Abstract
Context:
Studies examining whether vitamin D supplementation increases muscle mass or muscle-specific vitamin D receptor (VDR) concentration are lacking.
Objective:
Our objective was to determine whether vitamin D3 4000 IU/d alters muscle fiber cross-sectional area (FCSA) and intramyonuclear VDR concentration over 4 months.
Design and Setting:
This was a randomized, double-blind, placebo-controlled study in a single center.
Participants:
Participants were 21 mobility-limited women (aged ≥65 years) with serum 25-hydroxyvitamin D (25OHD) levels of 22.5 to 60 nmol/L.
Main Outcome Measures:
Baseline and 4-month FCSA and intramyonuclear VDR were measured from vastus lateralis muscle cross-sections probed for muscle fiber type (I/IIa/IIx) and VDR using immunofluorescence.
Results:
At baseline, mean (±SD) age was 78 ± 5 years; body mass index was 27 ± 5 kg/m2, 25OHD was 46.3 ± 9.5 nmol/L, and a short physical performance battery score was 7.95 ± 1.57 out of 12. At 4 months, 25OHD level was 52.5 ± 17.1 (placebo) vs 80.0 ± 11.5 nmol/L (vitamin D [VD]; P < .01), and change in 25OHD level was strongly associated with percent change in intramyonuclear VDR concentration-independent of group (r = 0.87, P < .001). By treatment group, percent change in intramyonuclear VDR concentration was 7.8% ± 18.2% (placebo) vs 29.7% ± 11.7% (VD; P = .03) with a more pronounced group difference in type II vs I fibers. Percent change in total (type I/II) FCSA was −7.4% ± 18.9% (placebo) vs 10.6% ± 20.0% (VD; P = .048).
Conclusion:
Vitamin D3 supplementation increased intramyonuclear VDR concentration by 30% and increased muscle fiber size by 10% in older, mobility-limited, vitamin D-insufficient women. Further work is needed to determine whether the observed effect of vitamin D on fiber size is mediated by the VDR and to identify which signaling pathways are involved.
Low vitamin D status has been associated with reduced muscle mass, strength, and performance in older adults (1–5). Several intervention studies have reported that vitamin D supplementation increases appendicular muscle strength and improves physical function particularly in older women with low vitamin D status (6–9). Experiments in vitro have examined potential mechanisms by which vitamin D acts on skeletal muscle cells (10–13). Administration of vitamin D, as 1,25-dihydroxyvitamin D, stimulated key pathways of muscle growth and differentiation in C2C12 myoblasts (10, 12, 14) and acted directly on these cells via a nuclear vitamin D receptor (VDR) (10, 11). These studies have also demonstrated that concentration of the intramyonuclear VDR increases after both 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D (25OHD) administration. Yet, clinical studies examining effects of parent vitamin D compounds on human muscle fibers and concentration of VDR in muscle, particularly in older adults, are lacking.
We conducted a pilot study to test the hypothesis that oral vitamin D3 4000 IU daily compared with placebo alters total and/or subtype muscle fiber cross-sectional area (FCSA) and intramyonuclear VDR concentration over a 4-month period in mobility-limited women aged 65 years and over with moderately low baseline vitamin D status. The study also aimed to examine the effects of vitamin D on the proportion of type I and/or II muscle fibers and urine nitrogen (UNi) excretion (a marker of muscle breakdown) and to confirm previously reported effects of vitamin D3 on muscle strength and physical function.
Subjects and Methods
Study design and subjects
This was a randomized, double-blind, placebo-controlled study conducted at the Metabolic Research Unit at the Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging at Tufts University. Subjects were randomized to take an oral vitamin D3 capsule 4000 IU daily or matching placebo for 4 months. The study obtained a fasting blood draw, a 24-hour urine collection, muscle performance measures, and a muscle biopsy of the vastus lateralis at baseline and 4 months. A 4-month duration was selected to evaluate changes in muscle tissue and simultaneously have a steady state in 25OHD level after the change in intake (15). The vitamin D3 4000 IU daily dose was chosen as a high yet safe dose of supplementation to minimize risk of underdosing (proof-of-principle study). According to the 2011 Institute of Medicine report, 4000 IU daily is the safe upper limit for supplementation (16).
Ambulatory, community-dwelling, postmenopausal women 65 years of age and over were recruited from direct mailings and advertisements. All subjects were required to maintain their usual level of physical activity and their habitual diet during the 4-month study to limit the impact of physical activity and dietary variation on skeletal muscle. Subjects with active parathyroid disease, chronic kidney disease, nephrolithiasis, malignancy, liver disease, malabsorption, diabetes, unstable heart disease, severe osteoarthritis, and neurodegenerative disease were excluded. Additional exclusions consisted of a vitamin D intake >400 IU/d or a 25OHD level <22.5 or >60 nmol/L; a calcium intake >1000 mg/d (due to risk of hypercalcemia) or an abnormal serum calcium or 24-hour urinary calcium >275 mg (due to risk of hypercalciuria); medications such as hormone replacement therapy in the last 6 months, oral glucocorticoids in the last month, diuretics, antiseizure medications, drugs to treat osteoporosis in the last year, and prescribed antiplatelet and anticoagulant medications; travel to latitudes below 35°N; and use of tanning beds, wheelchair, walker, and nasal oxygen.
We selected women who were defined as at moderate risk for disability based on a short physical performance battery (SPPB) score of ≤9 (out of a possible 12 points) (17), to target a population who would stand to benefit from this intervention. The SPPB involves a balance assessment (open, semitandem, and tandem stance), a timed 4-m walk, and a chair rise test (timed 5 rises) and is predictive of future disability.
Ninety-four women were screened, and 70 were excluded (38 scored >9 on the SPPB, 10 had 25OHD levels above or below target range, 4 used exclusion medications, 9 had excluded medical conditions, 1 was enrolled in another study, 1 had poor venous access, and 7 were not agreeable to study procedures). Twenty-four eligible subjects were randomized to receive placebo (n = 13) or vitamin D3 4000 IU daily (n = 11). Only 21 of these 24 subjects were included in the analyses because they had muscle biopsies at 4 months (1 subject in each group declined biopsy and 1 subject in the vitamin D group had insufficient muscle on final biopsy for analysis). The Tufts Medical Center-Tufts University Health Sciences Campus Institutional Review Board approved the study, and written informed consent was obtained from each subject.
Study capsules
Vitamin D3 and matching placebo capsules were purchased from Tishcon Corporation. The 4000-IU daily dose of vitamin D3 was independently analyzed by Covance, Inc to confirm content. To keep the randomization concealed, the placebo was an identical opaque capsule packed with microcrystalline cellulose. Adherence to study capsules was assessed via a daily compliance calendar, routine pill counts by nursing staff, and a follow-up 25OHD level at 4 months.
All subjects took vitamin D3 (or placebo) 4000 IU orally once daily in the morning immediately after breakfast. A safety spot urine was analyzed for calcium and creatinine on day 30 of the study. If the calcium to creatinine ratio exceeded 0.325 (corresponding to a 24-hour urine calcium [UCa] of 350 mg, the upper limit of the normal range) (18), the study pills were discontinued and a repeat spot urine and serum calcium level were drawn within the following 7 days. If the repeat UCa normalized and serum calcium was normal, pills could be resumed, but repeat testing on pills was performed during the following 2 and 4 weeks.
Biochemical measurements
Blood was drawn after a 12-hour overnight fast between 7:00 and 10:00 am. All samples from individual subjects were batched for analyses, with the exception of the spot UCa and urine creatinine (UCr) measurement on day 30 (a safety measurement). Serum 25OHD was measured with RIA kits from DiaSorin with coefficients of variation (CV) of 5.6% to 7.7%. The 24-hour UCr was measured on an automated clinical chemistry analyzer (Olympus AU400; Olympus America Inc). The CV for this assay ranged from 3.0% to 6.0%. The 24-hour UCa was measured by direct-current plasma emission spectroscopy (Beckman SpectraSpan VI Direct Current Plasma Emission Spectrophotometer; Beckman Instruments) with a CV of 3% to 5%. The 24-hour UNi was measured with a model FP-2000 nitrogen/protein analyzer (LECO), which employs a Dumas combustion method and detection using a thermal conductivity cell. It measures nitrogen with a precision of 15 ppm.
Muscle performance
Immediately after a standardized breakfast, muscle strength was quantitatively assessed by 1 repetition maximum (1RM) measures of knee extension in the nonbiopsied leg using Keiser pneumatic resistance training equipment. The 1RM is defined as the maximum load that could be moved only once throughout the full range of motion while maintaining proper form (19). After 1RM measurement and a 5-minute rest, assessment of individual knee extension average muscle power (in watts) was made. Performance of the average power measurement has been previously described (20). Briefly, subjects were instructed to complete 5 knee extension repetitions each separated by 30 seconds as quickly as possible through their full range of motion at both 40% and 70% of 1RM for knee extension. The average power at 40% and 70% of 1RM was recorded. Knee extension 1RM and average power measures were performed at baseline and at 4 months. The intraclass correlation coefficient for repeated 1RM testing of knee extension is 0.92 and for average power testing is 0.86 in previous reports from our group (20). The SPPB was performed at baseline and 4 months to capture change in physical function.
Muscle biopsy
Muscle biopsies were obtained from the nonexercised vastus lateralis muscle at the level of the midthigh under local anesthesia (xylocaine 1%) with a 5-mm Duchenne biopsy needle and suction approximately 1 hour after a standardized breakfast and 24 hours after the last dose of the study capsule. The specimens were mounted in a vinyl cryomold (Tissue-Tek) and secured using a viscous mounting medium (O.C.T.; Tissue-Tek) and then frozen in isopentane/liquid nitrogen slurry.
Immunohistochemistry
We employed a multistaining immunofluorescent technique to identify fiber type and intramyonuclear VDR as described in detail previously (21). Briefly, muscle tissue specimens were cut in 7-μm serial sections with a cryostat microtome (Leica CM1850; Leica Microsystems). Slides were probed with a primary mouse/antihuman VDR/NR1I1 monoclonal antibody (clone H4537; Perseus Proteomics, Inc), a type IIa (N2.261 IgG1) myosin heavy chain isoform-specific antibody, mouse antibodies against human type I (A4.951 IgG1; A4.840 IgM) and IIx (212F IgG1) myosin heavy chain, and a rabbit antihuman antibody (IgG) raised against laminin to facilitate identifying individual muscle fibers. Slides were mounted with 4′,6-diamidino-2-phenylindole-containing mounting medium to stain myonuclei (Vectashield H-1500; Vector Laboratories, Inc). Control sections were processed independently as described above, without primary, secondary or both antibodies (blank control). No staining signal higher than the natural self-fluorescence of the sections was observed (results not shown).
Fiber cross-sectional area
After the staining protocol, digital imaging was performed through ×100 final magnification. Nikon NIS-AR (3.01) was employed for data acquisition. Image processing, including identification of fiber type in all fibers on the muscle cross-section, was performed using Adobe Photoshop CS3. The circumference of each fiber was outlined using NIH ImageJ software (version 1.37) to generate FCSA. Criteria used in the selection of muscle fibers to measure FCSA has been described previously (22). Image analyses were performed by 3 coauthors (L.C., S.N., and M.D.M.) who were blinded to treatment assignment. As an assessment of agreement between operators for average FCSA measurement, the mean absolute deviation was less than 0.03. The relative proportion of type I, IIa, IIx, and hybrid (ie, IIa-x) muscle fibers was recorded in each cross-section manually using Adobe Photoshop CS3. Criteria for muscle fibers to be included in total cross-sectional fiber number has been described previously (22). Because many individual muscle cross-sections had a substantial number of hybrid fiber subtypes (ie, IIa-x), we were not able to examine changes in type IIa and IIx fibers separately, but instead analyzed changes in the broader fiber classifications of type I or II for our analysis.
Intramyonuclear VDR concentration
A subset of 14 subjects (placebo, n = 8; vitamin D, n = 6) had sufficient muscle tissue available for VDR concentration analyses. Nonoverlapping high-magnification (×400) fields selected on the basis of lack of tissue artifacts were used to count myonuclei. On average, the combined field areas included 40% of the muscle cross-section at baseline and 4 months. Because vastus lateralis muscle has a mixed muscle fiber type distribution, the fields examined had similar muscle fiber type I to type II ratios (30%–70%) to those of the whole sections. The mean (±SD) total myonuclei per subject evaluated for VDR was 576 ± 223 at baseline and 602 ± 349 at 4 months. Using a multistaining protocol (21), we colocalized 4′,6-diamidino-2-phenylindole myonuclei staining with VDR-positive staining. Bright-field overlaid images were used to aid assignment of VDR-positive myonuclei to a particular fiber. We measured the relative number of VDR-positive myonuclei within each muscle fiber subtype in all fields. We calculated the ratio of VDR-positive myonuclei to total myonuclei by muscle fiber subtype from fields evaluated. These analyses were performed by one of the coauthors (S.N.) who was blinded to treatment assignment.
Statistical analysis
Unblinding occurred after biochemical, muscle histology, and muscle performance analyses were completed. UCa and UNi were normalized to UCr excretion to control for incomplete 24-hour urine collections (23). Mean baseline values of clinical characteristics (age, body weight, body mass index [BMI], and daily calcium and protein intake), biochemical measurements (25OHD, UCa/Cr, and UNi/Cr), FCSA (total, type I, and type II), proportion of fiber type (type I, and type II), percent of VDR-positive myonuclei out of total myonuclei, and the mean changes in these values from baseline to the final 4-month visit were compared across groups with t tests for 2 independent samples. Pearson correlation coefficients were used to describe linear associations. Two-sided P values < .05 were considered to indicate statistical significance. Statistical analyses were conducted using SAS Enterprise Guide version 4.2 (SAS Institute, Inc). Based on a previous study (24), our planned sample size of 12 participants per group provided over 80% power to detect a difference in FCSA of 740 μm2 at the two-tailed 0.05 α-level.
Results
Subjects and intervention
Baseline characteristics of the 21 subjects are listed in Table 1. The vitamin D group was modestly younger and weighed more than the placebo group. Mean baseline serum 25OHD was 45.8 ± 10.8 nmol/L in the entire cohort and did not differ significantly by group (Table 2). As expected, there was a significant increase in 25OHD level in the vitamin D (36.4 ± 13.6 nmol/L) compared with placebo group (4.2 ± 11.9 nmol/L; P < .001).
Table 1.
Baseline Characteristics in the Total Sample (n = 21) and the Subset With VDR Measurements (n = 14)
| Characteristics | Total Sample |
Subset |
||
|---|---|---|---|---|
| Placebo (Mean ± SD) | Vitamin D3 (Mean ± SD) | Placebo (Mean ± SD) | Vitamin D3 (Mean ± SD) | |
| n | 12 | 9 | 8 | 6 |
| Caucasian, n | 11 | 7 | 8 | 5 |
| Age, y | 80 ± 5 | 76 ± 4a | 79 ± 3 | 77 ± 4 |
| Height, cm | 154.3 ± 9.9 | 162.2 ± 7.3 | 153.6 ± 8.4 | 159.1 ± 6.5 |
| Weight, kg | 60 ± 9 | 77 ± 15b | 58 ± 10 | 77 ± 16c |
| BMI, kg/m2 | 25 ± 3 | 29 ± 7 | 24 ± 3 | 31 ± 7d |
| Dietary protein intake, g/de | 82.7 ± 73.9 | 63.3 ± 30.1 | 50.2 ± 27.0 | 59.0 ± 21.5 |
| Dietary calcium intake, mg/de | 1316 ± 1257 | 963 ± 663 | 752.8 ± 246.8 | 635.5 ± 486.9 |
| SPPB score | 8.17 ± 1.27 | 7.56 ± 1.88 | 8.38 ± 0.92 | 8.33 ± 0.82 |
| 40% of 1RM average power, Wf | 39.71 ± 14.45 | 43.52 ± 16.02 | 38.01 ± 14.53 | 45.50 ± 14.84 |
| 70% of 1RM average power, Wf | 51.44 ± 19.78 | 48.08 ± 23.12 | 48.50 ± 13.43 | 48.19 ± 22.00 |
P = 0.03, value compared with placebo.
P = .005, value compared with placebo.
P = .02, value compared with placebo.
P = .046, value compared with placebo.
One subject in the placebo group did not complete the food frequency questionnaire.
One subject in the placebo group was unable to perform the knee extensor measure due to knee pain.
Table 2.
Serum and Urine Biochemistry, FCSA, and Relative Proportion of Fiber Subtype Measurements at Baseline and 4 Months in the 2 Groups (n = 12 in Placebo; n = 9 in Vitamin D Group)
| Baseline Mean ± SD | Final Mean ± SD | |
|---|---|---|
| Serum | ||
| 25OHD, nmol/L | ||
| Placebo | 48.3 ± 8.8 | 52.5 ± 17.1 |
| Vitamin D3 | 43.6 ± 10.3 | 80.0 ± 11.5a |
| 24-h urine corrected for creatinine | ||
| UNi/Cr, mmol/mmol | ||
| Placebo | 83.4 ± 22.7 | 84.8 ± 28.0 |
| Vitamin D3 | 80.7 ± 20.7 | 70.7 ± 18.1 |
| UCa/Cr, mmol/mol | ||
| Placebo | 326.1 ± 120.2 | 324.4 ± 207.2 |
| Vitamin D3 | 393.1 ± 208.5 | 440.3 ± 247.1 |
| FCSA | ||
| Total (type I and II) muscle, μm2 | ||
| Placebo | 2985 ± 455 | 2770 ± 705 |
| Vitamin D3 | 2836 ± 618 | 3101 ± 711 |
| Type I muscle, μm2 | ||
| Placebo | 3860 ± 635 | 3755 ± 959 |
| Vitamin D3 | 3624 ± 915 | 3860 ± 1223 |
| Type II muscle FCSA, μm2 | ||
| Placebo | 2212 ± 516 | 2171 ± 555 |
| Vitamin D3 | 2138 ± 436 | 2434 ± 520 |
| Relative percentages of muscle fiber subtypesc | ||
| Percent of type II muscle fibers, % | ||
| Placebo | 57.7 ± 11.2 | 64.5 ± 12.6 |
| Vitamin D3 | 59.9 ± 10.5 | 60.8 ± 8.3 |
Differs from placebo group at P < .001.
Differs from placebo group at P = .048.
The percentage of type I fibers is not shown as it is the inverse of the type II.
Biochemical measurements and muscle performance
Baseline urine biochemistry values did not differ significantly in the 2 groups (Table 2). Percent change in UNi/Cr was −10.9% ± 15.9% in the vitamin D group compared with 2.2% ± 17.3% in the placebo group (P = .09). Baseline (Table 1) and 4-month changes in 40% and 70% of 1RM average power in knee extension (data not shown) did not differ significantly in the 2 groups (P > .28). Likewise, changes in total SPPB score did not differ significantly by group (placebo, 0.67 ± 1.56, vs vitamin D, 0.67 ± 1.32, P = 1.0).
Muscle morphology
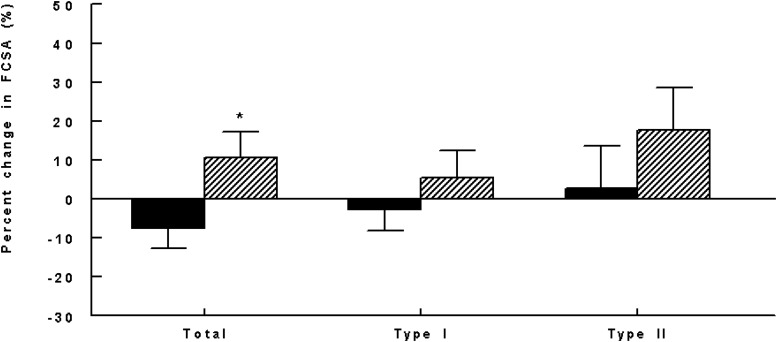
The 2 groups had similar mean baseline total, type I, and type II FCSA and relative proportion of type I and II fibers (Table 2). Percent change in total (combined type I and II) FCSA was 10.6% ± 20.0% in the vitamin D group and −7.4% ± 18.9% in the placebo (P = .048; Figure 1). Percent changes in type I and type II-specific FCSA did not differ significantly in the 2 groups (P > .30; Figure 1), although there was a suggestion of type II preference. There was no association of age or BMI with baseline or 4-month change in FCSA. Percent changes in the relative proportion of type I and type II fibers in the muscle cross-sections did not differ significantly by group (data not shown).
Figure 1.
Four-month percent changes in FCSA by fiber type and group (P = .048 [total], .363 [type I], and .356 [type II]). Black bars represent placebo; hatched bars represent vitamin D. *, P < .05.
Intramyonuclear VDR concentration
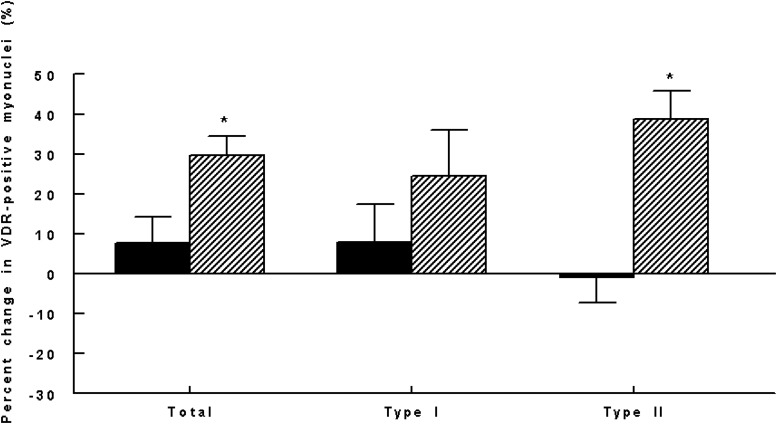
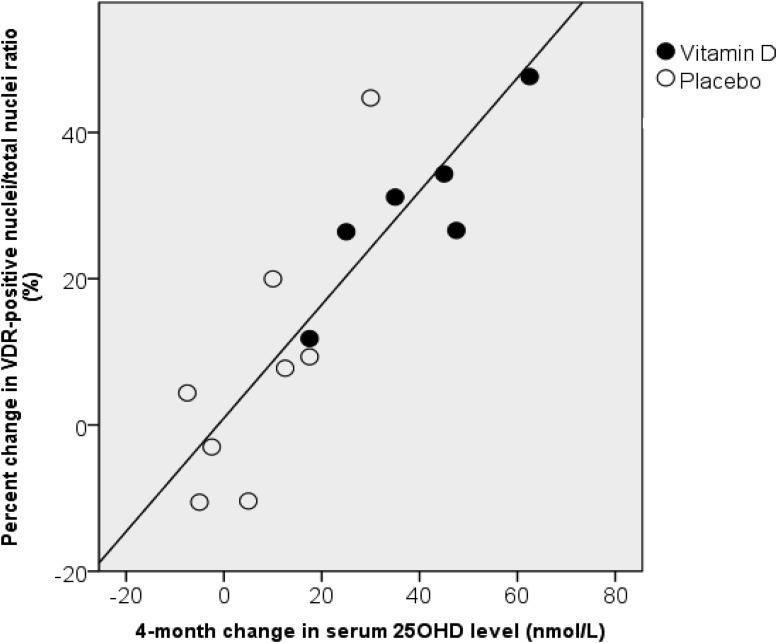
Clinical characteristics of the 14 subjects with VDR measurements are shown in Table 1. The ratio of VDR-positive myonuclei to total myonuclei was analyzed as a measure of intramyonuclear VDR concentration. At baseline, subjects in both groups had similar proportions of VDR-positive myonuclei on sampled cross-sections (Table 3). Percent change in VDR concentration was 29.7% ± 11.7% in the vitamin D group and 7.8% ± 18.2% in the placebo (P = .025; Figure 2). There was no association of age or BMI with baseline or 4-month change in VDR concentration. In the groups combined, the 4-month change in 25OHD level was strongly associated with percent change in intramyonuclear VDR concentration independent of group (r = 0.87, P < .001; Figure 3).
Table 3.
Baseline and Final Ratio of VDR-Positive Myonuclei to Total Myonuclei in the 2 Groups (n = 8 in Placebo; n = 6 in Vitamin D Group)
| Baseline Ratio Mean ± SD | Final Ratio Mean ± SD | |
|---|---|---|
| Total (type I + II fibers) | ||
| Placebo | 0.60 ± 0.03 | 0.65 ± 0.11 |
| Vitamin D3 | 0.61 ± 0.04 | 0.79 ± 0.03 |
| P value | .581 | .012 |
| Type I fibers | ||
| Placebo | 0.65 ± 0.07 | 0.69 ± 0.15 |
| Vitamin D3a | 0.66 ± 0.12 | 0.80 ± 0.05 |
| P value | .816 | .149 |
| Type II fibers | ||
| Placebo | 0.56 ± 0.07 | 0.56 ± 0.14 |
| Vitamin D3a | 0.52 ± 0.04 | 0.73 ± 0.09 |
| P value | .380 | .030 |
One subject did not have adequate ×400 digital images with fiber type staining and was not included.
Figure 2.
Four-month percent changes in intramyonuclear VDR concentration by fiber type and group (P = .025 [total], .301 [type I], and .002 [type II]). Black bars represent placebo; hatched bars represent vitamin D. *, P < .05.
Figure 3.
Association between change in 25OHD (nanomoles per liter) and the percent change in ratio of VDR-positive to total nuclei (r = 0.87, P < .001). ○, placebo; ●, vitamin D.
Fiber type-specific analyses revealed a significant increase in percent VDR-positive myonuclei in type II fibers after vitamin D3 vs placebo (P = .002; Figure 2). There was also an increase in VDR concentration in type I fibers in the vitamin D group, but it was not statistically significant at the .05 level when compared with the placebo group.
Adverse events
One subject in the vitamin D group had a transient high spot UCa/UCr ratio, which resolved on follow-up testing on study pills. No additional adverse events related to the study intervention were reported during the study.
Discussion
In our study of older mobility-limited women with moderately low vitamin D status, supplementation with vitamin D3 resulted in a 30% increase in intramyonuclear VDR protein concentration and a 10% increase in total (type I and II) muscle fiber size over a 4-month period. These findings, along with a trend toward lower UNi excretion, are consistent with the concept that vitamin D may promote muscle mass in this population at high risk for disability.
Intramyonuclear VDR concentration
The proportion of VDR-positive myonuclei at baseline in these older women was approximately 60%, which is consistent with a previous report (25) despite some differences in study design and a different VDR monoclonal antibody used for immunostaining. The previous cross-sectional study by Bischoff-Ferrari et al (25) examined the percentage of VDR-positive myonuclei in women with a broad baseline range in age (20–100 years) and serum 25OHD level (11–107 nmol/L) and who were undergoing either spine or hip surgery.
Our study is the first to show increased intramyonuclear VDR concentration after vitamin D supplementation in human muscle tissue samples. Recent data in C2C12 myoblasts also revealed significant increases in intramyonuclear staining of VDR protein occurring several days after a single administration of either 1,25-dihydroxyvitamin D3 or 25OHD3 (11). Previous studies in cell culture and animals (26, 27) have indicated that the content of VDR in target tissues is positively associated with the level of biological activity in response to vitamin D administration. Therefore, an increase in VDR content in myocytes after 4 months of vitamin D supplementation supports the concept that there may be sustained clinical effects of vitamin D supplements on muscle metabolism and/or function. Fiber type-specific analyses indicate that the pattern of changes in VDR concentration in type I and II fibers may be similar, but a larger study is needed to confirm this preliminary observation.
The presence of the VDR in human skeletal muscle cells has been a subject of debate mainly due to the absence of the VDR in a muscle tissue section from a subject using the D-6-specific VDR antibody (28). However, a previous study indicated that VDR signal was detectable in 8 fresh-frozen human muscle tissue sections using the VDR/NR1I1 monoclonal antibody, the D-6-specific VDR monoclonal antibody, and a third commercial antibody (21). Based on these results, we conclude that the VDR/NR1I1 is a reliable monoclonal antibody to use to detect VDR in fresh-frozen human muscle samples.
Muscle morphology
When comparing change in total (type I and II) muscle fiber area, the vitamin D group had greater gains in fiber size compared with placebo on average. There were similar trends in the fiber-specific analyses, yet these results were not statistically significant. To date, there are limited data on the impact of vitamin D on muscle fiber size. More than 3 decades ago, an uncontrolled study obtained muscle biopsies from 11 older osteoporotic women with profound vitamin D deficiency before and after treatment with 1α-hydroxyvitamin D and calcium for 3 to 6 months. Muscle cross-sections showed an increase in type IIa muscle FCSA (24). A more recent study found that treatment of older Japanese female stroke survivors with 1000 IU vitamin D2 daily increased mean type II muscle fiber diameter by >90% over a 2-year period in the nonparetic limb compared with placebo (29). In these rehabilitated women whose baseline 25OHD levels were <25 nmol/L, there was also a correlation between serum 25(OH)D level and type II muscle fiber diameter both at baseline and after 2 years of follow-up. Our study did not confirm these previous reports (24, 29) that also suggested an effect of vitamin D on relative proportion of type II muscle fibers, nor did it note significant differences in knee extension power and physical function as measured by the SPPB test. A larger sample size may be needed to detect significant differences in muscle strength and physical function.
Strengths and limitations
This pilot study had some important strengths, including the fact that the dose of vitamin D3 effectively and safely increased serum 25OHD levels to current optimal levels for bone outcomes in older adults (30). Our subjects' adherence to the intervention was high as demonstrated by the large group difference in 25OHD levels, and their dropout rate was low. We chose a parallel-arm, blinded, placebo-controlled design to study the vitamin D3 effects. The primary limitation of this pilot study was the small sample size, which likely prevented us from detecting some clinically meaningful effects, such as changes in muscle performance, and resulted in imbalances in calcium intake and body weight between the groups. However, these baseline differing factors (ie, calcium and weight) were not significantly correlated to baseline or changes in FCSA or VDR concentration in our sample. Our findings pertain to older, mobility-limited women with moderately low vitamin D status at baseline. We, therefore, cannot comment on the degree to which these findings may vary in men or premenopausal women. Finally, we cannot comment on whether the relatively high dose of 4000 IU per day was needed or whether a lower dose would have had similar effects on muscle fiber size and VDR concentration.
In conclusion, supplementation with vitamin D3 for 4 months in older mobility-limited women with moderately low vitamin D status increased intramyonuclear VDR concentration and muscle fiber size. Further work is needed to confirm these findings in a larger sample and to determine whether our two main findings are related, that is, whether vitamin D increases muscle fiber size by activating the VDR. Additionally, it will be important to identify the signaling pathways involved.
Acknowledgments
The A4.951, A4.840, and N2.261 monoclonal antibodies developed by H. M. Blau were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology, Iowa City, Iowa. The 212F monoclonal antibody was produced in-house from hybridomas generously donated by Dr Peter Merrifield, University of Ontario, Canada. We are grateful to the staff of the Metabolic Research Unit at the Jean Mayer U.S. Department of Agriculture (USDA) Human Nutrition Research Center on Aging (HNRCA) at Tufts University for assistance in carrying out on this study.
L.C. was supported by National Institutes of Health Grant KL2 RR025751 and the Gerald J. and Dorothy R. Friedman Foundation. The study was funded by the Jean Mayer USDA HNRCA at Tufts University and P30 AG031679 (Boston Claude Pepper Older Americans Independence Center). This material is based upon work supported by the USDA, Agricultural Research Service, under Agreement 58–1950-7–707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Disclosure Summary: L.C., S.N., M.D.M., D.A.R., and S.S.H. have nothing to disclose. H.B.-F. has received investigator-initiated funding from DSM Nutritional Products, WILD Nutrition, and Nestlé. R.A.F. has consulted for Merck, Eli Lilly, Essentient, Regeneron, Dairy Management Inc, and Nestlé and has received investigator-initiated funding from the Dairy Research Institute, Unilever, and Nestlé. B.D.-H. received investigator-initiated funding from Pfizer and is on the scientific advisory board at Pfizer and Cytochroma.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- FCSA
- fiber cross-sectional area
- 25OHD
- 25-hydroxyvitamin D
- 1RM
- 1 repetition maximum
- SPPB
- short physical performance battery
- VDR
- vitamin D receptor
- UCa
- urine calcium
- UCr
- urine creatinine
- UNi
- urine nitrogen.
References
- 1. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758 [DOI] [PubMed] [Google Scholar]
- 2. Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065 [DOI] [PubMed] [Google Scholar]
- 3. Kuchuk NO, Pluijm SM, van Schoor NM, et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–1250 [DOI] [PubMed] [Google Scholar]
- 4. Houston DK, Tooze JA, Davis CC, et al. Serum 25-hydroxyvitamin D and physical function in older adults: the Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2011;59:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772 [DOI] [PubMed] [Google Scholar]
- 6. Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118 [DOI] [PubMed] [Google Scholar]
- 7. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351 [DOI] [PubMed] [Google Scholar]
- 8. Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595 [DOI] [PubMed] [Google Scholar]
- 9. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20:315–322 [DOI] [PubMed] [Google Scholar]
- 10. Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011;152:2976–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol. 2012;303:C396–C405, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buitrago C, Arango N, Boland R. 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J Cell Biochem. 2012;113:1170–1181 [DOI] [PubMed] [Google Scholar]
- 13. Garcia LA, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C2C12 skeletal muscle cells. J Steroid Biochem Mol Biol. 2013;133:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou QG, Hou FF, Guo ZJ, Liang M, Wang GB, Zhang X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res Rev. 2008;24:459–464 [DOI] [PubMed] [Google Scholar]
- 15. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856 [DOI] [PubMed] [Google Scholar]
- 16. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011 [Google Scholar]
- 17. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 18. Gökçe C, Gökçe O, Baydinç C, et al. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med. 1991;151:1587–1588 [DOI] [PubMed] [Google Scholar]
- 19. McDonagh MJ, Davies CT. Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur J Appl Physiol Occup Physiol. 1984;52:139–155 [DOI] [PubMed] [Google Scholar]
- 20. Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199 [DOI] [PubMed] [Google Scholar]
- 21. Ceglia L, da Silva Morais M, Park LK, et al. Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J Mol Histol. 2010;41:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceglia L, Niramitmahapanya S, Price LL, Harris SS, Fielding RA, Dawson-Hughes B. An evaluation of the reliability of muscle fiber cross-sectional area and fiber number measurements in rat skeletal muscle. Biol Proced Online. 2013;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swaminathan R, Bradley JA, Hill GH, Morgan DB. The nitrogen to creatinine ratio in untimed samples of urine as an index of protein catabolism after surgery. Postgrad Med J. 1979;55:858–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sorensen OH, Lund B, Saltin B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1α-hydroxycholecalciferol and calcium. Clin Sci (Lond). 1979;56:157–161 [DOI] [PubMed] [Google Scholar]
- 25. Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269 [DOI] [PubMed] [Google Scholar]
- 26. Halloran BP, DeLuca HF. Appearance of the intestinal cytosolic receptor for 1,25-dihydroxyvitamin D3 during neonatal development in the rat. J Biol Chem. 1981;256:7338–7342 [PubMed] [Google Scholar]
- 27. Dokoh S, Donaldson CA, Haussler MR. Influence of 1,25-dihydroxyvitamin D3 on cultured osteogenic sarcoma cells: correlation with the 1,25-dihydroxyvitamin D3 receptor. Cancer Res. 1984;44:2103–2109 [PubMed] [Google Scholar]
- 28. Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152:354–363 [DOI] [PubMed] [Google Scholar]
- 29. Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192 [DOI] [PubMed] [Google Scholar]
- 30. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010 [DOI] [PubMed] [Google Scholar]