Abstract
Context:
GnRH agonists (GnRHa) are being used experimentally in an attempt to preserve fertility in young female cancer patients undergoing chemotherapy. Anti-Müllerian hormone (AMH) produced by ovarian granulosa cells may serve as a marker of ovarian reserve, but it is not clear whether this marker is useful during GnRHa treatment.
Objective:
The purpose of this study was to determine the effect of a depot GnRHa on AMH levels.
Design:
Depot leuprolide (3.75 mg) was administered in the midluteal phase (MLP) in healthy women. Assessments of AMH, FSH, LH, estradiol, and progesterone were performed in the early follicular phase (EFP) and MLP before GnRHa treatment and approximately 7, 14, and 30 days after GnRHa administration.
Setting:
The study was conducted in a university research center.
Patients:
Participants were 33 healthy, premenopausal women aged 18 to 45 years old with regular menses.
Results:
EFP and MLP AMH levels were similar before GnRHa administration. Relative to MLP AMH levels, AMH decreased 7 days after GnRHa administration by a median of 24% (P < .001) and then increased above pretreatment levels 14 and 30 days after GnRHa by 13% and 32%, respectively (P < .001). Changes in AMH levels did not correlate with changes in gonadotropins, estradiol, or progesterone.
Conclusions:
Significant changes in AMH levels occur in the first 4 weeks after depot leuprolide administration, suggesting that AMH may not be a reliable marker of ovarian reserve during this interval. Changes in AMH occurred independent of gonadotropin levels, supporting a direct effect of GnRHa on granulosa cell expression of AMH or an indirect effect of GnRHa on the development and/or dynamics of the follicle pool.
Anti-Müllerian hormone (AMH) is a glycoprotein expressed by granulosa cells in preantral and small antral ovarian follicles. AMH levels provide an indication of the size of the growing follicle pool, with expression disappearing as antral follicles develop (1). Because of the correlation of AMH levels with outcomes of assisted reproduction, fecundability, and menopause, AMH is a commonly used biomarker of ovarian reserve in reproductive-aged women (2–4).
In young female patients with cancer, GnRH agonists (GnRHa) are being used experimentally to preserve fertility (5). With administration of GnRHa, FSH levels cannot be used to measure ovarian function because of gonadotropin down-regulation. AMH levels appear relatively stable across the menstrual cycle (6) and with administration of birth control pills (7, 8), suggesting gonadotropin independence. AMH might therefore be a useful biomarker of ovarian reserve with GnRHa treatment.
The use of GnRHa for fertility preservation during chemotherapy is controversial because of inconclusive outcome data on fertility (9) and because the mechanism by which GnRHa may act to preserve fertility is unknown. The major known function of GnRHa is to suppress gonadotropins, acting indirectly on ovarian follicles. However, recent in vitro studies demonstrating that GnRH modulates expression of GnRH and GnRH receptors in human luteinized granulosa cells and induces granulosa cell apoptosis (10, 11) raise the possibility that GnRHa may also act directly at the ovary. Determining the effect of GnRHa on AMH levels is an essential prelude to use of AMH as a marker of ovarian reserve in cancer patients receiving GnRHa.
The study objective was to examine the response of AMH in normal women over a 4-week exposure to depot leuprolide, a GnRHa used widely in patients with cancer. Because small, growing ovarian follicles that express AMH are not gonadotropin dependent, we hypothesized that AMH levels would not be altered by the initial flare or subsequent down-regulation of pituitary gonadotropins by GnRHa.
Subjects and Methods
The study was approved by institutional review boards at Massachusetts General Hospital and University of California, San Diego. Participants were healthy, premenopausal women aged 18 to 45 years with monthly menstrual cycles, were ovulatory (midluteal serum progesterone [P4], >3 ng/dL), and had both ovaries. Exclusion criteria were pregnancy, lactation, hot flashes, hormonal contraception within 3 months, or abnormal prolactin or thyroid hormone levels. Women participating in the current analysis included all those enrolled in parent studies focused on changes in sleep patterns after GnRHa who had available sera for analysis (11, 12).
Participants were given one open-label im injection of depot leuprolide (3.75 mg) in the midluteal phase (MLP), timed 1 week after urinary LH levels and basal body temperature indicated ovulation. When the basal body temperature and LH surge were discrepant, the LH surge was used to determine the ovulation date. Participants provided blood samples in the early follicular phase (EFP), as determined by menstrual cycle day, and in the MLP of the menstrual cycle in which GnRHa was administered, as determined by days from ovulation. All women had an expected menstrual bleed approximately 1 week after the GnRHa injection. Blood samples were drawn approximately 7 (GnRHa+7), 14 (GnRHa+14), and 30 (GnRHa+30) days after GnRHa administration. Sera were frozen at −30°C before testing. AMH was measured by the AMH Gen II ELISA kit (Beckman Coulter). The interassay coefficient of variation (CV) was 4.7%. The limit of quantitation was 0.17 ng/mL. LH, FSH, and P4 were measured by chemiluminescent immunoassays (Architect; Abbott Diagnostics) with assay sensitivities of 0.3 IU/L, 0.7 IU/L, and 0.1 ng/mL and interassay CVs of <6%, <7%, and <5% for LH, FSH, and P4, respectively (6, 13). Estradiol (E2) was measured by liquid chromatography/mass spectrometry with an 8.6% interassay CV (Mayo Medical) (14).
Analyses were conducted using Stata (StataCorp). Because only AMH levels met normality assumptions, nonparametric testing was used for analysis. AMH levels were compared among all time points by the Friedman nonparametric repeated-measures test, followed by Wilcoxon signed rank tests for pairwise comparisons between MLP levels and levels from (1) pre-GnRHa EFP, (2) GnRHa+7, (3) GnRHa+14, and (4) GnRHa+30. MLP levels were used as baseline values for post-GnRHa changes because of temporal proximity to GnRHa exposure. Correlations between percent changes in AMH from midluteal levels to the 3 post-GnRHa intervals (GnRHa+7, GnRHa+14, and GnRHa+30) and percent changes in FSH, LH, E2, P4, and baseline covariates were assessed by Spearman ρ for continuous variables and linear regression for discrete variables. Statistical tests were 2-tailed. Values of P < .05 were considered significant. Data are presented as medians (interquartile range [IQR]) unless otherwise stated.
An a priori sample size calculation was based on prior studies of AMH in premenopausal women, where log-transformed mean AMH (SD) was 0.69 (0.75) ng/mL (4). With 33 participants, 80% power, and an α error of .05, the detectable difference would be 0.43 SD.
Results
There were 33 participants with a mean (SD) age of 30.3(8.5) years. Mean body mass index (BMI) (SD, range) was 25.7(4.7, 18.4–36.7) kg/m2. The cohort included 20 (61%) whites, 6 (18%) blacks, and 7 (21%) other/not reported; 6 (18%) were Hispanic. None had infertility. Average cycle length (SD) was 28 (2) days. Median (IQR) cycle days of EFP and MLP blood samples were 6 (3.5) and 22 (2), respectively. MLP AMH was significantly lower with older age (P = .02), non-Hispanic ethnicity (P = .01), and shorter cycle length (P = .01) but was not associated with BMI or race (P > .05). The median (IQR) days from GnRHa administration were 7 (1) days for GnRHa+7, 14 (1) days for GnRHa+14, and 30 (7) days for GnRHa+30 time points.
FSH, LH, E2, and P4 levels from all time points are depicted in Figure 1. Before GnRHa administration, FSH and LH levels were lower in the MLP than in the EFP (P < .001). Compared with MLP levels, FSH was reduced further after GnRHa administration at GnRHa+7 (P < .001) and GnRHa+14 (P < .001). At 30 days, there was partial recovery of pituitary function, because FSH levels approached MLP levels (P = .69). After GnRHa administration, LH levels at 14 and 30 days were significantly decreased compared with midluteal levels (P < .001). In response to GnRHa, E2 and P4 levels declined sharply from MLP levels (P < .001) with the lowest values apparent at 14 and 30 days after GnRHa.
Figure 1.
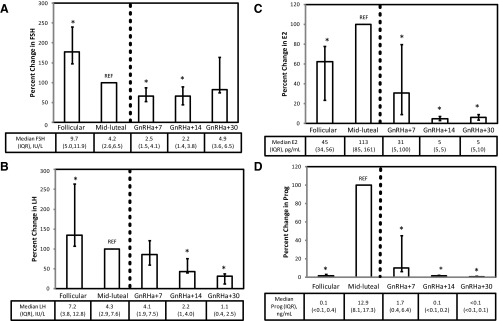
FSH (A), LH (B), estradiol (C), and progesterone (D) expressed as a percentage of MLP levels obtained in the follicular phase, MLP, and approximately 7 days (GnRHa+7), 14 days (GnRHa+14), and 30 days (GnRHa+30) after administration of the depot GnRHa leuprolide (3.75 mg im), indicated by the dashed vertical line (n = 33). REF, reference. Data are depicted as medians with 25th to 75th percentile values, and absolute levels are reported immediately below the graph. *, P < .0001 vs MLP level.
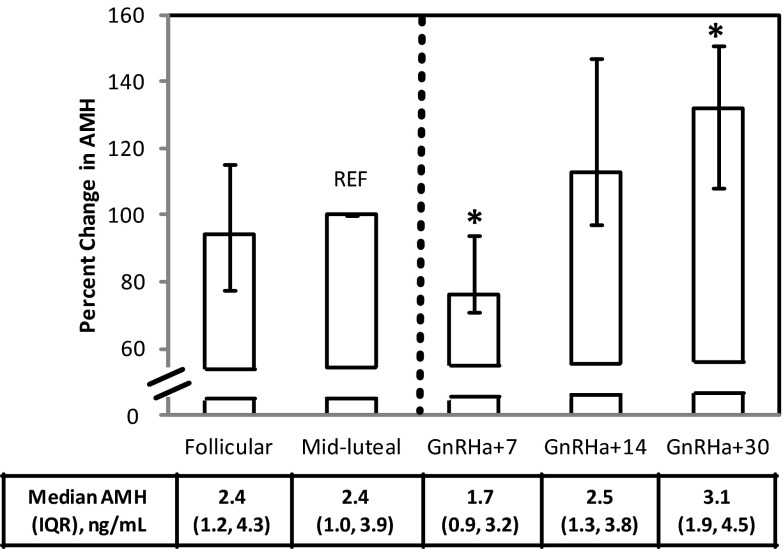
AMH levels were not significantly different between the follicular phase and MLP (median [IQR], 2.4 [3.2] ng/mL vs 2.4 [2.9] ng/mL, respectively; P = .67). After GnRHa administration, AMH levels declined significantly from the MLP (median [IQR], 2.4 [3.2] ng/mL) to GnRHa+7 (1.7 [2.3] ng/mL, P < .001), representing a median decrease of 24% (Figure 2). AMH levels subsequently rose to a median (IQR) of 2.5 (2.5) ng/mL at GnRHa+14 (P = .09) and 3.1 (2.6) ng/mL at GnRHa+30 (P < .001), 13% and 32% higher than midluteal values. The pattern of change with GnRHa exposure was similar within each group when participants were dichotomized according to the median MLP AMH (2.4 ng/mL) (data not shown). There were no associations of change in AMH in response to GnRHa with age, race, ethnicity, BMI, or cycle length (all P > .05).
Figure 2.
AMH expressed as a percentage of MLP levels in the follicular phase, MLP, and approximately 7 days (GnRHa+7), 14 days (GnRHa+14), and 30 days (GnRHa+30) after administration of the depot GnRHa leuprolide (3.75 mg im), indicated by the dashed vertical line (n = 33). REF, reference. Data are depicted as medians with 25th to 75th percentile values, and absolute levels are reported immediately below the graph. *, P < .001 vs MLP level.
The decline in AMH from the MLP to GnRHa+7 was associated with concurrent decreases in E2 (r = 0.46, P = .01) and P4 (r = 0.57, P = .002) but not with concomitant changes in FSH (r = −0.15, P = .43) or LH (r = −0.28, P = .13). Increases in AMH from 7 to 14 days and from 14 to 30 days after GnRHa administration were unrelated to concurrent changes in FSH, LH, E2, or P4 (all r < 0.26, all P > .18).
Discussion
In reproductive-aged women undergoing depot leuprolide treatment, AMH levels changed dynamically across the initial 4 weeks after midluteal administration. AMH levels reached the observed nadir after 7 days of GnRHa and then increased progressively to levels exceeding baseline midluteal levels by 14 and 30 days of GnRHa. Whereas lower AMH levels were observed in the MLP in older and in non-Hispanic women, this biphasic response of AMH response to GnRHa was observed regardless of age, pretreatment AMH levels, or ethnicity. These data indicate that AMH is not likely to be a reliable marker of ovarian reserve in patients with cancer over the first month of GnRHa treatment. Our data also show that changes in AMH levels did not correlate with concurrently measured LH and FSH levels, raising the possibility of a direct, gonadotropin-independent effect of GnRHa on granulosa cells.
The role of gonadotropins and GnRHa in modulation of AMH secretion is controversial. GnRHa administration causes an initial increase in LH and FSH, which can stimulate follicle development and E2 secretion (flare), followed by gonadotropin desensitization and sustained suppression of gonadotropins and ovarian steroids. The initial agonist-induced flare is minimized with luteal phase administration, as used in this protocol, because of suppression of the FSH flare by the negative feedback of E2 and inhibin A on FSH. AMH measured weekly in the first month after GnRHa administration demonstrated a biphasic response, helping to resolve apparent conflicting results of previous studies in which AMH was measured at different time points during GnRHa administration (15). In one study (2), there was no change in AMH 24 hours after GnRHa administration, despite marked elevations in FSH, E2, and inhibin B, whereas AMH levels were higher in association with suppressed LH, FSH, E2, and inhibin B levels after 2 weeks of daily GnRHa in a second study (15), consistent with the current findings. In contrast, AMH levels in 15 early-maturing girls were significantly suppressed 3 months after initiation of continuous GnRHa treatment for up to 1 year (19). Similarly, AMH levels were lower 3 and 6 months after institution of GnRHa in premenopausal patients with breast cancer in conjunction with LH, FSH, E2, and inhibin B suppression (n = 9) (17). Taken together, these data suggest a dynamic response of AMH to GnRHa in women, with an initial decline followed by an increase and perhaps a further subsequent decline in AMH in the setting of continued GnRHa administration.
In this study, AMH levels were not different in the follicular and luteal phases in the natural cycle, a finding consistent with prior studies showing that AMH levels are stable within an individual woman from one menstrual cycle to the next (6, 16, 18). Our short-term follow-up data emphasize that AMH levels follow a predictable, biphasic trajectory after GnRHa is administered, thereby limiting the utility of AMH as a marker of ovarian reserve in the first posttreatment month. In the context of cancer treatment, GnRHa may be coadministered with chemotherapy or started after chemotherapy completion. With gonadotoxic treatment (eg, chemotherapy), AMH levels first decline and, in a proportion of young women, subsequently rise after cessation of chemotherapy, suggesting ovarian recovery (17, 19). Our observation of a dynamic AMH response to GnRHa highlights the complexity of interpreting AMH levels in the first month after GnRHa whether administered concurrently or sequentially with chemotherapy.
The current study provides in vivo data to support a direct action of GnRHa on the ovary. Both pre- and post-GnRHa AMH levels suggest some degree of gonadotropin independence. Consistent with prior reports (6), AMH levels were stable between follicular and luteal phases, whereas FSH and LH levels were lower in the MLP. In addition, neither the initial decline nor subsequent rise in AMH levels after GnRHa administration correlated with changes in gonadotropins. GnRH receptors are expressed by human granulosa cells and up-regulated, in part, by GnRH (10). The AMH decrease after 7 days of treatment may have resulted from up-regulation of GnRH receptors combined with the antiproliferative and apoptotic effects of short-term GnRHa exposure on granulosa cells (10, 11). Rodent studies showing that granulosa cell GnRH receptor expression declines with longer GnRHa administration may explain the subsequent increase we observed in AMH levels starting at 14 days of GnRHa. The observed decrease in AMH at 7 days may have allowed an expansion of AMH-secreting preantral and small antral follicular pools, resulting in subsequent increases in AMH levels at days 14 and 30 (20). Whether GnRHa directly affects only granulosa cells or modulates follicle dynamics is key to discerning whether GnRHa administration is likely to protect fertility in patients with cancer undergoing chemotherapy.
In the current study, we did not detect a gonadotropin flare but did not sample until 7 days after GnRHa. Therefore, an alternative explanation for the observed AMH changes is that the initial decrease in AMH resulted from an undetected gonadotropin flare, which advanced follicular development in gonadotropin-responsive larger antral follicles, thereby reducing AMH (15). After the flare, suppression of FSH and LH precluded additional advancement of FSH-dependent follicles, thereby increasing the total number of gonadotropin-responsive follicles secreting AMH. More frequent blood sampling immediately after GnRHa administration to demonstrate a flare with a concomitant decrease in AMH levels would test this hypothesis.
An important strength of this study is that subjects served as their own controls, providing AMH levels from the EFP and MLP before GnRHa administration as evidence of stability of AMH across the menstrual cycle and in sharp contrast to the changes in AMH observed after GnRHa administration. Several limitations must be considered. Longer follow-up is needed to determine whether AMH levels become partially suppressed again with prolonged exposure, as suggested previously (18, 21). Follow-up data are important for interpreting ovarian reserve measurements in young female patients with cancer who are receiving prolonged GnRHa therapy. This study did not include antral follicle counts, so changes in follicular populations can only be surmised using AMH levels. However, others have not detected an increase in the number of smaller follicles concurrent with elevated levels of AMH after 14 days of GnRHa (15), suggesting that changes in the follicle population may occur below ultrasound resolution.
This study provides evidence that dynamic changes in AMH levels occur in the first 4 weeks after depot leuprolide administration, suggesting that AMH will not be a reliable marker of ovarian reserve in patients with cancer receiving GnRHa during this time period. The clinical question of how to measure ovarian reserve in women receiving prolonged GnRHa treatment remains unanswered. However, the finding that fluctuations in AMH levels occurred independent of gonadotropins, whether the result of a direct effect of GnRHa on granulosa cell AMH expression and/or an indirect effect of GnRHa on follicle pool development and/or dynamics, supports continued efforts to elucidate the role of GnRHa in fertility preservation.
Acknowledgments
This work was supported by the National Institutes of Health (grants HD058799 to H.I.S. and 5R01MH082922 to H.J.) and American Cancer Society (grant MRSG-08–110-01-CCE to H.I.S.).
Disclosure Summary: H.J. has received grant funding from Cephalon/Teva, serves on the advisory board for Noven Pharmaceuticals and is an unpaid consultant to Sunovion. H.I.S. has served on the advisory board for Ferring Pharmaceuticals. The other authors have nothing to disclose.
Footnotes
- AMH
- anti-Müllerian hormone
- BMI
- body mass index
- CV
- coefficient of variation
- EFP
- early follicular phase
- E2
- estradiol
- GnRHa
- GnRH agonist(s)
- IQR
- interquartile range
- MLP
- midluteal phase
- P4
- progesterone.
References
- 1. Weenen C, Laven JS, Von Bergh AR, et al. Anti-müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83 [DOI] [PubMed] [Google Scholar]
- 2. van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071 [DOI] [PubMed] [Google Scholar]
- 3. Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steiner AZ, Herring AH, Kesner JS, et al. Antimüllerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117:798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931 [DOI] [PubMed] [Google Scholar]
- 6. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063 [DOI] [PubMed] [Google Scholar]
- 7. Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81:245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90:395–400 [DOI] [PubMed] [Google Scholar]
- 9. Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269–276 [DOI] [PubMed] [Google Scholar]
- 10. Peng C, Fan NC, Ligier M, Väänänen J, Leung PC. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994;135:1740–1746 [DOI] [PubMed] [Google Scholar]
- 11. Joffe H, White DP, Crawford SL, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013;36(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenfield RL, Wroblewski K, Padmanabhan V, Littlejohn E, Mortensen M, Ehrmann DA. Antimüllerian hormone levels are independently related to ovarian hyperandrogenism and polycystic ovaries. Fertil Steril. 2012;98:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384 [DOI] [PubMed] [Google Scholar]
- 15. Jayaprakasan K, Campbell BK, Hopkisson JF, Clewes JS, Johnson IR, Raine-Fenning NJ. Effect of pituitary desensitization on the early growing follicular cohort estimated using anti-Mullerian hormone. Hum Reprod. 2008;23:2577–2583 [DOI] [PubMed] [Google Scholar]
- 16. van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25:221–227 [DOI] [PubMed] [Google Scholar]
- 17. Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–2592 [DOI] [PubMed] [Google Scholar]
- 18. Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–1840 [DOI] [PubMed] [Google Scholar]
- 19. Mörse H, Elfving M, Lindgren A, Wölner-Hanssen P, Andersen CY, Øra I. Acute onset of ovarian dysfunction in young females after start of cancer treatment. Pediatr Blood Cancer. 2013;60:676–681 [DOI] [PubMed] [Google Scholar]
- 20. Fanchin R, Schonäuer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003;18:328–332 [DOI] [PubMed] [Google Scholar]
- 21. Young J, Rey R, Couzinet B, Chanson P, Josso N, Schaison G. Antimüllerian hormone in patients with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1999;84:2696–2699 [DOI] [PubMed] [Google Scholar]