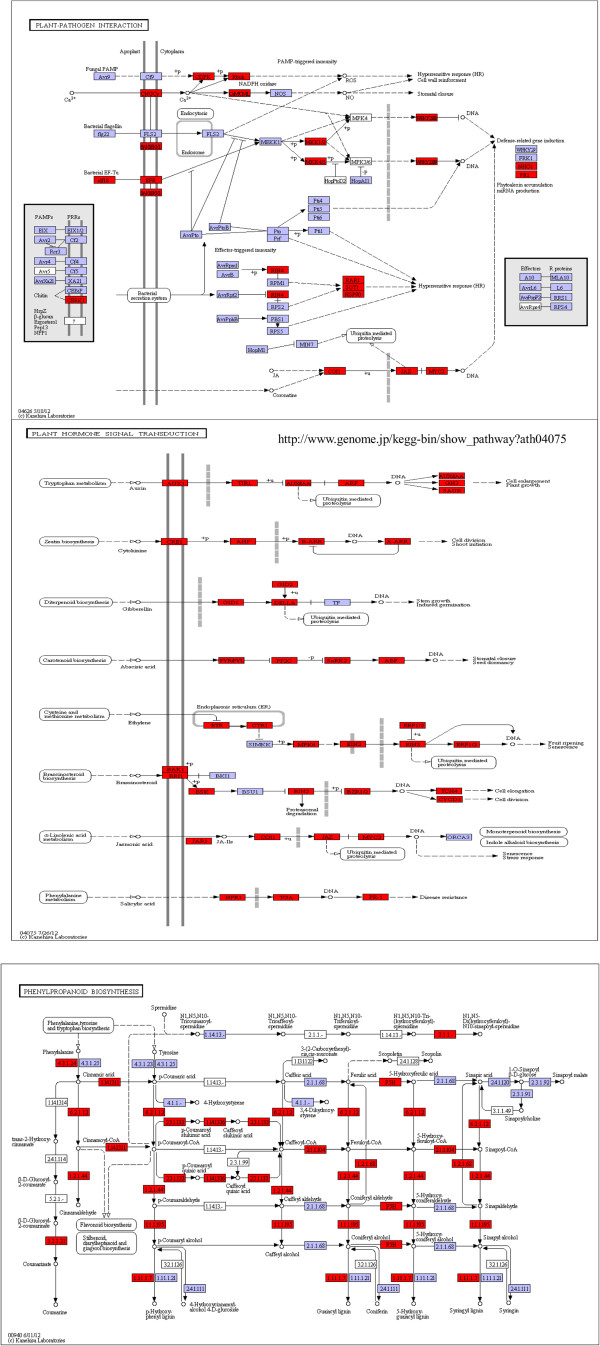
Examples of KEGG pathways for plant–pathogen interactions, plant hormone signal transduction, and phenylpropanoid biosynthesis are shown in Figure 6, where genes represented in the full-length cDNA library are highlighted (i.e., Ko04626, Ko04075, and Ko00940). These three pathways served as examples of pathways influenced by pathogen attacks or various abiotic stresses.
Figure 6.
Example of KEGG pathways found for full-length cDNA clone ESTs. Each box shows enzymes involved in each section of pathway. Genes highlighted in red were detected from our full-length cDNA library.
Comparisons of unigenes from other species of cotton and model plants
To identify which of the G. barbadense-specific sequences were similar to ESTs from other cotton species (G. hirsutum, G. raimondii, and G. arboretum), we used the unigenes as queries in a Blastn search against three databases. Overall, 680 unigenes (2.94%) had no significant matches with any sequence in the current EST databases for cotton (Figure 7A). That is, they probably are novel and belong to sequences that are specific to G. barbadense. In addition, we used Blastx to compare these unigenes with protein sequences from other species and found similarities of 87.1% between cotton and both Arabidopsis and Populus, 85.2% for Vitis, and 80.6% for Ricinus.
Figure 7.
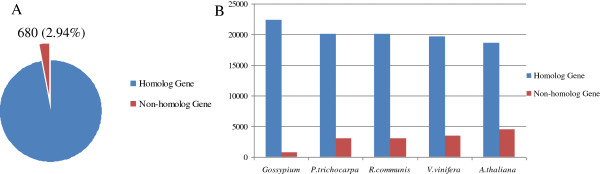
Comparisons of 23,126 unigenes from cotton and other plant species. Searches were performed against nucleotide databases for ESTs (A) or proteins (B) using Blastn or Blastx (E-value ≤10-5).
Disease resistance-related protein families
In examining the molecular biology of a defense system in cotton, our Blast analysis determined that 3027 unigenes were homologous to known defense-related genes from other plants (Additional file 3: Table S2). They could be divided into nine major groups: perception of PAMPs by PRRs, effector-triggered immunity (ETI), ion fluxes, transcription factors, oxidative burst, pathogenesis-related (PR) proteins, programmed cell death, plant hormones, and cell wall modification (Table 2). KEGG analysis revealed that these unigenes were significantly enriched in various known resistance-relevant metabolic or signaling pathways. This indicated that such genes and pathways were highly conserved between cotton and other plants. Thus, we deduced that our unigenes were closely associated with the defense response in cotton roots against the Verticillium pathogen.
Table 2.
Known unigenes expressed in response to V. dahliae infection in resistant G. barbadense
| Pathway | Unigene | Related functions |
|---|---|---|
| Perception of PAMPs by PRRs |
Chitin elicitor receptor kinase (CERK1) |
Chitin elicitor signaling |
| Elicitor-responsive proteins (ERG) |
Plant defense signaling |
|
| Proline-rich extensin-like receptor kinases (PERKs) |
Perception of PAMPs and induction of defense responses |
|
| BRI1-associated receptor kinase 1 (BAK1) |
Perception of PAMPs and induction of defense responses |
|
| Somatic embryogenesis receptor-like kinases (SERKs) |
Plant immune responses to pathogen attack |
|
| Plant receptor-like kinases (RLKs) |
Perception of PAMPs and induction of defense responses |
|
| Mitogen-activated protein kinase (MAPK) |
Downstream components in PTI |
|
| CC-NBS-LRR resistance protein (RPM1) |
Manages of signaling potential via intra-molecular negative regulation |
|
| Effector-triggered immunity (ETI) |
TMV resistance protein |
TMV-N mediated signal transduction pathway |
| Disease resistance protein (RPS2) |
Specifically recognizes effector protein from pathogen |
|
| NBS-LRR resistance gene (RPP8) |
Specifically recognizes effector protein from pathogen |
|
| RPM1 Interacting Protein 4 (RIN4) |
Negatively regulates disease resistance mediated by RPS2 |
|
| Ion Fluxes |
CaM-related proteins |
Calcium signal transducer |
| Plant cyclic nucleotide gated channels (CNGCs) |
Facilitates Ca2+ uptake into the cytosol in response to PAMP |
|
| Calmodulin (CaM) |
Calcium signal transducer |
|
| Calcium binding protein |
Calcium signal transducer |
|
| Calmodulin-like protein (CML) |
Calcium signal transducer |
|
| Calcineurin B-like proteins (CBL) |
Decoding of calcium transients |
|
| Transcription factors (TFs) |
ERF, EREBP-like |
Binding ethylene-responsive element |
| WRKY |
Regulates signaling and transcriptional reprogramming associated with plant defense responses |
|
| BHLH |
Regulates signaling and transcriptional reprogramming associated with plant defense responses |
|
| Histone promoter-binding protein (HBP)-1a |
Negatively regulates defense response |
|
| NADPH oxidase or respiratory burst oxidase |
Generation of superoxide |
|
| Oxidative burst |
Ascorbate peroxidase |
Detoxifies peroxides |
| Thioredoxin peroxidases |
Reduces various peroxides |
|
| Glutathione peroxidases (GPXs) |
Reduces H2O2, organic hydroperoxidases, and lipid peroxides |
|
| Cationic peroxidases |
Causes a disease resistance response |
|
| Protein disulfide-isomerase (PDI) |
Ubiquitous redox protein |
|
| Catalase |
Decomposition of hydrogen peroxide to water and oxygen |
|
| PR1C |
Confers resistance to pathogen and hallmarks of defense pathways |
|
| Pathogenesis-related (PR) proteins |
Beta-1,3-glucanase-like genes (PR2 homologs) |
Lyses cell walls of fungal pathogens |
| PR1 protein |
Confers resistance to pathogen and hallmarks of defense pathways |
|
| Chitinase (PR3 and 8 homologs) |
Lyses cell walls of fungal pathogens |
|
| Thaumatin-like protein (PR5) |
Inhibits hyphal growth and sporulation by various fungi |
|
| BAG-like genes: BCL-2-associated athanogenes |
Suppresses apoptosis |
|
| Dynamin-related proteins (DRP) |
Key regulators of PCD |
|
| Programmed cell death (PCD) |
Apoptosis Inducing Factor homolog (AIF) |
Chromatin condensation and DNA degradation |
| Nitric oxide synthase |
Catalyzes arginine to produce nitric oxide |
|
| Enhanced Disease Susceptibility 1(EDS1) |
Catalyzes arginine to produce nitric oxide |
|
| Non-expression of PR gene 1 (NPR1) |
Regulatory component in SA signaling |
|
| Plant hormones |
Pathogen-inducible salicylic acid glucosyltransferase (SGT1) |
Involved in the early disease response and the accumulation of glucosyl SA during pathogenesis |
| Phenylalanine ammonia lyase (PAL) |
Key enzyme in SA biosynthesis |
|
| Isochorismate synthase (ICS) |
Key enzyme in SA biosynthesis |
|
| Lipoxygenase (LOX) |
Key enzyme in jasmonic acid (JA) biosynthesis |
|
| Allene oxide synthase (AOS) |
Key enzyme in jasmonic acid biosynthesis |
|
| Jasmonate ZIM-motif (JAZ) proteins (TIFY10B) |
JA signaling |
|
| 1-aminocyclopropane-1-carboxylic acid oxidase (ACO) |
Key enzyme in ethylene biosynthesis |
|
| 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase |
Biosynthesis of derived secondary metabolites |
|
| Cell wall modification | Polyphenol oxidase |
Oxidation of phenol compounds |
| 4-coumarate-CoA ligase |
Phenylpropanoid metabolism |
|
| Glutathione-S-transferase (GST) |
Conjugates electrophilic molecules to glutathione (GSH) |
|
| Caffeic acid 3-O-methyltransferase |
Lignin biosynthesis |
|
| Extension |
Inhibits pathogen invasion |
|
| Cellulose synthase |
Callose synthesis |
|
| Sucrose synthase |
Sucrose synthesis |
|
| UDP-glucuronic acid decarboxylase 1 | Key factor in xylose formation |
When we examined the levels of relative expression in this non-normalized cDNA library, our sequencing results (Table 3) showed that the most frequent family was PR protein 10 (1537 ESTs), followed by the dirigent-like protein (384 ESTs), glutathione S-transferase (GST-C-Tau class; 331 ESTs) and meloidogyne-induced cotton protein (331 ESTs). Other abundant families included those related to Bet_V1-like protein (275 ESTs), transmembrane protein (234 ESTs), short chain alcohol dehydrogenase (207 ESTs), gibberellin 3-beta-dioxygenase (185 ESTs), glutathione S-transferase omega (176 ESTs), and metallothionein-like type 1 protein (160 ESTs).
Table 3.
Most frequent families found in the cotton library
| Description | Total ESTs |
|---|---|
|
PR protein class 10 |
1537 |
|
dirigent-like protein |
384 |
|
glutathione S-transferase (Tau class) |
331 |
|
meloidogyne-induced cotton protein |
331 |
|
Bet_V1-like protein |
275 |
|
transmembrane protein |
234 |
|
short chain alcohol dehydrogenase |
207 |
|
gibberellin 3-beta-dioxygenase |
185 |
|
glutathione S-transferase omega |
176 |
| metallothionein-like type 1 protein | 160 |
Differential expression analysis of unigenes after V. dahliae inoculation
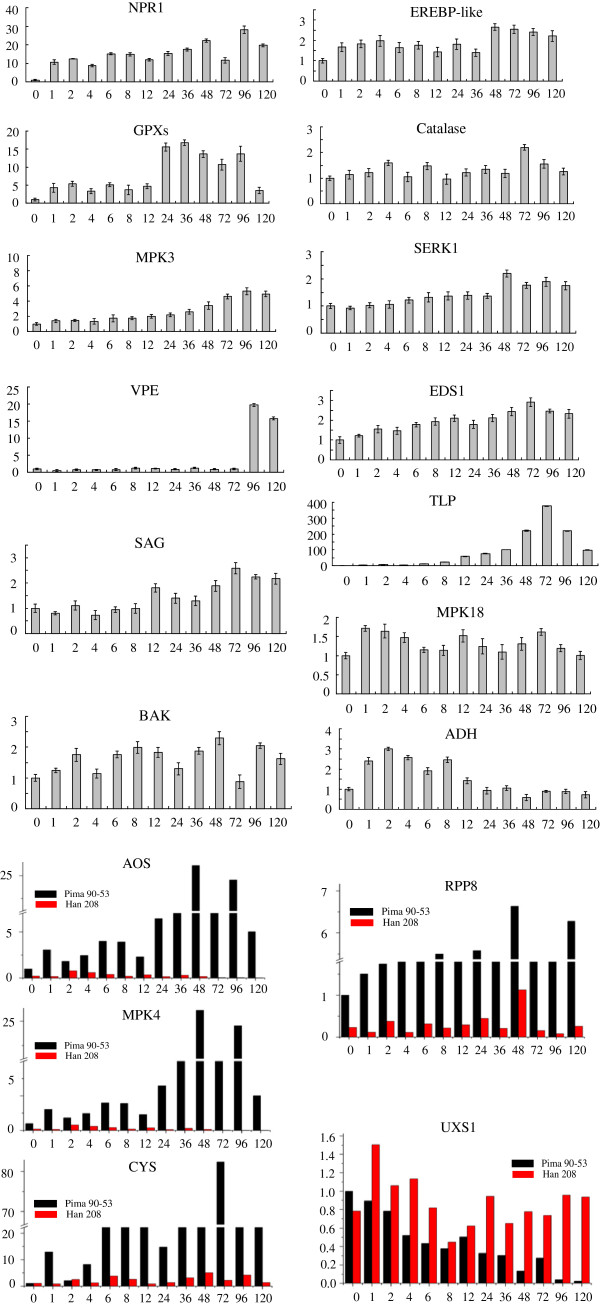
To analyze the global transcriptional changes in cotton infected with V. dahliae, we monitored 18 genes that function in eight metabolic pathways associated with immunity. Three patterns of expression emerged (Figure 8). First, transcripts from infected tissues could be markedly increased when compared with mock-inoculated samples. For example, the expression of TPL was rapidly induced within 2 h, with transcripts being >100-fold higher than the control at 36 hpi and peaking at >300-fold over the mock at 72 hpi. In addition, expression of CYS, VPE, GPXs, NPR1, AOS, and MPK4 was 20- to 80-fold greater when compared with the mock. The second pattern was manifested by 10 genes -- MPK3, MPK18, RPP8, EREBP-like, Catalase, SERK1, EDS1, ADH, SAG, and BAK – for which transcript abundance in infected tissue was enhanced by 2- to 6-fold. The third pattern was illustrated by UDP-glucuronic acid decarboxylase1 (UXS1), for which the transcription level was greatly decreased post-inoculation in the resistant G. barbadense 'Pima90-53’. Its expression was lowest at 120 hpi, being down-regulated by >10-fold. In the susceptible G. hirsutum 'Han208’, UXS1 expression was only slightly altered, with transcripts being maintained at nearly their original level throughout the monitoring period. These differences in expression between resistant and susceptible cultivars may have reflected the degree to which they are influenced by V. dahliae attacks.
Figure 8.
Detailed expression profiles of defense-related genes. Q-PCR analysis was conducted for transcription levels of selected genes in response to V. dahliae infection in mock-inoculated and fungal-inoculated roots at 1, 2, 4, 6, 8, 12, 24, 36, 48, 72, 96, and 120 hpi. Data within each column are means and standard errors (bar) for 3 independent Q-PCR experiments using 3 technical replicates; vertical bars indicate standard errors. Transcription level is represented as ratio of Ct value for studied gene, calibrated to mock-inoculated control and normalized to Ct value for GhUBQ14 and cotton actin.
We subjected a subset of genes to qPCR analysis during the interactions between V. dahliae and the two species. The transcriptomes were compared for differentially expressed AOS, MPK4, CYS, and RPP8. After inoculation occurred, expressions were either reduced or showed delays in their upregulation in tissues from the susceptible 'Han208’ when compared with the more resistant 'Pima90-53’ (Figure 8).
Identification of putative transcription factors
Putative TFs act as master switches by slightly modifying the expression of a series of genes. Therefore, they play critical roles in the regulation of cellular pathways during normal plant development or in response to biotic and abiotic stimuli. We identified these TFs by searching PlantTFDB2.0, a comprehensive database for 49 species that include 53,319 putative TFs and 58 families. A total of 4936 ESTs was identified in our library. The most abundant TF family was the NAC group (527), followed by G2-like (440), MYB (372), BHLH (331), bZIP (271) ERF, C3H, WRKY, C2H2, Dof, ARF, B3, GRAS, and Trihelix families (Table 4). Their distributions in G. barbadense and several related species are presented in Additional file 4: Table S3. Compared with other model species, frequencies were relatively higher in cotton for NAC (527; 10.67%), G2-like (440; 8.91%), C3H (265; 5.36%), Dof (169; 3.42%), s1fa-like (28; 0.57%), and STAT (13; 0.26%) but lower for ERF (270; 5.46%), HD-Zip (51; 1.03%), Wox (5; 0.1%), and ZF-HD (4; 0.08%). Except for NZZ/SPL and SAP, all of the other TF families were detected in our datasets. Yuan et al. [48] have previously reported some novel TF families within G. barbadense, including those for HMG, GARP-ARR-B, JUMONJI, Gif, E2F-DP, ABI3-VP1, ULT, C2C2-Dof, AS2, and ARID.
Table 4.
The most abundant putative transcriptional factors (TFs) in cotton resistant to Verticillium infection
| TF family | Description | Total ESTs |
|---|---|---|
|
NAC |
No apical meristem (NAM) protein |
527 |
|
G2-like |
Golden 2-like (GLK) |
440 |
|
MYB |
Myb-like DNA-binding domain |
372 |
|
BHLH |
Basic/helix-loop-helix domain |
331 |
|
MYB-related |
N-terminal myb-domain |
321 |
|
bZIP |
Basic leucine zipper (bZIP) motif |
271 |
|
ERF |
Single AP2/ERF domain |
270 |
|
C3H |
Zinc finger, C-x8-C-x5-C-x3-H type |
265 |
|
WRKY |
WRKY DNA-binding domain |
225 |
|
C2H2 |
Zinc finger, C2H2 type |
218 |
|
Dof |
DNA binding with one zinc finger |
169 |
|
ARF |
Auxin response factor |
157 |
|
B3 |
ERF-B3 family |
94 |
|
GRAS |
Three initially identified members GAI, RGA, and SCR |
84 |
|
Trihelix |
Trihelix DNA-binding domain |
76 |
|
FAR1 |
Far-red-impaired Response 1 |
56 |
|
HD-ZIP |
HD domain with leucine zipper motif |
51 |
|
M-type |
MADS-box transcription factors |
50 |
|
GATA |
Binds to DNA sequence "GATA" |
49 |
|
LBD |
Lateral organ boundary domain (LBD) gene family |
48 |
|
HB-other |
Homeobox domain |
46 |
| TCP | Non-canonical basic-Helix-Loop-Helix (bHLH) structure | 42 |
Identification of EST-SSRs
Molecular markers are valuable resources for constructing high-density genetics maps, facilitating crop breeding, and identifying traits of interest. Special EST-SSRs have high efficiency and incur a low cost, making them one of the best genetic markers for such systems. Here, 1212 SSRs were identified in 1169 (4.3%) unigenes. Among the repeat types, trinucleotide repeats were the most abundant SSRs (760, or 62.7% of the EST-SSRs), followed by dimeric SSRs (311; 25.7%), and tetrameric SSRs (98; 8.1%) (Additional file 5: Table S4). We previously reported the construction of a genome-wide map for cotton, in which these SSRs were used to add to the map density (Yang et al., [49]).
Discussion
Because allotetraploid cotton has a large and complex genome, researchers had not previously elucidated any comprehensive sequence information to describe the transcriptome related to defense responses against Verticillium dahliae. Here, we investigated such responses through full-length cDNA library construction and EST sequencing. Although two tetraploid species, G. hirsutum and G. barbadense, exist, few examinations of the latter have been made because it is less commonly cultivated. However, because it is more resistant to this wilt pathogen (Zhang et al., [50]d), we used G. barbadense 'Pima90-53’ in our tests. As an American type of Pima, this cultivar has proven to be a better germplasm resource than other available types (i.e., Egyptian or Mid-Asian types of G. barbadense) because of its desirable traits for immunity to Verticillium, fiber quality, and greater yield (Ma et al., [51], Wang et al., [52]). Its resistance and phenotypic characters have long been recognized in both field and greenhouse settings. Moreover, we have previously cloned several functional genes related to resistance and/or fiber quality from 'Pima90-53’, e.g., GbVe, GbWRKY1, and ADF1 (Chi et al., [53,54]; Liu et al., [55]; Pan et al., [56]; Zhang et al., [57]b; Zhang et al., [19]a; Zhang et al., [58]d). Therefore, we chose this specific Pima cotton for profiling the G. barbadense transcriptome. Our goal was to mine those genes for economically important traits so that we might potentially improve those traits in the Upland cotton G. hirsutum.
Infections with V. dahliae progress throughout the roots and into the rest of the plant, causing serious losses in both yield and quality. Because the root is the first barrier against such an attack, we selected this particular tissue for analysis. Fungal spores germinate and epidermal cells are often penetrated within the first 12 h (Fradin and Thomma, [59]). Complex perception, transduction, and exchange of signals usually occurs in the early stages of infection (Zhang and Klessig, [60]; Kunkel and Brooks, [61]; Jones and Dangl, [62]). Therefore, we sampled at 1, 2, 4, 6, 8, 12, 24, 36, 48, 72, 96, and 120 hpi to coincide with those crucial stages and to isolate early pathogen-responsive genes. We also utilized an experimental system that allowed for tight control of environmental conditions so that gene expression was not altered by any factors other than the pathogen. This enabled us to identify and monitor as many genes as possible from our library.
More than 46,192 high-quality ESTs were generated from our root cDNA library of Verticillium-infected G. barbadense seedlings. These ESTs were assembled into 23,126 unigenes. Annotation results showed that this library contained many previously reported key response genes, such as for PR protein, chitinase, members of the GST gene family, PAL, CYP, and NDR1 (McFadden et al., [63]; Li et al., [64]; Gao et al., [22]; Xu et al., [12]; Zhu et al., [65]; Ahmed et al., [66]). Our library also included several genes that function in the development of cotton fibers, e.g., SusA1, CesAs, UXS1, ADF1, tubulin, and aquaporin (Pan et al., [56]; Yuan et al., [48]; Jiang et al., [67]; Kim et al., [68]; Chi et al., [54]). Our findings suggested that defense-related genes were abundant in our library, and that genes contributing to fiber formation might also function in protecting G. barbadense against infection by V. dahliae. Therefore, this library is an important genomics resource for isolating genes with novel roles. We also demonstrated the importance of identify critical genes that code for different phenotypes, such as stress resistance or fiber quality. That is, researchers can now construct a subtracted library to find genes that are preferentially expressed in resistant lines. We previously completed an SSH library that used a resistant G. hirsutum cultivar and contained more than 200 genes that are differentially expressed between it and susceptible cultivars (Zhang et al. [69]). Future studies will incorporate microarrays to achieve this goal in related experiments.
When exposed to various environmental stimuli, plants utilize elaborate mechanisms to regulate cellular and molecular events so they can protect themselves with pre-formed defense barriers and induce appropriate responses. For example, pathogen-triggered immunity (PTI) constitutes the first layer of that response, restricting pathogen activity by blocking further colonization (Zhang et al., [70]). A second layer, ETI, specifically recognizes the effector by one of its NB-LRR proteins (Nürnberger et al., [71]). ETI is an accelerated response while PTI is amplified, resulting in disease resistance and, usually, a hypersensitive cell death response at the infection site. In our full-length cDNA library, groups such as LRR-RLKs, signalling-related genes, and TFs were expressed during the cotton defense response. All of them possibly contribute to these PTI- or ETI-related systems.
Most PTI-associated genes were obtained in our library, including the chitin elicitor receptor kinase (CERK1), which recognizes chitin oligosaccharides during plant–pathogen interactions. It acts as a representative general elicitor to induce defense responses in a wide range of plant cells (Kaku et al., [72]). We also identified chitinases, which, as PR proteins, play important roles in enhancing stress resistance in a variety of plants. These chitin-degrading enzymes hydrolyze b-(1, 4) linkages and are capable of degrading the cell walls of plant pathogenic fungi (Adams, [73]; Cheng et al., [74]; Lawrence and Novak, [75]) while releasing elicitors of defense reactions. Therefore, chitinases are thought to have crucial roles in plant defenses against biotic stresses. Based on this, we might conclude that chitinases and their elicitors confer pathogen resistance in cotton by hydrolyzing the cell walls of V. dahliae.
Another important PTI-related gene, Brassinosteroid Insensitive 1-Associated Kinase 1, or BAK1, was isolated here. Known as Somatic Embryo Genesis Receptor-Like Kinase 3 (SERK3), it is required by PRRs (Heese et al., [76]; Dodds and Rathjen, [77]). SERK3/BAK1 not only interacts with BRI1, which is involved in brassinosteroid signal transduction, but it also rapidly forms a complex with FLS2, which functions in reactions for plant disease resistance. For example, the BAK1/SERK4 homolog in Nicotiana has a direct role in elicitor perception of bacterial cold shock protein, flagellin, and elicitin, but not chitin (Heese et al., [76]). However, we must still investigate whether similar elicitors exist in V. dahliae and how the FLS2–BAK1 complex acts to confer pathogen immunity in resistant cotton plants.
In addition to the genes already mentioned, we obtained another important clue regarding wilt resistance in cotton. The major pollen allergen (Bet v1) family protein was one of the most abundant in our library. Proteins in this family have previously been identified under various biotic and abiotic stresses in birch, potato, pea, soybean, and cotton (Breiteneder et al., [78]; Matton and Brisson, [79]; Barratt and Clark, [80]; Crowell et al., [81]; Cheng et al., [74]). Family members may also have essential roles in the defense response by Sea Island cotton to Verticillium wilt (Chen et al., [16]). The mRNA transcripts examined in our study demonstrated that Bet v1 family genes were more abundant than any others, suggesting that this family is a vital component of the cotton response to Verticillium infection.
With critical roles in complex signaling cascades, phytohormones have been integrated into current models for defense responses (Bari and Jones, [82]; Grant and Jones, [83]). For example, the pathways for salicylic acid (SA), jasmonic acid (JA), ethylene (ET), and brassinosteroids are important regulators of expression by defense-related genes (Bari and Jones, [82]). In general, SA induces systemically acquired resistance and is implicated in plant tolerance to biotrophic pathogens (Spoel and Dong, [84]; Leon-Reyes et al., [85]). Both ET and JA are typically associated with defense responses to necrotrophic pathogens (Spoel et al., [86]; Bari and Jones, [82]). Cross-talk can occur between those SA- and JA/ET-mediated defense reactions to abiotic- and biotic-stress stimuli (Grant and Jones, [83]). Results from our Q-PCR analysis showed that expression patterns were the same for EDS1 and PAD4. Both genes were up-regulated upon inoculation, and their transcript levels were several-fold higher than that of the mock. This implied that the EDS1–PAD4 complex interferes with activation of innate cotton immunity. In addition, while identifying the biochemical pathways that were active during the response to V. dahliae inoculation, we discovered a plant hormone signal transduction pathway, within which exists an important branch: "SA→NPR1→TGA→PR-1→Disease resistance". The presence of this branch might suggest its role in the resistance response. However, in contrast to earlier conclusions (Leon-Reyes et al., [85]), we determined that SA is not involved in resistance to necrotrophic pathogens, such as V. dahliae. Therefore, we deduced that the process by which cotton becomes infected by Verticillium involves both biotrophic and necrotrophic stages.
The plant cell wall serves not only as a physical barrier, but as a defense barrier against pathogen penetration. Secondary metabolites play a fundamental role in the plant’s ability to fight against invading pathogens (Dubery and Smit, [87]; Naoumkina et al., [88]). That capacity is derived through multiple pathways, including those for the biosynthesis of phenylpropanoids, terpenoids, and cellulose. Most of the genes associated with those pathways, e.g., genes for caffeic acid, 3-O-methyltransferase, glutathione S–transferase, 4-coumarate:CoA ligase, UDP-glucuronic acid decarboxylase, cellulose synthase, and sucrose synthase. In this study, we detected a large phenylpropanoid pathway that encompassed those for flavonoid and lignin biosynthesis. We also obtained most of the genes that encode enzymes involved in the lignin pathway. For example, PAL, located in the core and entry of the phenylpropanoid pathway, is responsive to both biotic and abiotic stresses, including pathogen attack, and wounding (Huang et al., [89]). Likewise, POD has a role in reinforcing cell walls against the effects of pathogens or wounding through the polymerization of monolignols into lignin (Marjamaa et al., [90]). In Arabidopsis, Laccase4 and Laccase17 contribute to the constitutive lignification of stems, with the latter being involved in the deposition of G lignin units in fibers (Berthet et al., [91]). Therefore, all of these findings demonstrate that the phenylpropanoid pathway has an essential role in preventing the invasion or expansion of pathogens by reinforcing the cell wall. Nonetheless, future research should focus on the exact function of related genes within the phenylpropanoid pathway. Further characterization of those genes may provide valuable candidates for efforts toward the genetic improvement of cotton.
Conclusion
In this study, we characterized the root transcriptome of G. barbadense and provided gene resource that related to defense responses against V. dahliae. These findings provide a substantial contribution to existing sequence resources for cotton, and a strong basis for future genomic research. The putative signaling pathways generated in the present study revealed that the defense system of cotton may be a complex process. The findings of this study will hopefully accelerate research on resistance in cotton to V. dahliae and contribute to a better understanding of the cotton defense response to plant pathogens. Besides, the spatial and temporal expressions of defense-related genes require further study.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MZY conceived the experiment, coordinated and supervised the research, and drafted the manuscript. ZY and WXF had the main responsibility for full-length library construction and writing the manuscript. DZG, MQ, and ZGR were in charge of RNA isolations and assessment of RNA quality; ZSL assisted in the cultivation of Verticillium dahliae and sampling the tissues. ZY, ZGR, and LZK assisted in gene selection, RT-qPCR, and statistical analyses. WLQ and ZGY assisted in our sequence analysis. All authors read and approved the final manuscript.
Supplementary Material
Cotton naturally grew in the field. (A)G. barbadense cv. Pima90-53 displayed excellent resistance against V. dahliae.(B)G. hirsutum cv. Han208 naturally infected Verticillium wilt in the field. (C) Severe browning of vascular tissue in a longitudinal section of infected plants. (D) Typical sectorial necrosis from which V. dahliae mycelium may be re-isolated (E). Monospore was cultured on 25% potato dextrose agar. (F) Mycelium of V. dahliae was observed by optic microscope.
The unigenes used in Q-PCR analysis.
Unigenes of known disease/stress response functions discovered in response to V. dahliae infection in resistant G. barbadense cv. Pima90-53.
The most frequent sequences in our cDNA library.
Number of dinucleotide, trinucleotide and tetranucleotide repeats.
Contributor Information
Yan Zhang, Email: zhangyan7235@126.com.
Xing Fen Wang, Email: cotton@hebau.edu.cn.
Ze Guo Ding, Email: 171183145@qq.com.
Qing Ma, Email: 495441252@qq.com.
Gui Rong Zhang, Email: 1031935091@qq.com.
Shu Ling Zhang, Email: 806605739@qq.com.
Zhi Kun Li, Email: 38877452@qq.com.
Li Qiang Wu, Email: wuliqiang@hebau.edu.cn.
Gui Yin Zhang, Email: mhyzh@hebau.edu.cn.
Zhi Ying Ma, Email: mzhy@hebau.edu.cn.
Acknowledgments
This study was supported by funds for the early-stage basic research key project (No. 2011CB111609) and for the 863 project of China (No. 2013AA102601-5). The authors are grateful to Priscilla Licht for critical reading of the manuscript.
References
- Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV. Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol. 2009;47:39–62. doi: 10.1146/annurev-phyto-080508-081748. [DOI] [PubMed] [Google Scholar]
- Sink K, Grey WE. A root-injection method to assess Verticillium wilt resistance of peppermint (Mentha × piperita L) and its use in identifying resistant somaclones of cv Black Mitcham. Euphytica. 1999;106:223–230. doi: 10.1023/A:1003591908308. [DOI] [Google Scholar]
- Kamal ME. Integrated control of Verticillium wilt of cotton. Plant Dis. 1985;69:1025–1032. doi: 10.1094/PD-69-1025. [DOI] [Google Scholar]
- Wang XF, Wang YX, Zhang GY, Ma ZY. An integrated breeding technology for accelerating generation advancement and trait introgression in cotton. Plant Breed. 2011;130:569–573. doi: 10.1111/j.1439-0523.2011.01868.x. [DOI] [Google Scholar]
- Cai YF, He XH, Mo JC, Sun Q, Yang JP, Liu JG. Molecular research and genetic engineering of resistance to Verticillium wilt in cotton. Afr J Biotechnol. 2009;8:7363–7372. [Google Scholar]
- Tan XP, Liang WQ, Liu CJ, Luo P, Heinstein P, Chen XY. Expression pattern of (+)-delta-cadinene synthase genes and biosynthesis of sesquiterpene aldehydes in plants of Gossypium arboretum L. Planta. 2000;210:644–651. doi: 10.1007/s004250050055. [DOI] [PubMed] [Google Scholar]
- Luo P, Wang YH, Wang GD, Essenberg M, Chen XY. Molecular cloning and functional identification of (+)-delta-cadinene-8-hydroxylase, a cytochrome P450 mono-oxygenase (CYP706B1) of cotton sesquiterpene biosynthesis. Plant J. 2001;28:95–104. doi: 10.1046/j.1365-313X.2001.01133.x. [DOI] [PubMed] [Google Scholar]
- Xu YH, Wang JW, Wang S, Wang JY, Chen XY. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-delta-cadinene synthase-A. Plant Physiol. 2004;135:507–515. doi: 10.1104/pp.104.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit F, Dubery LA. Cell wall reinforcement in cotton hypocotyls in response to a Verticillium dahliae elicitor. Phytochemistry. 1997;44:811–815. doi: 10.1016/S0031-9422(96)00595-X. [DOI] [Google Scholar]
- Pomar F, Novo M, Bernal MA, Merino F, Barcelo AR. Changes in stem lignins (monomer composition and crosslinking) and peroxidase are related with the maintenance of leaf photosynthetic integrity during Verticillium wilt in Capsicum annuum. New Phytol. 2004;163:111–123. doi: 10.1111/j.1469-8137.2004.01092.x. [DOI] [PubMed] [Google Scholar]
- Gayoso C, Pomar F, Novo-Uzal E, Merino F, Martinez de Ilarduya O. The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PAL gene expression. BMC Plant Biol. 2010;10:232–251. doi: 10.1186/1471-2229-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhu LF, Tu LL, Liu LL, Yuan DJ, Jin L, Long L, Zhang XL. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J Exp Bot. 2011;62:5607–5621. doi: 10.1093/jxb/err245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MK, Lyon KJ, Lyon BR. Identification of disease response genes expressed in Gossypium hirsutum upon infection with the wilt pathogen Verticillium dahliae. Plant Mol Biol. 1999;40:289–296. doi: 10.1023/A:1006146419544. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Lu S, Xu YH, Wang JW, Chen XY. A cotton cDNA (GaPR-10) encoding a pathogenesis-related 10 protein with in vitro ribonuclease activity. Plant Sci. 2002;162:629–636. doi: 10.1016/S0168-9452(02)00002-X. [DOI] [Google Scholar]
- Wang YQ, Chen DJ, Wang DM, Huang QS, Yao ZP, Liu FJ, Wei XW, Li RJ, Zhang ZN, Sun YR. Over-expression of Gastrodia anti-fungal protein enhances Verticillium wilt resistance in coloured cotton. Plant Breed. 2004;123:454–459. doi: 10.1111/j.1439-0523.2004.01005.x. [DOI] [Google Scholar]
- Chen JY, Dai XF. Cloning and characterization of the Gossypium hirsutum major latex protein gene and functional analysis in Arabidopsis thaliana. Planta. 2010;231:861–873. doi: 10.1007/s00425-009-1092-2. [DOI] [PubMed] [Google Scholar]
- Munis MFH, Tu LL, Deng FL, Tan JF, Xu L, Xu S, Long L, Zhang XL. A thaumatin-like protein gene involved in cotton fiber secondary cell wall development enhances resistance against Verticillium dahliae and other stresses in transgenic tobacco. Biochem Biophys Res Commun. 2010;393:38–44. doi: 10.1016/j.bbrc.2010.01.069. [DOI] [PubMed] [Google Scholar]
- Tian J, Zhang XY, Liang BG, Li SW, Wu ZX, Wang QH, Leng CX, Dong JL, Wang T. Expression of baculovirus anti-apoptotic genes p35 and op-iap in cotton (Gossypium hirsutum L.) enhances tolerance to Verticillium Wilt. PLoS One. 2010;5:e14218. doi: 10.1371/journal.pone.0014218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BL, Yang YW, Chen TZ, Yu WG, Liu TL, Li HJ, Fan XH, Ren YZ, Shen DY, Liu L, Dou DL, Chang YH. Island cotton GbVe1 gene encoding a receptor-like protein confers resistance to both defoliating and non-defoliating isolates of Verticillium dahliae. PLoS One. 2012;7:e51091. doi: 10.1371/journal.pone.0051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Zhao JY, Zuo KJ, Cao YF, Ling H, Sun XF, Tang KX. Isolation and characterization of an ERF-like gene from Gossypium barbadense. Plant Sci. 2004;167:1383–1389. doi: 10.1016/j.plantsci.2004.07.012. [DOI] [Google Scholar]
- Qu ZL, Wang HY, Xia GX. GhHb1: a nonsymbiotic hemoglobin gene of cotton responsive to infection by Verticillium dahliae. Biochim Biophys Acta. 2005;1730:103–113. doi: 10.1016/j.bbaexp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gao XQ, Wheeler T, Li ZH, Kenerley CM, He P, Shan LB. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 2011;66:293–305. doi: 10.1111/j.1365-313X.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li FG, Liu CL, Zhang CJ, Zhang XY. Construction and analysis of cotton (Gossypium arboreum L.) drought-related cDNA library. BMC Res Notes. 2009;2:120. doi: 10.1186/1756-0500-2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JF, Sanogo S, Flynn R, Baral J, Bajaj S, Hughs S, Percy R. Germplasm evaluation and transfer of Verticillium wilt resistance from Pima (Gossypium barbadense) to upland cotton (G. hirsutum) Euphytica. 2012;187:147–160. doi: 10.1007/s10681-011-0549-0. [DOI] [Google Scholar]
- Wang KB, Wang ZW, Li FG, Ye WW, Wang JY, Song GL, Yue Z, Cong L, Shang HH, Zhu SL, Zou CS, Li Q, Yuan YL, Lu CR, Wei HL, Gou CY, Zheng ZQ, Yin Y, Zhang XY, Liu K, Wang B, Song C, Shi N, Kohel RJ, Percy RG, Yu JZ, Zhu YX, Wang J, Yu SX. The draft genome of a diploid cotton Gossypium raimondii. Nat Genet. 2012;44:1098–1103. doi: 10.1038/ng.2371. [DOI] [PubMed] [Google Scholar]
- Carninci P, Shabata Y, Hayatsu N, Itoh M, Shiraki T, Hirozane T, Watahiki A, Shibata K, Muramatsu M, Hayashizaki Y. Balanced size and long-size cloning of full-length, cap-trapped cDNAs into vectors of the novel lambda-FLC family allows enhanced gene discovery rate and functional analysis. Genomics. 2001;77:79–90. doi: 10.1006/geno.2001.6601. [DOI] [PubMed] [Google Scholar]
- Seki M, Shinozaki K. Functional genomics using RIKEN Arabidopsis thaliana full-length cDNAs. J Plant Res. 2009;122:355–366. doi: 10.1007/s10265-009-0239-3. [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Kamiya A, Ishida J, Satou M, Sakurai T, Nakajima M, Enju A, Akiyama K, Oono Y, Muramatsu M, Hayashizaki Y, Kawai J, Caninci P, Itoh M, Ishii Y, Arakawa T, Shibata K, Shinagawa A, Shinozaki K. Functional annotation of a full-length Arabidopsis cDNA collection. Science. 2002;296:141–145. doi: 10.1126/science.1071006. [DOI] [PubMed] [Google Scholar]
- Seki M, Satou M, Sakurai T, Akiyama K, Iida K, Ishida J, Nakajima M, Enju A, Narusaka M, Fujita M, Shinosaki K. RIKEN Arabidopsis full-length (RAFL) cDNA and its applications for expression profiling under abiotic stress conditions. J Exp Bot. 2004;55:213–223. doi: 10.1093/jxb/erh007. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Plata G, Rodríguez-Zapata F, Seki M, Salcedo A, Toyoda A, Ishiwata A, Tohme J, Sakaki Y, Shinozaki K, Manabu Ishitani M. Sequencing analysis of 20,000 full-length cDNA clones from cassava reveals lineage specific expansions in gene families related to stress response. BMC Plant Biol. 2007;7:66. doi: 10.1186/1471-2229-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R, Schlötterer C, Lelley T. SciRoKo: a new tool for whole genome microsatellite search and investigation. Bioinformatics. 2007;13:1683–1685. doi: 10.1093/bioinformatics/btm157. [DOI] [PubMed] [Google Scholar]
- Galeano CH, Fernández AC, Gómez M, Blair MW. Single strand conformation polymorphism based SNP and Indel markers for genetic mapping and synteny analysis of common bean (Phaseolus vulgaris L.) BMC Genom. 2009;10:629. doi: 10.1186/1471-2164-10-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Holmes MH. DNA sequence quality trimming and vector removal. Bioinformatics. 2001;17:1093–1104. doi: 10.1093/bioinformatics/17.12.1093. [DOI] [PubMed] [Google Scholar]
- Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LD, Yuan DJ, Zhang JW, Wang SP, Zhang QF. A new method for EST clustering. Acta Genet Sin. 2003;30:147–153. [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli C, Jongeneel CV, Bucher P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Intl Conf Intell Syst Mol Biol. 1999. pp. 138–148. [PubMed]
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, Eilbeck K, Lewis S, Marshall B, Mungall C, Richter J, Rubin GM, Blake JA, Bult C, Dolan M, Drabkin H, Eppig JT, Hill DP, Ni L, Ringwald M, Balakrishnan R, Cherry JM, Christie KR, Costanzo MC, Dwight SS, Engel S, Fisk DG, Hirschman JE, Hong EL, Nash RS. et al. Gene Ontology Consortium: The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32:258–261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Götz S, Garcia-Gomez JM, Terol J, Talon M. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M. Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol. 2010;10:49. doi: 10.1186/1471-2229-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Guo AY, Chen X, Gao G, Zhang H, Zhu QH, Liu XC, Zhong YF, Gu X, He K, Luo J. PlantTFDB: a comprehensive plant transcription factor database. Nucleic Acids Res. 2008;36:966–969. doi: 10.1093/nar/gkm841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jin J, Tang L, Zhao Y, Gu X. PlantTFDB 2.0: update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res. 2011;39:1114–1117. doi: 10.1093/nar/gkq1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp PD, Paley S, Romero P. The Pathway Tools software. Bioinformatics. 2002;18:S225–S232. doi: 10.1093/bioinformatics/18.suppl_1.S225. [DOI] [PubMed] [Google Scholar]
- Yuan DJ, Tu LL, Zhang XL. Generation, annotation and analysis of first large-scale expressed sequence tags from developing fiber of Gossypium barbadense L. PLoS One. 2011;6:e22758. doi: 10.1371/journal.pone.0022758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Wang ZW, Zhang GY, Pan YX, Wu LQ, Li ZK, Wang XF, Ma ZY. Construction of molecular genetic map and QTL analysis of fiber quality in cotton (Gossypium hirsutum L.) Acta Agron Sin. 2009;35:2159–2166. doi: 10.3724/SP.J.1006.2009.02159. [DOI] [Google Scholar]
- Zhang WW, Jian GL, Jiang TF, Wang SZ, Qi FJ, Xu SC. Cotton gene expression profiles in resistant Gossypium hirsutum cv. Zhongzhimian KV1 responding to Verticillium dahliae strain V991 infection. Mol Biol Rep. 2012;39(10):9765–9774. doi: 10.1007/s11033-012-1842-2. [DOI] [PubMed] [Google Scholar]
- Ma ZY, Wang XF, Zhang GY, Liu SQ, Sun JZ, Liu JL. Genetic studies of Verticillium wilt resistance among different types of sea island cottons. Acta Agron Sin. 2000;26:315–321. [Google Scholar]
- Wang XF MAJ, Wang WS, Zheng YM, Zhang GY, Liu CJ, Ma ZY. Construction and characterization of the first bacterial artificial chromosome library for the cotton species Gossypium barbadense L. Genome. 2006;49(11):1393–1398. doi: 10.1139/g06-113. [DOI] [PubMed] [Google Scholar]
- Chi JN, Wang XF, Zhou HM, Zhang GY, Sun YX, Li ZK, Ma ZY. Molecular cloning and characterization of the actin-depolymerizing factor gene in Gossypium barbadense. Genes Genet Syst. 2008;83:383–391. doi: 10.1266/ggs.83.383. [DOI] [PubMed] [Google Scholar]
- Chi JN, Han YC, Wang XF, Wu LZ, Zhang GY, Ma ZY. Overexpression of the Gossypium barbadense actin-depolymerizing factor 1 gene mediates biological changes in transgenic tobacco. Plant Mol Biol Rep. 2013;2013 doi:10.1007/s11105-013-0557-4. [Google Scholar]
- Liu HW, Wang XF, Pan YX, Shi RF, Zhang GY, Ma ZY. Mining cotton fiber strength candidate genes based on transcriptome mapping. Chin Sci Bull. 2009;54(24):4651–4657. doi: 10.1007/s11434-009-0708-z. [DOI] [Google Scholar]
- Pan YX, Wang XF, Liu HW, Zhang GY, Ma ZY. Molecular cloning of three UDP-glucuronate decarboxylase genes that are preferentially expressed in Gossypium fibers from elongation to secondary cell wall synthesis. J Plant Biol. 2010;53:367–373. doi: 10.1007/s12374-010-9124-9. [DOI] [Google Scholar]
- Zhang Y, Wang XF, Yang S, Chi JN, Zhang GY, Ma ZY. Cloning and characterization of a Verticillium wilt resistance gene from Gossypium barbadense and functional analysis in Arabidopsis thaliana. Plant Cell Rep. 2011;30:2085–2096. doi: 10.1007/s00299-011-1115-x. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Wang XF, Zhang Y, Liu JF, Wu LZ, Zhang DM, Ma ZY. GbWRKY1, a novel cotton (Gossypium barbadense) WRKY gene isolated from a bacteriophage full-length cDNA library, is induced by infection with Verticillium dahliae. Indian J Biochem Biophys. 2012;49:405–413. [PubMed] [Google Scholar]
- Fradin EF, Thomma BPHJ. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol. 2006;7:71–86. doi: 10.1111/j.1364-3703.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001;6:520–527. doi: 10.1016/S1360-1385(01)02103-3. [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/S1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- McFadden HG, Chapple R, De Feyter R, Dennis E. Expression of pathogenesis-related genes in cotton stems in response to infection by Verticillium dahliae. Physiol Mol Plant Pathol. 2001;58:119–131. doi: 10.1006/pmpp.2001.0320. [DOI] [Google Scholar]
- Li ZK, Wang XF, Ma J, Zhang GY, Ma ZY. Cloning and characterization of a tau glutathione S-transferase subunit encoding gene in Gossypium hirsutum. Genes Genet Syst. 2008;83:219–225. doi: 10.1266/ggs.83.219. [DOI] [PubMed] [Google Scholar]
- Zhu CF, Wang YX, Li YB, Bhatti KH, Tian YC, Wu JH. Overexpression of a cotton cyclophilin gene (GhCyp1) in transgenic tobacco plants confers dual tolerance to salt stress and Pseudomonas syringae pv. tabaci infection. Plant Physiol Biochem. 2011;49:1264–1271. doi: 10.1016/j.plaphy.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ahmed NU, Park JI, Jung HJ, Kang KK, Hur YK, Lim YP, Nou IS. Molecular characterization of stress resistance-related chitinase genes of Brassica rapa. Plant Physiol Biochem. 2012;58:106–115. doi: 10.1016/j.plaphy.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Guo WZ, Zhu HY, Ruan YL, Zhang TZ. Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant Biotechnol J. 2012;10:301–312. doi: 10.1111/j.1467-7652.2011.00662.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Triplett BA, Zhang HB, Lee MK, Hinchliffe DJ, Li P, Fang DD. Cloning and characterization of homeologous cellulose synthase catalytic subunit 2 genes from allotetraploid cotton (Gossypium hirsutum L.) Gene. 2012;494:181–189. doi: 10.1016/j.gene.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Wang SF, Zhang GY, Wu LQ, Chi JN, Li ZK, Ma ZY. EST analysis of suppression subtractive hybridization library from upland cotton resistant cultivar infected by Verticillium dahliae. Cotton Sci. 2010;22(1):17–22. [Google Scholar]
- Zhang J, Zhou JM. Plant immunity triggered by microbial molecular signatures. Mol Plant. 2010;3:783–793. doi: 10.1093/mp/ssq035. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA. 2006;103:11086–11091. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DJ. Fungal cell wall chitinases and glucanases. Microbiology. 2004;150:2029–2035. doi: 10.1099/mic.0.26980-0. [DOI] [PubMed] [Google Scholar]
- Cheng HM, Jian GL, Ni WC, Yang HH, Wang ZX, Sun WJ, Zhang BL, Wang XF, Ma C, Jia SR. Increase of Fusarium- and Verticillium-resistance by transferring chitinase and glucanase gene into cotton. Sci Agric Sin. 2005;38:1160–1166. [Google Scholar]
- Lawrence SD, Novak NG. Expression of poplar chitinase in tomato leads to inhibition of development in Colorado potato beetle. Biotechnol Lett. 2006;28:593–599. doi: 10.1007/s10529-006-0022-7. [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, Scheiner O, Breitenbach M. The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–1938. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matton DP, Brisson N. Cloning, expression, and sequence conservation of pathogenesis-related gene transcripts of potato. Mol Plant Microbe Interact. 1989;2:325–331. doi: 10.1094/MPMI-2-325. [DOI] [PubMed] [Google Scholar]
- Barratt DHP, Clark JA. Proteins arising during the late stages of embryogenesis in Pisum sativum L. Planta. 1991;184:14–23. doi: 10.1007/BF00208230. [DOI] [PubMed] [Google Scholar]
- Crowell DN, John ME, Russell D, Amasino RM. Characterization of a stress-induced, developmentally regulated gene family from soybean. Plant Mol Biol. 1992;18:459–466. doi: 10.1007/BF00040662. [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JD. Role of plant hormones in plant defense responses. Plant Mol Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Grant MR, Jones JD. Hormone (dis) harmony moulds plant health and disease. Science. 2009;324:750–752. doi: 10.1126/science.1173771. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RA, Ritsema T, Pieterse CM. Ethylene modulates the role of nonexpressor of pathogenesis-related genes1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009;149:1797–1809. doi: 10.1104/pp.108.133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA. 2007;104:18842–18847. doi: 10.1073/pnas.0708139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubery IA, Smit F. Phenylalanine ammonia-lyase from cotton (Gossypium hirsutum) hypocotyls: properties of the enzyme induced by a Verticillium dahliae phytotoxin. Biochim Biophys Acta. 1994;1207:24–30. doi: 10.1016/0167-4838(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Naoumkina MA, Zhao Q, Gallego-Giraldo L, Dai X, Zhao PX, Dixon RA. Genome-wide analysis of phenylpropanoid defence pathways. Mol Plant Pathol. 2010;11:829–846. doi: 10.1111/j.1364-3703.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010;153:1526–1538. doi: 10.1104/pp.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjamaa K, Kukkola EM, Fagerstedt KV. The role of xylem class III peroxidases in lignification. J Exp Bot. 2009;60:367–376. doi: 10.1093/jxb/ern278. [DOI] [PubMed] [Google Scholar]
- Berthet S, Demont-Caulet N, Pollet B, Bidzinski P, Cézard L, Bris PL, Borrega N, Hervé J, Blondet E, Balzergue S, Lapierre C, Jouanin L. Disruption of Laccase4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell. 2011;23:1124–1137. doi: 10.1105/tpc.110.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cotton naturally grew in the field. (A)G. barbadense cv. Pima90-53 displayed excellent resistance against V. dahliae.(B)G. hirsutum cv. Han208 naturally infected Verticillium wilt in the field. (C) Severe browning of vascular tissue in a longitudinal section of infected plants. (D) Typical sectorial necrosis from which V. dahliae mycelium may be re-isolated (E). Monospore was cultured on 25% potato dextrose agar. (F) Mycelium of V. dahliae was observed by optic microscope.
The unigenes used in Q-PCR analysis.
Unigenes of known disease/stress response functions discovered in response to V. dahliae infection in resistant G. barbadense cv. Pima90-53.
The most frequent sequences in our cDNA library.
Number of dinucleotide, trinucleotide and tetranucleotide repeats.