Abstract
Background
Irritable bowel syndrome (IBS) is a common chronic functional gastrointestinal disorder. Post-infectious IBS (PI-IBS) is a subset of IBS that accounts for a large proportion of IBS patients. The PI-IBS symptoms meet the Rome criteria for IBS with diarrhoea (IBS-D) or IBS with mixed bowel habits (IBS-M). A low-grade inflammation has been reported to occur in PI-IBS. Abnormalities in intestinal endocrine cells have been reported in both sporadic IBS and PI-IBS.
Case presentation
A 20-year-old female with a diagnosis of IBS with constipation (IBS-C), according to Rome III criteria, contracted Campylobacter-induced gastroenteritis, after which her symptom pattern changed to IBS-M. She showed an intestinal low-grade inflammation that was manifested by an increase in the number of intraepithelial and lamina propria leucocytes and lymphocytes and an increase in the density of mast cells in lamina propria. There was also an increase in the density of intestinal serotonin and peptide YY (PYY) cells and a decrease in the density of rectal somatostatin cells. Follow-up of the patient at 4-months post-infection revealed reduction of IBS symptoms and an improvement in her quality of life. However, 6 months following the Campylobacter infection, the patient switched back from IBS-M to IBS-C, probably due to recovery from PI-IBS. The patient was treated with prucalopride, which is serotonin 5HT4 receptor agonist. Six months later following this treatment, the symptoms were reduced and the quality of life improved in the reported patient.
Conclusions
Gastroenteritis in patients with IBS-C causes a post-infectious, low-grade inflammation. Interaction between immune-cells and intestinal endocrine cells increases the density of certain endocrine cells, which in turn might be responsible for the change in the symptom pattern, the milder symptoms and the improvement in the quality of life seen in the reported patient. The findings in this case raise the question as to whether intestinal infections are responsible for the previously reported switching of IBS from one subtype to another over time.
Keywords: Campylobacter, Irritable bowel syndrome, Peptide YY, Quality of life, Serotonin, Somatostatin
Background
Irritable bowel syndrome (IBS) is a common chronic functional gastrointestinal disorder, that is characterized by frequent abdominal pain/discomfort, abdominal bloating/distension and an altered stool pattern [1-4]. Post-infectious IBS (PI-IBS) is a subset of IBS, and is characterized as a sudden onset of IBS symptoms following gastroenteritis in individuals who have had no gastrointestinal complaints [5]. The proportion of patients developing IBS following gastroenteritis varied between studies, from 3.7% to 36% [5]. Patients with IBS are more common in patients presenting with bacterial gastroenteritis to primary care physician than community controls [6]. This may indicate that IBS patients are predisposed to bacterial gastroenteritis, or that they tend to seek their doctor for bowel symptoms more often than the background population. Human infections caused by Campylobacter jejuni are a leading cause of food-borne enteritis, the bacteria usually being transmitted by the ingestion of undercooked poultry, or contact with farm animals. This infection leads to PI-IBS in 9-13% of cases [5,7-9]. The symptoms of PI-IBS meet the Rome criteria for IBS with diarrhoea (IBS-D) or IBS with mixed bowel habits (IBS-M) [10-12].
In IBS, there appears to be a general depletion of gastrointestinal endocrine cells, and especially serotonin and PYY cells [13,14], whereas in PI-IBS there is an increase in the density of these cells, especially serotonin and PYY cells [1,5,11]. Furthermore, a low-grade inflammation has been reported in PI-IBS, which is manifested by increased intraepithelial lymphocytes and an infiltration of mast cells in the lamina propria of the large intestine [5,15-17]. It has been suggested that the alterations in the population of gastrointestinal endocrine cells and the low-grade inflammation play a role in the pathogeneses of both sporadic and PI-IBS [1,5,13].
Case presentation
A 20-year-old female was investigated for recurrent abdominal pain, abdominal distension, constipation and nausea. She had a bowel movement every 7–10 days, with straining at defecation and hard or lumpy stools. She was non-smoker and was not currently taking any medications. This patient had suffered from these symptoms since her childhood. Her mother had similar symptoms and had a diagnosis of IBS. Her symptoms affected her schoolwork and isolated her socially; she has been hospitalized on many occasions. The patient submitted to a complete physical examination and was investigated by means of blood (full blood count, electrolytes, calcium, and inflammatory markers), liver, and thyroid function tests. She also underwent gastroscopy with duodenal biopsy sampling and colonoscopy with segmental biopsy sampling. The findings of all these examinations and tests were normal. The patient fulfilled Rome III criteria and was thus given the diagnosis of IBS with constipation (IBS-C). She was asked to complete the three following questionnaires (Table 1): Birmingham IBS Symptom scores, Short-Form Nepean Dyspepsia Index (SF-NDI) measuring the reduction in quality of life and Irritable Bowel Syndrome quality of life (IBS-QOL) [18-20]. She was then submitted to a non-pharmacological treatment program at our clinic, which includes provision of information and reassurance, dietary guidance, regular exercise and regular intake of probiotics [21]. Her symptoms subsequently reduced and her quality of life improved.
Table 1.
Symptoms and quality of life in the patient before, during and after Campylobacter infection
|
Questionnaire |
Before infection |
During infection |
After infection |
|||
|---|---|---|---|---|---|---|
| 2 months | 4 months | 6 months | 12 months | |||
| Birmingham |
|
|
|
|
|
|
| Total score |
30 |
35 |
26 |
18 |
29 |
4 |
| Pain |
6 |
15 |
4 |
3 |
6 |
2 |
| Diarrhoea |
4 |
20 |
12 |
8 |
5 |
0 |
| Constipation |
20 |
0 |
10 |
7 |
20 |
2 |
| SF-NDI |
29 |
48 |
20 |
17 |
28 |
13 |
| IBS-QOLa | 61 | 50 | 84 | 86 | 60 | 94 |
Six months after infection, the patient was treated with 2 mg prucalopride daily.
Birmingham, Birmingham Irritable Bowel Syndrome Symptom Questionnaire; SF-NDI, Short-Form Nepean Dyspepsia Index; IBS-QOL, Irritable Bowel Syndrome Quality Of Life Questionnaire.
aPercentage of the total score.
Seven months later, the patient was referred to the causal department because of a 3-day history of bloody diarrhoea occurring between 10 to 15 times daily, extreme fatigue and dehydration. She did not have a fever and with the exception of C-reactive protein (CRP), which was 17 mg/l (normal range 0–10 mg/l), her blood tests were normal. Colonoscopy revealed severe colonic inflammation with erythema, oedema, friable mucosae, haemorrhagic spots and ulcers. Biopsy samples taken during colonoscopy revealed preserved crypt architecture. However, a focal increase in the density of immune cells in the lamina propria and focal cryptitis and crypt abscesses were observed. Stool culture was positive for Campylobacter jujeni. The patient was treated with 400-mg metronidazole, twice daily for 2 weeks.
The findings of a physical examination and blood tests performed at follow-up visits at the outpatient clinic 2, 4, 6 and 12 months after Campylobacter infection were normal. Colonoscopy at 2 and 4 months visits revealed a normal endoscopic appearance. Moreover, the patient’s general condition was improved. Her symptom pattern had changed and she experienced an improvement in her quality of life (Table 1). Reassessment of her symptoms according to Rome III criteria put the patient into the IBS-M subtype. Six months following the Campylobacter infection, the patient suffered from abdominal pain, abdominal distension, constipation and nausea in the same degree as before the infection. She was treated with 2 mg prucalopride daily. Six months later, the patient’s symptom was reduced and her quality of life improved (Table 1).
Colonic and rectal biopsy samples obtained during colonoscopy before, during, and 2 and 4 months after Campylobacter infection were fixed overnight in 4% buffered paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. The sections were immunostained with the avidin-biotin –complex (ABC) method using Vectastain ABC-kit and 3,3′-diaminobenzidine (DAB) peroxidase Substrate Kit (Vector laboratories). The sections were incubated with the primary antiserum/antibody at room temperature for 2 h. The sections were then washed in PBS buffer and incubated with biotinylated swine anti-mouse (in the case of monoclonal antibodies) or anti-rabbit IgG (in the case of polyclonal antibodies) diluted 1:200 for 30 min at room temperature. After washing the slides in PBS buffer, the sections were incubated for 30 min with avidin-biotin-peroxidase complex diluted 1:100, and then immersed in 3,3′-diaminobenzidine (DAB) peroxidase substrate, followed by counterstaining in hematoxylin. The following primary antisera/antibodies were used: monoclonal mouse anti-N-terminal of purified Chromogranin A (Dako, code no. M869), monoclonal mouse anti-serotonin (Dako, code no. 5HT-209), polyclonal anti-porcine peptide PYY (Alpha-Dagnostica, code PYY 11A), polyclonal rabbit anti-synthetic-human PP (Diagnostic Biosystems, code no. #114), polyclonal rabbit anti-porcine glicentin/glucagon (Acris Antibodies, code BP508), polyclonal rabbit anti-synthetic-human somatostatin (Dako, code no. A566); monoclonal mouse anti-human CD45 (Dako, code no. M0701), monoclonal mouse anti-human CD47 (Dako, code no. I5647), monoclonal mouse anti-human CD68 (Dako, code no. M0814) and monoclonal mouse anti-human mast cell tryptase (Dako, code no. M7052). CD45 is considered as a leucocyte common antigen and is expressed exclusively on cells of the hematopoietic system and their progenitors. CD57 is expressed by subsets of NK cells and CD8+ lymphocytes, and by a small percentage of CD4+/CD45R0+ T lymphocytes. CD68 labels human monocytes, macrophages and myeloid cells. Human mast cell tryptase comprise a family of trypsin-like neutral serine proteases that are predominantly expressed in mast cells. The total leucocytes, lymphocytes and mast cells, as well as chromogranin A, serotonin, peptide YY (PYY), and somatostatin cells. The densities of these cells were quantified by computerized image analysis using Olympus cellSens imaging software (version 1.7) on a computer linked to an Olympus microscope type BX 43 with an Olympus camera (DP 26). A ×40 objective was used, for which each frame (field) on the monitor represented a tissue area of 0.14 mm2 of the tissue. The number intraepithelial leucocytes cells and the endocrine cells as well as the area of the epithelial cells were measured in each field. The number of leucocytes, lymphocytes, and mast cells in lamina propria were counted per microscopic field. All measurements were done in 10 randomly chosen fields for each individual.
The densities of both intraepithelial and lamina propria leucocytes and lymphocytes were increased in both the colon and rectum at 2 and 4 months after the Campylobacter infection (Figure 1), as were the number of mast cells in the lamina propria in both the colon and rectum (Figure 2, Tables 2 and 3). The total number of endocrine cells in the colon and rectum prior to Campylobacter infection (as detected by chromogranin A staining) was low, but within the normal limits (Tables 4 and 5). This is in agreement with previously published results in IBS-C patients [22,23]. Although chromogranin A is used as a common marker for peptide hormone containing cells, chromogranin A immunoreactivity varies between gastrointestinal segments and even within population of the same endocrine cell type [24]. It has been found that chromogranin A- immunoreactive cells are not representative of the entire population of endocrine cells and that they are the least numerous of all of the endocrine cells combined [25]. The densities of serotonin and PYY cells had increased in both the colon and rectum during, 2 and 4 months post-infection (Figure 3). However, somatostatin cell density in the rectum was reduced in the rectum during and after Campylobacter infection.
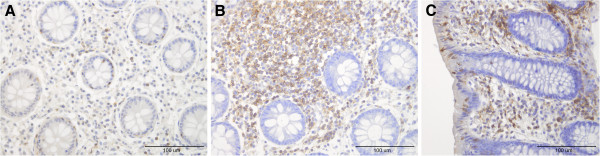
Figure 1.
Leucocytes in the patient before Campylobacter infection (A), during (B) and 4-months after (C) Campylobacter infection.
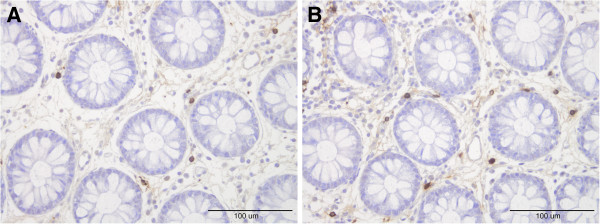
Figure 2.
Mast cells in the lamina propria before Campylobacter infection (A) and (B) 4- months after Campylobacter infection.
Table 2.
Number of colonic intraepithelial (IE) and lamina propria (LP) immune cells before, during and after Campylobacter infection
| Cell type | Before infection | During infection |
After infection |
Controlsc 95% confidence interval | |
|---|---|---|---|---|---|
| 2 months | 4 months | ||||
| Leucocytes in LPa |
69 |
268 |
102 |
199 |
81-118 |
| Leucocytes in IEb |
110 |
224 |
162 |
150 |
78-115 |
| Lymphocytes in LPa |
3 |
39 |
2 |
2 |
0-5 |
| Lymphocytes in IEb |
1 |
6 |
7 |
8 |
0-2 |
| Mast cellsa | 7 | 17 | 11 | 12 | 6-10 |
Quantifications of cells were conducted in ten randomly chosen fields using the Olympus CellSense software.
aNumber of cells per field.
bNumber of cells per mm2 of epithelium.
cThe control group comprised 27subjects (16 females and 11 males; mean age 52 years, range 20–69 years) who had submitted to colonoscopy for the following reasons: gastrointestinal bleeding, where the source of bleeding was identified as haemorrhoids (n=18), or angiodysplasia (n=2), and health worries resulting from a relative being diagnosed with colon carcinoma (n=7).
Table 3.
Densities of rectal IE and LP immune endocrine cells before, during and after Campylobacter infection
| Cell type | Before infection | During infection |
After infection |
Controls 95% confidence interval | |
|---|---|---|---|---|---|
| 2 months | 4 months | ||||
| Leucocytes in LP |
71 |
298 |
104 |
202 |
82–112 |
| Leucocytes in IE |
105 |
224 |
172 |
153 |
81–120 |
| Lymphocytes in LP |
1 |
42 |
2 |
2 |
0-6 |
| Lymphocytes in IE |
2 |
7 |
7 |
9 |
0-2 |
| Mast cells | 9 | 19 | 14 | 15 | 9-12 |
Quantifications and controls are the same as in Table 2.
Table 4.
Endocrine cell densities in the colon before, during and after Campylobacter infection
| Cell type | Before infection | During infection |
After infection |
Controls 95% confidence interval | |
|---|---|---|---|---|---|
| 2 months | 4 months | ||||
| Chromogranin A |
7 |
59 |
50 |
20 |
32-43 |
| Serotonin |
5 |
32 |
32 |
28 |
27-32 |
| PYY | 4 | 29 | 20 | 15 | 6-10 |
Quantifications and controls are the same as in Table 2.
Table 5.
Densities of rectal endocrine cells before, during and after Campylobacter infection
| Cell type | Before infection | During infection |
After infection |
Controls 95% confidence interval | |
|---|---|---|---|---|---|
| 2 months | 4 months | ||||
| Chromogranin A |
35 |
154 |
50 |
65 |
108–136 |
| Serotonin |
21 |
83 |
32 |
43 |
32–51 |
| PYY |
16 |
49 |
24 |
29 |
54–67 |
| Somatostatin | 22 | 9 | 3 | 15 | 14–20 |
Quantifications and controls are the same as in Table 2.
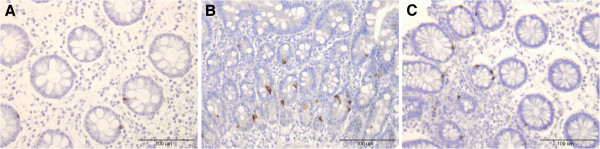
Figure 3.
Serotonin immunoreactive cells before (A), during (B) and 4 months after (C) Campylobacter infection.
Discussion
Consistent with previously published observations, the present case developed a low-grad inflammation following Campylobacter infection [1,5,11,16,26-31]. An increase in the densities of intestinal endocrine cells, and especially serotonin and PYY cells, has been reported in Crohn’s disease, ulcerative colitis and lymphocytic colitis [32-35]. An increase in the density of intestinal endocrine has also been described in PI-IBS [5,11,15,16,28,30,31,33]. Several studies have shown that inflammation and immune cells affect the neuroendocrine system of the gut (the endocrine/immune axis) [1,36]. It seems that infection/inflammation induces an increase in the population of certain gut endocrine cells through an interaction between those cells and immune cells [1,36].
The pattern of symptoms in the present patient changed from IBS-C to IBS-M with much less abdominal pain. Serotonin activates the submucosal sensory branch of the enteric nervous system, and controls gastrointestinal motility and chloride secretion via inter-neurons and motor neurons [13,37-42]. PYY delays gastric emptying, inhibits gastric and pancreatic secretion, and is a major ileal brake mediator [13,43,44]. Moreover, PYY inhibits prostaglandin (PG) E2 and vasoactive intestinal peptide (VIP), both of which stimulate intestinal secretion [13,45-47]. Administration of PYY inhibits diarrhoea in experimental animals by reducing intestinal fluid secretion and slowing colon transit [13,48]. Somatostatin inhibits intestinal contraction, and inhibits gut exocrine and neuroendocrine secretion [13]. It is therefore conceivable, that the changes in the present patient’s symptoms are attributable to the reported changes in the density of the endocrine cells.
It is not uncommon for IBS patients to switch from one subtype to another over time [49-52]. The patient presented here switched from the IBS-C subtype to the IBS-M subtype following a bout of gastroenteritis, and it is possible that intestinal infection was the underlying cause of this switch. However, 6 months following the Campylobacter infection, the patient switched back from IBS-M to IBS-C. Campylobacter jejuni produces a range of toxins including cytolethal distending toxin (24), which first produces secretory diarrhoea in the small intestine early in the illness, after which there is invasion of the distal ileum and colon to produce an inflammatory ileocolitis, which can extend all the way to the rectum [53]. It has been reported that PI-IBS symptoms following Campylobacter infection decline with time [54-56]. It is conceivable, therefore, to conclude that the patient returning to her original symptoms represent a recovering form PI-IBS.
The symptoms were reduced and the quality of life improved in the patient following the treatment with prucalopride, which is a highly selective serotonin 5HT4 receptor agonist that has been shown to stimulate gut motility [57]. The patient disclosed a low density of colonic serotonin cells, which is in line with previously published observations in IBS patients [14]. This may explain why a serotonin agonist was effective in the treatment of the reported patient.
Conclusions
Gastroenteritis due to Campylobacter infection in patients with IBS-C causes low-grade inflammation and changes in the densities of intestinal endocrine cells. These changes may be responsible for the change in symptom pattern and the switch from IBS-C to IBS-M that were observed in the reported patient. The patient switched back to IBS-C, 6 months following the Campylobacter infection, probably as a recovery from IP-IBS. Furthermore, treatment with serotonin agonist was successful in the reported patient, who disclosed reduced colonic serotonin cell density.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ME planned the study, recruited and followed-up the patients, performed three of the four colonoscopies, quantified the immune and endocrine cells, analysed the data and drafted the manuscript. TM contributed to patient follow-up, performed one of the four colonoscopies, contributed to the data analysis of and writing this manuscript. DG contributed to the data analysis and writing this manuscript. JGH checked the data, reviewed the manuscript and contributed to discussions. TH checked the data, reviewed the manuscript and contributed to discussions. All of the authors read and approved the final version of this manuscript.
Contributor Information
Magdy El-Salhy, Email: magdy.el-salhy@helse-fonna.no.
Tarek Mazzawi, Email: tarek.ramzi.elia.mazzawi@helse-fonna.no.
Doris Gundersen, Email: doris.Irene.Gundersen@helse-fonna.no.
Jan G Hatlebakk, Email: jan.hatlebakk@helse-bergen.no.
Trygve Hausken, Email: trygve.hausken@helse-bergen.no.
Acknowledgement
This study was supported by a grant from Helse-Fonna.
References
- El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Chromogranin a cell density as a diagnostic marker for lymphocytic colitis. Dig Dis Sci. 2012;57:3154–3159. doi: 10.1007/s10620-012-2249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agreus L, Svardsudd K, Nyren O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Thompson WG, Heaton KW. Functional bowel disorders in apparently healthy people. Gastroenterology. 1980;79:283–288. [PubMed] [Google Scholar]
- Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. et al. U.S. householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil. 2012;18(3):258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry SD, Stansfield R, Jelley D, Gregory W, Phillips E, Barton JR, Welfare MR. Does bacterial gastroenteritis predispose people to functional gastrointestinal disorders? a prospective, community-based, case–control study. Am J Gastroenterol. 2003;98:1970–1975. doi: 10.1111/j.1572-0241.2003.07664.x. [DOI] [PubMed] [Google Scholar]
- Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, Reddy S, Ahuja SD, Helfrick DL, Hardnett F. et al. Risk factors for sporadic campylobacter infection in the United States: a case–control study in foodnet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- Kapperud G, Skjerve E, Bean NH, Ostroff SM, Lassen J. Risk factors for sporadic campylobacter infections: results of a case–control study in southeastern Norway. J Clin Microbiol. 1992;30:3117–3121. doi: 10.1128/jcm.30.12.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314(7083):779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53(8):1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/S1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- Roalfe AK, Roberts LM, Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC gastroenterology. 2008;8:30. doi: 10.1186/1471-230X-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther. 2001;15(2):207–216. doi: 10.1046/j.1365-2036.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–411. doi: 10.1023/A:1018831127942. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Lillebo E, Reinemo A, Salmelid L, Hausken T. Effects of a health program comprising reassurance, diet management, probiotics administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterology insights. 2010;2:21–26. [Google Scholar]
- El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439. doi: 10.3109/00365521.2010.503965. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Mazzawi T, Gundersen D, Hausken T. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol Med Report. 2012;6:1223–1225. doi: 10.3892/mmr.2012.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin Y, Muller-Koppel L, Aunis D, Bader MF, Grube D. Chromogranin A (CgA) in the gastro-entero-pancreatic (GEP) endocrine system II. CgA in mammalian entero-endocrine cells. Histochemistry. 1989;92(4):265–275. doi: 10.1007/BF00500540. [DOI] [PubMed] [Google Scholar]
- Sandstrom O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech Ageing Dev. 1999;108:39–48. doi: 10.1016/S0047-6374(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Weston AP, Biddle WL, Bhatia PS, Miner PB Jr. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–1595. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O’Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, pyschological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection- an observation in a small case control study. Younsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta histochemica. 2002;104(2):185–192. doi: 10.1078/0065-1281-00641. [DOI] [PubMed] [Google Scholar]
- Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Tari A, Teshima H, Sumii K, Haruma K, Ohgoshi H, Yoshihara M, Kajiyama G, Miyachi Y. Peptide YY abnormalities in patients with ulcerative colitis. Japanese journal of medicine. 1988;27(1):49–55. doi: 10.2169/internalmedicine1962.27.49. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. High densities of serotonin and peptide YY cells in the colon of patients with lymphocytic colitis. World J Gastroenterol. 2012;18(42):6070–6075. doi: 10.3748/wjg.v18.i42.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Tack JF, Janssens J, Vantrappen G, Wood JD. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am J Physiol. 1992;263:G838–846. doi: 10.1152/ajpgi.1992.263.6.G838. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. discussion 280. [PMC free article] [PubMed] [Google Scholar]
- Michel K, Sann H, Schaaf C, Schemann M. Subpopulations of gastric myenteric neurons are differentially activated via distinct serotonin receptors: projection, neurochemical coding, and functional implications. J Neurosci. 1997;17:8009–8017. doi: 10.1523/JNEUROSCI.17-20-08009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake–inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read NW, McFarlane A, Kinsman RI, Bates TE, Blackhall NW, Farrar GB, Hall JC, Moss G, Morris AP, O’Neill B. et al. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology. 1984;86:274–280. [PubMed] [Google Scholar]
- Goumain M, Voisin T, Lorinet AM, Ducroc R, Tsocas A, Roze C, Rouet-Benzineb P, Herzog H, Balasubramaniam A, Laburthe M. The peptide YY-preferring receptor mediating inhibition of small intestinal secretion is a peripheral Y(2) receptor: pharmacological evidence and molecular cloning. Mol Pharmacol. 2001;60:124–134. doi: 10.1124/mol.60.1.124. [DOI] [PubMed] [Google Scholar]
- Souli A, Chariot J, Voisin T, Presset O, Tsocas A, Balasubramaniam A, Laburthe M, Roze C. Several receptors mediate the antisecretory effect of peptide YY, neuropeptide Y, and pancreatic polypeptide on VIP-induced fluid secretion in the rat jejunum in vivo. Peptides. 1997;18:551–557. doi: 10.1016/S0196-9781(97)00069-7. [DOI] [PubMed] [Google Scholar]
- Whang EE, Hines OJ, Reeve JR Jr, Grandt D, Moser JA, Bilchik AJ, Zinner MJ, McFadden DW, Ashley SW. Antisecretory mechanisms of peptide YY in rat distal colon. Dig Dis Sci. 1997;42:1121–1127. doi: 10.1023/A:1018869116284. [DOI] [PubMed] [Google Scholar]
- Moriya R, Shirakura T, Hirose H, Kanno T, Suzuki J, Kanatani A. NPY Y2 receptor agonist PYY(3–36) inhibits diarrhea by reducing intestinal fluid secretion and slowing colonic transit in mice. Peptides. 2010;31:671–675. doi: 10.1016/j.peptides.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Morris CB, Hu Y, Toner BB, Diamant N, Leserman J, Shetzline M, Dalton C, Bangdiwala SI. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128(3):580–589. doi: 10.1053/j.gastro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Mearin F, Balboa A, Badia X, Baro E, Caldwell E, Cucala M, Diaz-Rubio M, Fueyo A, Ponce J, Roset M. et al. Irritable bowel syndrome subtypes according to bowel habit: revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003;15(2):165–172. doi: 10.1097/00042737-200302000-00010. [DOI] [PubMed] [Google Scholar]
- Mearin F, Baro E, Roset M, Badia X, Zarate N, Perez I. Clinical patterns over time in irritable bowel syndrome: symptom instability and severity variability. Am J Gastroenterol. 2004;99:113–121. doi: 10.1046/j.1572-0241.2003.04023.x. [DOI] [PubMed] [Google Scholar]
- Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Fluctuation of gastrointestinal symptoms in the community: a 10-year longitudinal follow-up study. Aliment Pharmacol Ther. 2008;28:1013–1020. doi: 10.1111/j.1365-2036.2008.03813.x. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Geboes K, Ponette E, Coremans G, Vantrappen G. Acute infective colitis caused by endemic pathogens in western Europe: endoscopic features. Endoscopy. 1982;14:212–219. doi: 10.1055/s-2007-1021624. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Spence M. To "lump" or to "split" the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosom Med. 2006;68:463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- Spence MJ, Moss-Morris R. The cognitive behavioural model of irritable bowel syndrome: a prospective investigation of patients with gastroenteritis. Gut. 2007;56:1066–1071. doi: 10.1136/gut.2006.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. quiz 660. [DOI] [PubMed] [Google Scholar]
- Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation–a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315–328. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]