Abstract
Context:
Chinese-American women have bone microarchitectural features that confer greater bone stiffness compared to white women, but the physiology underlying these findings has not been investigated.
Objective:
The purpose of the study was to assess racial differences in serum sclerostin and bone turnover markers (BTMs), and to explore their associations with each other, volumetric bone mineral density (BMD), and bone microarchitecture in Chinese-American and white women.
Design and Setting:
We conducted a cross-sectional study at a university hospital.
Participants:
We studied 138 women.
Results:
Serum osteocalcin was 19–28% lower in pre- and postmenopausal Chinese-American vs white women, respectively (both P < .01). C-Terminal telopeptide of type I collagen (CTX) level was 18–22% lower in pre- and postmenopausal Chinese-American vs white women (both P < .05). Pre- vs postmenopausal differences in osteocalcin and CTX were greater in white vs Chinese-American women. Sclerostin levels were similar in both races, but BTMs were differentially associated with sclerostin by race and menopausal status. BTMs were not correlated with sclerostin in Chinese-Americans. CTX and bone-specific alkaline phosphatase were positively associated with sclerostin (r = 0.353, r = 0.458; both P < .05) in white premenopausal women. In contrast, in postmenopausal white women, the associations of sclerostin with amino-terminal propeptide of type I procollagen, isoform 5b of tartrate-resistant acid phosphatase, and CTX were negative (all P < .05). Adjusting for covariates, sclerostin was positively associated with areal BMD in both races.
Conclusions:
Lower BTMs in Chinese-American women and greater age-related differences in BTMs among white women provide a physiological framework to account for racial differences in BMD, microarchitecture, and fracture.
Asian women have a lower risk of hip and forearm fractures, despite lower areal bone mineral density (BMD) by dual-energy x-ray absorptiometry (DXA) (1–4). Because DXA provides a two-dimensional BMD measurement (ie, areal BMD in g/cm2), it is affected by bone size and/or weight and height. DXA tends to underestimate areal BMD in those with small body/bone size. Thus, the smaller body size of Asians (on average) helps to explain their lower areal BMD (5). High-resolution peripheral quantitative computed tomography (HRpQCT) is a technique that measures bone area, volumetric BMD (vBMD), and bone microarchitecture noninvasively. Our prior work as well as that of others utilizing this methodology indicates key racial differences in vBMD and microstructure that were not obvious from DXA but help to clarify the reduced risk for some types of fractures in Asian women (6–8). We have previously shown that pre- and postmenopausal Chinese-American women have smaller bone size than white women, but they have thicker, denser cortices and thicker, more plate-like trabeculae at the radius and tibia as measured by HRpQCT (7–9). Together, these properties confer greater whole bone stiffness as estimated by micro-finite element analysis compared to white women (10). The underlying physiological mechanisms to account for these differences in bone structure are unknown.
Measurement of bone turnover markers (BTMs) provides a noninvasive approach to assess the bone-remodeling process (11). Additionally, the recent development of validated assays for a key regulator of bone formation, serum sclerostin, has provided insight into the remodeling dynamics of a number of skeletal disorders. Sclerostin is an osteocyte-secreted protein encoded by the SOST gene. As an inhibitor of the anabolic Wnt signaling pathway, it provides a regulatory brake to osteoblast-mediated bone formation (12, 13). Sclerosteosis and van Buchem disease, both human disorders of increased bone mass, are due to loss-of-function mutations within the coding and regulatory regions of the SOST gene, respectively (14, 15). Equivalent phenotypic increases in bone mass from SOST gene knockout animal experiments confirm the observations in human disorders of SOST (16). Inhibition of sclerostin with a monoclonal antibody increases bone mass in both animals and humans (17–19). This finding is consistent with the central role of SOST in controlling bone formation.
Recent work has elucidated the role of sclerostin in aging and several disorders of bone metabolism. For example, circulating sclerostin levels are higher in postmenopausal than premenopausal white women, lower in primary hyperparathyroidism compared to hypoparathyroidism and euparathyroid controls, and higher in disuse osteoporosis vs ambulatory subjects (20–24). Additionally, sclerostin levels correlate positively with age and negatively with free estrogen index and PTH levels in postmenopausal women (20, 21). Further studies have shown that estrogen administration, body mass index (BMI), and physical activity contribute to circulating sclerostin levels (25–27). Higher sclerostin levels are associated with a greater risk for hip fracture independent of traditional risk factors in older women, but this study was limited to white women (28). It is possible that other variables influence sclerostin regulation, including race. Neither racial differences in sclerostin nor racial differences in the relationship of sclerostin with BTMs or microarchitecture have been studied.
This study was conducted to determine whether racial differences in serum sclerostin concentrations and BTMs help explain racial variation in bone mass and microarchitecture between Chinese-American and white women. To this end, there were two aims: 1) to determine whether circulating sclerostin levels and markers of bone turnover are different in Chinese-American as compared to white women; and 2) to investigate relationships between sclerostin levels, markers of bone turnover, BMD, and HRpQCT measures of bone microarchitecture in this cohort.
Subjects and Methods
Subjects
Sixty-one postmenopausal (29 white and 32 Chinese-American) and 77 premenopausal (35 white and 42 Chinese-American) women were studied. Participants in the current report represent those included in our studies utilizing HRpQCT who were not taking oral contraceptives and in whom serum was available for measurement of BTMs and sclerostin. Participants were recruited by newspaper and internet advertisements, by flyers, and directly at primary care physician offices as previously described (7, 8). Inclusion criteria were self-reported Chinese-American or white descent. Specific country of birth was not an inclusion/exclusion criterion. This cohort represents a convenience sample of women born in the United States, China, and other countries. The premenopausal age range, 29–40 years old, was selected in order to study women who had reached peak bone mass and in whom a perimenopausal transition or menopause had not yet influenced skeletal metabolism. The postmenopausal age range, 59–70 years old, was selected in order to study women who were well past the perimenopausal transition period but who had not yet reached an age when comorbid conditions and/or medications would be likely to affect bone metabolism. Women were screened by history and biochemical evaluation for conditions or medications known to affect bone metabolism. All participants were healthy, ambulatory women, and none of the participants were professional or semiprofessional athletes, nor did any of the women have impaired mobility. Exclusion criteria included untreated hyperthyroidism; renal or liver dysfunction; current pregnancy or lactation; intestinal malabsorption due to any cause; history of malignancy other than nonmelanomatous skin cancer; metabolic bone diseases such as primary or secondary hyperparathyroidism; HIV disease; organ transplantation; fragility fracture; and drug exposure affecting bone metabolism (current or past use of glucocorticoids, tacrolimus, cyclosporine, methotrexate, teriparatide, calcitonin, or aromatase inhibitors; current use or use within the last 12 mo of hormone replacement therapy, raloxifene, or bisphosphonates; and any bisphosphonate use of >6 mo duration). Premenopausal women taking oral contraceptives were specifically excluded from this analysis. All participants gave written, informed consent. Study participants were compensated for study participation and travel expenses within the guidelines of the Columbia University Medical Center Institutional Review Board, which approved this study.
Clinical evaluation
Information regarding past medical history and medications was obtained. Weight and height were measured by balance beam and a wall-mounted, calibrated Harpenden stadiometer, respectively. Physical activity was assessed as sport index using the Modified Baecke Questionnaire (29).
Biochemical testing
Intact PTH and 25-hydroxyvitamin D (25OHD) were measured by chemiluminescence assay and liquid chromatography tandem mass spectrometry, respectively, in a reference laboratory (Quest Diagnostics; normal range for PTH, 10–65 pg/mL). Morning fasting blood was collected and centrifuged. Serum was divided into aliquots and immediately frozen at −80°C. Samples were thawed once and run together at the same time in duplicate. Amino-terminal propeptide of type I procollagen (PINP) was measured by RIA (Orion Diagnostica); intra- and interassay precision are 6.5 and 8.3%, respectively (manufacturer's normal ranges, postmenopausal women, 16–96 μg/L; premenopausal women, 19–83 μg/L). Bone-specific alkaline phosphatase (BSAP) activity was measured by enzyme immunoassay (Quidel Corporation); intra- and interassay precisions are 6 and 8%, respectively (manufacturer's normal ranges, women ≥ age 45 y, 14.4–42.7 U/L; women ages 25–44 y, 11.6–29.6 U/L). N-Mid-osteocalcin was measured by ELISA (Immunodiagnostic Systems Ltd); intra- and interassay precisions are 4.7 and 9.0%, respectively (manufacturer's normal ranges, postmenopausal women, 12.8–55.0 ng/mL; premenopausal women, 8.4–33.9 ng/mL). Isoform 5b of tartrate-resistant acid phosphatase (TRAP-5b) was measured by ELISA (Immunodiagnostic Systems Ltd); intra- and interassay precisions are 5 and 9%, respectively (manufacturer's normal ranges, postmenopausal women, 1.49–4.89 U/L; premenopausal women, 1.03–4.15 U/L). C-Terminal telopeptide of type I collagen (CTX) was measured by ELISA (Immunodiagnostic Systems Ltd); intra- and interassay precisions are 5.4 and 8.1%, respectively (manufacturer's normal ranges, postmenopausal women, 0.142–1.351 ng/mL; premenopausal women, 0.112–0.738 ng/mL). Sclerostin was measured by ELISA (Human Sclerostin EIA) developed by the TECOmedical group; intra- and interassay precisions are 3.2 and 9.4%, respectively (manufacturer's mean observed values, postmenopausal women, 0.69 ± 0.26 ng/mL; premenopausal women, 0.56 ± 0.4 ng/mL).
Bone mineral density
Areal BMD was measured by DXA at the lumbar spine (L1–L4), total hip, femoral neck, and distal one-third radius using a QDR 4500A machine (Hologic). Participants were measured on the same densitometer using the same software, scan speed, and technologist certified by the International Society of Clinical Densitometry. In vivo precision, determined according to the standard method, at this facility is 1.28% at the lumbar spine, 1.36% at the hip, and 0.70% at the distal one-third radius (30).
vBMD and microarchitecture were measured at the nondominant forearm and tibia using an HRpQCT instrument (XtremeCT; Scanco Medical AG), as described previously (31–33). The following variables are reported at the radius and tibia: mean cortical thickness (Ct.Th), cortical and trabecular vBMD (Ct.vBMD and Tb.vBMD), trabecular number (Tb.N) and separation (Tb.Sp), and trabecular thickness (Tb.Th). In vivo reproducibility values for HRpQCT variables at our center (coefficient of variability) are: 0.55 to 1.06% for density measures, 0.16 to 1.25% for area measures, 0.91 to 1.53% for Ct.Th, and 3.65 to 5.22% for trabecular microarchitecture variables.
Statistical analysis
Data are expressed as mean ± SD. Comparisons of group differences between the Chinese-American and the Caucasians were evaluated by Wilcoxon rank sum test. Levels of BTMs and sclerostin levels were first compared between the two racial groups using generalized linear models without adjustment and then again with adjustment for age, PTH, and BMI. Covariate selection (age, PTH, and BMI) was based on the reported effects of these factors on sclerostin levels and/or bone markers (20–22). Values were not adjusted for physical activity because there was no association between physical activity and sclerostin in our cohort. Both unadjusted and adjusted P values are reported to show the magnitude and direction of the influence of covariates on the comparisons. The strength of the association of sclerostin with BTMs and HRpQCT variables was assessed using Spearman correlation coefficients before and after partialling the contribution of the covariates. Generalized linear models with race, menopausal status, and their interaction as fixed effects were used to test the between-race differences between their pre- and postmenopausal cohorts. For all analyses, a two-tailed P < .05 was considered to indicate statistical significance. No adjustments were made for statistical tests applied to multiple dependent variables. Statistical analysis was performed using SAS version 9.3 (SAS Institute).
Results
As shown in Table 1, there were no differences in age between Chinese-American and white women within the pre- or postmenopausal groups. Premenopausal and postmenopausal Chinese-American women weighed less and were shorter than white women. Serum 25OHD levels were lower in both pre- and postmenopausal Chinese-American women than in white women. Premenopausal white women had a higher physical activity score than premenopausal Chinese-American women (Table 1).
Table 1.
Group Characteristics
| Premenopausal Chinese | Premenopausal White | Chinese vs White P Value | Postmenopausal Chinese | Postmenopausal White | Chinese vs White P Value | |
|---|---|---|---|---|---|---|
| n | 42 | 35 | 32 | 29 | ||
| Age, y | 34 ± 4 | 34 ± 4 | .59 | 61 ± 2 | 62 ± 3 | .3 |
| Weight, kg | 56 ± 10 | 66 ± 19 | .001 | 59 ± 8 | 69.1 ± 13 | .0015 |
| Height, m | 1.62 ± 0.06 | 1.65 ± 0.06 | .017 | 1.58 ± 0.05 | 1.64 ± 0.05 | .0002 |
| BMI, kg/m2 | 21 ± 4 | 24 ± 6 | .019 | 24 ± 3 | 26 ± 5 | .1 |
| Physical activity (Baecke sport index) | 1.3 ± 0.7 | 1.5 ± 0.6 | .01 | 1.5 ± 0.6 | 1.4 ± 0.6 | .7 |
Data are presented as mean ± SD.
Markers of bone turnover
As shown in Table 2, serum osteocalcin levels were 20 and 28% lower in Chinese-American pre- and postmenopausal women, respectively, compared to white women in both age groups (both P < .01). Serum CTX was also 17 and 23% lower in pre- and postmenopausal Chinese-American, respectively, vs white women (both P < .05). There were no racial differences in PINP, BSAP, or TRAP-5b in either the premenopausal or postmenopausal groups. Adjustment for age, BMI, and PTH level did not change the direction or significance of the BTM comparisons.
Table 2.
Markers of Mineral Metabolism and Bone Turnover (Unadjusted)
| Premenopausal Chinese-American | Premenopausal White | Chinese-American vs White P Value | Postmenopausal Chinese-American | Postmenopausal White | Chinese-American vs White P Value | |
|---|---|---|---|---|---|---|
| n | 42 | 35 | 32 | 29 | ||
| PTH, pg/mL | 37 ± 13 | 33 ± 14 | .09 | 35 ± 10 | 39 ± 13a | .32 |
| 25OHD, ng/mL | 24 ± 9 | 32 ± 12 | .004 | 31 ± 8b | 38 ± 14 | .047 |
| PINP, μg/L | 45 ± 11 | 50 ± 16 | .4 | 50 ± 18 | 56 ± 17 | .22 |
| BSAP, U/L | 22 ± 7 | 23 ± 8 | .3 | 31 ± 10c | 29 ± 8b | .22 |
| Osteocalcin, ng/mL | 12.8 ± 3 | 16.0 ± 6 | .002 | 13.4 ± 5 | 18.7 ± 6 | .002 |
| TRAP-5b, U/L | 2.8 ± 0.7 | 2.9 ± 0.7 | .44 | 3.7 ± 1b | 4.0 ± 0.9c | .13 |
| CTX, ng/L | 444 ± 177 | 537 ± 191 | .03 | 537 ± 236 | 693 ± 239b | .013 |
| Sclerostin, ng/mLd | 0.788 ± 0.19 | 0.732 ± 0.22 | .14 | 1.054 ± 0.22c | 1.023 ± 0.25c | .62 |
Data are presented as mean ± SD.
P ≤ .05;
P < .01;
P < .0001, for comparison of within-race post- and premenopausal groups.
To convert to picomoles/liter, multiply by 44.
Serum CTX, BSAP, and TRAP-5b were higher in postmenopausal than premenopausal white women, but only BSAP and TRAP-5b levels were higher in postmenopausal than premenopausal Chinese-American women (Table 2). The within-race postmenopausal vs premenopausal differences in osteocalcin (17 vs 5%; P = .03) and CTX (29 vs 21%; P = .06), were greater in white women than Chinese-American women.
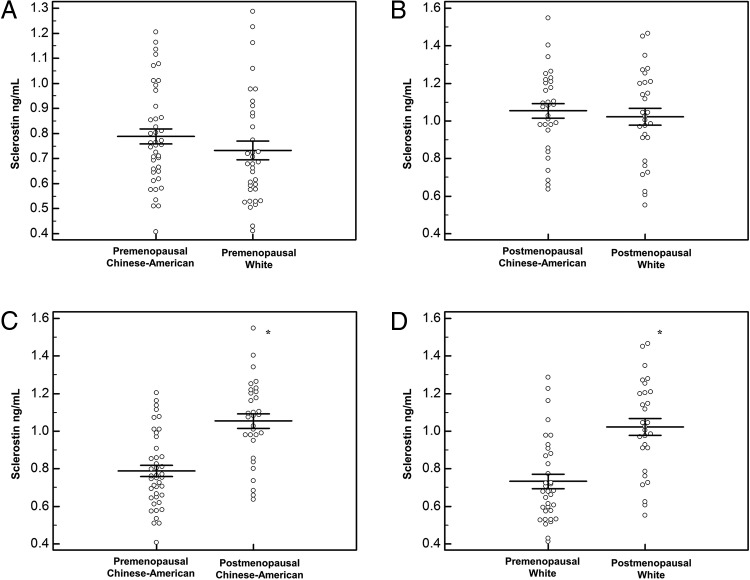
Sclerostin
As shown in Table 2 and Figure 1, there were no racial differences in sclerostin in either pre- or postmenopausal women before or after adjustment for confounders (age, BMI, PTH). Nonetheless, postmenopausal women in each race had higher levels of sclerostin than premenopausal women (Figure 1). There were no race–menopause interactions. When BMD measured by DXA (lumbar spine, total hip, or distal one-third radius) or HRpQCT (total vBMD, Tb.vBMD, or Ct.vBMD) was added to separate adjusted models, sclerostin levels were also not different between white and Chinese-American women.
Figure 1.
Comparison of circulating sclerostin levels in premenopausal Chinese-American and white women (A); postmenopausal Chinese-American and white women (B); Chinese-American pre- and postmenopausal women (C); and white pre- and postmenopausal women (D). *, P < .05.
Relationship between sclerostin and markers of bone turnover
As shown in Table 3, in the entire cohort, sclerostin was positively associated with BSAP, but this association was no longer statistically significant after adjustment for confounders. Because the relationship between sclerostin and BTMs has been reported to vary between younger and older women, we assessed associations for each race-menopause subgroup separately. There was no association between sclerostin and markers of bone turnover among pre- or postmenopausal Chinese-American women before or after adjustment for covariates. After adjusting for age, BMI, and PTH, serum sclerostin positively correlated with BSAP (r = 0.458; P < .01) and CTX (r = 0.353; P < .05) among premenopausal white women. In contrast, sclerostin negatively correlated with PINP (r = −0.670; P < .001), TRAP-5b (r = −0.467; P < .05), and CTX (r = −0.537; P < .01) in postmenopausal white women. Including BMD as a covariate in the adjusted analyses did not change the direction or significance of results.
Table 3.
Correlation (r) of Sclerostin Levels with Markers of Bone Turnover
| All | Premenopausal Chinese-American | Premenopausal White | Postmenopausal Chinese-American | Postmenopausal White | |
|---|---|---|---|---|---|
| PINP | 0.056/0.023 | −0.085/−0.009 | 0.200/0.290 | 0.265/0.359 | −0.493b/−0.670c |
| BSAP | 0.226b/0.108 | −0.028/−0.021 | 0.260/0.458b | −0.168/−0.03 | −0.234/−0.202 |
| Osteocalcin | −0.063/−0.07 | −0.205/−0.128 | −0.005/0.234 | −0.001/0.086 | −0.215/−0.366 |
| TRAP-5b | 0.156/0.004 | −0.225/−0.174 | 0.075/0.257 | −0.090/−0.060 | −0.268/−0.467a |
| CTX | 0.046/0.001 | −0.174/−0.050 | 0.112/0.353a | 0.090/0.206 | −0.306/−0.537b |
Data are expressed as unadjusted/adjusted values.
P ≤ .05;
P < .01;
P < .001, for null hypothesis r = 0; values are adjusted for age, BMI, and PTH.
Relationship between sclerostin and areal BMD
After adjustment for covariates, in the entire cohort there were positive associations between sclerostin and areal BMD at the lumbar spine (r = 0.262; P < .01), total hip (r = 0.339; P < .001), and femoral neck (r = 0.292; P < .001) (Table 4). Among premenopausal women of both races, sclerostin was positively correlated with areal BMD at the lumbar spine, femoral neck, and total hip, but not the one-third radius, both before and after adjustment for covariates in both racial groups. Among postmenopausal women, sclerostin was only associated with distal one-third radius BMD in Chinese-American women before adjustment for covariates (r = 0.460; P < .01). After adjusting for confounders, sclerostin was positively associated with total hip (r = 0.539; P < .01) and femoral neck BMD (r = 0.395; P < .05) in postmenopausal white women, and with distal one-third radius BMD in postmenopausal Chinese-American women (r = 0.480; P < .05).
Table 4.
Correlation (r) of Sclerostin Levels With BMD and Microarchitecture
| BMD | All | Premenopausal Chinese-American | Premenopausal White | Postmenopausal Chinese | Postmenopausal White |
|---|---|---|---|---|---|
| DXA | |||||
| Lumbar spine | 0.065/0.262b | 0.441b/0.406a | 0.393a/0.362a | 0.317/0.321 | −0.012/0.285 |
| Total hip | 0.125/0.339c | 0.361a/0.344a | 0.475b/0.507b | 0.288/0.325 | 0.268/0.539b |
| Femoral neck | 0.015/0.292c | 0.487b/0.565c | 0.357a/0.371a | 0.200/0.266 | 0.231/0.395a |
| Distal 1/3 radius | −0.132/0.148 | 0.171/0.249 | 0.177/0.147 | 0.460b/0.480a | 0.047/0.151 |
| HRpQCT | |||||
| Radius | |||||
| Total vBMD | −0.173a/−0.006 | 0.060/0.066 | 0.006/−0.090 | −0.252/−0.272 | −0.329/−0.266 |
| Ct.vBMD | −0.243b/−0.075 | 0.166/0.060 | −0.149/−0.156 | −0.313/−0.282 | −0.263/−0.177 |
| Ct.Th | −0.168a/−0.029 | 0.070/0.027 | −0.060/−0.134 | −0.336/−0.340 | −0.234/−0.144 |
| Tb.vBMD | −0.061/0.143 | 0.126/0.212 | 0.340a/0.248 | 0.172/0.146 | −0.108/−0.051 |
| Tb.N | −0.060/0.086 | −0.020/0.084 | 0.346a/0.320 | 0.303/0.316 | −0.156/−0.050 |
| Tb.Th | −0.030/0.141 | 0.158/0.166 | 0.240/0.125 | −0.0/−0.023 | 0.031/0.034 |
| Tb.Sp | 0.069/−0.099 | 0.010/−0.103 | −0.373a/−0.324 | −0.298/−0.285 | 0.167/0.078 |
| Tibia | |||||
| Total vBMD | −0.108/0.153 | 0.290/0.307 | 0.325/0.299 | 0.087/0.054 | −0.330/−0.289 |
| Ct.vBMD | −0.285c/0.041 | 0.460b/0.370a | 0.151/0.095 | −0.036/−0.043 | −0.175/−0.135 |
| Ct.Th | −0.144/0.088 | 0.275/0.222 | 0.080/0.049 | 0.168/0.144 | −0.293/−0.255 |
| Tb.vBMD | −0.033/0.208a | 0.196/0.339a | 0.419a/0.374a | 0.081/0.087 | −0.230/−0.190 |
| Tb.N | −0.045/0.061 | −0.004/0.097 | 0.307/0.332 | 0.081/0.034 | 0.026/0.168 |
| Tb.Th | 0.112/0.193a | 0.198/0.278 | 0.335a/0.301 | 0.081/−0.099 | −0.293/−0.379 |
| Tb.Sp | 0.039/−0.085 | −0.046/−0.166 | −0.339a/−0.348a | −0.083/−0.038 | 0.011/−0.131 |
Data are expressed as unadjusted/adjusted values.
P ≤ .05;
P < .01;
P < .001, for null hypothesis r = 0; values are adjusted for age, BMI, and PTH.
Relationship between sclerostin and vBMD and microarchitecture by HRpQCT
As shown in Table 4, among Chinese-American premenopausal women, unadjusted sclerostin positively correlated with tibial Ct.vBMD (r = 0.460; P = .002). This association remained significant after adjustment for covariates (r = 0.370; P = .02). In addition, sclerostin positively correlated with Tb.vBMD (r = 0.339; P = .03) at the tibia in the Chinese-American premenopausal group after adjustment. In the premenopausal white group, unadjusted sclerostin was associated with tibial Tb.vBMD (r = 0.419; P = .01), Tb.Th (r = 0.335; P = .05), and Tb.Sp (r = −0.339; P = .05); and with radius Tb.vBMD (r = 0.340; P = .05), Tb.N (r = 0.346; P = .04), and Tb.Sp (r = −0.373; P = .03), but after adjusting for confounders only the association with tibia Tb.vBMD and Tb.Sp remained significant (P = .04 and P = .05, respectively). Sclerostin was not associated with vBMD or microarchitecture among postmenopausal Chinese-American or white women before or after adjustment for covariates.
Discussion
We assessed racial differences in circulating sclerostin levels and markers of bone turnover to further delineate possible physiological mechanisms underlying differences in bone density and bone microarchitecture between Chinese-American and white women. This is the first study to examine racial/ethnic differences in circulating sclerostin levels. We found that sclerostin levels did not differ by race in either pre- or postmenopausal women. Consistent with other published reports, we found circulating sclerostin levels to be higher in postmenopausal women compared to premenopausal women of both races (20, 21).
Little data exist regarding racial differences in BTMs between white and Asian women, and there are no data concerning dynamic indices of bone remodeling from bone biopsy studies. Consistent with our results, one study reported lower osteocalcin and urinary N-telopeptide levels in late pre- and perimenopausal Chinese-American compared to white women (34). Although the results reported here confirm prior observations in this regard and extend them to postmenopausal women, our study is the first to comprehensively investigate multiple markers of bone formation and resorption in these racial/ethnic groups. Osteocalcin and CTX levels were lower in Chinese-American women. These findings suggest a lower rate of bone remodeling in Chinese-American women, which in turn could lead to greater vBMD and microstructural advantages. Within-race age-related differences in BTMs tended to be greater in white vs Chinese-American women. Whether this could represent a more robust increase in bone resorption with aging/menopause for white vs Chinese-American women will need to be assessed with longitudinal studies. A number of studies indicate that higher markers of bone turnover reflect an increased risk of fragility fracture, independent of BMD (35–37), and thus the lower markers in Chinese-American vs white women and greater “increase” in BTMs in white women are also consistent with the lower risk for certain types of fracture in Chinese-American women.
Sclerostin was found to correlate with BTMs only in white women, but the direction of the association differed by menopausal status. Adjusted correlations indicated a positive association between sclerostin and BTMs in premenopausal white women, whereas in postmenopausal white women the association was negative. Published data regarding associations between markers of bone turnover and sclerostin are inconsistent. However, studies that include patients with high turnover conditions known to promote uncoupling (ie, postmenopausal osteoporosis, primary hyperparathyroidism, immobilization) typically indicate negative associations between markers of bone turnover and serum sclerostin (23, 24). In contrast, in hypoparathyroidism, a metabolic bone disorder characterized by low bone turnover, sclerostin is positively associated with markers of bone turnover (24). Thus, the direction of the association between sclerostin and bone markers and the relevance of sclerostin to influence bone formation may depend upon whether bone turnover is increased or decreased.
In some previous publications, sclerostin has been noted to be influenced by physical activity (23, 25). Conversely, in our study physical activity was not associated with sclerostin levels. The reasons underlying these inconsistencies are unclear but may be due to between-study differences in the assessment of physical activity or the variability of physical activity.
Although similar levels of sclerostin were found in Chinese-American vs white women, we did not find associations between markers of bone turnover and sclerostin in Chinese-Americans. As described above, this finding could be due to the relatively low bone turnover noted in Chinese-American women. Alternatively, this finding could suggest racial differences in skeletal sensitivity to sclerostin. Further work is needed to examine the underlying mechanisms, which were not directly assessed by our study.
Sclerostin was positively associated with BMD in both racial groups. Positive associations between sclerostin and BMD and bone microstructural variables by HRpQCT have been previously described (21, 38, 39). Higher levels of sclerostin in those with the greatest bone density are thought to reflect, at least in part, greater osteocyte density. The lack of association between sclerostin and markers of bone turnover in addition to positive association with bone density in Chinese-American women also supports the concept that there may be racial differences in skeletal sensitivity to the actions of sclerostin. Nonetheless, the serum levels of sclerostin were still correlated with indices reflecting osteocyte mass.
Our study has several limitations. The cohort studied was a small convenience sample of women, and thus the results could have been influenced by selection bias. Although we excluded women taking either oral contraceptive pills or hormone replacement therapy, we did not measure estrogen levels that may have been a helpful covariate in our analyses. Furthermore, bone marker measurements are subject to considerable variability, but we took precautions to limit external factors known to cause variation in bone marker measures such as collecting morning fasting samples and conducting the assays in replicate fashion (40). Finally, it is not known how well circulating levels of sclerostin reflect tissue levels or effects. Despite these limitations, this study provides the first data assessing possible racial differences in sclerostin levels. Additionally, our investigation provides a comprehensive comparison of BTMs in Chinese-American and white women.
In summary, our results provide evidence that there are racial differences in BTMs between Chinese-American and white women. Furthermore, sclerostin is differentially associated with BTMs by race and menopausal status. Whether this suggests that different racial groups may vary in their responses to therapies targeting sclerostin is unclear. Although limited in scope, our findings suggest that there are racial differences in bone remodeling between Chinese-American and white women. Lower indices of bone remodeling help explain racial differences in bone density and microarchitecture as well as the clinical observations regarding fracture incidence in these two cohorts.
Acknowledgments
We gratefully thank Dr. Clyde Wu for his vision and support.
This work was supported by National Institutes of Health Grant K23 AR053507, National Osteoporosis Foundation grant, the Mary and David Hoar Fellowship Program of the New York Community Trust, and the New York Academy of Medicine.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- BSAP
- bone-specific alkaline phosphatase
- BTM
- bone turnover marker
- Ct.Th
- cortical thickness
- Ct.vBMD
- cortical vBMD
- CTX
- C-terminal telopeptide of type I collagen
- DXA
- dual-energy x-ray absorptiometry
- HRpQCT
- high-resolution peripheral quantitative computed tomography
- 25OHD
- 25-hydroxyvitamin D
- PINP
- amino-terminal propeptide of type I procollagen
- Tb.N
- trabecular number
- Tb.Sp
- trabecular separation
- Tb.Th
- trabecular thickness
- Tb.vBMD
- trabecular vBMD
- TRAP-5b
- isoform 5b of tartrate-resistant acid phosphatase
- vBMD
- volumetric BMD.
References
- 1. Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194 [DOI] [PubMed] [Google Scholar]
- 2. Walker MD, Babbar R, Opotowsky AR, et al. A referent bone mineral density database for Chinese American women. Osteoporos Int. 2006;17:878–887 [DOI] [PubMed] [Google Scholar]
- 3. Russell-Aulet M, Wang J, Thornton JC, Colt EW, Pierson RN., Jr 1993 Bone mineral density and mass in a cross-sectional study of white and Asian women. J Bone Miner Res. 8:575–582 [DOI] [PubMed] [Google Scholar]
- 4. Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol. 1997;146:502–509 [DOI] [PubMed] [Google Scholar]
- 5. Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3057–3067 [DOI] [PubMed] [Google Scholar]
- 6. Wang XF, Wang Q, Ghasem-Zadeh A, et al. Differences in macro- and microarchitecture of the appendicular skeleton in young Chinese and white women. J Bone Miner Res. 2009;24:1946–1952 [DOI] [PubMed] [Google Scholar]
- 7. Walker MD, Liu XS, Stein E, et al. Differences in bone microarchitecture between postmenopausal Chinese-American and white women. J Bone Miner Res. 2011;26:1392–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker MD, McMahon DJ, Udesky J, Liu G, Bilezikian JP. Application of high-resolution skeletal imaging to measurements of volumetric BMD and skeletal microarchitecture in Chinese-American and white women: explanation of a paradox. J Bone Miner Res. 2009;24:1953–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu XS, Walker MD, McMahon DJ, et al. Better skeletal microstructure confers greater mechanical advantages in Chinese-American women versus white women. J Bone Miner Res. 2011;26:1783–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker MD, Liu XS, Zhou B, et al. Premenopausal and postmenopausal differences in bone microstructure and mechanical competence in Chinese-American and white women. J Bone Miner Res. 2013;28:1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brixen K, Eriksen EF. Validation of biochemical markers of bone turnover. In: Seibel MJ, Robins SP, Bilezikian JP, eds. Dynamics of Bone and Cartilage Metabolism. 2nd ed San Diego, CA: Academic Press; 2006:583–594 [Google Scholar]
- 12. Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844 [DOI] [PubMed] [Google Scholar]
- 13. van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327 [DOI] [PubMed] [Google Scholar]
- 14. Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10:537–543 [DOI] [PubMed] [Google Scholar]
- 15. Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869 [DOI] [PubMed] [Google Scholar]
- 17. Li X, Warmington KS, Niu QT, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010;25:2647–2656 [DOI] [PubMed] [Google Scholar]
- 18. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26 [DOI] [PubMed] [Google Scholar]
- 19. Ominsky MS, Li C, Li X, et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26:1012–1021 [DOI] [PubMed] [Google Scholar]
- 20. Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010;95:1991–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mödder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Lierop AH, Witteveen JE, Hamdy NA, Papapoulos SE. Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol. 2010;163:833–837 [DOI] [PubMed] [Google Scholar]
- 23. Gaudio A, Pennisi P, Bratengeier C, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–2253 [DOI] [PubMed] [Google Scholar]
- 24. Costa AG, Cremers S, Rubin MR, et al. Circulating sclerostin in disorders of parathyroid gland function. J Clin Endocrinol Metab. 2011;96:3804–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amrein K, Amrein S, Drexler C, et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97:148–154 [DOI] [PubMed] [Google Scholar]
- 26. Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mödder UI, Clowes JA, Hoey K, et al. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011;26:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arasu A, Cawthon PM, Lui LY, et al. ; Study of Osteoporotic Fractures Research G Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab. 2012;97:2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 30. Bonnick SL, Johnston CC, Jr, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–110 [DOI] [PubMed] [Google Scholar]
- 31. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515 [DOI] [PubMed] [Google Scholar]
- 32. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–399 [DOI] [PubMed] [Google Scholar]
- 33. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–433 [DOI] [PubMed] [Google Scholar]
- 34. Finkelstein JS, Sowers M, Greendale GA, et al. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3051–3056 [DOI] [PubMed] [Google Scholar]
- 35. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538 [DOI] [PubMed] [Google Scholar]
- 36. Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20:1813–1819 [DOI] [PubMed] [Google Scholar]
- 37. Johnell O, Kanis JA, Black DM, et al. Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res. 2004;19:764–772 [DOI] [PubMed] [Google Scholar]
- 38. Sheng Z, Tong D, Ou Y, et al. Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south Chinese postmenopausal women. Clin Endocrinol (Oxf). 2012;76:797–801 [DOI] [PubMed] [Google Scholar]
- 39. Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int. 2013;24:489–494 [DOI] [PubMed] [Google Scholar]
- 40. Vasikaran S, Eastell R, Bruyère O, et al. ; IOF-IFCC Bone Marker Standards Working Group Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420 [DOI] [PubMed] [Google Scholar]