Abstract
Context:
Although numerous epidemiologic studies have documented associations between osteoporosis and cardiovascular disease, the mechanisms underlying this association remain to be clarified. One hypothesis is that hyperlipidemia may be a common predisposing factor to both atherosclerotic heart disease and bone fragility.
Objective:
To evaluate this, we compared bone mineral density (BMD) between subjects with and without the R3500Q APOB mutation, the cause of familial defective apolipoprotein B-100, which has been previously shown to markedly increase low-density lipoprotein cholesterol (LDL-C). We hypothesized that R3500Q carriers would have lower BMD due to lifetime, elevated LDL-C.
Design:
This was a a cross-sectional study in the Old Order Amish (OOA) population.
Participants:
The R3500Q APOB mutation is present at a high frequency (∼6% vs <0.5%) in the OOA population due to a founder effect. Therefore, we conducted analysis on 1097 Amish individuals of whom 125 were R3500Q carriers.
Main Outcome Measure:
BMD was measured by dual-energy x-ray absorptiometry.
Results:
After adjusting for age, age2, sex, body mass index, and family structure, carriers for the Q risk allele had significantly lower BMD than noncarriers at the femoral neck (P = .037), lumbar spine (P = .035) and whole body (P = .016). Adjusting for LDL-C attenuated the association between R3500Q genotype and BMD but did not completely explain the relationship. Subgroup analyses showed no significant interactions with sex, age, or presence of metabolic syndrome.
Conclusion:
These results use the unique genetic architecture of the OOA population to provide a novel line of evidence supporting a causal role for elevated LDL-C in lowering BMD.
Although epidemiologic investigations have shown osteoporosis to be correlated with both cardiovascular disease mortality and clinical indicators of cardiovascular disease (1), the mechanisms linking these disorders have not yet been established. One hypothesis is that hyperlipidemia may predispose one to both atherosclerotic heart disease and bone fragility, although epidemiologic support for this hypothesis is equivocal with associations between serum lipids and bone mineral density (BMD) reported in some (2–6), but not all (7–9), studies. A major limitation in discerning the relationship of hyperlipidemia and BMD at the population level is that hyperlipidemia tends to cluster with other characteristics of the metabolic syndrome that can impact bone, such as obesity and diabetes, so that when associations are observed, it is difficult to disentangle the effects of hyperlipidemia from other variables.
To differentiate the effects of hyperlipidemia on bone from other metabolic abnormalities, we have measured BMD in 125 Amish individuals carrying the mutation for familial defective apolipoprotein-B100 (FDB). This disorder is caused by a mutation in apolipoprotein B (APOB) that is characterized by isolated, high levels of low-density lipoprotein cholesterol (LDL-C). FDB is most commonly caused by a substitution mutation (R3500Q) that results in a conformational change in the APOB-100 glycoprotein, reduces its binding affinity to the LDL receptor, and thus impedes LDL clearance (10, 11). The R3500Q mutation appears to be confined to Caucasian individuals with frequency estimated at ∼1 in 500 in North America and Central Europe, although estimates from northwestern Switzerland are as high as 1 in 200 (12, 13). Recent work by our group identified the R3500Q mutation as a major predictor of LDL-C and coronary artery calcification (CAC) in the Old Order Amish (OOA) and determined that this mutation is observed in this population at a much higher carrier frequency (∼12%) likely due to a founder effect (14). In the OOA, each copy of the variant (Q) allele is associated with an ∼58 mg/dL higher level of LDL-C. The large number of R3500Q carriers in this population provides a unique opportunity to evaluate the isolated effects of high LDL-C on BMD. Because high LDL-C levels are evident in R3500Q variant carriers even in young adulthood and because the variant is not associated with other characteristics of the metabolic syndrome, we reasoned that any differences in BMD between R3500Q carriers and noncarriers would likely be mediated through LDL-C.
Subjects and Methods
The OOA community in Lancaster County, Pennsylvania, was founded in the late 18th century by a small group of immigrants (∼400) from Switzerland (15). The Lancaster County OOA community now numbers ∼30 000, virtually all of whom are descendants of the original set of immigrants. The community is characterized by its social cohesiveness and a relatively homogeneous lifestyle.
Our group has been studying the genetic determinants of complex diseases and their risk factors in this community since 1993. To date, we have screened ∼4500 Amish adults for risk factors related to cardiovascular disease (16), diabetes (17), and osteoporosis (18). Participants of our studies have generally included relatively healthy volunteers and their family members. Due to the availability of extensive genealogical records (19), we are able to link virtually all of these participants into a single 14-generation pedigree. This report is based on 1097 OOA men and women in whom APOB R3500Q genotype and BMD and LDL-C measurements were available. Our primary analysis was to test whether the APOB R3500Q variant was associated with BMD in OOA. Secondary analyses were performed to examine the effects of sex, age, CAC, and features of the metabolic syndrome on the relationship between APOB R3500Q genotype and BMD.
Areal BMD (grams per square centimeter) was measured by dual-energy x-ray absorptiometry at the proximal femur, lumbar spine, one-third radius, and whole body using a Hologic 4500W (Hologic). Daily phantom measurements were obtained to detect measurement shifts over time, and the coefficients of variation for the repeat measures of the sites included in this analysis were between 1.5% and 0.8%. Serum lipid and high-density lipoprotein cholesterol (HDL-C) were measured by Quest Diagnostics, and LDL-C was calculated using the Friedewald formula. CAC was assessed in the left main, left anterior descending, circumflex, and right coronary arteries by electron-beam computed tomography (Imatron C-150 scanner), and the Agatston method was used to quantify CAC (20). The presence of metabolic syndrome was determined using National Cholesterol Education Program Adult Treatment Panel III guidelines (21), which define metabolic syndrome as having 3 or more of the following risk factors: central obesity, elevated serum triglycerides, low HDL-C, high blood pressure, and impaired fasting glucose. Genotyping of the APOB R3500Q mutation (rs5742904, OMIM 107730.0009) was completed using TaqMan chemistry (Applied Biosystems) and a 94.6% genotyping call rate and 98.9% genotyping replication rate was observed.
Frequencies, means, and SDs were calculated for selected participant characteristics by APOB genotype. Statistical analyses were performed using SOLAR (The Southwest Foundation for Biomedical Research) under the variance components analytical framework to account for the family structure of the founder population (22). Age, sex, and body mass index (BMI) were associated with BMD on univariate analysis and were therefore included as covariates in models assessing the association of genotype with BMD, with additional adjustment for LDL-C in some models. An age-squared term was also included to better model the nonlinear relationship between BMD and age. Subgroup analyses to determine whether differences in age, sex, CAC, or metabolic syndrome status mediated the relationship between genotype and BMD were performed. Differences in the genotype effects on BMD between subgroups were evaluated by inclusion of a genotype-by-subgroup interaction term in the association model. Power calculations were conducted using Quanto assuming an allele frequency of 6%, a mean BMD of 0.95 g/cm2, an SD of 0.15, and a two-sided α of 0.05(23). We estimated that our sample provided 80% power to detect differences in BMD of at least 0.27 SDs between the 972 noncarriers and 125 carriers of APOB R3500Q.
Institutional Review Board approval was obtained from the University of Maryland at Baltimore, and all participants provided informed consent.
Results
On average, the 1097 subjects in our analysis were 54.3 (range 22–95) years old, and 52.3% of the subjects were female. The APOB Q allele frequency in this sample was 6% with 121 individuals being heterozygous (RQ) and 4 being homozygous for the Q allele. As previously reported by our group (14) and illustrated in Table 1, the R3500Q variant is strongly associated with LDL-C level and CAC score but not with other lipid measures or body size.
Table 1.
Population Characteristics by Genotype Groupa
| Genotype |
|||
|---|---|---|---|
| R/R | Q/R | Q/Qe | |
| n | 972 | 121 | 4 |
| Age, y | 54 (14) | 53 (15) | 50–84 |
| Female sex, % (frequency) | 52% (505) | 54% (66) | 75% (3) |
| Current smoker,b % (frequency) | 8% (80) | 11% (13) | 0% (0) |
| BMI, kg/m2 | 27.8 (5.0) | 27.7 (4.6) | 23.2–30.0 |
| Height, cm | 165 (9) | 165 (9) | 153–165 |
| Weight, kg | 75.2 (14.1) | 75 (13) | 59–75 |
| LDL, mg/dLc | 132 (33) | 198 (49) | 169–323 |
| HDL, mg/dL | 58 (15) | 54 (15) | 40–65 |
| TG, mg/dL | 86 (50) | 87 (57) | 57–145 |
| Use of cholesterol-lowering drugs, % (frequency) | 4% (38) | 10% (12) | 50% (2) |
| Median CAC (Q1, Q3)d | 1.03 (0, 106) | 129 (1.03, 585) | 0–1998 |
| BMD, g/cm2 | |||
| Femoral neck | 0.825 (0.138) | 0.814 (0.132) | 0.612–0.707 |
| Lumbar spine | 0.950 (0.153) | 0.927 (0.141) | 0.730–0.938 |
| 1/3 radius | 0.756 (0.108) | 0.736 (0.103) | 0.506–0.757 |
| Whole body | 1.124 (0.117) | 1.106 (0.116) | 0.855–0.972 |
Abbreviations: Q1, quartile 1; Q3, quartile 3; TG, triglyceride.
Unless otherwise noted, mean and SD are presented.
All individuals reporting current smoking are males; in Amish culture, tobacco use among women is prohibited.
LDL values for Q/Q subjects were 169, 171, 245, and 323 mg/dL.
CAC measures were available in only 861 participants (R/R, n = 772; Q/R, n = 85; Q/Q, n = 4).
Ranges are shown for Q/Q genotype instead of mean and SD due to small sample size.
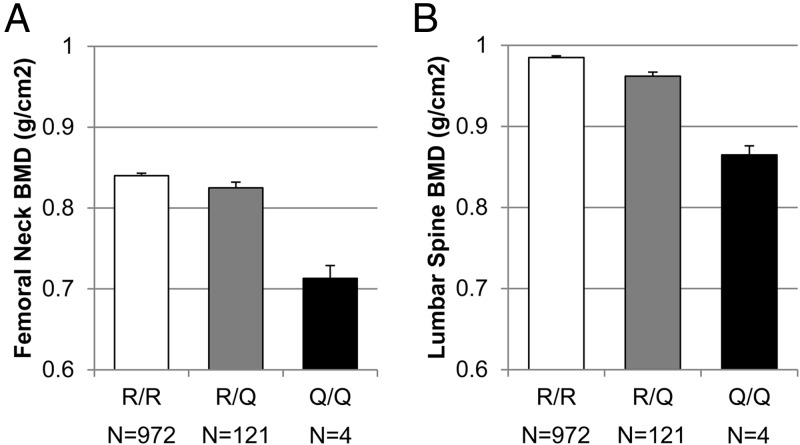
Results of the additive association analyses between the R3500Q genotype and BMD are provided in Figure 1 and Table 2 (Model 1). After adjusting for age, age2, sex, and BMI and accounting for family structure, the APOB Q risk allele was associated with lower BMD at the femoral neck (P = .037) and lumbar spine (P = .035) as well as whole-body BMD (P = .016). Carriers of the Q allele had on average 1.7% to 2.3% lower BMD at the femoral neck, spine, and whole body than normal (RR) homozygotes. The APOB Q risk allele was not significantly associated (P = .171) with lower BMD at the one-third radius in adjusted models. R3500Q genotype remained associated (P < .05) with femoral neck, lumbar spine, and whole-body BMD in models adjusted for only sex, age, and age2 (Table 2, model 2). R3500Q genotype was associated (P < .05) with femoral neck, lumbar spine, whole-body, and one-third radius BMD in unadjusted models that account only for family structure.
Figure 1.
BMD by APOB genotype. Age-, age2-, sex-, and BMI-adjusted BMD is presented by APOB genotype for the femoral neck (A) and lumbar spine (B). Adjusted means and SEs are presented.
Table 2.
Association Between R3500Q APOB and BMD
| n | Model 1, Adjusted for Age, Age2, Sex, BMI |
Model 2, Adjustment for Age, Age2, Sex |
Model 3, Adjusted for Age, Age2, Sex, BMI, LDL |
||||
|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | ||
| BMD, g/cm2 | |||||||
| Femoral neck | 1097 | −0.022 (0.011) | .037 | −0.024 (0.012) | .041 | −0.026 (0.012) | .029 |
| Lumbar spine | 1097 | −0.029 (0.013) | .035 | −0.029 (0.014) | .045 | −0.025 (0.015) | .097 |
| Forearm | 930 | −0.010 (0.007) | .171 | −0.011 (0.007) | .132 | −0.008 (0.008) | .320 |
| Whole body | 809 | −0.025 (0.011) | .016 | −0.027 (0.011) | .015 | −0.021 (0.012) | .084 |
The β, SE, and P values were calculated using an additive genetic model.
We conducted a sensitivity analysis excluding the 4 individuals with the QQ genotype. In models adjusted for age, age2, sex, and BMI and accounting for family structure, the R3500Q genotype remained significant at the lumbar spine and whole body (P < .05). R3500Q was no longer significant for the femoral neck (P = .07) and not significant at the one-third radius (P = .24).
We then undertook several secondary sets of analyses. We first considered whether LDL-C was correlated with BMD independently of APOB genotype and/or whether the association of the APOB genotype could be mediated entirely by LDL-C levels. These analyses revealed that LDL-C was not significantly correlated with BMD at any site (femoral neck, P = .75; lumbar spine, P = .16; forearm, P = .31; or whole body, P = .07) in an age-, age2-, sex-, and BMI-adjusted models and ignoring APOB genotype. In models including LDL-C as an additional covariate, APOB genotype (Table 2, model 3) remained significantly associated with BMD at the femoral neck (P = .029) with a slightly larger effect size. However, the APOB genotype was no longer associated at the lumbar spine (P = .097) or whole body (P = .084), although a similar magnitude of effect was observed.
We next performed subgroup analyses to assess whether the effect of the APOB Q allele was stronger in particular subgroups. Sex-stratified analyses indicated that the genotype effect on BMD did not differ significantly between the sexes, although the effects tended to be 2 to 3 times higher in women than in men (all interaction P values > .13; data not shown). To determine whether genotype effects on BMD might be more pronounced at younger ages (which would reflect potential APOB genotype effects on peak bone mass), or older ages (which would reflect potential APOB genotype effects on peak bone mass and/or bone loss), we compared the genotype effect on BMD between subjects greater than vs less than age 45 years. Again, none of the genotype-by-age interactions achieved statistical significance for any of the BMD sites. Hypothesizing that the genotype effect might be stronger in those with metabolic syndrome because of its known association with lipid oxidation, we compared the genotype effect between those with (n = 94) and without (n = 992) metabolic syndrome but observed no significant interactions. Finally, models including an interaction term for genotype by presence of CAC were carried out to determine whether CAC was an effect modifier for this association in a subset of 861 individuals with both BMD and CAC measures. There was no significant interaction between CAC presence and genotype for femoral neck, lumbar spine, or whole-body BMD. A statistically significant interaction (P = .03) was observed for radius BMD; however, this interaction was in the absence of an association with main effects (ie, there was no association with either APOB genotype or CAC presence alone) and should be interpreted cautiously because most APOB R3500Q carriers had CAC (n = 58) compared with the small number not having CAC (n = 18).
Discussion
Our results show an association of the APOB R3500Q variant with low BMD at the femoral neck, lumbar spine, and total-body sites, with the risk allele associated with a lowering of BMD by 1.7% to 2.3%. The R3500Q variant is associated with an ∼58 mg/dL higher LDL-C, with carriers having perhaps a lifetime burden of high LDL-C indicated by the fact that the effect size remained constant across all ages of our study population (14). Possible effects of hyperlipidemia on BMD have been debated for some time, and although some support has been generated from in vitro and animal models, conclusive data from human studies has been lacking.
Under the principles of Mendelian randomization, causality can be inferred under the following conditions (24): 1) there is no reverse causality (ie, low BMD does not alter genotype); 2) there are no unmeasured common causes of genotype and outcome (ie, the APOB-BMD association is not spurious due to population stratification); or 3) all causal pathways from genotype to outcome pass entirely through LDL-C (ie, the effects of APOB on BMD are caused by other metabolic abnormalities associated with APOB and not from elevated LDL-C). Of these conditions, the first two seem to hold true. Reverse causality is unlikely because the functional consequences of the R3500Q variant on binding affinity of APOB to the LDL receptor are well established. Likewise, population stratification is unlikely in our study because the OOA are a genetically homogeneous population. It is more difficult to establish unequivocally that assumption 3 holds, ie, that the effect of the R3500Q variant on BMD is due entirely to LDL-C. A key limitation of Mendelian randomization is that genetic variants with multiple (ie, pleiotropic) effects could lead to erroneous conclusions (25). However, as we have previously reported (14), R3500Q carriers appear to have an isolated defect of high LDL-C levels (and consequently increased CAC); they do not differ from noncarriers in terms of BMI, smoking, or other cardiovascular risk factors (blood pressure, HDL-C, triglycerides, or diabetes mellitus). Although we did observe that adjusting for LDL-C attenuated the relationship between APOB genotype and BMD, it did not completely explain the relationship, and we do not interpret this as evidence that LDL-C is not the only mechanism involved in this relationship, but rather that in FDB carriers, a single measure of LDL-C is not sufficient to capture the total lifetime LDL-C burden on atherosclerosis and possibly, as indicated in this study, BMD as well. CAC, which is strongly associated with R3500Q genotype, can confound dual-energy x-ray absorptiometry measurement by artificially increasing lumbar spine BMD (26). Although the artificially higher BMD could impact our lumbar spine results, this would likely bias our findings toward the null and would not impact the association observed at other skeletal sites. If R3500Q carriers knew they were at higher risk for cardiovascular disease, there could be differences in lifestyle factors that would confound the relationship between genotype and BMD. However, the OOA population has limited access to preventative health care compared with non-Amish individuals, and many of the carriers we have identified were previously unaware of their high LDL-C. Even if undiagnosed, it is plausible that cardiovascular disease symptoms in R3500Q carriers could result in subtle lifestyle differences that we are unable to detect.
Although our study implies a causal relationship between LDL-C and BMD, a key question to be addressed is how LDL-C influences BMD. Previous research suggests at least 2 possible mechanisms that are not mutually exclusive. The vascularization hypothesis is based on the fact that the bone microenvironment is highly vascularized, which may make it liable for deposition of lipoproteins and oxidized lipid products (1, 27). In fact, early histological studies observed lipid deposition in bone vasculature (28), and Oil red O staining of human, cortical osteoporotic femur bone has shown positive staining for lipid within the perivascular space of Haversian canals (29). A second potential pathway is that hyperlipidemia may have direct impacts on osteoblast activity. Minimally oxidized, but not native, LDL-C inhibits differentiation, alkaline phosphatase activity, and calcification of preosteoblasts but has opposite effects on calcifying vascular cells that are believed to lead to vascular calcification (27). Further impacting bone formation, lipid oxidation products can increase peroxisome proliferator-activated receptor-gamma and decrease WNT signaling resulting in an environment where progenitor mesenchymal stem cells may be more likely to differentiate into adipocytes instead of osteoblasts (30). Recent work by Pirih and colleagues (31) extends these observations and demonstrates that hyperlipidemic mice on a high-fat diet had worse bone mechanical strength and lower levels of the bone biomarker procollagen type I N-terminal propeptide, which indicate reduced bone formation. There is also evidence that hyperlipidemia promotes bone resorption. In studies of wild-type and LDLR-knockout C57BL/6J mice on either normal or high-fat diet, a higher LDL was positively correlated with tartrate-resistant acid phosphatase positivity and resorption pit formation, indicating higher osteoclast activity (r = 0.77 and 0.54, respectively) (29).
A recent study of 22 familial hypercholesterolemia (FH) patients (age 20–71 years) with the same mutation in LDLR did not observe lower BMD compared with controls matched on age, sex, and geographical region (32). Although we would have anticipated a similar association of BMD in FH as observed in our FDB carriers, our study had approximately 5 times as many APOB Q allele carriers and may have observed these associations because it was adequately powered. Although lower BMD was not observed in the FH patients, the authors did observe that a higher degree of aortic calcification was negatively correlated with serum osteocalcin, urinary calcium, and estimated glomerular filtration rate and posited that this may be an indication of modified osteoblast function and altered mineral homeostasis in mutation carriers with more severe cardiovascular disease (32).
The significance of the association we have observed between LDL-C and BMD is not in promoting the R3500Q variant as an important cause of decreased bone health or osteoporosis risk (due to the low frequency of this mutation in non-Amish populations), but rather in providing support for a biological connection between lipid homeostasis and bone. Although our study does not allow us to definitively point to LDL-C as being the cause of decreased BMD because there may be other unmeasured variables associated with the R3500Q mutation having direct effects on BMD that we did not measure, it does provide a unique opportunity to look for an association between long-term high LDL-C levels and low BMD. Furthermore, even if LDL-C is a cause of low BMD, our study does not address whether it is the proximal cause or whether it leads to a cascade of downstream effects that alter bone metabolism. Despite these caveats, our data provide, perhaps, unique evidence supporting a lipid-BMD connection.
Acknowledgments
We thank the Amish liaisons, field staff, and participants for their important contributions to this project.
This work was supported by Research Grants R01-HL088119, R01-HL069313, R01-AR046838, U01-HL072515, U01-GM074518–04, and U01-HL105198 from the National Institutes of Health (NIH), Scientist Development Grant 0830146N (to H.S.) and Grant-in-Aid 0855400E (to B.D.M.) from the American Heart Association. L.M.Y.-A. was supported by F32 AR059469 from National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH. Partial support was also received from the Mid-Atlantic Nutrition and Obesity Center (P30 DK072488). Construction and maintenance of the Anabaptist Genealogy Database is covered under an Institutional Review Board-approved protocol at the NIH (Dr Leslie Biesecker, Principal Investigator).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APOB
- apolipoprotein B
- BMD
- bone mineral density
- BMI
- body mass index
- CAC
- coronary artery calcification
- FDB
- familial defective apolipoprotein-B100
- FH
- familial hypercholesterolemia
- HDL-C
- high-density lipoprotein cholesterol
- LDL-C
- low-density lipoprotein cholesterol
- OOA
- Old Order Amish.
References
- 1. Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. 2008;5:19–34 [PMC free article] [PubMed] [Google Scholar]
- 2. Cui LH, Shin MH, Chung EK, et al. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int. 2005;16:1975–1981 [DOI] [PubMed] [Google Scholar]
- 3. Poli A, Bruschi F, Cesana B, Rossi M, Paoletti R, Crosignani PG. Plasma low-density lipoprotein cholesterol and bone mass densitometry in postmenopausal women. Obstet Gynecol. 2003;102:922–926 [DOI] [PubMed] [Google Scholar]
- 4. Tankó LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. 2003;32:8–14 [DOI] [PubMed] [Google Scholar]
- 5. Wu LY, Yang TC, Kuo SW, et al. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. 2003;29:317–325 [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi T, Sugimoto T, Yano S, et al. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–217 [DOI] [PubMed] [Google Scholar]
- 7. Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tanko LB. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownbill RA, Ilich JZ. Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Womens Health (Larchmt). 2006;15:261–270 [DOI] [PubMed] [Google Scholar]
- 9. Samelson EJ, Cupples LA, Hannan MT, et al. Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham Osteoporosis Study. Bone. 2004;34:557–561 [DOI] [PubMed] [Google Scholar]
- 10. Borén J, Ekström U, Agren B, Nilsson-Ehle P, Innerarity TL. The molecular mechanism for the genetic disorder familial defective apolipoprotein B100. J Biol Chem. 2001;276:9214–9218 [DOI] [PubMed] [Google Scholar]
- 11. Whitfield AJ, Barrett PH, van Bockxmeer FM, Burnett JR. Lipid disorders and mutations in the APOB gene. Clin Chem. 2004;50:1725–1732 [DOI] [PubMed] [Google Scholar]
- 12. Innerarity TL, Mahley RW, Weisgraber KH, et al. Familial defective apolipoprotein B-100: a mutation of apolipoprotein B that causes hypercholesterolemia. J Lipid Res. 1990;31:1337–1349 [PubMed] [Google Scholar]
- 13. Miserez AR, Muller PY. Familial defective apolipoprotein B-100: a mutation emerged in the mesolithic ancestors of Celtic peoples? Atherosclerosis. 2000;148:433–436 [DOI] [PubMed] [Google Scholar]
- 14. Shen H, Damcott CM, Rampersaud E, et al. Familial defective apolipoprotein B-100 and increased low-density lipoprotein cholesterol and coronary artery calcification in the Old Order Amish. Arch Intern Med. 170:1850–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee WJ, Pollin TI, O'Connell JR, Agarwala R, Schäffer AA. PedHunter 2.0 and its usage to characterize the founder structure of the Old Order Amish of Lancaster County. BMC Med Genet. 2010;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell BD, McArdle PF, Shen H, et al. The genetic response to short-term interventions affecting cardiovascular function: rationale and design of the Heredity and Phenotype Intervention (HAPI) Heart Study. Am Heart J. 2008;155:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsueh WC, Mitchell BD, Aburomia R, et al. Diabetes in the Old Order Amish: characterization and heritability analysis of the Amish Family Diabetes Study. Diabetes Care. 2000;23:595–601 [DOI] [PubMed] [Google Scholar]
- 18. Streeten EA, McBride DJ, Pollin TI, et al. Quantitative trait loci for BMD identified by autosome-wide linkage scan to chromosomes 7q and 21q in men from the Amish Family Osteoporosis Study. J Bone Miner Res. 2006;21:1433–1442 [DOI] [PubMed] [Google Scholar]
- 19. Agarwala R, Biesecker LG, Schäffer AA. Anabaptist genealogy database. Am J Med Genet C Semin Med Genet. 2003;121C:32–37 [DOI] [PubMed] [Google Scholar]
- 20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832 [DOI] [PubMed] [Google Scholar]
- 21. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421 [PubMed] [Google Scholar]
- 22. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gauderman WJ, Morrison JM. Quanto 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies, http://hydra.usc.edu/gxe, 2006
- 24. Glymour MM, Tchetgen EJ, Robins JM. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davey Smith G, Ebrahim S. What can Mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ. 2005;330:1076–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reid IR, Evans MC, Ames R, Wattie DJ. The influence of osteophytes and aortic calcification on spinal mineral density in postmenopausal women. J Clin Endocrinol Metab. 1991;72:1372–1374 [DOI] [PubMed] [Google Scholar]
- 27. Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687 [DOI] [PubMed] [Google Scholar]
- 28. Ramseier E. Atherosclerotic lesions of the bone arteries. Virchows Arch. 1962;336:77–86 [Google Scholar]
- 29. Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–e10 [DOI] [PubMed] [Google Scholar]
- 30. Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-γ expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pirih F, Lu J, Ye F, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27:309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Awan Z, Alwaili K, Alshahrani A, Langsetmo L, Goltzman D, Genest J. Calcium homeostasis and skeletal integrity in individuals with familial hypercholesterolemia and aortic calcification. Clin Chem. 2010;56:1599–1607 [DOI] [PubMed] [Google Scholar]