Abstract
Context:
Endometriosis is characterized by progesterone resistance and hyperactivity of the AKT and MAPK pathways. Kinases can cause posttranslational modifications of the progesterone receptor (PR) to influence cellular localization and protein stability.
Objective:
The objective of this study was to determine whether the increased AKT or MAPK kinase-1/2 (MEK1/2) activity observed in endometriotic stromal cells (OSIS) from ovarian endometriomas influences levels of PR protein. In turn, the effects of inhibiting AKT or MEK1/2 in the presence of the progestin R5020 on cell viability were investigated.
Results:
Inhibiting AKT with MK-2206 or MEK1/2 with U0126 for 24 hours in the absence of R5020 increased total and nuclear PRA and PRB protein levels in OSIS but not in eutopic endometrial stromal cells from disease-free patients from disease-free patients. MK-2206 and R5020 decreased OSIS viability and increased apoptosis. Trends toward decreased volumes of sc grafted endometriosis tissues were demonstrated with MK-2206 and progesterone.
Conclusions:
Inhibition of AKT or MEK1/2 increased total and nuclear PR protein in OSIS. MK-2206 and R5020 decreased OSIS viability and increased apoptosis. The AKT and MAPK pathways may be potential molecular targets for the treatment of endometriosis.
Endometriosis is an estrogen-dependent inflammatory disease defined by the presence of endometrial glands and stroma outside of the uterine cavity (1). It affects 6% to 10% of women of reproductive age in the United States (2), and its prevalence is as high as 50% to 60% among women with chronic pelvic pain or infertility (3, 4). The pathogenesis of endometriosis is poorly understood; therefore, current options for the treatment of endometriosis-associated pain are limited (5–9).
Expanding the current treatment options for endometriosis requires a better understanding of the molecular defects associated with development of the disease. Progesterone resistance has been demonstrated in the ectopic and eutopic endometrial stromal cells of women with endometriosis and is believed to contribute to disease pathogenesis as well as suboptimal response to progestin therapy (10–14). In addition to progesterone resistance, other molecular defects likely contribute to dysregulated proliferation and apoptosis in endometriosis. For example, the pro-proliferative and antiapoptotic phosphatidylinositol 3-kinase (PI3K)/AKT and MAPK signaling pathways have been shown to be hyperactive in endometriosis (15–17). Increased levels of the phosphorylated activated forms of AKT and ERK1/2 have been demonstrated in eutopic endometrial stromal cells from women with endometriosis compared with those from disease-free women (18, 19). MAPK and AKT have been shown to regulate progesterone receptor (PR) in breast and endometrial cancer cells; therefore, it is likely that a similar mechanism could contribute to decreased PR and progesterone resistance in endometriosis (20–22). We hypothesized that hyperactive AKT and MAPK promote nuclear export and decreased levels of PR in endometriotic stromal cells. Inhibition of AKT with the inhibitor MK-2206 or MEK1/2 with the inhibitor U0126 would therefore increase PR levels and decrease proliferation and viability of endometriotic stromal cells.
Patients and Methods
Tissue acquisition
This study was approved by the Northwestern University Institutional Review Board. Written informed consent was obtained from all patients. Endometrioma cyst walls were obtained from patients undergoing ovarian cystectomy or salpingo-oophorectomy for endometriosis at Prentice Women's Hospital. Eutopic endometrium was obtained from patients without endometriosis undergoing hysterectomy for benign indications. All patients reported regular menses. Patients who had undergone hormonal therapy or had been pregnant within the 3 months before surgery were excluded. All samples were histologically confirmed by the pathologist.
Stromal cell isolation and cell culture
Stromal cells were isolated as previously described (23). Endometriotic stromal cells were given the designation OSIS, and eutopic endometrial stromal cells from disease-free patients were designated ESC. Cells were cultured in DMEM/F12 (1:1) (Invitrogen) with 10% fetal bovine serum (FBS) and penicillin (100 U/mL) with streptomycin (100 U/mL) at 37°C in a humidified atmosphere with 5% CO2. Culture medium was changed every 3 days. Cells were used for experiments until passage 7, at which point they were discarded. Before treatment with hormones or inhibitors, cells were washed 3 times with PBS and the medium changed to phenol red-free DMEM/F12 (1:1) with 2% stripped FBS and penicillin (100 U/mL) with streptomycin (100 U/mL) for 24 hours. The medium was replaced and cells pretreated with a designated concentration of MK-2206 (Merck), U0126 (Promega), or dimethylsulfoxide (vehicle) for 2 hours followed by incubation with 100nM promegestrone (R5020; PerkinElmer Life Sciences) or ethanol (vehicle) for 24 hours. Additional studies with ESC were done using 1nM estradiol (E2) along with R5020 and MK-2206 treatments.
Immunoblotting
OSIS and ESC were washed with ice-cold PBS and lysed in mammalian protein extraction reagent (Thermo Scientific) supplemented with protease and phosphatase inhibitors (Sigma). Cytoplasmic and nuclear extracts were isolated with the NE-PER nuclear protein extraction kit (Thermo Scientific). Protein concentrations were determined with the micro-BCA protein assay kit (Thermo Scientific). Equal amounts of protein (at least 30 μg) were resolved on a 10% polyacrylamide gel and transferred to a polyvinyl difluoride membrane. The membranes were blocked with 5% BSA in Tris-buffered saline with 0.1% Tween 20 for 1 hour at room temperature and then incubated overnight at 4°C in 1% BSA with antibodies against PR (Dako), phospho-AKT (Ser473), total AKT, phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204), p44/42 MAPK (ERK1/2), cleaved caspase-3 (Cell Signaling), histone deacetylase 1, α-tubulin (Millipore), or β-actin (Sigma). The blots were washed with Tris-buffered saline with 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated goat antirabbit or antimouse secondary antibodies (1:10 000) for 1 hour and then detected with a chemiluminescent detection kit (Thermo Scientific).
RNA extraction and quantitative real-time RT-PCR
Total RNA from OSIS or ESC was isolated with TRIzol reagent (Sigma) using the manufacturer's protocol. Genomic DNA contamination was minimized with a DNA-free RNA kit (Zymo Research). One microgram of RNA was reverse transcribed in a final volume of 20 or 50 μL to generate cDNA with the Superscript III first-strand synthesis system (Invitrogen). Quantitative real-time PCR was performed with the ABI 7000 sequence detection system and the ABI Power Sybr Green gene expression detection system (Applied Biosystems Inc) to quantify PRB and total PR (PRA and PRB) mRNA. The 18S rRNA was used as an internal control. All PCR were run for 40 cycles (95°C for 15 seconds, 60°C for 1 minute) after a 10-minute incubation at 95°C. The fold change in expression of each gene was calculated with the change in cycle threshold value method (ΔΔCt) (24).
Small interfering RNA knockdown
OSIS cells were cultured to 50% confluence and then transfected with a nontargeting negative control small interfering RNA (siRNA) (Dharmacon) or siRNAs against AKT1, AKT2, and AKT3 (Dharmacon) at a final concentration of 35nM with Lipofectamine RNAiMAX (Invitrogen) in Opti-MEM reduced serum medium (Invitrogen). A no-siRNA control was also added to demonstrate no significant effect of siRNA to AKT levels (Supplemental Figure 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org). Cells were incubated for 6 hours, washed with PBS, and incubated for 48 hours in phenol red-free DMEM/F12 (1:1) with 2% stripped FBS and penicillin (100 U/mL) with streptomycin (100 U/mL). Transfected cells were collected and lysed for immunoblotting.
Cell viability and proliferation
OSIS or ESC were plated in 96-well plates at 5 × 104 cells per well and allowed to attach overnight. Cells were then washed with PBS and incubated overnight in phenol red-free DMEM/F12 (1:1) with 2% stripped FBS and penicillin (100 U/mL) with streptomycin (100 U/mL). Media were again replaced and cells were pretreated for 2 hours with MK-2206, U0126, or vehicle followed by a 24-hour incubation with 100nM R5020 or vehicle. The WST-1 cell proliferation assay kit (Clontech) was used to measure cell viability according to the manufacturer's protocol. The absorbance of the wells was measured using a microtiter plate rater (Synergy HT model no. SIAFRID; Bio-Tek) at wavelengths of 420 and 605 nm. Fold change relative to vehicle control was calculated. The 5-bromo-2′-deoxyuridine (BrdU) incorporation immunoassay (Roche Applied Sciences) was used to measure cell proliferation according to the manufacturer's protocol. The absorbance of the wells was measured at wavelengths of 370 and 492 nm. Fold change relative to vehicle control was calculated.
Tissue culture for grafting
Human endometriotic cyst walls were obtained on day 1 and dissected into 1-mm3 fragments. Tissue fragments were cultured in DMEM/F12 (1:1) (Invitrogen) with 2% stripped FBS, 10nM E2, and penicillin (100 U/mL) with streptomycin (100 U/mL) at 37°C in a humidified atmosphere with 5% CO2 for 24 hours before injection into mice.
Xenograft mouse model
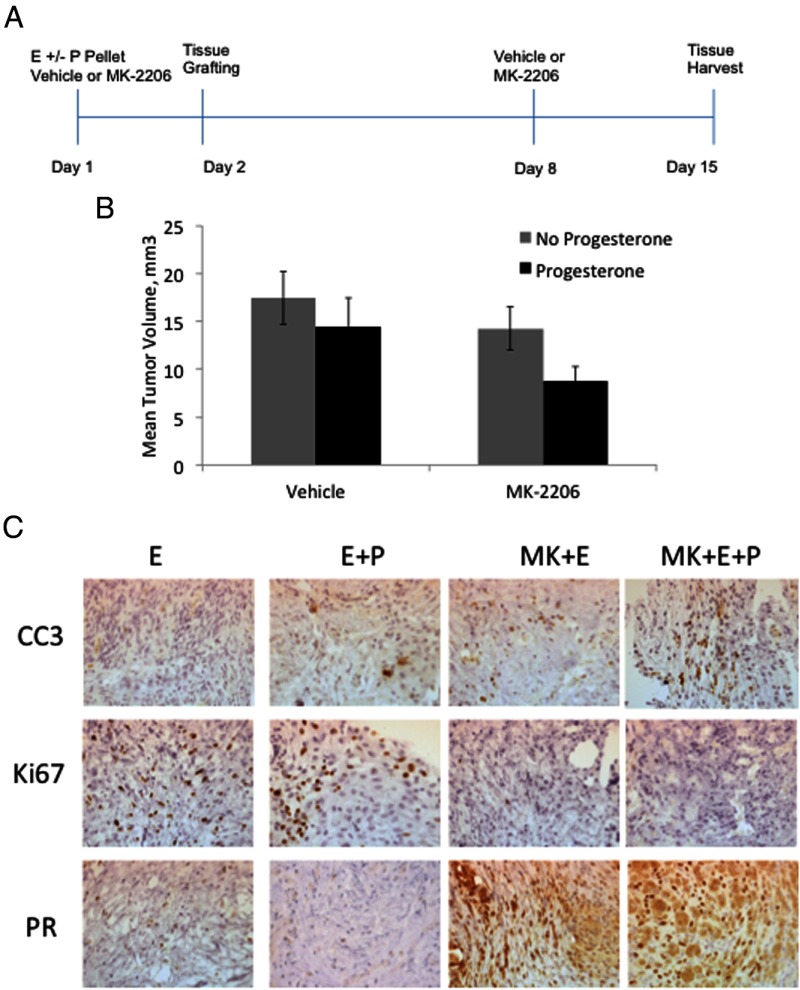
A xenograft mouse model was adapted from the model previously described by Bruner-Tran et al (25–27). All animal experiments were approved by Northwestern University Animal Care Committee. Briefly, 5-week-old CD-1 nude mice were obtained from Charles River Laboratories and surgically ovariectomized. On day 1, E2 pellets (1.7 mg/60 days sustained release, or 28.3 μg/d) were implanted sc in all mice. Additionally, half of the mice received sc progesterone pellets (50 mg/60 days sustained release, or 833 μg/d). Mice were treated with either MK-2206 (360 mg/kg) or vehicle (0.3 mL 30% Captisol) via oral gavage. On day 2, mice were injected sc with human endometriotic tissue fragments (10 fragments suspended in 500 μL PBS injected in each flank). Mice were subsequently treated with either 360 mg/kg MK-2206 or vehicle by oral gavage on day 8. Mice were killed and tissue pellets harvested on day 15. Tumor volume was calculated with the following formula: volume = 0.5 × length × width2.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde for 24 hours and embedded in paraffin. Embedded tissues were cut into 4-μm sections and then mounted on glass slides. Tissue sections were processed for immunohistochemistry at the Mouse Histology and Phenotyping Core at Northwestern University. Immunohistochemical staining for PR (Dako), Ki67 (Dako), and cleaved caspase-3 (Cell Signaling) was performed. Incubation with a secondary biotinylated antibody (Jackson ImmunoResearch) was done, followed by staining with the ABC kit (Vector Laboratories).
Statistical analysis
Statistical analyses were performed with SPSS version 19 (IBM Corporation). Quantitative real-time RT-PCR data were analyzed with randomized block ANOVA. WST-1, BrdU, and tumor volume data were analyzed with factorial ANOVA. If no statistically significant interaction was present, main effects were analyzed. In the case of a statistically significant main effect, a one-sided Dunnett's test for multiple comparisons was then used to compare each treatment group to the vehicle group. A P value < .05 was considered statistically significant.
Results
Inhibition of AKT or MEK1/2 increases PR protein in OSIS but not ESC
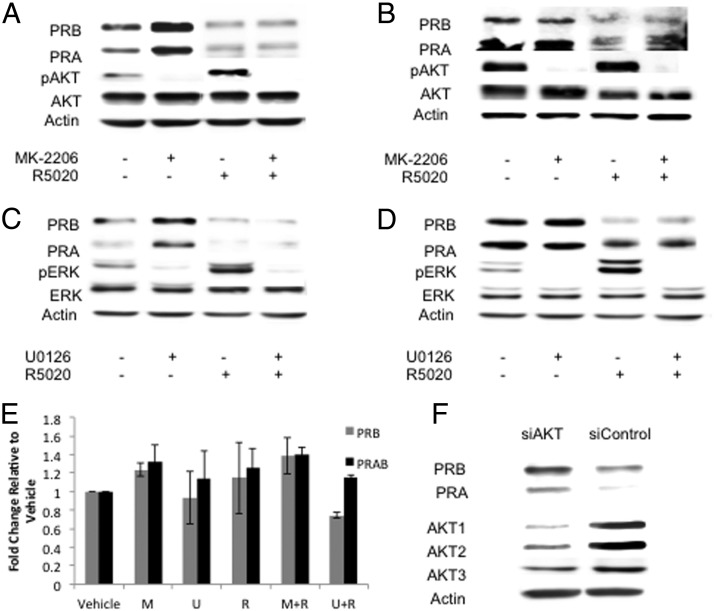
It has been shown that the AKT and MAPK pathways are overactive in endometriosis, both in the ectopic and eutopic endometrial tissue (15–19). To determine whether the increased activation of AKT or MEK1/2 influences levels of PR protein, OSIS and ESC were pretreated with 100nM MK-2206, 10μM U0126, or vehicle for 2 hours. They were then treated with 100nM R5020 or vehicle for 24 hours. Levels of PRA and PRB protein increased after treatment with MK-2206 in OSIS in the absence of R5020. In the presence of R5020, PR levels decreased overall with no difference after MK-2206 treatment (Figure 1A). ESC PR protein levels did not change after MK-2206 treatment in the presence or absence of R5020 (Figure 1B). After treatment with U0126, OSIS PRA and PRB protein levels increased; however, in the presence of R5020, PR levels were low and did not change after U0126 treatment (Figure 1C). ESC PR protein levels did not change after treatment with U0126 in the presence or absence of R5020 (Figure 1D).
Figure 1.
Inhibition of AKT or MEK1/2 increases PR protein in OSIS but not ESC. Stromal cells from disease-free eutopic endometrium (ESC) or ovarian endometriomas (OSIS) were pretreated with either 100nM MK-2206 or 10μM U0126 for 2 hours. They were then incubated in the presence of 100nM R5020 or vehicle for 24 hours and A–D, Western blots for PR were performed. PR protein levels for OSIS after MK-2206 ± R5020 (A), ESC after MK-2206 ± R5020 (B), OSIS after U0126 ± R5020 (C), and ESC after U0126 ± R5020 (D). Western blots are representative of experimental replicates from 5 patients. E, PRB and PRAB mRNA levels were measured after treatment with MK-2206 or U0126 in the presence or absence of R5020. Data are expressed as fold change relative to vehicle and are the mean ± SEM of 3 experimental replicates. F, AKT knockdown using siRNA for AKT1, AKT2, and AKT3 (siAKT) or nonspecific siRNA (siControl) was performed and levels of PR protein measured. Data are representative of experimental replicates from 3 patients.
To determine whether AKT and MAPK regulate PR at the transcriptional level, quantitative real-time RT-PCR was performed to analyze PRB and PRAB (total PR) mRNA. As shown in Figure 1E, PRB and PRAB mRNA levels did not significantly change after treatment with MK-2206 or U0126 (P = .384 for PRB; P = .275 for PRAB). To provide additional evidence for the role of AKT in PR regulation, we used siRNA to knock down endogenous expression of AKT1, AKT2, and AKT3 in OSIS. Cells were lysed and Western blot performed for PR. Both PRA and PRB protein levels increased after siRNA knockdown of AKT (Figure 1F).
It has been reported that endometriotic stromal cells in culture synthesize and secrete E2 (28), which could contribute to the differential regulation of PR upon inhibition of AKT and MAPK in OSIS and ESC. ESC were treated with 1nM E2 with or without MK-2206 and R5020. In response to E2 only, there was a subtle increase in PR protein levels that was increased further when cotreated with MK-2206 (Supplemental Figure 2). Addition of R5020 decreased levels of PR. These data suggest that the secretion of E2 by OSIS may be involved in the upregulation of PR with MK-2206.
Inhibition of AKT or MEK1/2 increases nuclear PR protein in OSIS but not ESC
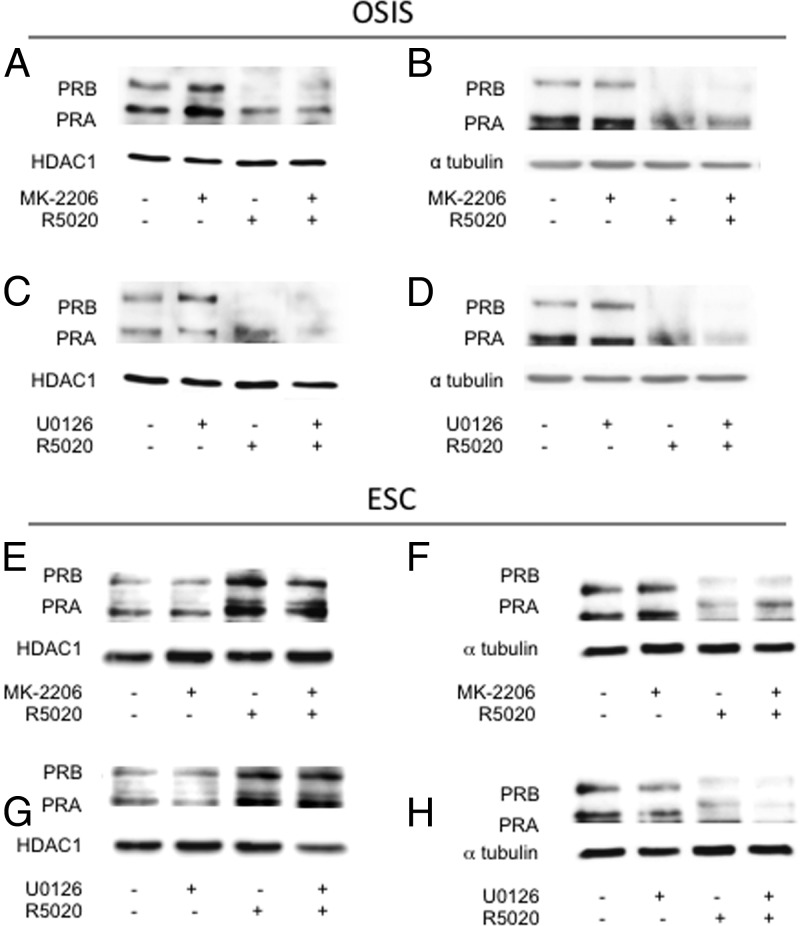
PR localization in response to inhibition of AKT or MEK1/2 was analyzed in OSIS and ESC in the presence and absence of R5020. Nuclear PRA and PRB protein levels increased in OSIS after treatment with MK-2206 (Figure 2A) or U0126 (Figure 2C) in the absence of R5020, with little change of PR in the cytoplasm (Figure 2, B and D). In ESC, levels of PR in the nuclear and cytoplasmic fractions remained unchanged after MK-2206 or U0126 treatment (Figure 2, E–H).
Figure 2.
Inhibition of AKT and MEK1/2 increases nuclear PR protein in OSIS. OSIS and ESC were pretreated with 100nM MK-2206, 10μM U0126, or vehicle for 2 hours. They were then incubated with 100nM R5020 or vehicle for 24 hours. Nuclear and cytoplasmic extracts were isolated and Western blots for PR were performed: A, nuclear fractions from OSIS cells treated with MK-2206 ± R5020; B, cytoplasmic fractions from OSIS treated with MK-2206 ± R5020; C, nuclear fractions from OSIS treated with U01206 ± R5020; D, cytoplasmic fractions from OSIS treated with U0126 ± R5020; E, nuclear fractions from ESC treated with MK-2206 ± R5020; F, cytoplasmic fractions from ESC treated with MK-2206 ± R5020; G, nuclear fractions from ESC treated with U01206 ± R5020; H, cytoplasmic fractions from ESC treated with U0126 ± R5020. Data are representative of experimental replicates from 3 patients.
MK-2206 decreases cell proliferation of OSIS but not ESC
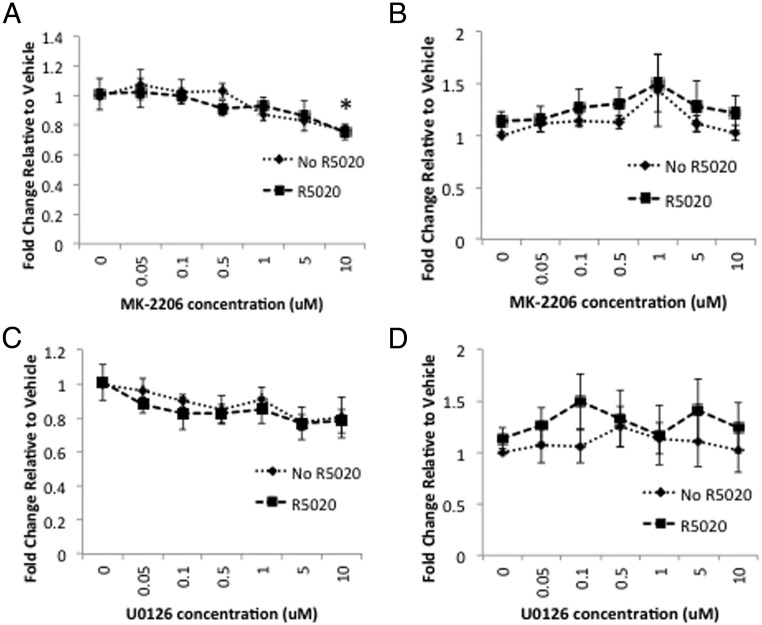
OSIS and ESC proliferation was assessed using the BrdU assay in response to various concentrations of MK-2206 or U0126 in combination with R5020. In OSIS, factorial ANOVA demonstrated no significant interaction between MK-2206 and R5020 (P = .898) (Figure 3A). A significant main effect on proliferation was demonstrated with MK-2206 (P = .002), but there was no significant main effect of R5020 (P = .658). One-sided Dunnett's test for multiple comparisons demonstrated a trend toward decreased proliferation with 5μM MK-2206 (P = .058) and significantly decreased proliferation at 10μM (P = .003). In ESC, factorial ANOVA demonstrated no significant interaction between MK-2206 and R5020 (P = .999) and no significant main effect of either MK-2206 (P = .336) or R5020 (P = .153) (Figure 3B). In response to U0126, there was no significant interaction between U0126 and R5020 (P = .994) in OSIS. There was a trend toward decreased proliferation with U0126 (P = .078) but no significant main effect of R5020 (P = .370) (Figure 3C). In ESC, there was no significant interaction between U0126 and R5020 (P = .975) and no significant main effect of either U0126 (P = .926) or R5020 (P = .092) (Figure 3D). Thus, these data demonstrate significantly decreased proliferation with MK-2206 and a trend toward decreased proliferation with U0126 in OSIS. R5020 did not influence proliferation.
Figure 3.
A–D, MK-2206 decreases proliferation of OSIS but not ESC. OSIS (A and C) or ESC (B and D) were treated with increasing concentrations of MK-2206 (A and B) or U0126 (C and D) with or without 100nM R5020 and proliferation measured using the BrdU assay. Data are expressed as fold change relative to vehicle and are the mean ± SEM of experimental replicates from 3 patients. *, P < .05 by one-sided Dunnett's test for multiple comparisons.
MK-2206 and R5020 decrease viability of OSIS and ESC
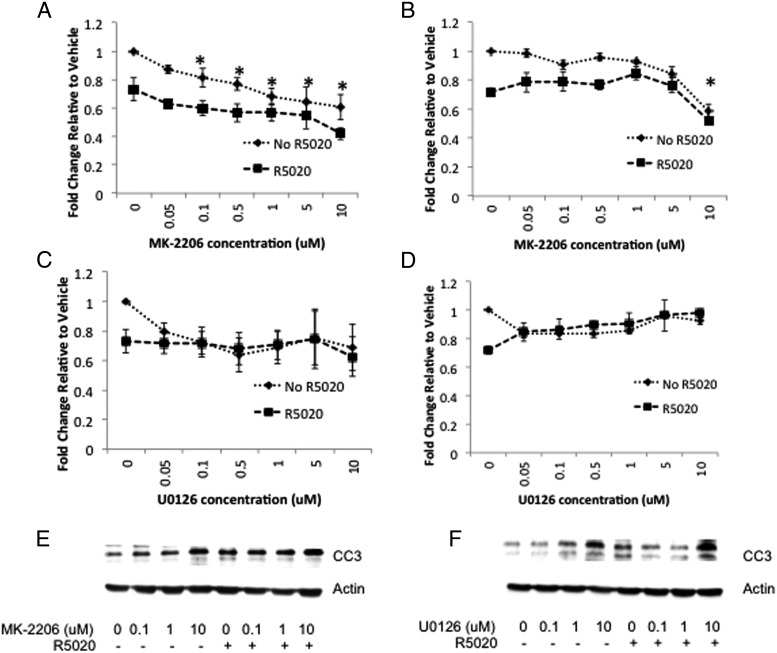
The effects of inhibiting AKT and MEK1/2 in the presence of R5020 on OSIS and ESC viability were investigated. Statistical analysis of data from OSIS treated with MK-2206 revealed no significant interaction between MK-2206 and R5020 (P = .817) (Figure 4A). Significant main effects were seen for MK-2206 (P < .0001) and R5020 (P < .0001). One-sided Dunnett's test for multiple comparisons revealed a statistically significant decrease in viability with concentrations of MK-2206 as low as 100nM compared with vehicle (P = .04) (Figure 4A). In ESC, factorial ANOVA revealed no significant interaction between MK-2206 and R5020 (P = .121). Significant main effects were seen for MK-2206 (P < .0001) and R5020 (P < .0001). One-sided Dunnett's test for multiple comparisons determined that the decrease in viability with MK-2206 compared with vehicle was statistically significant only at the highest concentration, 10μM (P < .0001) (Figure 4B). Interestingly, the response to U0126 was different. There was no statistically significant interaction between U0126 and R5020 (P = .664) or main effect of U0126 (P = .717) or R5020 (P = .171) on viability (Figure 4C). Similarly in ESC, no significant main effects of U0126 (P = .065) or R5020 (P = .716) on viability occurred.
Figure 4.
A–D, MK-2206 and R5020 independently decrease OSIS and ESC viability. OSIS (A and C) or ESC (B and D) were treated with increasing concentrations of MK-2206 (A and B) or U0126 (C and D) with or without 100nM R5020, and cell viability was measured using the WST assay. Data are expressed as fold change relative to vehicle and are the mean ± SEM of experimental replicates from 3 patients. *, P < .05 by one-sided Dunnett's test for multiple comparisons. E and F, Cleaved caspase-3 levels were measured by Western blot in OSIS after MK-2206 (E) or U0126 (F) in the presence or absence of R5020. Data are representative of experimental replicates from 3 patients.
MK-2206, U0126, and R5020 increase cleaved caspase-3 in OSIS
To determine whether MK-2206 or U0126 in combination with R5020 induced apoptosis, levels of cleaved caspase-3 in OSIS cells were measured by Western blot. In the absence of R5020, treatment with MK-2206 resulted in a dose-dependent increase in cleaved caspase-3 in OSIS. The increase became evident at 100nM MK-2206, and there was an additive increase in cleaved caspase-3 with R5020 (Figure 4E). In the absence of R5020, treatment with U0126 resulted in an increase in cleaved caspase-3 that was evident at 1μM. In the presence of R5020, the increase in cleaved caspase-3 was evident only at the highest concentration of U0126 (10μM). Furthermore, R5020 treatment in the presence of U0126 did not result in an additive increase in cleaved caspase-3 (Figure 4F). In contrast, there were very low levels of cleaved caspase-3 in ESC that did not change with MK-2206 (Supplemental Figure 3A) or U0126 (Supplemental Figure 3B) treatment, in the presence or absence of R5020.
MK-2206 and R5020 decrease volume of endometriosis grafts
To test the effects of MK-2206 and R5020 on human endometriotic tissues ex vivo, tissue fragments from ovarian endometriomas were injected sc into nude ovariectomized mice, as described by Bruner-Tran et al (25–27). Each mouse received 2 injections of tissue, 1 in each flank. The mice were assigned to 1 of 4 treatment arms: E2 (E), E + progesterone (P), E+MK-2206, E+P+MK-2206 (Figure 5A). E2 was included in all treatment arms for establishment of endometriotic lesions, as previously described (25). Two weeks later, mice were killed and graft volumes obtained. Factorial ANOVA demonstrated no significant interaction between MK-2206 and progesterone (P=0.628). Trends toward decreased tumor volume were noted with MK-2206 (P = .077) and progesterone (P = .087) (Figure 5B). Immunohistochemical analysis of the tissue grafts showed that Ki67 levels were highest with E or E+P, whereas treatment with MK-2206 decreased levels of Ki67 (Figure 5C). Levels of cleaved caspase-3 (CC3) were very low in E and E+P-treated grafts, whereas MK-2206 increased CC3 levels, especially in the presence of P. Finally, levels of PR were very low in E and E+P-treated grafts, whereas PR levels increased substantially in MK-treated grafts. Both nuclear and cytoplasmic levels of PR increased.
Figure 5.
MK-2206 and R5020 decrease size of endometriosis grafts. A, Estradiol plus progesterone (E+P) hormone pellets were implanted sc in nude mice and then treated orally with 360 mg/kg MK-2206 (MK) or vehicle. The next day, endometriosis tissue fragments were injected sc into both flanks of the mice. MK-2206 was given orally once a week for 2 weeks. Graft volumes were measured, and immunohistochemical staining was done on the grafts. B, Independent trends toward decreased mean tumor volume were noted with MK-2206 and progesterone treatment. Data are expressed as mean ± SEM from 78 lesions in 39 mice from 4 separate experiments. C, Immunohistochemical staining for CC3, Ki67, and PR was performed. Data are representative of experimental replicates from 4 patients.
Discussion
In this study, we have demonstrated that AKT and, to a limited extent, MEK1/2 promote proliferation and survival of endometriotic cells and tissue. In addition, we have shown that these pathways influence PR protein levels and localization and could thereby be one explanation as to why PR levels are low in endometriosis, promoting a progesterone-resistant phenotype.
The AKT and MAPK pathways are hyperactivated in endometriosis (15–19) and thought to contribute to the pathogenesis of the disease. Inhibition of AKT using MK-2206 decreased proliferation, decreased viability, and increased apoptosis, whereas U0126 had a less dramatic effect on proliferation and apoptosis of OSIS. The progestin R5020 also decreased cell viability and increased apoptosis in OSIS. Although the role of progestins in differentiation of the stromal cells is well established (17, 29, 30), its role in mediating stromal cell death is unclear. Rather, the withdrawal of hormones after prolonged treatment initiates the apoptotic pathway mediated by Forkhead box O1 (31). Studies that demonstrate progestin-mediated apoptosis in the endometrium or ectopic lesions show that apoptosis occurs in the glandular epithelial cells and not the stroma (32, 33). During the luteal phase of the menstrual cycle, there is very little apoptosis in the stroma (34). Thus, the apoptosis observed with 100nM R5020 in OSIS is intriguing, and the mechanisms are not clear.
We initially hypothesized that due to the increase in PR protein upon inhibition of AKT and MEK1/2, there would be a synergistic effect on proliferation and viability with progestin and MK-2206 or U0126. R5020 did not affect proliferation, either independently or synergistically with MK-2206 or U0126. However, R5020 independently decreased OSIS cell viability in the presence of MK-2206. This contrasted to OSIS cells treated with U0126, where no decrease was observed with R5020. Mechanistically, this could be directly linked to the posttranslational modifications of PR in response to active signaling pathways. Lange and colleagues (35, 36) have previously demonstrated that phosphorylation of PR on Ser294 by MAPKs results in nuclear export, PR targeting to the ubiquitination-proteasome pathway, and subsequent degradation. We are currently elucidating posttranslational modifications of PR by AKT that could reveal differential regulation of PR compared with MAPK.
Interestingly, we observed an increase in phospho-AKT and phospho-ERK with R5020 treatment in both endometrial and endometriotic stromal cells for reasons that are unclear. Previously, we demonstrated that R5020 can rapidly activate AKT in a nongenomic manner in leiomyoma cells (37). Others have shown that the p85 subunit of PI3K can interact with PR in a cell-free glutathione S-transferase pulldown assay (38). The nongenomic role of progesterone in the activation of Src kinase has been well characterized in breast cancer cells (38). This has been shown to occur through physical interaction of the polyproline sequence motif in the N-terminal domain of PR to the SH3 domain of Src, which in turn activates the Src/Ras-Raf/Mek-1/MAPK signaling pathway. The mechanism by which phospho-AKT and phospho-ERK increase with R5020 could involve similar mechanisms in endometrial stromal cells but would need further investigation. Nevertheless, inhibition of AKT and ERK would prevent this effect of progestins.
In our study, the presence of R5020 downregulated PR whether cells were treated with MK-2206 or U0126, suggesting that the downregulation of PR involves mechanisms independent of AKT or MEK1/2 in OSIS. Alternatively, it is also possible that our in vitro findings were influenced by the lack of E2 in our cell culture system. First, E2 upregulates PR gene expression and promotes progesterone action in normal endometrium (39–41); thus, the lack of transcriptional induction of PR coupled with downregulation by R5020 could have primarily contributed to the downregulation observed in the cells. In support of this, there was no significant difference in PR protein in the tissue grafts between E- and E+P-treated mice, albeit the levels were low. In addition, we observed a sustained upregulation of PR when AKT was inhibited in the E+P+MK-2206-treated grafts (Figure 5C). Furthermore, E2 could have contributed to increased PR levels in OSIS treated with MK-2206, because endometriotic stromal cells have been shown to synthesize and secrete E2, whereas ESC do not (28). Thus, local E2 production may be important. In support of this, ESC treated with exogenous E2 in combination with MK-2206 did indeed increase PR levels (Supplemental Figure 2).
Previously, we reported that inhibition of AKT in the Ishikawa endometrial cancer cell line increased PR levels (22). In addition, the combination of MK-2206 and progesterone decreased tumor volume to a greater extent than each treatment alone. In our endometriosis tissue grafts, volumes of the grafts were the smallest when treated with MK-2206 and progesterone, consistent with our previous findings; however, these data did not reach statistical significance (P = .087). An increase in replicates may be required to achieve statistical significance. Nevertheless, an increase in cleaved caspase-3 was observed as well as decreased Ki67 with MK-2206 and progesterone.
Another study demonstrated that the PI3K/AKT pathway promotes progesterone resistance in endometrial cancer (21). The authors showed that medroxyprogesterone acetate and the PI3K inhibitor LY294002 decreased endometrial cancer cell growth. Thus, there is increasing evidence that the hyperactivated AKT pathway promotes progesterone resistance, which is prevalent in gynecopathologies such as endometriosis.
To our knowledge, this is the first report to demonstrate that the AKT and MAPK pathways may contribute to PR deficiency in endometriosis. Our data show that inhibiting these pathways increases PR levels, decreases proliferation, and increases apoptosis of endometriotic stromal cells and tissues. It is possible that inhibiting these pathways in women with the disease might similarly improve progesterone response and decrease survival of endometriotic stromal cells, resulting in decreased pain and improved fertility outcomes. The specificity with which these inhibitors act on endometriotic cells compared with normal stromal cells suggests that cells with hyperactivated AKT are more responsive to the inhibitors. In the normal context, these pathways are tightly controlled at many levels, such as transient stimulation by growth factors, regulation by phosphatases, and turnover of kinases. However, in endometriotic stromal cells, the AKT and MAPK pathways are hyperactivated. Thus, the efficacy of the inhibitors could be fine tuned in the future to specifically target those cells exhibiting high activation of these pathways, with minimal effects on normal, growing cells. Phase II clinical trials are currently investigating the use of MK-2206 and several MEK1/2 inhibitors for the treatment of various cancers. Given the current lack of an effective treatment for endometriosis, our study suggests that further research on the potential use of AKT and MEK1/2 inhibitors for endometriosis treatment is warranted.
Acknowledgments
We are grateful to Dr. Kaylon Bruner-Tran for providing expertise in the sc grafting of endometriosis tissues, Xunqin Yin for helping with tissue collection, and the Mouse Histology and Phenotyping Core facilities at the Robert Lurie Cancer Center at Northwestern University.
This work was supported by NIHR01HD044715 and Friends of Prentice Grants Initiative. The MK-2206 was generously provided by Merck Sharp and Dohme Corp. and the National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-bromo-2′-deoxyuridine
- CC3
- cleaved caspase-3
- E2
- estradiol
- ESC
- eutopic endometrial stromal cells
- FBS
- fetal bovine serum
- OSIS
- endometriotic stromal cells
- PI3K
- phosphatidylinositol 3-kinase
- PR
- progesterone receptor
- siRNA
- small interfering RNA.
References
- 1. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279 [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein DP, deCholnoky C, Emans SJ, Leventhal JM. Laparoscopy in the diagnosis and management of pelvic pain in adolescents. J Reprod Med. 1980;24:251–256 [PubMed] [Google Scholar]
- 4. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258 [DOI] [PubMed] [Google Scholar]
- 5. Kauppila A, Rönnberg L. Naproxen sodium in dysmenorrhea secondary to endometriosis. Obstet Gynecol. 1985;65:379–383 [PubMed] [Google Scholar]
- 6. Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update. 2006;12:179–189 [DOI] [PubMed] [Google Scholar]
- 7. Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril. 1993;60:75–79 [PubMed] [Google Scholar]
- 8. Bianchi S, Busacca M, Agnoli B, Candiani M, Calia C, Vignali M. Effects of 3 month therapy with danazol after laparoscopic surgery for stage III/IV endometriosis: a randomized study. Hum Reprod. 1999;14:1335–1337 [DOI] [PubMed] [Google Scholar]
- 9. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis and treatment. Drugs. 2001;61:1735–1750 [DOI] [PubMed] [Google Scholar]
- 10. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881 [DOI] [PubMed] [Google Scholar]
- 11. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103 [DOI] [PubMed] [Google Scholar]
- 12. Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17beta-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol. 2007;196:391.e1–e7; discussion 391.e7–e8 [DOI] [PubMed] [Google Scholar]
- 13. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 14. Aghajanova L, Tatsumi K, Horcajadas JA, et al. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod. 2011;84:801–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFκB, and β-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23:1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M. 17βE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFκB-dependent pathway. Mol Cell Endocrinol. 2010;317:31–43 [DOI] [PubMed] [Google Scholar]
- 17. Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab. 2012;97:E35–E43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cinar O, Seval Y, Uz YH, et al. Differential regulation of Akt phosphorylation in endometriosis. Reprod Biomed Online. 2009;19:864–871 [DOI] [PubMed] [Google Scholar]
- 19. Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology. 2009;150:4701–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu C, Zhang Z, Yu Y, et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci. 2011;102:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pant A, Lee II, Lu Z, Rueda BR, Schink J, Kim JJ. Inhibition of AKT with the orally active allosteric AKT inhibitor, MK-2206, sensitizes endometrial cancer cells to progestin. PLoS One. 2012;7:e41593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649 [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–4791 [DOI] [PubMed] [Google Scholar]
- 27. Bruner-Tran KL, Zhang Z, Eisenberg E, Winneker RC, Osteen KG. Down-regulation of endometrial matrix metalloproteinase-3 and -7 expression in vitro and therapeutic regression of experimental endometriosis in vivo by a novel nonsteroidal progesterone receptor agonist, tanaproget. J Clin Endocrinol Metab. 2006;91:1554–1560 [DOI] [PubMed] [Google Scholar]
- 28. Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453 [DOI] [PubMed] [Google Scholar]
- 30. Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372 [DOI] [PubMed] [Google Scholar]
- 31. Labied S, Kajihara T, Madureira PA, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44 [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez GC, Rimel BJ, Watkin W, et al. Progestin treatment induces apoptosis and modulates transforming growth factor-β in the uterine endometrium. Cancer Epidemiol Biomarkers Prev. 2008;17:578–584 [DOI] [PubMed] [Google Scholar]
- 33. Meresman GF, Augé L, Barañao RI, Lombardi E, Tesone M, Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertility and sterility. 2002;77:1141–1147 [DOI] [PubMed] [Google Scholar]
- 34. Jones RK, Searle RF, Stewart JA, Turner S, Bulmer JN. Apoptosis, bcl-2 expression, and proliferative activity in human endometrial stroma and endometrial granulated lymphocytes. Biol Reprod. 1998;58:995–1002 [DOI] [PubMed] [Google Scholar]
- 35. Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–157 [DOI] [PubMed] [Google Scholar]
- 36. Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642 [DOI] [PubMed] [Google Scholar]
- 37. Hoekstra AV, Sefton EC, Berry E, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boonyaratanakornkit V, Scott MP, Ribon V, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280 [DOI] [PubMed] [Google Scholar]
- 39. Schultz JR, Petz LN, Nardulli AM. Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol. 2003;201:165–175 [DOI] [PubMed] [Google Scholar]
- 40. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-α, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902 [DOI] [PubMed] [Google Scholar]