Abstract
The saccule is a vestibular sensory organ that depends upon regulation of its luminal fluid, endolymph, for normal transduction of linear acceleration into afferent neural transmission. Previous studies suggested that endolymph in the saccule was merely derived from cochlear endolymph. We developed and used a preparation of isolated mouse saccule to measure transepithelial currents from the extramacular epithelium with a current density probe. The direction and pharmacology of transepithelial current was consistent with Na+ absorption by the epithelial Na+ channel (ENaC) and was blocked by the ENaC-specific inhibitors benzamil and amiloride. Involvement of Na+,K+-ATPase and K+ channels was demonstrated by reduction of the current by ouabain and the K+ channel blockers Ba2+, XE991, and 4-AP. Glucocorticoids upregulated the current via glucocorticoid receptors. Dexamethasone stimulated the current after 24 h and the stimulation was blocked by mifepristone but not spironolactone. No acute response was observed to elevated cAMP in the presence of amiloride nor to bumetanide, a blocker of Na+,K+,2Cl− cotransporter. The results are consistent with a canonical model of corticosteroid-regulated Na+ absorption that includes entry of luminal Na+ through apical membrane Na+ channels and active basolateral exit of Na+ via a Na+ pump, with recycling of K+ at the basolateral membrane via K+-permeable channels. These observations provide our first understanding of the active role played by saccular epithelium in the local regulation of the [Na+] of endolymph for maintenance of our sense of balance.
Introduction
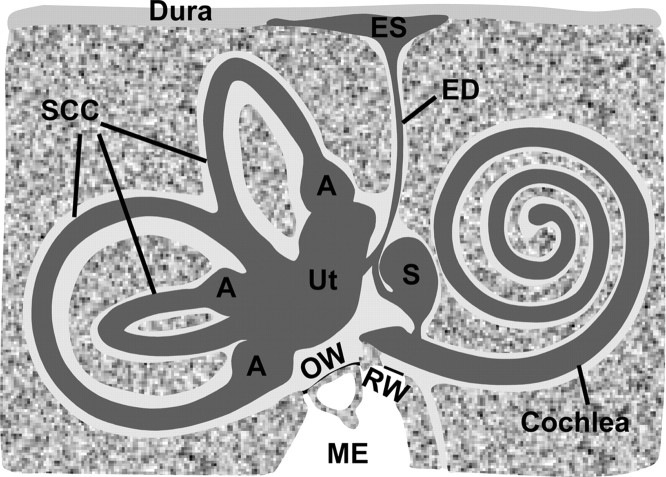
The saccule and utricle are vestibular organs that transduce linear acceleration to nerve impulses, which propagate to the brain and contribute to balance. The saccule is situated in the endolymphatic system between the base of the cochlea and the rest of the vestibular labyrinth (Fig. 1). Acceleration produces movement relative to surrounding structures of the otoconial membrane, which is coupled to the stereocilia of the hair cells in the maculae. Displacement of the stereocilia modulates transduction channels near the tips of the stereocilia, which results in receptor potential changes at the base of the hair cells that modulate transmitter release into the synapses with the vestibular nerve (Moser et al., 2006). K+ (∼150 mm) is the most abundant ion in the luminal fluid (endolymph) of the saccule and is many times higher than in the basolateral fluid (perilymph; ∼5 mm). In contrast, the other major cations in saccular endolymph, Na+ (∼3 mm) and Ca2+ (∼90 μm) (Sellick and Johnstone, 1972; Salt et al., 1989), are much lower than in perilymph (150 mm Na+ and 0.7 mm Ca2+) (Wangemann and Schacht, 1996).
Figure 1.
Structure of the inner ear. The inner ear comprises the endolymphatic space and perilymphatic spaces, which are filled with endolymph ([K+] = ∼150 mm; [Na+] = ∼1–10 mm) and perilymph ([K+] = ∼5 mm; [Na+] = ∼150 mm), respectively. The saccule (S) is situated in the endolymphatic system between the base of the cochlea and the rest of the vestibular labyrinth [utricle (Ut), ampulla (A), and semicircular canals (SCC)]. ES, Endolymphatic sac; ED, endolymphatic duct; OW, oval window; RW, round window; ME, middle ear. Endolymph composition of the saccule was previously thought to be completely derived from cochlear endolymph by longitudinal diffusion; the present study demonstrates that at least Na+ composition of the saccule is regulated locally by absorptive transport. The drawing was adapted with permission from Lim (2002) (their Fig. 2–4).
The production and maintenance of these ion concentration differences is essential for normal function of the vestibular organs. Although K+ is the primary current-carrying ion species for transduction, Na+ and Ca2+ concentrations in endolymph must be maintained for control of hair cell function since the transduction channels are cation permeable, but not K+ selective (Jørgensen and Kroese, 1994). Flooding or fluctuations of the hair cell cytosol with Na+ and Ca2+ would be expected to lead to cellular dysfunction and vertigo.
It is well established that the utricle, ampullae, and common crus of the semicircular canals all contain vestibular dark cell epithelia that secrete K+, while the saccule is devoid of vestibular dark cells (Kimura, 1969; Burnham and Stirling, 1984; Marcus and Shen, 1994; Marcus and Shipley, 1994). In fact, the saccular epithelium, outside of the sensory macular area, has been reported to consist of a single, simple cuboidal cell type (Smith, 1970). Based on these and other observations (Sellick and Johnstone, 1972), it was proposed that saccular endolymph originates from the cochlea by longitudinal flow and/or diffusion and is not produced in this organ. However, [Na+] in saccular endolymph (∼3 mm) is different from other inner ear organs: higher than in the cochlea (∼1 mm) but lower than in the endolymphatic sac (∼129 mm) and utricle (∼10 mm) (Sellick and Johnstone, 1972; Wangemann and Schacht, 1996), suggesting that the saccule may have its own [Na+] regulatory mechanism.
We hypothesized that saccular extramacular epithelium is responsible for active Na+ absorption from endolymph. We sought functional evidence of electrogenic transepithelial ion transport by extramacular epithelium of adult mouse saccule and examined whether it is controlled by glucocorticoids via the glucocorticoid receptor (GR) and/or the mineralocorticoid receptor (MR) by electrophysiologic and pharmacologic means. The experimental strategy used in the current study of transepithelial transport by the saccule is based on that used for the study of Na+ transport across many epithelia, including others in the inner ear (Pondugula et al., 2004; Pondugula et al., 2006; Kim et al., 2009).
Materials and Methods
Tissue preparation.
C57BL/6 mice (4–8 weeks old) were anesthetized with 4% tribromoethanol (0.016 ml/g of body weight, i.p.) and killed by decapitation under a protocol approved by the Institutional Animal Care and Use Committee of Kansas State University. The cochlea was removed from the temporal bone and the whole membranous labyrinth of the vestibule was carefully removed (Fig. 2A). The saccule was separated with microscissors from the utricle (Fig. 2A,B), and saccular extramacular epithelium was carefully separated from the saccular macula with one side remaining attached to the longest edge of the macula (Fig. 2B,C). The tissue was then folded with the apical side of the epithelium facing outward (Fig. 2C,D). The tissue was mounted in a perfusion chamber on the stage of an inverted microscope (Axiovert 200; Carl Zeiss) and continuously perfused at 37°C at an exchange rate of 1.1 times/s (Fig. 2E). For investigation of the effect of dexamethasone via GR and/or MR, dissected saccules were divided into four groups and incubated for 24 h in DMEM-F12 medium (Invitrogen) supplemented with 100 U/ml penicillin (Sigma) and 100 μg/ml streptomycin (Sigma) at 37°C in a 5% CO2 atmosphere with saturated humidity. Each of two groups was incubated in the presence or absence of 100 nm cyclodextrin-encapsulated dexamethasone (Sigma), a concentration that corresponds to the physiological and therapeutic concentration of dexamethasone (Balis et al., 1987; Braat et al., 1992) and to the concentration that produced a peak response from semicircular canal epithelial cells (Pondugula et al., 2004). The other two groups were treated with 100 nm dexamethasone in the presence and absence of either the GR antagonist mifepristone (100 nm, Sigma) or MR antagonist spironolactone (100 nm, Sigma). Inhibitors of the receptors were added concurrently with the agonist for 24 h. After 24 h, incubated saccules were prepared for current density measurement as described above.
Figure 2.
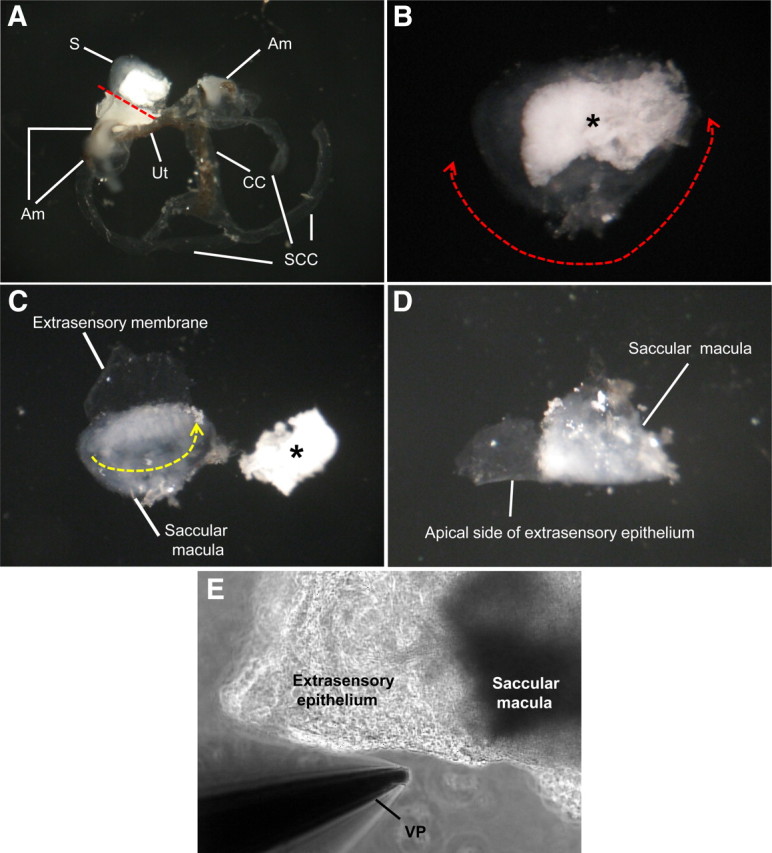
Preparation of saccular extramacular membrane for the measurement of current density. A, Separated membranous labyrinth of vestibule. The saccule is separated with microscissors from the utricle (red dotted line). B, Isolated saccule and separation of extramacular membrane from saccular macula. The extramacular membrane is separated along red dotted line with one side attached to the longest edge of the macula. C, Tissue folding. The tissue is folded along the yellow dotted line with the apical side of the epithelium facing out. D, Prepared tissue before mounting on stage of microscope. E, Photo of mounted tissue for current measurement. S, Saccule; Am, ampulla; Ut, utricle; CC, common crus; SCC, semicircular canal; *otoconia of saccule; VP, vibrating probe.
Voltage-sensitive vibrating probe.
The vibrating probe technique was chosen to measure transepithelial current under short circuit conditions because of the small dimensions of saccular extramacular membrane. The technique was identical to that described previously (Lee et al., 2001). Briefly, current density was monitored by vibrating a platinum-iridium wire microelectrode that was insulated with parylene-C (Micro Probe) and coated with Pt black on the exposed tip. The electrode tip of the probe was vibrated at two frequencies between 400 and 700 Hz along horizontal (x) and vertical (z) axes by piezoelectric bimorph elements (Applicable Electronics) and was positioned at 4 ± 2 μm from the apical surface of the saccular extramacular membrane. The x-axis was perpendicular to the face of the epithelium. A platinum-black electrode served as reference in the bath chamber. The signals from the oscillators driving the probe were also connected to a dual-channel phase-sensitive detector (Applicable Electronics) and the signals from the phase-sensitive detectors were digitized (16 bit) at a rate of 0.5 Hz. The electrode was positioned where current density showed a maximum x value and minimum z value; data are derived from the x direction current density and were plotted with Origin software, version 7.0. (OriginLab Software).
Solutions and chemicals.
In all electrophysiological experiments, both sides of the epithelium were perfused with a perilymph-like physiologic saline containing (in mm) 150 NaCl, 3.6 KCl, 1 MgCl2, 0.7 CaCl2, 5 glucose, and 10 HEPES, pH 7.4. Amiloride, benzamil, ethylisopropylamiloride (EIPA), forskolin, 3-isobutyl-1-methylxanthine (IBMX), ouabain, bumetanide, glibenclamide, clotrimazole, iberiotoxin, mifepristone, and spironolactone were all purchased from Sigma and dissolved in dimethylsulfoxide (DMSO; Sigma), which were then diluted to 0.1% DMSO or less in the physiologic saline before application. DMSO at this concentration had no effect on the current density. Dexamethasone (Sigma), 8-bromoadenosine 3′,5′-cyclic monophosphate (bromo-cAMP; Sigma), 8-bromoguanosine 3′,5′-cyclic monophosphate (bromo-cGMP; Sigma), tetra-ethyl-ammonium chloride (TEA; Sigma), 4-aminopyridine (4-AP; Sigma), apamin (Sigma), BaCl2 dihydrate (Fluka), and XE991 (Tocris Bioscience) were directly dissolved in physiologic saline just before use. TEA-Cl was substituted equimolar for NaCl and current density was corrected for the 4.5% increase in the resistivity of the solution. The effect of TEA-Cl (30 mm) was compared with that of equimolar NMDG-Cl (Fluka) to control for any effect of the reduced concentration of Na+. The current density measured with NMDG-Cl was corrected for the 6.8% increase in the resistivity of the solution. For pH values <6, MES buffer was substituted equimolar for HEPES buffer, and pH was adjusted to pH 6, 5, and 4, respectively.
Data analysis.
Data are presented as averaged recordings over time with the SE indicated at intervals for clarity and as mean values of each experimental condition ± SE from n observations. The tabulated currents for each condition were obtained by averaging samples (collected at 2 Hz) over 15 s, beginning 30 s before changes in perfusion or before the end of the drug washout. The control values before and after drug perfusion were averaged when treatments were clearly reversible to compensate for possible drift (e.g., see Figs. 4, 5B, 6A–D, 7A). Significance of current density changes between the control and individual experimental conditions were calculated with the paired t test. Significance among the control, dexamethasone-treated, dexamethasone + mifepristone-treated, and dexamethasone + spironolactone-treated groups was calculated with one-way repeated-measures ANOVA and Holm–Sidak posttests. Differences were considered significant for p < 0.05. Concentration dependence of the effects was analyzed using the Hill equation: I = Imax [Ch/(IC50h + Ch)] + Ioffset, where Imax is current in the presence of saturating concentration of drug, IC50 is the concentration that produces a half-maximal effect, C is the concentration of drug, h is the Hill coefficient, and Ioffset is the residual current during maximal inhibition by the drug. Data were fitted for each experiment separately, and then the mean values ± SE were estimated.
Figure 4.
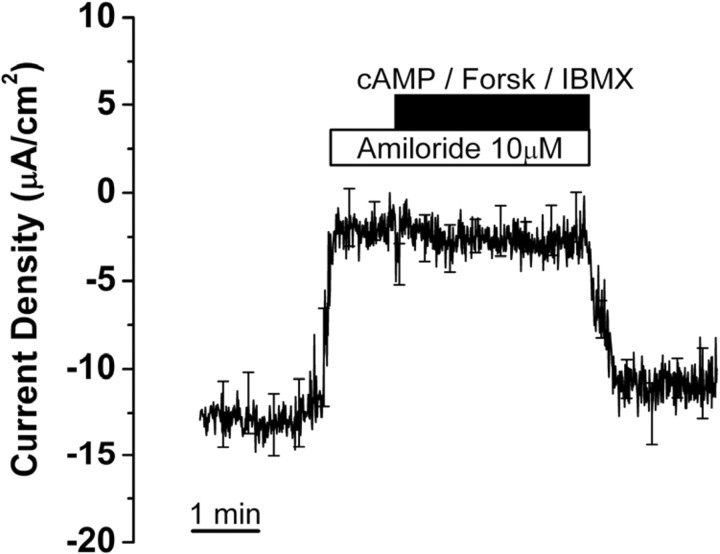
Effect of stimulators of cAMP-dependent chloride transport on current from saccular extramacular epithelium. Recording is digital averages of all experiments; SE is drawn only at intervals for clarity; n = 5. The following concentrations were used: cAMP (bromo-cAMP), 500 μm; forskolin (Forsk), 10 μm; IBMX, 125 μm.
Figure 5.
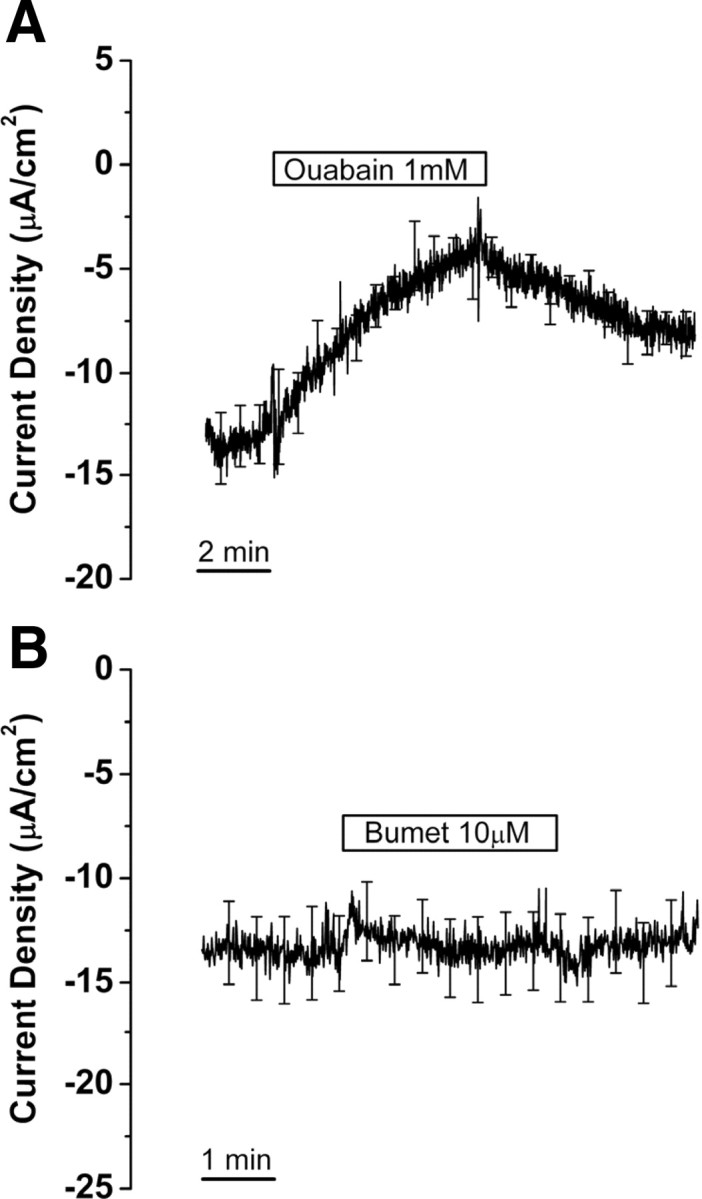
Effect of Na+,K+-ATPase and Na+,K+,2Cl− cotransporter inhibitors on current from saccular extramacular epithelium. Current is shown in the absence and presence of ouabain (A) and bumetanide (Bumet) (B). Recordings are digital averages of all experiments; SE is drawn only at intervals for clarity; n = 8, each.
Figure 6.
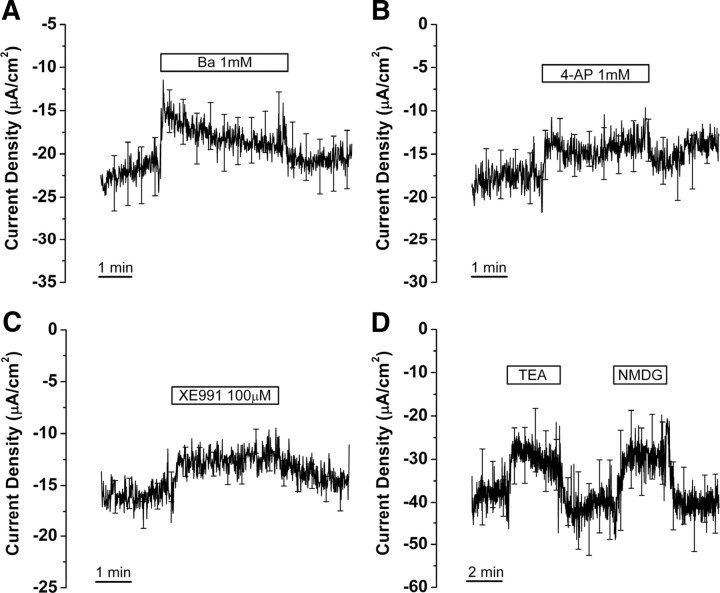
Effect of K+ channel blockers on current from saccular extramacular epithelium. Current changes after perfusion of Ba2+ (A; n = 10), 4-aminopyridine (4-AP) (B; n = 6), XE991 (C; n = 11), tetra-ethyl-ammonium (TEA; 30 mm), and NMDG (30 mm) (D; n = 5) are shown.
Figure 7.
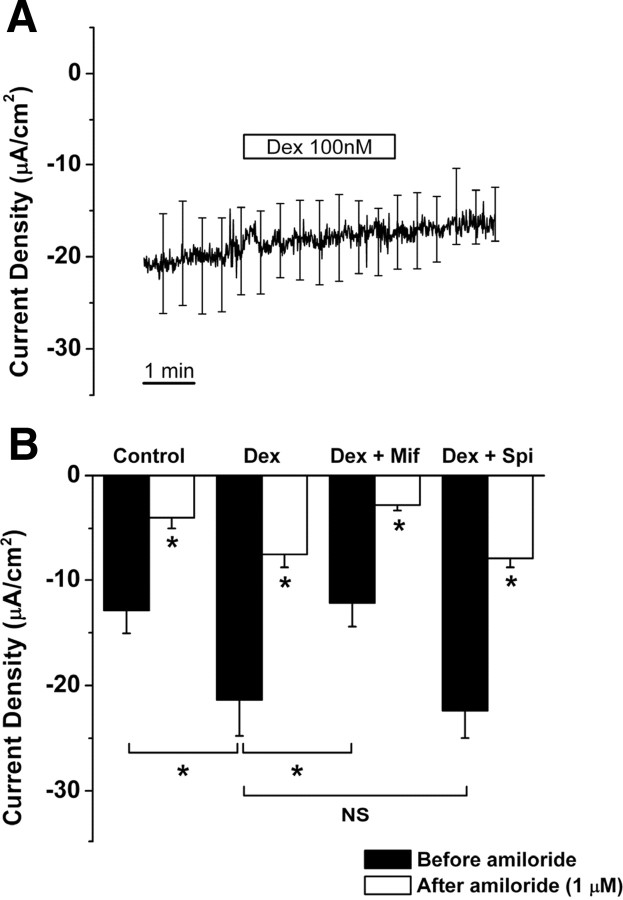
Dexamethasone acts at GR to regulate current density in saccular extramacular epithelial cell. A, Test for nongenomic (acute) effect of dexamethasone (Dex; 100 nm). B, Changes of current density under the conditions of control, dexamethasone (Dex; 100 nm), dexamethasone + GR antagonist mifepristone (Mif; 100 nm), and dexamethasone + MR antagonist spironolactone (Spi; 100 nm). Values are means ± SE; n = 5. NS, Not significant, dexamethasone versus dexamethasone + spironolactone. *p < 0.05, control versus dexamethasone, dexamethasone versus dexamethasone + mifepristone, and before amiloride versus during amiloride (1 μm) in all conditions.
Results
The current from the apical side of saccular extramacular epithelium in physiologic saline was −18.6 ± 1.1 μA/cm2 (n = 82), which could be accounted for by cation absorption and/or anion secretion.
Amiloride-sensitive current
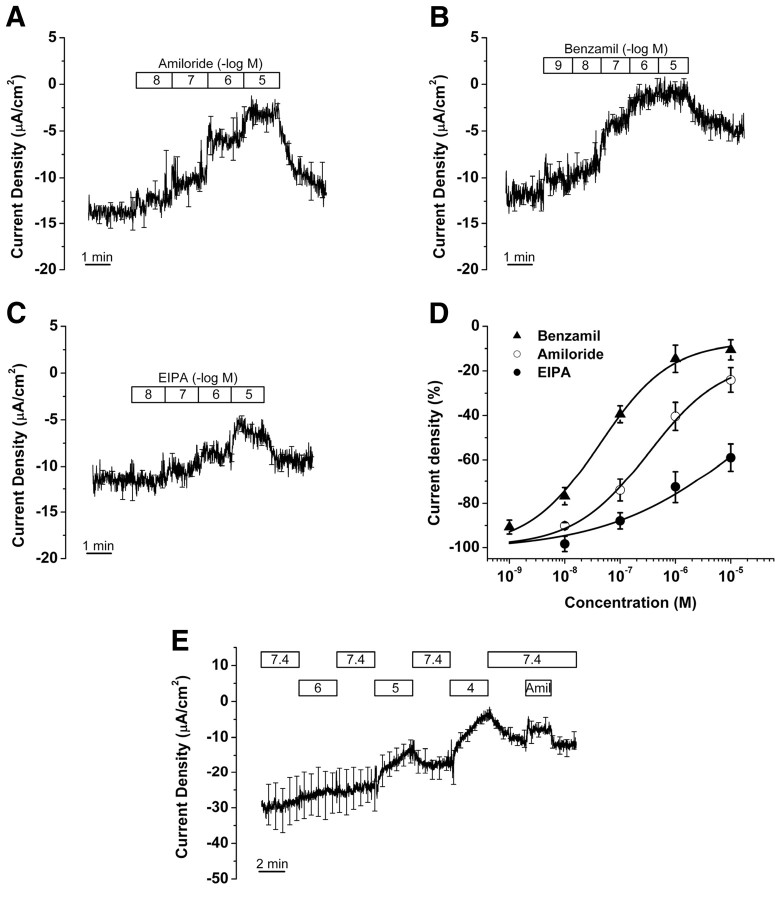
Perfusion of amiloride and its analogs (benzamil and EIPA) decreased current density in a dose-dependent manner, with a potency order of benzamil > amiloride ≫ EIPA (Fig. 3A–C). The concentration–response curves were fitted with the Hill equation (Fig. 3D; supplemental Table S1, available at www.jneurosci.org as supplemental material). The IC50 values for amiloride and benzamil are 0.3 and 0.04 μm, with Ioffset for benzamil of 2.6%. The fit of EIPA inhibition to the Hill equation was not well defined, but clearly showed a low sensitivity of the currents (IC50 = ∼25 μm) to this drug. These data provide a pharmacologic fingerprint for Na+ absorption mainly via ENaC (Kleyman and Cragoe, 1990; Garty, 1994). The benzamil data suggested that there was a small amiloride-insensitive residual current at high drug concentration. To examine the contribution of cyclic nucleotide-gated (CNG) cation channels in amiloride-sensitive Na+ transport, the tissues were perfused with bromo-cGMP (300 μm). Perfusion of bromo-cGMP caused no significant change of the current (−16.0 ± 2.1 to −15.1 ± 2.6 μA/cm2, n = 3).
Figure 3.
Effect of Na+ transport inhibitors on current from saccular extramacular epithelium. A, Amiloride (n = 5); bars show duration of perfusion at concentrations (molar) expressed as the negative log of the number in the bar. B, Benzamil (n = 5). C, EIPA (n = 5). D, Concentration–response relationships. E, pH 6, 5, and 4 and 1 μm amiloride (n = 4); bars show the duration of perfusion at the pH values in the bar. Amiloride (Amil; 1 μm) was perfused at pH 7.4. Graphs in A–C and E are derived from averages at each time point at 500 ms intervals of all experiments; the SE bars are drawn only at larger intervals for clarity.
Test for cAMP-mediated current
The tissues were superfused with bromo-cAMP (500 μm), forskolin (10 μm), an agonist for adenylyl cyclase, and IBMX (125 μm), an inhibitor of phosphodiesterase, in the continued presence of apical amiloride (10 μm) to elevate cytosolic cAMP levels in the absence of interfering Na+ currents. Superfusion of bromo-cAMP/forskolin/IBMX for 2.5 min did not cause a significant change of current density (−2.5 ± 1.4 to −2.9 ± 1.4 μA/cm2, n = 5) (Fig. 4). The best-characterized and molecularly identified Cl− channel that is stimulated by cAMP via protein kinase A is CFTR. We found no evidence for cAMP-stimulated anion currents in saccular extramacular epithelium.
Contributions of Na+,K+-ATPase and Na+,K+,2Cl− cotransporter to current density.
We investigated whether a ouabain-sensitive Na+,K+-ATPase and a bumetanide-sensitive Na+,K+,2Cl− cotransporter could be involved in Na+ absorption via saccular extramacular epithelium.
Functional Na+,K+-ATPase consists of an α and a β subunit, of which there are four α and three β subunits (Kim et al., 2009). Different combinations of isoforms display a range of affinities for their substrates Na+ and K+ and a wide range of affinities for the specific inhibitor ouabain (Segall et al., 2001; Pierre et al., 2008). Further, individual tissues can express more than one α and more than one β isoform; for example, in the ear it was observed that Reissner's membrane expresses α1, α2, β1, β2, and β3 (Kim et al., 2009). To test for the functional contribution of any isoforms of Na+,K+-ATPase, we used a concentration of ouabain, 1 mm, that is known to block even the relatively insensitive inner ear ion transport system in vestibular dark cells of the utricle (Marcus et al., 1987). Perfusion of ouabain (1 mm) for 6 min caused a decrease of current density by 60.0 ± 7.2% (−10.0 ± 0.9 to −4.0 ± 0.6 μA/cm2, n = 8) (Fig. 5A).
Bumetanide is the most potent inhibitor available of the Na+,K+,2Cl− cotransporter and is highly effective in the ear and other epithelial and neural systems at the concentration (10 μm) we used (Chen and Sun, 2005; Marcus et al., 1987). Higher concentrations are known to be capable of blocking some Cl− channels (Reddy and Quinton, 1999) and K+-Cl− cotransporters (Payne, 1997). Perfusion of bumetanide (10 μm) for 3 min caused no change of current density (−13.5 ± 1.9 to −13.4 ± 1.9 μA/cm2, n = 8) (Fig. 5B). These results demonstrate the involvement of Na+,K+-ATPase but not Na+,K+,2Cl− cotransporter in Na+ transport.
Effects of K+ channel blockers
A spectrum of inhibitors of K+ channels, ranging from highly specific (e.g., iberiotoxin) to those with broad effect (e.g., Ba2+) were tested for their effectiveness in reducing the current density from saccular extramacular epithelium. There were agents at both ends of the spectrum that were effective and ineffective (Table 1). The results argue for the participation of a limited number of candidate K channels in saccule ion transport, although specific isoforms were not identified.
Table 1.
Effect of K+ channel inhibitors on current density
| Drug | Target | Conc. (m) | Effect | Reference |
|---|---|---|---|---|
| Ba2+ | Kir and others | 10−3 | −12% | 1 |
| 4-Aminopyridine | Kv | 10−3 | −11% | 2 |
| XE991 | KCNQ | 10−4 | −19% | 3 |
| Glibenclamide | KATP channel | 10−5 | NC | 4 |
| Apamin | sK channels | 10−7 | NC | 5 |
| Clotrimazole | IKCa | 10−5 | NC | 6 |
| Iberiotoxin | KCa channels | 10−7 | NC | 7 |
| Extracellular low pH | Some K2P channels | 10−6 (pH 6) | NC | 8 |
| TEA | Many K+ channels | 3 × 10−3 | NC | 9 |
NC, No change; Conc., concentration.
1, Krapivinsky et al. (1998); 2, Wang et al. (2009); 3, Moser et al. (2008); 4, Quayle et al. (1997); 5, Stocker (2004); 6, Devor et al. (1997) and Dutta et al. (2009); 7, Tanaka et al. (2004); 8, Duprat et al. (1997, 2005), Rajan et al. (2000, 2005), and Morton et al. (2005); 9, Safronov et al. (1996), Cai et al. (1998), and Hadley et al. (2000).
Three K+ channel blockers used in this study were effective, although none blocked the current from saccular extramacular epithelium >20%. Ba2+ (1 mm) and 4-AP (1 mm), which are nonspecific K+ channel blockers, decreased current density by 11.6 ± 4.7% (−20.1 ± 2.9 to −17.7 ± 2.7 μA/cm2, n = 10) and 11.3 ± 6.8% (−15.0 ± 1.9 to −13.6 ± 2.2 μA/cm2, n = 6), respectively (Fig. 6A,B). XE991 (100 μm), which blocks KCNQ channels (Moser et al., 2008), decreased current density by 19.4 ± 3.1% (−14.5 ± 1.0 to −11.9 ± 1.3 μA/cm2, n = 11) (Fig. 6C).
Other K+ channel blockers [glibenclamide for KATP channel (10 μm, n = 11) (Quayle et al., 1997); apamin (100 nm, n = 6), clotrimazole (10 μm, n = 4), iberiotoxin (100 nm, n = 4) for KCa channels (Devor et al., 1997; Stocker, 2004; Tanaka et al., 2004); acidic physiologic saline (pH 6, n = 5) (Fig. 3E) for some K2P channels (Duprat et al., 1997, 2005; Rajan et al., 2000, 2005; Morton et al., 2005)] did not cause significant changes in current. Perfusion of pH 5 and pH 4 physiologic saline slowly decreased current by 33.0 ± 5.9% (−20.1 ± 3.9 to −13.5 ± 2.6 μA/cm2, n = 4) and 68.3 ± 4.7% (−13.1 ± 2.4 to −4.1 ± 1.5 μA/cm2, n = 4), respectively (Fig. 3E). The slow response of the current to acidic pH steps suggests action via a change in intracellular pH, which would be consistent with inhibition of ENaC (Chalfant et al., 1999). Another nonspecific K+ channel blocker TEA (30 mm) decreased current density by 25.2 ± 11.3% (−37.7 ± 6.6 to −28.3 ± 6.8 μA/cm2, n = 5) (Fig. 6D); however, substitution of 30 mm NMDG-Cl for NaCl also decreased the current density by 33.7 ± 9.0% (−40.7 ± 7.6 to −27.5 ± 7.3 μA/cm2, n = 5) (Fig. 6D), which is not significantly different from the effect of equimolar TEA. Therefore, the decrease in current density could be accounted for by low [Na+] rather than TEA.
Genomic effect of dexamethasone on transepithelial Na+ transport
Acute application of dexamethasone (100 nm for 3 min) did not cause a significant change of current density (−18.4 ± 4.16 to −17.1 ± 3.66 μA/cm2, n = 4) (Fig. 7A). However, current density increased significantly after 24 h incubation of the tissue in the presence of dexamethasone (100 nm) (control: −12.9 ± 2.2 μA/cm2, n = 5; with dexamethasone: −21.4 ± 3.4 μA/cm2, n = 5) (Fig. 7B). The GR antagonist mifepristone (100 nm) significantly reduced the current density from dexamethasone-treated saccular extramacular epithelium, whereas the MR antagonist spironolactone (100 nm) had no significant effect (control: −12.9 ± 2.2 μA/cm2, n = 5; with dexamethasone + mifepristone: −12.2 ± 2.2 μA/cm2, n = 5; with dexamethasone + spironolactone: −22.4 ± 2.6 μA/cm2, n = 5) (Fig. 7B). Application of amiloride (1 μm) decreased the current density of each group >60% (control: 69.9 ± 4.3%; with dexamethasone: 64.6 ± 2.9%; with dexamethasone + mifepristone: 76.2 ± 2.4%; with dexamethasone + spironolactone: 64.4 ± 1.3%) (Fig. 7B), which demonstrates that current increased by dexamethasone is primarily Na+-dependent current via amiloride-sensitive Na+ channels. These findings suggest that dexamethasone increases the transepithelial Na+ transport via genomic regulation selectively via GR and not via MR.
Discussion
We demonstrated for the first time Na+ absorption by mouse saccular extramacular epithelium that is mediated by apical ENaC, basolateral Na+,K+-ATPase, and K+-permeable channels (Fig. 8), and that is regulated by glucocorticoids and not by mineralocorticoids. These results provide an understanding of an important transport function of the saccule in the homeostasis of endolymphatic Na+. Na+ homeostasis in the vestibular labyrinth is essential for maintaining endolymph volume and hair cell function since elevation of luminal [Na+] would be expected to increase endolymph volume by osmotic driving force and to load the sensory hair cells with Na+ through the cation-permeable transduction channels, leading to cell swelling, dysfunction, and balance disorder. The maintenance of saccular [Na+] is challenged by passive diffusion of Na+ from perilymph through paracellular pathways in the epithelium and by diffusion within the endolymph along the lumen from the endolymphatic sac and utricle and from the cochlea (Fig. 8).
Figure 8.
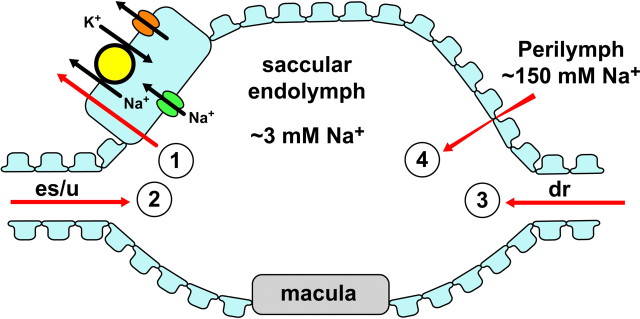
Model of Na+ absorption by saccule extramacular epithelium. The saccule is bounded by an epithelial monolayer with one tubular connection that bifurcates to the endolymphatic sac (es) and utricle (u) and another tubular connection to the cochlea via the ductus reuniens (dr). Na+ moves into and out of the saccule by at least four routes (red arrows). 1, Na+ is removed from the lumen by active transepithelial transport as illustrated in the expanded cell (upper left). 2, Na+ diffuses in from the utricle and sac, which have [Na+] higher than the saccule. 3, Na+ diffuses into the saccule from the cochlea, which has a slightly lower [Na+] than the saccule, but also a large positive voltage with respect to the saccule. The net electrochemical driving force on Na+ is in the direction cochlea to saccule, but the magnitude of the flux is expected to be small due to the low [Na+]. 4, Na+ diffuses between the epithelial cells from the perilymph to the saccular endolymph. Na+ from endolymph moves into the cells through Na+-permeable channels in the apical membrane. This pathway has the pharmacological fingerprint of ENaC, but may include other channels. Na+ exits the cell at the basolateral membrane by active extrusion via the Na+-pump, Na+,K+-ATPase. K+ brought into the cell on the Na+-pump leaves the cell across the same membrane by electrodiffusion via K+-permeable channels. The rate of absorption is stimulated by activation of glucocorticoid receptors (GR). Candidate isoforms of the transporters are discussed in the text.
Na+ entry across the apical membrane
The epithelial Na+ channel (ENaC) is likely the primary molecular entity that conducts Na+ into the cell. It is conceivable, however, that additional amiloride-sensitive channels are also expressed in saccular extramacular epithelium since the Hill coefficients for amiloride and benzamil are <1 (∼0.7) and the IC50 value of amiloride (∼0.3 mm) is slightly higher than that of heterologously expressed αβγ-ENaC (∼0.1 μm) (Canessa et al., 1994).
Nonselective cation channels may coexist with ENaC in the saccular extramacular epithelial cell as recently reported in H441 cells that contain both a nonselective cation channel of unknown molecular identity (but not CNG channels) and showed a similar (composite) IC50 value of amiloride (∼0.6 μm) (Albert et al., 2008). We examined if amiloride-sensitive CNG channels contribute to Na+ transport in saccular extramacular epithelium using a membrane-permeable analog of cGMP, but could not find functional evidence for those channels. It is also conceivable that SGK, which regulates corticosteroid-dependent ENaC expression (Lang et al., 2006), increased the IC50 of amiloride as reported previously (Böhmer et al., 2000) since Na+ transport in saccular extramacular epithelium is glucocorticoid-dependent and is likely to express the common signaling kinase SGK.
Na+ exit and K+ recycling across the basolateral membrane
The driving force for Na+ absorption in saccular extramacular epithelium is generated by basolateral Na+,K+-ATPase. K+ brought into the cell by Na+,K+-ATPase would then be recycled via K+ channels in the same membrane. The molecular identities of the K+ channels cannot be unambiguously determined since the current was not inhibited fully by any of several K+ channel blockers and because of the poor selectivity of most of the effective inhibitory agents. Nonetheless, the constellation of effective and ineffective agents (Table 1) suggests the contribution of Kv channels (including KCNQs) blocked by 4-AP and XE991, Kir channels (not KATP channel) blocked by Ba2+, and some K2P channels that are not blocked by acidic pH.
Our observations argue against the participation of KATP, KCa, and some acid-sensitive K2P channels such as KCNK1, KCNK3, KCNK5, KCNK9, and KCNK17, considering the ineffectiveness of the drugs glibenclamide (Quayle et al., 1997), clotrimazole (Devor et al., 1997), iberiotoxin (Tanaka et al., 2004), apamin (Stocker, 2004), and pH 6 solution (Duprat et al., 1997, 2005; Rajan et al., 2000, 2005; Morton et al., 2005). The findings that the K+ channel inhibitors together blocked no more than 42% of the current may point to a nonselective cation channel or K+,Cl− cotransporter in the basolateral membrane through which K+ recycled.
Absence of electrogenic PKA-activated Cl− secretion
The direction of current measured from the apical side of saccular extramacular epithelium could be accounted for by cation absorption and/or anion secretion. The majority of current was decreased by amiloride analogues where the order of potency was benzamil > amiloride ≫ EIPA, which suggests that transepithelial Na+ transport via ENaC mostly contributes to the current. Some Na+-absorbing epithelia, including semicircular canal duct, are capable of bidirectional salt transport and secrete Cl− under stimulation of the cAMP signaling pathway (Mall et al., 1999; Reddy et al., 1999; Chan et al., 2000; Kunzelmann et al., 2000; Milhaud et al., 2002; Choi et al., 2006). In contrast, no immediate response of current to a mixture known to raise intracellular cAMP levels was observed from the saccule. This result suggests that the majority of the transepithelial current from saccular extramacular epithelium is derived solely from Na+ absorption via ENaC.
Regulation by corticosteroid receptors
Corticosteroid receptors have been reported in several inner ear epithelia (Rarey and Luttge, 1989; Pitovski et al., 1993a). The mineralocorticoid signaling system has perhaps received the most attention with respect to regulation of epithelial Na+ transport genes in the kidney and other tissues (Bhargava and Pearce, 2004). Indeed, there have been studies that have investigated this pathway in the inner ear (ten Cate and Rarey, 1991; Pitovski et al., 1993b; Trune et al., 2006).
Recently, glucocorticoids were reported to regulate ion homeostasis of inner ear fluids via genomic pathways in semicircular canal (Pondugula et al., 2004, 2006), Reissner's membrane (Kim et al., 2009), and nongenomic pathways in stria vascularis (Lee and Marcus, 2002). In the present report, Na+ absorption in saccular extramacular epithelium was found to be stimulated by glucocorticoids via genomic regulation through GR, not through MR, as determined by the long time scale of stimulation and the differential effects of GR and MR antagonists.
The similar transport systems and regulation by glucocorticoids in saccule, Reissner's membrane and semicircular canal supports the importance of this regulatory pathway throughout the inner ear since the cochlea, saccule, and utricle/canals constitute three anatomically distinct parts of the endolymphatic system despite the fine tubular connections among them (Fig. 1). Treatments based on glucocorticoid administration would therefore be expected to have beneficial effects throughout the ear.
Similarities and differences among ENaC-mediated transport epithelia
Despite the similar ENaC-mediated transport among the saccule, Reissner's membrane, and canal duct, these cells are not identical and therefore not merely the same cell type growing in different inner ear organs. The properties of the mouse saccular extramacular epithelium in transepithelial Na+ transport established in the present study are similar to those of mouse Reissner's membrane epithelium (Kim et al., 2009), although there are differences in the K+ channel pharmacologic fingerprint (supplemental Table S2, available at www.jneurosci.org as supplemental material). Mouse Reissner's membrane is insensitive to 4-AP but sensitive to acidic extracellular pH. The rat semicircular canal duct epithelium is also insensitive to 4-AP (supplemental Table S2, available at www.jneurosci.org as supplemental material). Further, there are differences among these epithelia in the fingerprint for Cl− secretion via the cAMP-stimulated CFTR Cl− channel. The transepithelial current across both the saccule and Reissner's membrane is acutely insensitive to elevation of cAMP, in contrast to the semicircular canal duct, which has both amiloride-sensitive currents and cAMP-stimulated Cl− secretion. Consistent with that observation, the CFTR stimulator genistein increased the current from the canal duct cells (Milhaud et al., 2002). All three of these Na+-absorptive inner ear epithelia are regulated by glucocorticoids.
Physiological significance
Na+ homeostasis in the vestibular labyrinth is thought to be essential for maintaining endolymph volume and hair cell function as described above. Swelling of the endolymphatic space (hydrops) is often associated with Meniere's syndrome, which is characterized by vertigo, hearing loss, and tinnitus. Saccular dysfunction, such as Tumarkin crisis or vestibular drop attack, in Meniere's syndrome has been observed by vestibular evoked myogenic potentials (Timmer et al., 2006; Ozeki et al., 2008) and is associated with saccular hydrops (Lin et al., 2006).
Our findings of steroid-regulated transepithelial Na+ absorption in saccular extramacular epithelium suggest that the epithelium is likely to contribute to the maintenance of the low concentration of Na+ in saccular endolymph and may partly explain the success in many Meniere's syndrome patients of local treatment by synthetic glucocorticoids (Barrs et al., 2001).
Insight into the relevance of the transepithelial currents reported here can be gained by calculating the corresponding Na+ flux and estimating the resulting decrease in saccular Na+ concentration ([Na+]) and volume over time. Using reasonable assumptions, we estimated (supplemental material, available at www.jneurosci.org) a transport rate that would reduce endolymphatic [Na+] at the rate of 6 mm/h. A presumed pathologically high [Na+] of 21 mm (Silverstein and Takeda, 1977; Morgenstern et al., 1984) could be reduced to normal (3 mm) (Sellick and Johnstone, 1972) in only 3 h and the volume of saccular endolymph would be reduced at the rate of 4%/h.
Footnotes
This work was supported by National Institutes of Health Grants R01-DC00212 and P20-RR017686. We thank Dr. Philine Wangemann for helpful discussions.
References
- Albert AP, Woollhead AM, Mace OJ, Baines DL. AICAR decreases the activity of two distinct amiloride-sensitive Na+-permeable channels in H441 human lung epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2008;295:L837–L848. doi: 10.1152/ajplung.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202–207. doi: 10.1200/JCO.1987.5.2.202. [DOI] [PubMed] [Google Scholar]
- Barrs DM, Keyser JS, Stallworth C, McElveen JT., Jr Intratympanic steroid injections for intractable Meniere's disease. Laryngoscope. 2001;111:2100–2104. doi: 10.1097/00005537-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Bhargava A, Pearce D. Mechanisms of mineralocorticoid action: determinants of receptor specificity and actions of regulated gene products. Trends Endocrinol Metab. 2004;15:147–153. doi: 10.1016/j.tem.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Böhmer C, Wagner CA, Beck S, Moschen I, Melzig J, Werner A, Lin JT, Lang F, Wehner F. The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol Biochem. 2000;10:187–194. doi: 10.1159/000016349. [DOI] [PubMed] [Google Scholar]
- Braat MC, Oosterhuis B, Koopmans RP, Meewis JM, Van Boxtel CJ. Kinetic-dynamic modeling of lymphocytopenia induced by the combined action of dexamethasone and hydrocortisone in humans, after inhalation and intravenous administration of dexamethasone. J Pharmacol Exp Ther. 1992;262:509–515. [PubMed] [Google Scholar]
- Burnham JA, Stirling CE. Quantitative localization of Na-K pump sites in the frog sacculus. J Neurocytol. 1984;13:617–638. doi: 10.1007/BF01148082. [DOI] [PubMed] [Google Scholar]
- Cai S, Garneau L, Sauvé R. Single-channel characterization of the pharmacological properties of the K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J Membr Biol. 1998;163:147–158. doi: 10.1007/s002329900379. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Chalfant ML, Denton JS, Berdiev BK, Ismailov II, Benos DJ, Stanton BA. Intracellular H+ regulates the alpha-subunit of ENaC, the epithelial Na+ channel. Am J Physiol. 1999;276:C477–C486. doi: 10.1152/ajpcell.1999.276.2.C477. [DOI] [PubMed] [Google Scholar]
- Chan LN, Wang XF, Tsang LL, Liu CQ, Chan HC. Suppression of CFTR-mediated Cl− secretion by enhanced expression of epithelial Na+ channels in mouse endometrial epithelium. Biochem Biophys Res Commun. 2000;276:40–44. doi: 10.1006/bbrc.2000.3426. [DOI] [PubMed] [Google Scholar]
- Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res. 2005;27:280–286. doi: 10.1179/016164105X25243. [DOI] [PubMed] [Google Scholar]
- Choi JY, Son EJ, Kim JL, Lee JH, Park HY, Kim SH, Song MH, Yoon JH. ENaC- and CFTR-dependent ion and fluid transport in human middle ear epithelial cells. Hear Res. 2006;211:26–32. doi: 10.1016/j.heares.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Gerlach AC, Frizzell RA, Bridges RJ. Inhibition of intestinal Cl− secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol. 1997;273:C531–C540. doi: 10.1152/ajpcell.1997.273.2.C531. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Girard C, Jarretou G, Lazdunski M. Pancreatic two P domain K+ channels TALK-1 and TALK-2 are activated by nitric oxide and reactive oxygen species. J Physiol. 2005;562:235–244. doi: 10.1113/jphysiol.2004.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta AK, Khimji AK, Sathe M, Kresge C, Parameswara V, Esser V, Rockey DC, Feranchak AP. Identification and functional characterization of the intermediate conductance Ca2+-activated K+ channel (IK-1) in biliary epithelium. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00223.2009. Advance online publication. Retrieved Dec. 1, 2009. doi: 10.1152/ajpgi.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H. Molecular properties of epithelial, amiloride-blockable Na+ channels. FASEB J. 1994;8:522–528. doi: 10.1096/fasebj.8.8.8181670. [DOI] [PubMed] [Google Scholar]
- Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br J Pharmacol. 2000;129:413–415. doi: 10.1038/sj.bjp.0703086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen F, Kroese AB. Ionic selectivity of the mechano-electrical transduction channels in the hair cells of the frog sacculus. Acta Physiol Scand. 1994;151:7–16. doi: 10.1111/j.1748-1716.1994.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim KX, Raveendran NN, Wu T, Pondugula SR, Marcus DC. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner's membrane epithelium. Am J Physiol Cell Physiol. 2009;296:C544–C557. doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura RS. Distribution, structure, and function of dark cells in the vestibular labyrinth. Ann Otol Rhinol Laryngol. 1969;78:542–561. doi: 10.1177/000348946907800311. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Cation transport probes: the amiloride series. Methods Enzymol. 1990;191:739–755. doi: 10.1016/0076-6879(90)91045-8. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Eng L, Krapivinsky L, Yang Y, Clapham DE. A novel inward rectifier K+ channel with unique pore properties. Neuron. 1998;20:995–1005. doi: 10.1016/s0896-6273(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Schreiber R, Nitschke R, Mall M. Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflugers Arch. 2000;440:193–201. doi: 10.1007/s004240000255. [DOI] [PubMed] [Google Scholar]
- Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Nongenomic effects of corticosteroids on ion transport by stria vascularis. Audiol Neurootol. 2002;7:100–106. doi: 10.1159/000057657. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DH. The structure and function of hearing organ. In: Kim CS, editor. Otorhinolaryngology head and neck surgery. Seoul, Korea: Il Jo Gak; 2002. p. 21. [Google Scholar]
- Lin MY, Timmer FCA, Oriel BS, Zhou G, Guinan JJ, Kujawa SG, Herrmann BS, Merchant SN, Rauch SD. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope. 2006;116:987–992. doi: 10.1097/01.mlg.0000216815.75512.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol. 1999;277:G709–G716. doi: 10.1152/ajpgi.1999.277.3.G709. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Shen Z. Slowly activating, voltage-dependent K+ conductance is apical pathway for K+ secretion in vestibular dark cells. Am J Physiol. 1994;267:C857–C864. doi: 10.1152/ajpcell.1994.267.3.C857. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Shipley AM. Potassium secretion by vestibular dark cell epithelium demonstrated by vibrating probe. Biophys J. 1994;66:1939–1942. doi: 10.1016/S0006-3495(94)80987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Marcus NY, Greger R. Sidedness of action of loop diuretics and ouabain on nonsensory cells of utricle: a micro-Ussing chamber for inner ear tissues. Hear Res. 1987;30:55–64. doi: 10.1016/0378-5955(87)90183-3. [DOI] [PubMed] [Google Scholar]
- Milhaud PG, Pondugula SR, Lee JH, Herzog M, Lehouelleur J, Wangemann P, Sans A, Marcus DC. Chloride secretion by semicircular canal duct epithelium is stimulated via β2-adrenergic receptors. Am J Physiol Cell Physiol. 2002;283:C1752–C1760. doi: 10.1152/ajpcell.00283.2002. [DOI] [PubMed] [Google Scholar]
- Morgenstern C, Mori N, Amano H. Pathogenesis of experimental endolymphatic hydrops. Acta Otolaryngol Suppl. 1984;406:56–58. doi: 10.3109/00016488309123003. [DOI] [PubMed] [Google Scholar]
- Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci U S A. 2005;102:16102–16106. doi: 10.1073/pnas.0506870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser SL, Harron SA, Crack J, Fawcett JP, Cowley EA. Multiple KCNQ potassium channel subtypes mediate basal anion secretion from the human airway epithelial cell line Calu-3. J Membr Biol. 2008;221:153–163. doi: 10.1007/s00232-008-9093-9. [DOI] [PubMed] [Google Scholar]
- Moser T, Brandt A, Lysakowski A. Hair cell ribbon synapses. Cell Tissue Res. 2006;326:347–359. doi: 10.1007/s00441-006-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki H, Iwasaki S, Murofushi T. Vestibular drop attack secondary to Meniere's disease results from unstable otolithic function. Acta Otolaryngol. 2008;128:887–891. doi: 10.1080/00016480701767390. [DOI] [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Pierre SV, Sottejeau Y, Gourbeau JM, Sánchez G, Shidyak A, Blanco G. Isoform specificity of Na-K-ATPase-mediated ouabain signaling. Am J Physiol Renal Physiol. 2008;294:F859–F866. doi: 10.1152/ajprenal.00089.2007. [DOI] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Drescher DG. High affinity aldosterone binding sites (type I receptors) in the mammalian inner ear. Hear Res. 1993a;69:10–14. doi: 10.1016/0378-5955(93)90088-i. [DOI] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Kerr TP, Drescher DG. Aldosterone mediates an increase in 3H ouabain binding at Na+, K+-ATPase sites in the mammalian inner ear. Brain Res. 1993b;601:273–278. doi: 10.1016/0006-8993(93)91720-d. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol. 2004;286:F1127–F1135. doi: 10.1152/ajprenal.00387.2003. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Raveendran NN, Ergonul Z, Deng Y, Chen J, Sanneman JD, Palmer LG, Marcus DC. Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat. Physiol Genomics. 2006;24:114–123. doi: 10.1152/physiolgenomics.00006.2005. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Liu GX, Preisig-Müller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel—an extracellular histidine as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Rarey KE, Luttge WG. Presence of type I and type II/IB receptors for adrenocorticosteroid hormones in the inner ear. Hear Res. 1989;41:217–221. doi: 10.1016/0378-5955(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM. Bumetanide blocks CFTR GCl in the native sweat duct. Am J Physiol. 1999;276:C231–C237. doi: 10.1152/ajpcell.1999.276.1.C231. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Bischoff U, Vogel W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. J Physiol. 1996;493:393–408. doi: 10.1113/jphysiol.1996.sp021391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Inamura N, Thalmann R, Vora A. Calcium gradients in inner ear endolymph. Am J Otolaryngol. 1989;10:371–375. doi: 10.1016/0196-0709(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Segall L, Daly SE, Blostein R. Mechanistic basis for kinetic differences between the rat alpha 1, alpha 2, and alpha 3 isoforms of the Na,K-ATPase. J Biol Chem. 2001;276:31535–31541. doi: 10.1074/jbc.M103720200. [DOI] [PubMed] [Google Scholar]
- Sellick PM, Johnstone BM. The electrophysiology of the saccule. Pflugers Arch. 1972;336:28–34. doi: 10.1007/BF00589139. [DOI] [PubMed] [Google Scholar]
- Silverstein H, Takeda T. Endolymphatic sac obstruction. Biochemical studies. Ann Otol Rhinol Laryngol. 1977;86:493–499. doi: 10.1177/000348947708600408. [DOI] [PubMed] [Google Scholar]
- Smith CA. The extrasensory cells of the vestibule. In: Paparella MM, editor. Biochemical mechanisms in hearing and deafness. Springfield, IL: Thomas; 1970. pp. 171–185. [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Koike K, Alioua A, Shigenobu K, Stefani E, Toro L. Beta 1-subunit of MaxiK channel in smooth muscle: a key molecule which tunes muscle mechanical activity. J Pharmacol Sci. 2004;94:339–347. doi: 10.1254/jphs.94.339. [DOI] [PubMed] [Google Scholar]
- ten Cate WJ, Rarey KE. Plasma membrane modulation of ampullar dark cells by corticosteroids. Arch Otolaryngol Head Neck Surg. 1991;117:96–99. doi: 10.1001/archotol.1991.01870130102025. [DOI] [PubMed] [Google Scholar]
- Timmer FCA, Zhou G, Guinan JJ, Kujawa SG, Herrmann BS, Rauch SD. Vestibular evoked myogenic potential (VEMP) in patients with Meniere's disease with drop attacks. Laryngoscope. 2006;116:776–779. doi: 10.1097/01.mlg.0000205129.78600.27. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006;212:22–32. doi: 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wong NC, Cheng Y, Kehl SJ, Fedida D. Control of voltage-gated K+ channel permeability to NMDG+ by a residue at the outer pore. J Gen Physiol. 2009;133:361–374. doi: 10.1085/jgp.200810139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Schacht J. Homeostatic mechanisms in the cochlea. In: Dallos P, Popper AN, Fay RR, editors. The cochlea. New York: Springer; 1996. pp. 130–185. [Google Scholar]