Abstract
Context:
There is no doubt that vitamin D must be activated to the hormonal form 1,25-dihydroxyvitamin D to achieve full biological activity or that many tissues participate in this activation process—be it endocrine or autocrine. We believe that not only is 25-hydroxyvitamin D important to tissue delivery for this activation process, but also that intact vitamin D has a pivotal role in this process.
Objective:
In this review, evidence on the vitamin D endocrine/autocrine system is presented and discussed in relation to vitamin D-binding protein affinity, circulating half-lives, and enzymatic transformations of vitamin D metabolites, and how these affect biological action in any given tissue.
Conclusions:
Circulating vitamin D, the parent compound, likely plays an important physiological role with respect to the vitamin D endocrine/autocrine system, as a substrate in many tissues, not originally thought to be important. Based on emerging data from the laboratory, clinical trials, and data on circulating 25-hydroxyvitamin D amassed during many decades, it is likely that for the optimal functioning of these systems, significant vitamin D should be available on a daily basis to ensure stable circulating concentrations, implying that variation in vitamin D dosing schedules could have profound effects on the outcomes of clinical trials because of the short circulating half-life of intact vitamin D.
Extraordinary advances in vitamin D research have occurred since Adolf Windaus was awarded the Nobel Prize for its discovery in 1928 (1). Over the next several decades vitamin D was studied mostly in dietary terms and later in metabolic studies, culminating in the discovery of 25-hydroxyvitamin D [25(OH)D] by the Deluca lab in 1968 (2). Intense study of 25(OH)D over the next 2 years resulted in the discovery of the hormonal form of vitamin D—1,25-dihydroxyvitamin D [1,25(OH)2D]—by various research groups (3–6). At that point, research into any potential biological function/contribution of circulating intact vitamin D basically ceased because 1,25(OH)2D became the major focus, followed by a later change in focus in the mid to late 1980s when circulating 25(OH)D was deemed the best nutritional status indicator for vitamin D and was shown to be associated with secondary hyperparathyroidism and cancer incidence in a way that circulating 1,25(OH)2D was not (7, 8). Thus, outcome relationships with circulating 25(OH)D have been an intense focus of research while the potential role of the parent compound intact vitamin D in the circulation has not been studied in the same way, especially because measuring this compound in the circulation has been very difficult until recently.
There is no debate that to become fully active, vitamin D must be metabolized to 1,25(OH)2D; however, what has not been fully appreciated in this metabolic scheme is the importance of cellular accessibility of the parent compound vitamin D, as well as that of 25(OH)D. The current literature shows clearly that vitamin D is vastly more accessible than 25(OH)D for internalization into any given cell type with the exception of cells expressing the megalin-cubilin system (9), the kidney and parathyroid gland, that provide the vitamin D endocrine system. In contrast, most other cell types depend on their paracrine/autocrine environment, but the availability of circulating 25(OH)D to these cells is reduced because it is tightly bound to the vitamin D binding protein (VDBP). Intact vitamin D is bound to VDBP much less strongly (10–12), with ample evidence in the literature to support this concept, showing that only the “free” metabolite can enter the cell easily for activation (13, 14). This fact becomes very important when designing clinical studies for vitamin D in the selection of dosing regime, ie, whether to give supplements at daily, weekly, monthly, or longer intervals, because such different regimes could well explain much of the discordance in outcomes that has been observed in interventional studies on infection and cancer (15–27). These data might prove more consistent with consideration of the provision of intact vitamin D as a major substrate for local activation of vitamin D in target tissues, in addition to the availability of circulating 25(OH)D. A further problem is that it is not yet known how much vitamin D is required and what is the dosing frequency to ensure a stable circulating concentration of intact vitamin D that is adequate for optimal tissue function.
Control of Circulating Concentrations of Vitamin D, 25(OH)D, and 1,25(OH)2D and Tissue Delivery
The control of circulating concentrations of vitamin D, 25(OH)D, and 1,25(OH)2D is a complex matter, affected by many disease states such as malabsorption syndromes, aberrant vitamin D metabolism as in sarcoidosis and/or disruptions in the calcium homeostatic system, etc (28–30). Although these are all important, they are beyond the scope of this short review and are not considered further; consideration is given only to what happens to these compounds under normal conditions when vitamin D is obtained through the diet or UV light induction and how they enter normal cells.
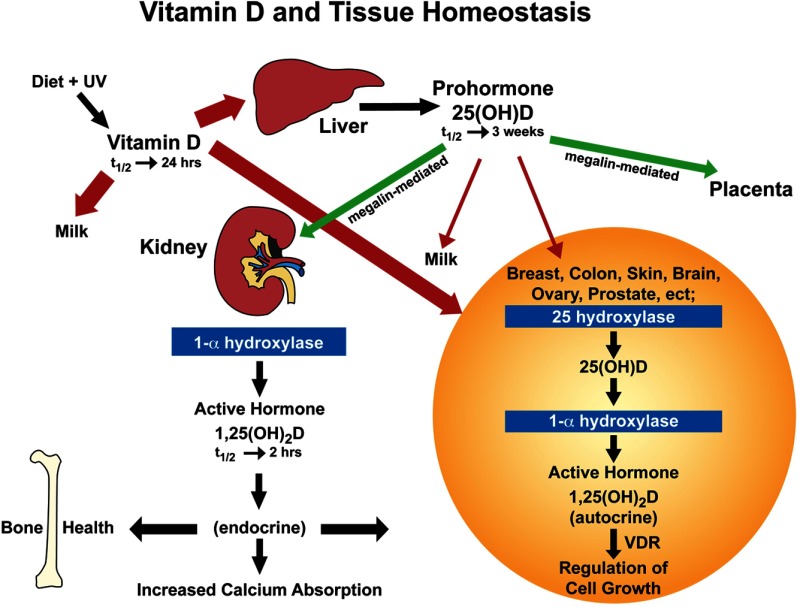
In humans, vitamin D3 is naturally obtained when sunlight in the UVB range strikes the skin and causes 7-dehydrocholesterol to be converted into vitamin D3, which then diffuses into the circulation through the capillary bed (31). Vitamin D also is obtained orally through the diet as either vitamin D2 or D3. As far as can be determined from the literature, this absorption process is primarily diffusion-based, is dependent on bile acid solubilization, and is not saturable (32, 33). When vitamin D3 enters the circulation after UV exposure, it is primarily associated with VDBP. In contrast, after intestinal absorption, it is coupled with both VDBP and lipoproteins (34). Vitamin D from either route is delivered primarily to the liver, where 25(OH)D is produced, becomes associated with VDBP, and is discharged into the circulation (35). However, vitamin D is circulated not only to the liver but also to all tissues in the body; many of these tissues are now known to contain not only the activating hydroxylase but also the vitamin D 25-hydroxylase that converts vitamin D into 25(OH)D, thus achieving autocrine production of 25(OH)D in those tissues (36–40) (Figure 1). We believe this to be an underappreciated and very important event that has not yet been adequately considered or investigated.
Figure 1.
Diagram of the metabolic processes providing vitamin D and its metabolites to various tissues of the body. Tissue distribution of vitamin D and 25(OH)D based on simple diffusion (red arrows) or endocytosis (green arrows). Endocytosis requires the tissue-specific megalin-cubilin system, whereas simple diffusion is primarily controlled by the dissociation constant of the vitamin D compound for the VDBP. Bolder red lines indicate greater diffusion rates due to a higher dissociation constant. t1/2, half life.
On reaching the circulation, the primary determinant of how long a vitamin D metabolite will stay in circulation is its affinity for VDBP (10–12). Vitamin D, 25(OH)D, and 1,25(OH)2D have vastly different dissociation constants with regard to VDBP: for 25(OH)D, it is approximately 10−9 m, and for vitamin D and 1,25(OH)2D, it is approximately 10−7 m (10–12); in addition, for vitamin D, it is probably reduced to approximately 10−8 m by its relative insolubility in vitro (41). These dissociation constants also contribute to the circulating half-lives of these compounds, where for 25(OH)D, it is weeks; for vitamin D, 1 day; and for 1,25(OH)2D, a few hours (42). These dissociation constants also dictate the “free” concentration of compound that is available to diffuse across a cell membrane into cells to be metabolized or to modulate cell activity (see Figure 1). In the case of these three compounds, the “free” circulating concentrations are greater for 1,25(OH)2D than for intact vitamin D, which in turn are larger than that of 25(OH)D, matching their relative circulating half-lives.
Besides simple cellular diffusion of free compound, there exists another important tissue transport mechanism for these steroids—the megalin-cubilin endocytotic system (9). This system is key in the delivery of 25(OH)D to the 25-hydroxyvitamin D-1-α-hydroxylase in the kidney (9), and it also exists in the parathyroid glands, so that its important role in the endocrine function of vitamin D is self-evident (43). The megalin-cubilin system also functions in the placenta (43), which we will revisit later. However, where tissues lack this endocytotic system, diffusion of vitamin D compounds in relation to free circulating concentrations becomes inherently important. Interestingly, VDBP-knockout animal models show normal survival when given dietary vitamin D on a daily basis (44, 45); because vitamin D metabolite cellular access could only be by diffusion in these animals, this shows that the parent compound vitamin D is normally transferred in wild-type animals through simple membrane diffusion.
Dosing with Vitamin D and Relative Sustainability of the Compounds in the Circulation
Vitamin D is introduced into the human body in two ways, orally or by UV exposure. UV exposure is the natural way by which humans receive their vitamin D, but now, due to a variety of lifestyle changes, it is mostly obtained from oral sources. Adams et al (46) first described the magnitude and time course by which vitamin D3 is produced and released into the circulation. This work clearly demonstrated that the human body could produce and release thousands of IU of vitamin D3 into the circulation within 24 hours of modest UV exposure (46), whereas orally ingested vitamin D3 appears in the circulation at a more rapid pace, with a 12-hour peak rather than a 24-hour peak (34, 46, 47). Furthermore, there is a different plasma distribution of vitamin D in oral vs UV-stimulated release (34); yet, the circulating half-life of the compound by either route is of the order of 12–24 hours (34). Because of this short half-life, even large bolus vitamin D doses of 50 000 to 100 000 IU are cleared from the circulation within a week, making vitamin D basically undetectable in the circulation (46–49). It was largely assumed that after a large bolus dose or UV exposure the rapidly disappearing vitamin D was stored in adipose tissue to be released at a later time. This has recently been shown not to occur, however, because it does not reappear in the systemic circulation at detectable concentrations (49). Early work has demonstrated vitamin D to be excreted in bile, feces, and urine (50). Thus, the only known way to sustain constant circulating vitamin D concentrations is by daily supplementation and/or chronic UV exposure (51, 52).
From a supplementation standpoint, one would have to supply an adult with a dose that would achieve increases in circulating vitamin D. For instance, a 400 IU/d dose for several months will achieve little or no detectable effect on the circulating concentration of vitamin D (51, 53). However, if one supplements adults for extended periods with 2000–6000 IU/d vitamin D3, stable circulating concentrations of vitamin D are maintained in the range of 10–40 ng/mL in a linear fashion (51, 53), although what constitutes a minimal dosage of vitamin D for achieving an optimal circulating vitamin D concentration remains to be determined.
What is the time course for the systemic appearance of 25(OH)D after acute or chronic dosing of vitamin D3? 25(OH)D does not appreciably increase in the systemic circulation until about 24 hours after oral supplementation with 100 000 IU vitamin D2, and then by only 1–4 ng/mL (54). Chronic daily dosing of vitamin D will result in a slow, sustained rise in circulating 25(OH)D that will reach a steady state at 3–4 months (55, 56), whereas acute, interval, or large bolus dosing with vitamin D results in a variety of appearance and disappearance rates (15, 49, 57–60).
Given the pharmacokinetics of vitamin D and 25(OH)D, investigators considering performing a clinical trial with vitamin D should consider the above information and what the circulating profiles of vitamin D and 25(OH)D might be on dosing of 400, 1000, 2000, or 4000 IU/d; 28 000 IU/wk; 120 000 IU/mo; 360 000 IU every 3–4 months; or some higher annual dose. All of these schedules have been utilized in various clinical trials (15–27, 51, 53, 57–77). All of these dosing schedules appear to be well tolerated in normal adults from a toxicity standpoint because none results in hypercalcemia or hypercalciuria (15–27, 51, 53, 57–77). However, these doses will differ greatly in their impact on circulating concentrations of vitamin D and 25(OH)D; daily doses of vitamin D result in stable circulating concentrations of both compounds (51), whereas weekly or longer interval dosing will result in large fluctuations in circulating vitamin D but stable concentrations of 25(OH)D (77–79). Indeed, any high-dose, long-interval dosing schedule can be considered pharmacological rather than physiological.
Biological Roles in Human Physiology Unique to the Parent Compound, Vitamin D
Lactation and pregnancy
If circulating vitamin D truly has unique roles independent of circulating 25(OH)D, what might they be? The clearest example of this occurs during human lactation. Our laboratory has been involved in this area of investigation during the last three decades. We published the first comprehensive report that quantified vitamin D and its various metabolites in human milk (80) and remain active in this area of work. Our interest in this area was sparked during undergraduate training (B.W.H.) when human milk was described as the perfect food for the newborn infant, except that it was deficient in vitamin D and breastfeeding could result in rickets to the nursing infant. Sadly, this fact is still true today in that the solely breastfed infant can exhibit severe vitamin D deficiency (81). For dieticians, nutritionists, and primary medical practitioners, the solution was simple—provide supplemental vitamin D drops for the nursing infant and the problem will be solved (82). But why should this problem exist, and how was it avoided in early humans?
Our initial work on the vitamin D content of human milk showed it to be woefully inadequate in vitamin D, confirming earlier work using bioassay methodology (83). In hindsight, this error was easy to make because only 25(OH)D was assayed and detected in human milk, and the parent vitamin D could not be measured due to inadequate methodology (80, 84). The basic problem, though, was that there was little circulating vitamin D in the mothers because they had poor oral intake and little UV exposure. Thus, there was little vitamin D for transfer into the mother's milk, and incorrect conclusions about human milk emerged as a result (80), which we corrected only when we performed experiments demonstrating that both UV exposure and increased maternal vitamin D supplementation could produce profound increases in both circulating and milk concentrations of vitamin D but minimal changes in concentrations of 25(OH)D (85, 86).
Our most intriguing observation on this topic was on a hypoparathyroid patient taking 100 000 IU/d vitamin D2 who went through two normal pregnancies and deliveries and breastfed her infants. Her milk contained nearly 8000 IU/L of antirachitic activity, mostly as vitamin D2 with relatively little 25(OH)D2 content (86); her milk contained vitamin D2 at 28% of the circulating concentration as compared to 25(OH)D2 at only 1.3% of the circulating concentration (86), an observation confirmed in lactating women with “normal” vitamin D status (87). Thus, the parent compound is clearly transferred from the circulation into the breast milk much more efficiently than 25(OH)D; a simple calculation suggests that for every 1000 IU/d vitamin D3 provided to a lactating woman, about 80 IU/L will appear in her breast milk. Thus, to provide 400–500 IU/d for their infants, nursing mothers will appear to need to acquire 6000 IU/d of vitamin D3, something not yet investigated because, until recently, recommended intakes for nursing mothers were 400 IU/d, and intakes above 2000 IU/d were considered harmful (88).
In 2001, Vieth et al (55) demonstrated that the 2000 IU/d upper safe limit for vitamin D assigned by the Institute of Medicine (IOM) in 1997 was incorrect and that daily intakes of vitamin D up to 4000 IU/d were indeed safe (55, 56). We utilized the findings of the 1999 Vieth study (89) to gain Institutional Review Board approval for a series of studies that clearly demonstrated our hypothesis (53, 90, 91). In addition, in our initial pilot studies, we found that maternal adherence (compliance) with vitamin D supplementation exceeded 90%, whereas adherence rates for giving infant vitamin D drops was only 70% (53, 90)—data that allowed us to proceed to a National Institute of Child Health and Human Development 6-year, two-site, double-blind, randomized controlled trial (RCT) that fully confirmed our initial observation (91).
Thus, our proposed approach safely addressed the needs of both the lactating woman and her recipient breastfeeding infant while reconfirming that the use of infant vitamin D supplementation is far below the universal supplementation recommended by the American Academy of Pediatrics that should begin within the first few days after birth (82): ie, of the 319 exclusively breastfeeding mother-infant dyads, 31 (9.7%) were receiving a vitamin D supplement at 1 month of age (our unpublished data). Sadly, if one follows the 2010 IOM recommendations for lactating women of 400 IU/d (92), we will see the same problems as in 1975 when we posed the initial question, “How can ingesting human milk cause vitamin D deficiency?” Breastfeeding is, therefore, a clear example of a situation where circulating vitamin D is critical in assuring vitamin D provision for the infant and where circulating 25(OH)D does not substitute for the intact vitamin.
Why should the transfer of vitamin D rather than 25(OH)D be favored in the mammary gland? We postulate that it is simply controlled by the relative freedom of vitamin D, as compared to 25(OH)D, from VDBP binding, which allows more vitamin D than 25(OH)D to diffuse across cell membranes into the milk. This situation is in contrast to the relative transfer of 25(OH)D and vitamin D from mother to fetus across the placenta. Here, 25(OH)D is much better able to navigate the crossing because fetal concentrations of 25(OH)D are about 70% that of the mother (93), whereas vitamin D concentrations are only about 10% of maternal concentrations (51), similar to the mammary gland (93, 94). What are the mechanisms here? Again, no one really knows, but a good hypothesis is that vitamin D is going across again by simple diffusion, whereas 25(OH)D is being specifically transported attached to the VDBP through the megalin-cubilin endocytotic system, which is very active in the placenta (43) and is key to the conversion of 25(OH)D to 1,25(OH)2D, as it is in the kidney (9).
Other tissues
It is possible that circulating vitamin D has a physiological function other than simple diffusion advantages in tissues other than the mammary gland, as suggested by some recent evidence. Such a mechanism would take advantage of the ability of vitamin D to diffuse into cells and then become activated by already known enzymatic steps because the cytochrome P450 enzymes that convert vitamin D to 25(OH)D are dispersed throughout the body, eg, in prostate, skin, intestine, brain, and of course, in liver (36–39). It is important to reemphasize that 25-hydroxyvitamin D-1-α-hydroxylase is even more widely dispersed in tissues (95) so that intact vitamin D can be metabolized to the active hormone in these tissues; being widely dispersed in various tissues, it is likely that they serve some useful biological function.
Of all cell types in the body already examined for vitamin D-axis enzymes, dermal keratinocytes are the best characterized (39). Indeed, the isolation and molecular cloning of the 25-hydroxyvitamin-D-1-α-hydroxylase was first achieved using keratinocytes (96), which are known to metabolize the intact compound into hormonal 1,25(OH)2D (39, 96). This function is unsurprising because huge amounts of vitamin D3 are made in the skin in response to UV light exposure. This process enhances anti-inflammatory and antimicrobial protection. When this system is short of vitamin D3 due to avoidance of sunlight or wearing sunscreen, serum intact vitamin D content falls (97).
Orally administered vitamin D3 can substitute for UVB replete keratinocytes; Hata et al (98) gave 4000 IU/d vitamin D3 orally to subjects for a period of 21 days and found marked increases in cathelicidin expression in keratinocytes from normal patients and those with atopic dermatitis, with only modest rises in circulating 25(OH)D concentrations from 22.5 to 35.5 ng/mL. Circulating vitamin D3 concentrations were not measured. This cathelicidin response might result from the small rise in circulating 25(OH)D, but might well have been due to the much larger rise in circulating vitamin D3 that would have occurred on such doses (from < 2 ng/mL to concentrations > 20 ng/mL) (51), able to diffuse into the keratinocyte ready for activation (39, 96).
Although a recent short-term RCT using a daily dose of vitamin D3 was negative (99), other studies using smaller oral doses of vitamin D3 have reported clinical improvement in atopic dermatitis (75, 100, 101), the best example of which is a recent study by Samochocki et al (101) where patients were given 2000 IU/d vitamin D3 for 3 months and significant clinical improvement in atopic dermatitis was seen with increases in circulating 25(OH)D to 13 ng/mL from 7 ng/mL (101), although immeasurable baseline vitamin D would have increased to approximately 10 ng/mL (51), providing significant increases in substrate vitamin D3 to the diseased keratinocyte.
Recent work demonstrates that intact vitamin D has effects on other tissues; for example, bolus doses of vitamin D2 (100 000 IU) decrease circulating hepcidin rapidly (M. Hewison, B. W. Hollis, unpublished data, 2013). Hepcidin is an antibacterial protein that regulates the absorption, tissue distribution, and extracellular concentration of iron by suppressing ferroportin-mediated export of cellular iron and is produced by hepatocytes and monocytes (M. Hewison, B. W. Hollis, unpublished data, 2013). This report demonstrated that circulating hepcidin began to decline 2 hours after a bolus of vitamin D2 before circulating 25(OH)D begins to rise and at the time orally administered intact vitamin D appears in the circulation (M. Hewison, B. W. Hollis, unpublished data, 2013).
Further evidence about the role of vitamin D in cancer suppression/elimination comes from our interventional trial on the progression of low-grade prostate cancer (27). In this study, men with low-grade prostate cancer were given 4000 IU/d vitamin D3 for 1 year. After this, repeat biopsies staged by a pathologist blinded to treatment showed improved outcome (in comparison with matched historical controls) (27), with increases in circulating 25(OH)D from 32 to 66 ng/mL. The men with the highest baseline circulating 25(OH)D concentrations showed the greatest benefit in clinical outcome with supplementation, whereas tissue slice analysis of prostate glands from men receiving 4000 IU/d vitamin D3 for 2 months before prostatectomy, using matrix-assisted laser desorption/ionization mass spectrometry, exhibited large changes in membrane lipids (B. W. Hollis, S. Gattoni-Celli, unpublished data). Again, the relative role of changes in 25(OH)D vs those of intact vitamin D on tumor progression remains to be tested.
Studies of the type reported by Bischoff-Ferrari et al (103) could answer many of the questions posed here. These investigators provided study subjects with 20 μg/d of either vitamin D3 or 25(OH)D3 and examined a variety of outcome variables after 4 months; circulating 25(OH)D3 was raised more than vitamin D3, but doses could be adjusted to allow comparison of their relative biological efficiency, although such studies would have to be performed outside the United States because we no longer have any available source of 25(OH)D3.
Dosing of Vitamin D and How It May Affect Study Outcomes
In 2010, the IOM committee on vitamin D suggested that the only data useful for advising changes in policy would have to originate from RCTs (92); however, RCTs are extremely expensive, and for vitamin D compounds, there is no financial incentive for their use. Furthermore, designing RCTs for nutrients is more challenging than for new medications because nutrients are normally taken in the diet, and for vitamin D, it is provided by diet as well as sunlight, confounding RCT data (104). Thus, we tend to see small trials performed on specific topics or in specific diseases (23–27, 64–76) or at certain times in the lifecycle, such as pregnancy (51, 62, 105) and lactation (53, 90, 106).
Infection
In addition to vitamin D trials for skeletal outcomes, the role of vitamin D in infectious disease currently is a hot topic of investigation (15–22, 63, 107, 108). Treatment/prevention of infectious disease with vitamin D must depend on its autocrine roles. Three high-profile negative studies recently have been reported (15, 22, 63). Each used periodic bolus dosing of vitamin D (15, 22, 63), so that a lack of continuing availability of intact vitamin D may have confounded the findings suggested by other work (18, 19) where, of six positive RCTs investigating vitamin D and infection (16–21), five utilized a daily dosing schedule (16, 18–21) for a range of infections, including pulmonary tuberculosis (15–17), respiratory infections (19, 20, 22, 63), influenza (18), and gingivitis (21). Indeed, a recent meta-analysis of RCTs with respect to vitamin D supplementation and respiratory tract infections demonstrated vitamin D to be an effective treatment (109) and suggested that vitamin D should be given in a daily dose, as is the premise of this review. The vitamin D doses in these studies that were effective were given daily, in line with the present review; furthermore, the effective doses were higher than those required for skeletal health and above recent IOM recommendations (92).
Musculoskeletal, cancer, cardiovascular, diabetes, multiple sclerosis, neurology, and pregnancy
Although all are agreed that vitamin D is required for skeletal health, an area of controversy that still persists is how much circulating 25(OH)D is required to maintain optimal skeletal homeostasis (102). Here again, long interval vitamin D dosing has proved ineffective and has even appeared to be harmful to bone health (77).
In evaluating recent vitamin D supplementation studies, it is clear that as a group they are diverse in the types of subjects and patients supplemented and in the dosing schedules used; yet, most demonstrate benefits from using vitamin D at 1200 to 40 000 IU/d, in many different conditions (16–21, 24–27, 64–76). Again, using higher vitamin D dosing than IOM bone recommendations (110), important outcomes are reported, including decreases in cancer rates (24), regression of established prostate cancer (27), reduction of blood pressure in African Americans (68), improved insulin sensitivity (69–71), and stabilization of Parkinson's disease in some patients (65). One of the most impressive study results was the ability of vitamin D3 to prevent multiple sclerosis in a highly susceptible population (74); again, it may be noted that these studies used daily doses of vitamin D of 1200 to 40 000 IU and with no adverse events reported (15–22, 24–27, 57, 58, 60–76). Conversely, there are notable high-profile RCTs that failed to produce positive results (15, 22, 23, 25, 63), including the Women's Health Initiative study that failed to show vitamin D could prevent cancer (23); however, this study provided only approximately 280 IU/d vitamin D3, which would have had no effect on any circulating vitamin D metabolites (55, 56, 89). Another RCT failure with respect to cancer was the Trivedi et al (25) study that administered 100 000 IU vitamin D3 on a quarterly basis, although it did demonstrate positive effects on skeletal outcomes (25). The failure of these studies with respect to cancer outcomes was predictable from the arguments we present in this review. What such studies do, however, is to support the premise that any dosing schedule that does not allow a stable circulating concentration of the parent compound will not provide an optimal autocrine environment for vitamin D metabolism in non-bony tissues.
This review considers the autocrine function of vitamin D, which is sensitive to both circulating vitamin D and 25(OH)D and where negative outcomes could be predicted due to failure to maintain circulating vitamin D over time, as with monthly, quarterly, or yearly bolus vitamin D dosing. Although these studies do not prove our view of the direct role of intact vitamin D in the tissues, they do suggest that caution and careful planning of dosing in future RCTs is required to avoid possible confounding from interval dosing.
Conclusions
Circulating vitamin D, the parent compound for tissue vitamin D activation, likely has an important direct physiological role beyond what was originally anticipated through the local tissue autocrine system. Based on emerging data from the laboratory and from clinical trials and on available knowledge of vitamin D axis metabolism, it appears likely that for the optimal benefits of vitamin D supplementation, enough vitamin D should be provided on a daily basis to ensure that stable circulating concentrations are maintained over time. This view implies that schedules for vitamin D dosing could have profound effects on the outcomes of clinical trials, due to the short circulating half-life of vitamin D. Although further work is needed to assess the impact of intact vitamin D concentrations on clinical outcomes, the information so far available suggests that care is required in the design of dosing regimes in future RCTs, both in terms of the doses given and the intervals at which they are given.
Acknowledgments
This work was funded in part by the National Institute of Child Health and Human Development (Grants R01 HD043921 and HD47511); National Institutes of Health (NIH) Grant RR01070; the South Carolina Clinical and Translational Research Institute, with an academic home at the Medical University of South Carolina; NIH/National Center for Research Resources Grant UL1 RR029882; National Center for Advancing Translational Sciences Grant UL1 TR000062; the Thrasher Research Fund; and the Kellogg Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25(OH)D
- 25-hydroxyvitamin D
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- RCT
- randomized controlled trial
- VDBP
- vitamin D binding protein.
References
- 1. Wolf G. The discovery of vitamin D: the contribution of Adolf Windaus. J Nutr. 2004;134:1299–1302 [DOI] [PubMed] [Google Scholar]
- 2. Blunt JW, Tanaka Y, DeLuca HF. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc Natl Acad Sci USA. 1968;61:1503–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764–766 [DOI] [PubMed] [Google Scholar]
- 4. Lawson DE, Fraser DR, Kodicek E, Morris HR, Williams DH. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971;230:228–230 [DOI] [PubMed] [Google Scholar]
- 5. Holick MF, Schnoes HK, DeLuca HF. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci USA. 1971;68:803–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popják G. 1,25-Dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science. 1971;173:51–54 [DOI] [PubMed] [Google Scholar]
- 7. Gloth FM, 3rd, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686 [DOI] [PubMed] [Google Scholar]
- 8. Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178 [DOI] [PubMed] [Google Scholar]
- 9. Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515 [DOI] [PubMed] [Google Scholar]
- 10. Haddad JG, Hillman L, Rojanasathit S. Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1976;43:86–91 [DOI] [PubMed] [Google Scholar]
- 11. Vieth R, Kessler MJ, Pritzker KP. Species differences in the binding kinetics of 25-hydroxyvitamin D3 to vitamin D binding protein. Can J Physiol Pharmacol. 1990;68:1368–1371 [DOI] [PubMed] [Google Scholar]
- 12. Kissmeyer A, Mathiasen IS, Latini S, Binderup L. Pharmacokinetic studies of vitamin D analogues: relationship to vitamin D binding protein (DBP). Endocrine. 1995;3:263–266 [DOI] [PubMed] [Google Scholar]
- 13. Gascon-Barré M, Huet PM. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244:E266–E271 [DOI] [PubMed] [Google Scholar]
- 14. Keenan MJ, Holmes RP. The uptake and metabolism of 25-hydroxyvitamin D3 and vitamin D binding protein by cultured porcine kidney cells (LLC-PK1). Int J Biochem. 1991;23:1225–1230 [DOI] [PubMed] [Google Scholar]
- 15. Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ganmaa D, Giovannucci E, Bloom BR, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr. 2012;96:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis. 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260 [DOI] [PubMed] [Google Scholar]
- 19. Camargo CA, Jr, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130:e561–567 [DOI] [PubMed] [Google Scholar]
- 20. Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:pii:e001663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiremath VP, Rao CB, Naik V, Prasad KV. Anti-inflammatory effect of vitamin D on gingivitis: a dose-response randomised control trial. Oral Health Prev Dent. 2013;11:61–69 [DOI] [PubMed] [Google Scholar]
- 22. Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696 [DOI] [PubMed] [Google Scholar]
- 24. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591 [DOI] [PubMed] [Google Scholar]
- 25. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wagner D, Trudel D, Van der Kwast T, et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and Ki67 labeling in prostate cancer patients. J Clin Endocrinol Metab. 2013;98:1498–1507 [DOI] [PubMed] [Google Scholar]
- 27. Marshall DT, Savage SJ, Garrett-Mayer E, et al. Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J Clin Endocrinol Metab. 2012;97:2315–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell NH, Stern PH, Pantzer E, Sinha TK, DeLuca HF. Evidence that increased circulating 1 α, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979;64:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heubi JE, Hollis BW, Specker B, Tsang RC. Bone disease in chronic childhood cholestasis. I. Vitamin D absorption and metabolism. Hepatology. 1989;9:258–264 [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen H, Wong M, Bikle D, Goodman DB. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972;51:2502–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol. 1981;77:51–58 [DOI] [PubMed] [Google Scholar]
- 32. Hollander D, Muralidhara KS, Zimmerman A. Vitamin D-3 intestinal absorption in vivo: influence of fatty acids, bile salts, and perfusate pH on absorption. Gut. 1978;19:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hollis BW, Lowery JW, Pittard WB, 3rd, Guy DG, Hansen JW. Effect of age on the intestinal absorption of vitamin D3-palmitate and nonesterified vitamin D2 in the term human infant. J Clin Endocrinol Metab. 1996;81:1385–1388 [DOI] [PubMed] [Google Scholar]
- 34. Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ponchon G, Kennan AL, DeLuca HF. “Activation” of vitamin D by the liver. J Clin Invest. 1969;48:2032–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26:2567–2572 [PubMed] [Google Scholar]
- 37. Karlgren M, Miura S, Ingelman-Sundberg M. Novel extrahepatic cytochrome P450s. Toxicol Appl Pharmacol. 2005;207:57–61 [DOI] [PubMed] [Google Scholar]
- 38. Hosseinpour F, Wikvall K. Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J Biol Chem. 2000;275:34650–34655 [DOI] [PubMed] [Google Scholar]
- 39. Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids. 2001;66:399–408 [DOI] [PubMed] [Google Scholar]
- 40. Zhu J, DeLuca HF. Vitamin D 25-hydroxylase—four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–36 [DOI] [PubMed] [Google Scholar]
- 41. Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21:81–86 [DOI] [PubMed] [Google Scholar]
- 42. Smith JE, Goodman DS. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971;50:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marzolo MP, Farfán P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res. 2011;44:89–105 [DOI] [PubMed] [Google Scholar]
- 44. Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149:3656–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Safadi FF, Thornton P, Magiera H, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306:722–725 [DOI] [PubMed] [Google Scholar]
- 47. Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42:644–649 [DOI] [PubMed] [Google Scholar]
- 48. Argao EA, Heubi JE, Hollis BW, Tsang RC. D-α-tocopheryl polyethylene glycol-1000 succinate enhances the absorption of vitamin D in chronic cholestatic liver disease of infancy and childhood. Pediatr Res. 1992;31:146–150 [DOI] [PubMed] [Google Scholar]
- 49. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447–E452 [DOI] [PubMed] [Google Scholar]
- 50. Avioli LV, Lee SW, McDonald JE, Lund J, DeLuca HF. Metabolism of vitamin D3–3H in human subjects: distribution in blood, bile, feces, and urine. J Clin Invest. 1967;46:983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsuoka LY, Wortsman J, Hollis BW. Suntanning and cutaneous synthesis of vitamin D3. J Lab Clin Med. 1990;116:87–90 [PubMed] [Google Scholar]
- 53. Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1:59–70 [DOI] [PubMed] [Google Scholar]
- 54. Lark RK, Lester GE, Ontjes DA, et al. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am J Clin Nutr. 2001;73:602–606 [DOI] [PubMed] [Google Scholar]
- 55. Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–294 [DOI] [PubMed] [Google Scholar]
- 56. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210 [DOI] [PubMed] [Google Scholar]
- 57. Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lips P, Binkley N, Pfeifer M, et al. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010;91:985–991 [DOI] [PubMed] [Google Scholar]
- 59. Cipriani C, Romagnoli E, Scillitani A, et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab. 2010;95:4771–4777 [DOI] [PubMed] [Google Scholar]
- 60. Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–4591 [DOI] [PubMed] [Google Scholar]
- 61. Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–437 [DOI] [PubMed] [Google Scholar]
- 62. Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab. 2013;98:2337–2346 [DOI] [PubMed] [Google Scholar]
- 63. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333–1339 [DOI] [PubMed] [Google Scholar]
- 64. Vieth R, Kimball S, Hu A, Walfish PG. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Suzuki M, Yoshioka M, Hashimoto M, et al. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97:1004–1013 [DOI] [PubMed] [Google Scholar]
- 66. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759 [DOI] [PubMed] [Google Scholar]
- 67. Harris RA, Pedersen-White J, Guo DH, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Forman JP, Scott JB, Ng K, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555 [DOI] [PubMed] [Google Scholar]
- 70. Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97:774–781 [DOI] [PubMed] [Google Scholar]
- 72. Kimball S, Vieth R, Dosch HM, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab. 2011;96:2826–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Soilu-Hänninen M, Aivo J, Lindström BM, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–571 [DOI] [PubMed] [Google Scholar]
- 74. Derakhshandi H, Etemadifar M, Feizi A, et al. Preventive effect of vitamin D3 supplementation on conversion of optic neuritis to clinically definite multiple sclerosis: a double blind, randomized, placebo-controlled pilot clinical trial. Acta Neurol Belg. 2013;113:257–263 [DOI] [PubMed] [Google Scholar]
- 75. Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159:245–247 [DOI] [PubMed] [Google Scholar]
- 76. Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol. 2013;40:265–272 [DOI] [PubMed] [Google Scholar]
- 77. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822 [DOI] [PubMed] [Google Scholar]
- 78. Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–1742 [DOI] [PubMed] [Google Scholar]
- 79. Hollis BW. Short-term and long-term consequences and concerns regarding valid assessment of vitamin D deficiency: comparison of recent food supplementation and clinical guidance reports. Curr Opin Clin Nutr Metab Care. 2011;14:598–604 [DOI] [PubMed] [Google Scholar]
- 80. Hollis BW, Roos BA, Draper HH, Lambert P. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–1248 [DOI] [PubMed] [Google Scholar]
- 81. Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118:603–610 [DOI] [PubMed] [Google Scholar]
- 82. Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152 [DOI] [PubMed] [Google Scholar]
- 83. Harris RS, Bunker JW. Vitamin D potency of human breast milk. Am J Public Health Nations Health. 1939;29:744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Greer FR, Ho M, Dodson D, Tsang RC. Lack of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in human milk. J Pediatr. 1981;99:233–235 [DOI] [PubMed] [Google Scholar]
- 85. Greer FR, Hollis BW, Cripps DJ, Tsang RC. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. J Pediatr. 1984;105:431–433 [DOI] [PubMed] [Google Scholar]
- 86. Greer FR, Hollis BW, Napoli JL. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J Pediatr. 1984;105:61–64 [DOI] [PubMed] [Google Scholar]
- 87. Hollis BW, Pittard WB, 3rd, Reinhardt TA. Relationships among vitamin D, 25-hydroxyvitamin D, and vitamin D-binding protein concentrations in the plasma and milk of human subjects. J Clin Endocrinol Metab. 1986;62:41–44 [DOI] [PubMed] [Google Scholar]
- 88. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academies Press; 1997 [PubMed] [Google Scholar]
- 89. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856 [DOI] [PubMed] [Google Scholar]
- 90. Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80:1752S–1758S [DOI] [PubMed] [Google Scholar]
- 91. Wagner CL, Howard CR, Hulsey T, et al. Results of NICHD two-site maternal vitamin D supplementation randomized controlled trial during lactation. In: Proceedings from the Pediatric Academic Societies; May 4–7, 2013; Washington, DC Abstract 1650.4 [Google Scholar]
- 92. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hollis BW, Pittard WB. Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–657 [DOI] [PubMed] [Google Scholar]
- 94. Hillman LS, Haddad JG. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974;84:742–749 [DOI] [PubMed] [Google Scholar]
- 95. Zehnder D, Bland R, Williams M, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 α-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894 [DOI] [PubMed] [Google Scholar]
- 96. Fu GK, Lin D, Zhang MY, et al. Cloning of human 25-hydroxyvitamin D-1 α-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–1970 [DOI] [PubMed] [Google Scholar]
- 97. Hollis BW, Roos BA, Lambert PW. Vitamin D in plasma: quantitation by a nonequilibrium ligand binding assay. Steroids. 1981;37:609–619 [DOI] [PubMed] [Google Scholar]
- 98. Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hata TR, Audish D, Kotol P, et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis [published online ahead of print May 3, 2013]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Amestejani M, Salehi BS, Vasigh M, et al. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol. 2012;11:327–330 [PubMed] [Google Scholar]
- 101. Samochocki Z, Bogaczewicz J, Jeziorkowska R, et al. Vitamin D effects in atopic dermatitis. J Am Acad Dermatol. 2013;69:238–244 [DOI] [PubMed] [Google Scholar]
- 102. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–49 [DOI] [PubMed] [Google Scholar]
- 103. Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27:160–169 [DOI] [PubMed] [Google Scholar]
- 104. Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol. 2012;4:95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208:137.e1–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed Med. 2006;1:27–35 [DOI] [PubMed] [Google Scholar]
- 107. Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567 [DOI] [PubMed] [Google Scholar]
- 108. Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65:106R–113R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bergman P, Lindh AU, Björkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]