Abstract
Context:
An end of fast insulin ≥3 μIU/mL and a proinsulin concentration ≥5 pmol/L have been suggested as useful cutoffs for the diagnosis of insulinoma.
Objective:
The main objective was to evaluate the diagnostic performance of an end of fast insulin concentration ≥3 μIU/mL and an end of fast proinsulin concentration ≥5 pmol/L.
Design:
The design was a case-control series.
Setting:
The setting was a tertiary-care center.
Patients:
Fifty-six subjects with a positive 48-hour supervised fast had an insulinoma between June 2000 and April 2011. During this same time period, a diagnosis of insulinoma was excluded in 29 subjects who underwent a supervised fast.
Intervention:
48-hour supervised fast.
Main Outcome Measure:
The main outcome measures were serum insulin concentration and plasma proinsulin concentration.
Results:
Ninety-one percent of the patients with an insulinoma had a measured insulin concentration ≥5 μIU/mL at the end of fast. The sensitivity increased to 98% if the threshold to define inadequate insulin suppression was lowered to ≥3 μIU/mL. The median (interquartile range) end of fast proinsulin was 100 (53–270) pmol/L for cases and 6.8 (4.2–12.0) pmol/L for controls. An end of fast proinsulin value of ≥5 pmol/L could not distinguish cases from controls (59% false positive rate). All patients with an insulinoma (sensitivity 100%) and none of the control subject (specificity 100%) had end of fast proinsulin concentration ≥27 pmol/L.
Conclusions:
Using a current insulin assay 9% of insulinoma cases end the supervised fast with an insulin concentration below 5 μIU/mL. Inadequate insulin suppression defined using a threshold of ≥3 μIU/mL increases the sensitivity of the test. The value of the proinsulin test lies in its unique ability to distinguish cases from controls. A proinsulin concentration of ≥22 pmol/L best discriminates cases from controls. Reliance on an end of fast proinsulin cutoff value of 5 pmol/L does not augment sensitivity but greatly reduces specificity of the test.
Normal pancreatic β cells suppress insulin secretion in response to a decrease in ambient glucose concentration. In subjects with insulin-producing tumors, serum insulin levels remain inappropriately high in the fasting state as plasma glucose concentration falls. Reviews of historical case series of patients with insulin-producing tumors (1, 2) suggested a serum insulin concentration of ≥5–6 μIU/mL in subjects with symptomatic, fasting, hypoglycemia-defined inadequate insulin suppression. In these large series, a threshold of ≥5–6 μIU/mL captured ≥95% of subjects with insulin-producing tumors thereby satisfying one criterion of a good diagnostic test (ie, high sensitivity). Since this cutoff was proposed, insulin immunoassays have become more specific and exhibit less cross-reactivity with proinsulin and its degradation products. In the era of specific assays, case reports of patients with insulin-producing tumors presenting with end of fast serum insulin concentration of <5 μIU/mL (ie, false negative cases) have been published (3–5). Practice guidelines (6) have recommended lowering the threshold that defines inadequate suppression to a serum insulin concentration of 3 μIU/mL or greater but the diagnostic value of the new proposed threshold has not been tested.
It has long been recognized that concentrations of circulating plasma proinsulin obtained in the fasted state are high in patients with insulinomas (1, 7–11). Before the availability of commercial proinsulin assays, proinsulin was detected by indirect means and proinsulin levels were quantified as a percentage of total immunoreactive insulin. In the last 20 years immunoassays that measure proinsulin concentration directly have become widely available. A review (1) of cases evaluated at our center before the time directly available assays came into use confirmed that in ∼90% of subjects with insulinoma proinsulin levels at the end of the fast exceeded the upper limit of normal levels for healthy individuals. Using correlation analysis, the proinsulin threshold defining an abnormally high proinsulin level on the indirect assay was determined to correspond to a directly measured proinsulin concentration of ≥22 pmol/L. In the Endocrine Society Clinical Practice Guideline (6) an end of fast proinsulin value of ≥5 pmol/L has been suggested as useful diagnostic cutoff.
The aim of this study was to evaluate the diagnostic performance of an end of fast serum insulin concentration of ≥3 μIU/mL and an end of fast plasma proinsulin concentration of ≥5 pmol/L in the diagnosis of insulin-producing tumors using currently available assays for a cohort of patients seen at the National Institutes of Health (NIH) from 2000 to 2011.
Materials and Methods
Study population
All subjects included in this series provided written informed consent to participate in the NIH Clinical Center hypoglycemic disorders protocol (ClinicalTrials.gov Identifier: NCT00001276). To identify cases and controls, all supervised fasts performed at NIH from June 2000 to April 2011 were retrospectively reviewed. This time period was selected to avoid case overlap between this and a series of previously published cases (1, 12). Fifty-six patients with a positive initial 48-hour supervised fast and a confirmed insulin-producing tumor were identified. Forty-four had localized nonrecurrent disease, six had malignant disease, and six had an insulinoma occurring in the setting of multiple endocrine neoplasia type 1. Controls were made up of 29 subjects who had a negative inpatient workup for hypoglycemic disorders during this same time period and were disease-free at follow-up (21 had no underlying hypoglycemic disorder, 4 were not found to have hypoglycemia but had a diagnosis of multiple endocrine neoplasia, and 4 patients were diagnosed with a factitious disorder).
Demographic and other baseline characteristics were similar between groups and representative of the spectrum of adult patients evaluated for hypoglycemic disorders at our center. In both groups, women accounted for most subjects (66 and 76%, respectively). Cases were on average older than controls (mean ± SD; 48.3 ± 15.9 y vs 41.4 ± 12.0 y). Renal function measured using serum creatinine was normal (0.84 ± 0.2 mg/dL vs 0.78 ± 0.2 mg/dL). Weight (83.4 ± 19.9 kg vs 79.1 ± 17.9 kg) and body mass index (30.0 ± 6.6 kg/m2 vs 28.8 ± 6.1 kg/m2) were similar in cases and controls.
All participants were admitted to the NIH clinical center the night before the start of the supervised fast. On the morning of the fast subjects ordered a standard hospital breakfast. Baseline glucose, insulin, C-peptide, proinsulin, anti-insulin antibody titer, and a sulfonylurea screen were obtained within 30 minutes following ingestion of the meal. Glucose, insulin, and C-peptide were obtained at regular timed intervals thereafter. When the patient was observed by medical personnel to have hypoglycemic symptoms and coincident low plasma glucose, all baseline laboratory analyses were repeated and carbohydrate was administered to resolve symptoms.
Clinical assays
Serum insulin and C-peptide concentration were measured by immunochemiluminescense (Siemens Immulite 2500) in the NIH Clinical Center Laboratory Department. The reference range for the insulin assay is 6.0–27.0 μIU/mL; the intra-assay coefficient of variation (CV) range is between 3.3 and 5.5% and the interassay CV range is between 4.1 and 7.3%. The reference range for the C-peptide assay is 0.9–7.1 ng/mL; the intra-assay CV range is between 1.9 and 3.2%, and the interassay CV range is between 2.9 and 5.4%. Plasma proinsulin concentration was measured by immunochemiluminescense at the Mayo Clinic Medical Laboratories. The reference range for the assay is 3–20 pmol/L. The intra-assay CV range is 4.4–5.1% and the interassay CV range is 7.2–8.8%.
Statistical analyses
Descriptive data for categorical variables are summarized using frequencies or percentages. Descriptive data for continuous measures are summarized using mean, SD, or median and interquartile range. Receiver operating characteristic (ROC) curves were generated by logistic regression modeling using JMP statistical software.
Results
Fast characteristics
Fast characteristics in patients with insulin-producing tumors were consistent with previously published reports. Whipple's triad was observed by medical personnel for 79% (44/56) and 100% (56/56) of insulinoma cases at the end of 24 and 48 hours, respectively. The mean ± SD plasma glucose at the end of the fast was 36.4 ± 6.5 mg/dL (SI units: 1.9 ± 0.4 mmol/L). Anti-insulin antibody titers were below the limit of quantification in both groups. In three of the controls the sulfonylurea screen was positive. In one of the controls the patient admitted to injecting insulin surreptitiously.
Insulin concentration at the end of the fast
In our current series relying on a new-generation insulin assay, an end of fast insulin concentration of ≥5 μIU/mL identifies 91% of cases (ie, 51/56). If the threshold to define inadequate insulin suppression at the end of the fast is lowered to ≥3 μIU/mL, as suggested by the Endocrine Society Guideline, the sensitivity of the test increases to 98% (55/56). Although most cases will be captured using this threshold, a small number of patients with insulin-producing tumors (ie, 2% in this series) will have an end of fast insulin concentration below 3 μIU/mL. Approximately 40% (10/25) of normal controls (ie, false positive rate) had an end of fast insulin ≥3 μIU/mL (note: data excludes four surreptitious cases).
Proinsulin concentration at the end of the fast
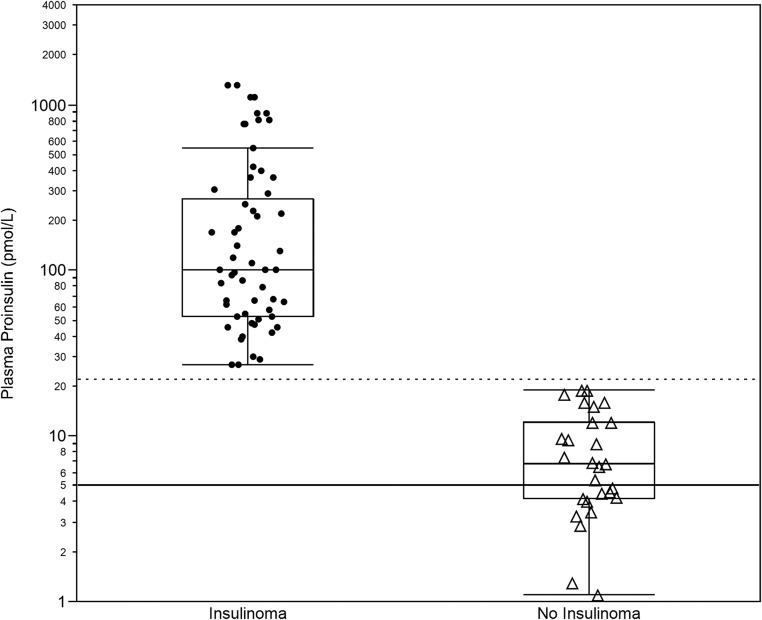
Figure 1 depicts the end of fast proinsulin concentration for patients with insulin-producing tumors and for controls in our series. The graph confirms that proinsulin concentrations at the end of the supervised fast are higher in subjects with insulinomas. The median end of fast plasma proinsulin concentration was 100 and 6.8 pmol/L in patients with insulin-producing tumors and in controls, respectively. Fifty percent of cases had end of fast proinsulin concentrations in the range of 53–270 pmol/L, whereas 50% of controls had end of fast proinsulin values in the range of 4.2–12.0 pmol/L. The difference in the end of fast proinsulin concentration between patients with and without an insulinoma is highly significant (Wilcoxon Rank Sum test P value <.0001). Most importantly, the figure shows an absence of overlap between the minimum observed end of fast proinsulin concentration in cases (27 pmol/L) and the maximum observed end of fast proinsulin concentration in controls (19 pmol/L). Proinsulin concentration at the end of fast, in this series, completely discriminates cases from controls.
Figure 1.
End of fast proinsulin concentrations. The figure displays the distribution of the proinsulin concentration (log scale) observed at the end of a 48-hour supervised fast in 56 cases with insulin-producing tumors (Insulinoma) and 29 control subjects with no insulin-producing tumors (No Insulinoma) and their respective box-plots. (solid line) 5 pmol/L cutoff; (dotted line) 22 pmol/L cutoff.
Table 1 summarizes the diagnostic performance of two ends of fast proinsulin concentration cutoff values using our series of patients. The first cutoff value, ≥5 pmol/L, is the cutoff proposed by the Endocrine Society Clinical Practice Guideline. This cutoff captures 100% of cases (ie, 100% sensitivity) but cannot distinguish cases (ie, true positives) from controls (ie, true negatives). Fifty-nine percent of subjects without an insulinoma in our series were observed to have an end of fast proinsulin value of ≥5 pmol/L. This high false positive rate makes this cutoff value of limited diagnostic utility. The second cutoff value shown in Table 1 is the point in our data that yields the highest sensitivity and lowest false positive rate. Because end of fast proinsulin data from cases and controls did not overlap in our series, this point was the lowest observed plasma proinsulin concentration for cases (ie, 27 pmol/L). This cutoff captures all cases (sensitivity 100%) and excludes all controls (specificity 100%). Raising the proinsulin threshold from 5 to 27 pmol/L does not lower sensitivity and greatly increases specificity. These data show that in this series a proinsulin concentration of ≥27 pmol/L more accurately predicts the presence of an insulin-producing tumor than a proinsulin concentration of ≥5 pmol/L.
Table 1.
Diagnostic Performance End of Fast Proinsulin
| End of Fast Proinsulin | ≥5 pmol/L | ≥27 pmol/L |
|---|---|---|
| Sensitivity | 100% | 100% |
| False negative | 0% | 0% |
| Specificity | 41% | 100% |
| False positive | 59% | 0% |
The diagnostic performance for two end of fast proinsulin concentration cutoff values in 56 patients with insulin producing tumors and 25 control subjects is shown. A proinsulin concentration of 5 pmol/L is the cutoff value proposed by the Endocrine Society practice guideline. The second proinsulin concentration cutoff value (ie, 27 pmol/L) yields the highest sensitivity and the lowest false positive rate in this series of patients.
Nonfasting proinsulin concentration
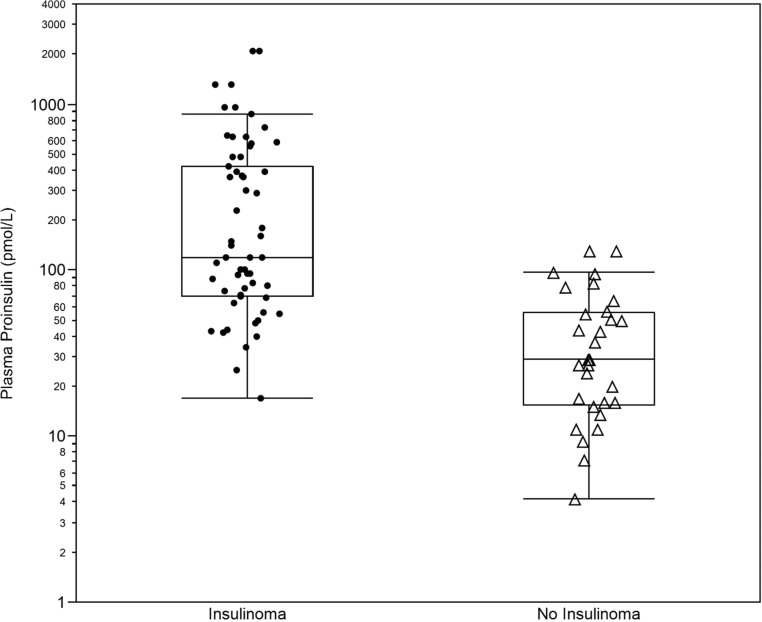
In Figure 2, nonfasting proinsulin concentrations are plotted for cases and controls. The median (interquartile range) proinsulin concentration at the beginning of the fast was 120 pmol/L (70–420 pmol/L) and 29 pmol/L (16–56 pmol/L) for patients with and without insulinomas, respectively. Although differences between the two groups remain statistically significant, overlap between cases and controls is seen. The nonfasting proinsulin cutoff value yielding the largest area under the ROC curve is a concentration of 67 pmol/L. At this concentration sensitivity is 78% and the false positive rate is 17%. One hundred percent specificity (ie, 0% false positive) was observed at proinsulin concentration in excess of 100 pmol/L. Proinsulin concentration obtained in the nonfasting state cannot reliably predict the presence of an insulinoma.
Figure 2.
Start of fast proinsulin concentrations. The figure displays the distribution of the proinsulin concentrations (log scale) observed at the start of a 48-hour supervised fast in 56 cases with insulin-producing tumors (Insulinoma) and 29 control subjects with no insulin-producing tumors (No Insulinoma) and their respective box-plots.
C-peptide concentration at the end of the fast
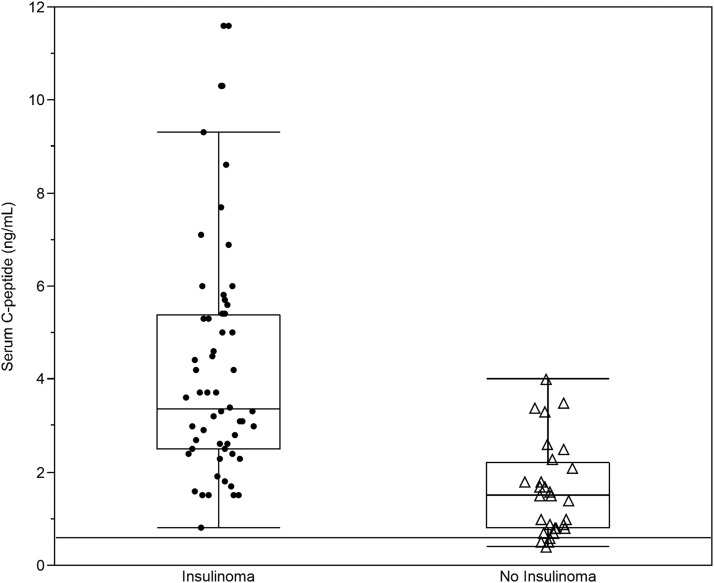
End of fast C-peptide concentrations for cases and controls are shown in Figure 3. All patients with insulin-producing tumors had an end of fast C-peptide concentration above the cutoff suggested by the Endocrine Society Clinical Practice Guideline of 0.6 ng/mL (ie, sensitivity 100%). A C-peptide level of 0.6 ng/mL was also observed in most control subjects (ie, false positive rate 90%). The C-peptide concentration found to discriminate cases best from controls on ROC curve analysis was 2.3 ng/mL. This cutoff had a sensitivity of 84% and a false positive rate of 24%.
Figure 3.
End of fast C-peptide concentrations. The figure displays the distribution of the C-peptide concentrations observed at the end of a 48-hour supervised fast in 56 cases with insulin-producing tumors (Insulinoma) and 29 control subjects with no insulin-producing tumors (No Insulinoma) and their respective box-plots. (solid line) Concentration of 0.6 ng/mL.
Discussion
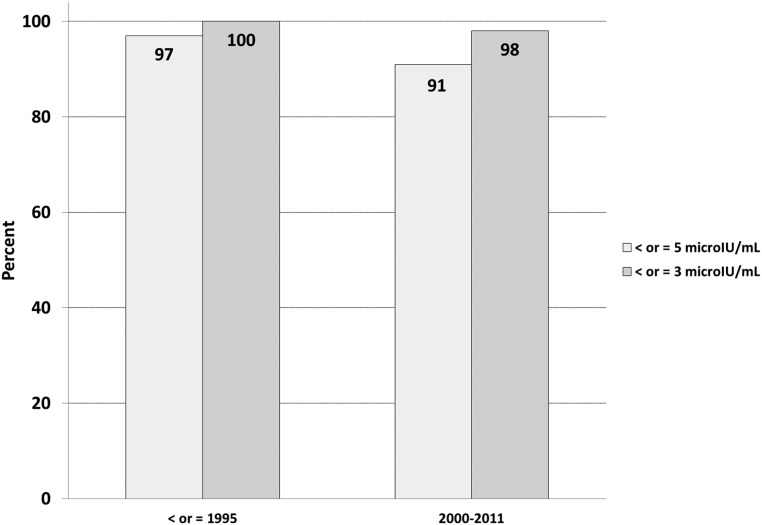
Figure 4 compares the diagnostic sensitivity of an end of fast insulin cutoff value of 5 and 3 μIU/mL through time and across assay techniques at the NIH clinical research center. Note that in the series of cases published in 1995 (1), insulin was quantified using a RIA technique. In this series, an insulin concentration of 5 μIU/mL or greater in patients observed to have symptomatic hypoglycemia captured 97% of subjects with an insulin-producing tumor. In the era of new insulin assays only 91% of patients with an insulin-producing tumor ended the supervised fast with a serum insulin concentration above 5 μIU/mL. These results suggest that the cutoff value of ≥5 μIU/mL applied to newer insulin assays could lead to misinterpretation of the fast result as falsely negative in up to 9% of subjects with insulin-producing tumors. Lowering the cutoff value to 3 μIU/mL increases the sensitivity of the test but does not completely eliminate the presence of an insulin-producing tumor (ie, false negatives). This finding is consistent with published reports describing patients with insulin-producing tumors and diagnosed on the basis of an elevated proinsulin level alone (3–5).
Figure 4.
Sensitivity of an end of fast insulin value of >5 μIU/mL and >3 μIU/mL for NIH cases before 1995 (RIA) and for current cases (newer “specific” insulin assay).
Insulin levels are dependent on glucose levels and it is not surprising that in the absence of other data an end of fast insulin value ≥3 μIU/mL lacks specificity and cannot discriminate cases from control. The specificity of the test increases when other data captured coincidently are considered (ie, symptoms and the observed end of fast plasma glucose levels). A recent article has reported on the diagnostic utility of a test that combines the end of fast insulin and glucose values (ie, the amended insulin to glucose ratio) in a large series of patients (13).
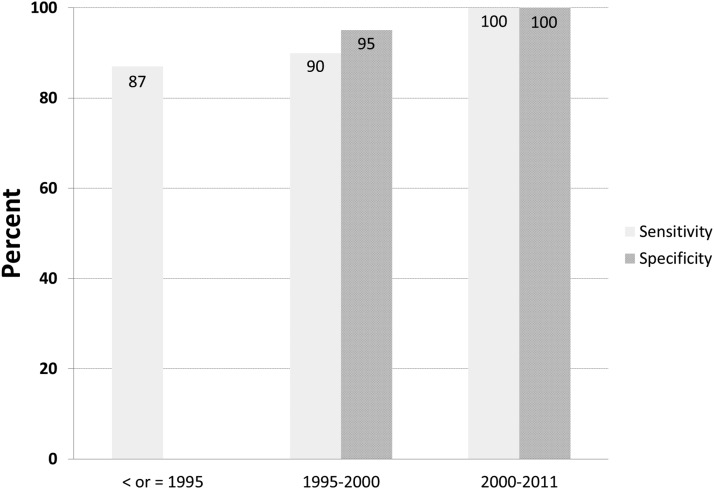
Our data confirm the value of proinsulin in the diagnosis of insulin-producing tumors. It has been recognized for well over four decades that the presence of an insulinoma is associated with high levels of circulating proinsulin (7–10). This observation appears consistent with histopathology findings, which suggest insulin processing, storage, and/or secretion may be abnormal in insulin-producing tumors (14–16). Using correlation analysis, we had suggested a proinsulin concentration of 22 pmol/L measured on the direct proinsulin assay correlated to the value used to define proinsulin elevation on the indirect historical assay. Figure 5 summarizes the diagnostic utility of an elevated fasting proinsulin, defined as a directly measured fasting proinsulin concentration above 22 pmol/L, across three independent case series of patients who underwent a supervised fast at our center over time. The case series from 1995 (1) and 1995–2000 (12) have been previously published. The data in our new series are entirely consistent with the two previously published series and show that proinsulin concentration at the end of the fast is greater than 22 pmol/L in most patients harboring an insulin-producing tumor. We conclude that this cutoff value provides adequate sensitivity.
Figure 5.
Diagnostic performance of an end of fast proinsulin >22 pmol/L or equivalent across three nonoverlapping series of NIH patients evaluated with a supervised fast. These data represent 176 patients with insulin-producing tumors and 50 control subjects. (Proinsulin prior to 1995 was not directly measured; values obtained using the indirect proinsulin assay were converted to concentrations using a correlation analysis between the indirect and direct proinsulin assays.)
More importantly, few control subjects end the supervised fast with a proinsulin concentration above 22 pmol/L. We conclude that an elevated end of fast plasma proinsulin level is the most specific, single indicator, of the presence of an insulin-producing tumor. Defining an elevated proinsulin using a fasting proinsulin cutoff value of 5 pmol/L is not useful because this threshold does not yield greater sensitivity compared to 22 pmol/L and cannot discriminate cases from controls (ie, low specificity).
We have found the proinsulin test to be particularly useful in the setting of a positive supervised fast when fasting insulin levels are observed to be low or suppressed. In this situation an elevated proinsulin level suggests an insulinoma. In our series, seven patients who had an insulin-producing tumor had an end of fast insulin value below 5 μIU/mL. Four of these seven patients had an end of fast insulin value below 3 μIU/mL. The lowest observed end of fast proinsulin concentration in these patients was 42 pmol/L. In the 14 controls who also had suppressed insulin to below 5 μIU/mL at the end of the fast the highest observed proinsulin value was 16 pmol/L. Another case illustrates the potential usefulness of proinsulin. The patient had a fasting glucose, insulin, and C-peptide pattern suggestive of surreptitious insulin injection (ie, a six-fold rise in insulin concentration midway through the fast and an absent concomitant rise in C-peptide concentration). The patient was discharged home with a presumptive diagnosis of factitious hypoglycemia. The result of the end of fast proinsulin test became available after discharge and was found to be 66 pmol/L. The patient underwent an exploratory laparotomy for recurrent hypoglycemia and an insulinoma was identified.
The end of fast proinsulin concentration was not useful in distinguishing malignant from nonmalignant disease in this series. Considerable overlap was observed between nonmalignant (minimum to maximum: 27–890 pmol/L) and malignant (minimum to maximum: 45–1300 pmol/L) end of fast proinsulin concentrations. Although conclusions regarding the utility of the end of fast proinsulin test in this series are limited due to the small number of malignant cases (n = 6), it is consistent with our past clinical experience.
In conclusion, a proinsulin concentration above 22 pmol/L at the end of an adequately supervised fast is both sensitive and specific and highly suggestive of the presence of an insulin-producing tumor.
Acknowledgments
We thank the 5NW nursing staff and physicians involved in the care of the patients presented in this report.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- coefficient of variation
- NIH
- National Institutes of Health
- ROC
- receiver operating characteristic.
References
- 1. Gorden P, Skarulis MC, Roach P, et al. Plasma proinsulin-like component in insulinoma: a 25-year experience. J Clin Endocrinol Metab. 1995;80(10):2884–2887 [DOI] [PubMed] [Google Scholar]
- 2. Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma—incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66(7):711–719 [DOI] [PubMed] [Google Scholar]
- 3. Alsever RN, Roberts JP, Gerber JG, Mako ME, Rubenstein AH. Insulinoma with low circulating insulin levels: the diagnostic value of proinsulin measurements. Ann Intern Med. 1975;82(3):347–350 [DOI] [PubMed] [Google Scholar]
- 4. Chia CW, Saudek CD. The diagnosis of fasting hypoglycemia due to an islet-cell tumor obscured by a highly specific insulin assay. J Clin Endocrinol Metab. 2003;88(4):1464–1467 [DOI] [PubMed] [Google Scholar]
- 5. Gómez-Pérez FJ, Cuevas-Ramos D, Valdés PA, Aguilar-Salinas CA, Mehta R, Rull JA. Beta-cell adenomas without hyperinsulinemia with use of highly specific insulin radioimmunoassays: case report and review of literature. Endocr Pract. 2010;16(4):660–663 [DOI] [PubMed] [Google Scholar]
- 6. Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728 [DOI] [PubMed] [Google Scholar]
- 7. Gorden P, Sherman B, Roth J. Proinsulin-like component of circulating insulin in the basal state and in patients and hamsters with islet cell tumors. J Clin Invest. 1971;50(10):2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sherman BM, Gorden P, Roth J, Freychet P. Circulating insulin: th proinsulin-like properties of “big” insulin in patients withou islet cell tumors. J Clin Invest. 1971;50(4):849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutman RA, Lazarus NR, Penhos JC, Fajans S, Recant L. Circulating proinsulin-like material in patients with functioning insulinomas. N Engl J Med. 1971;284(18):1003–1008 [DOI] [PubMed] [Google Scholar]
- 10. Sherman BM, Pek S, Fajans SS, Floyd JC, Jr, Conn JW. Plasma proinsulin in patients with functioning pancreatic islet cell tumors. J Clin Endocrinol Metab. 1972;35(2):271–280 [DOI] [PubMed] [Google Scholar]
- 11. Steiner DF, Oyer PE. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci USA. 1967;57(2):473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirshberg B, Livi A, Bartlett DL, et al. Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab. 2000;85(9):3222–3226 [DOI] [PubMed] [Google Scholar]
- 13. Nauck MA, Meier JJ. Diagnostic accuracy of an “amended” insulin-glucose ratio for the biochemical diagnosis of insulinomas. Ann Intern Med. 2012;157(11):767–775 [DOI] [PubMed] [Google Scholar]
- 14. Creutzfeldt W, Arnold R, Creutzfeldt C, Deuticke U, Frerichs H, Track NS. Biochemical and morphological investigations of 30 human insulinomas. Correlation between the tumour content of insulin and proinsulin-like components and the histological and ultrastructural appearance. Diabetologia. 1973;9(3):217–231 [DOI] [PubMed] [Google Scholar]
- 15. Creutzfeldt W, Creutzfeldt C, Frerichs H, Track NS, Arnold R. Histochemistry, ultrastructure and hormone content of human insulinomas. Horm Metab Res. 1976;suppl 6:7–18 [PubMed] [Google Scholar]
- 16. Creutzfeldt W, Arnold R, Creutzfeldt C, Frerichs H, Track NS. Immunohistological, ultrastructural and biochemical investigations of human insulinomas. Isr J Med Sci. 1972;8(6): 756 [PubMed] [Google Scholar]