Abstract
Purpose
The lymphatic system plays crucial roles in tissue fluid balance, trafficking of immune cells, and the uptake of dietary lipid from the intestine. Given these roles there has been an interest in targeting lymphatics through oral lipid-based formulations or intradermal delivery of drug carrier systems. However the mechanisms regulating lipid uptake by lymphatics remain unknown. Thus we sought to modify a previously developed in vitro model to investigate the role of ATP in lipid uptake into the lymphatics.
Methods
Lymphatic endothelial cells were cultured on a transwell membrane and the effective permeability to free fatty acid and Caco-2 cell-secreted lipid was calculated in the presence or absence of the ATP-inhibitor sodium azide. Results: ATP inhibition reduced Caco-2 cell-secreted lipid transport, but not dextran transport. FFA transport was ATP-dependent primarily during early periods of ATP-inhibition, while Caco-2 cell-secreted lipid transport was lowered at all time points studied. Furthermore, the transcellular component of transport was highly ATP-dependent, a mechanism not observed in fibroblasts, suggesting these mechanisms are unique to lymphatics. Total transport of Caco-2 cell-secreted lipid was dose-dependently reduced by ATP inhibition, and transcellular lipoprotein transport was completely attenuated.
Conclusion
The transport of lipid across the lymphatic endothelium as demonstrated with this in vitro model occurs in part by an ATP-dependent, transcellular route independent of passive permeability. It remains to be determined the extent that this mechanism exists in vivo and future work should be directed in this area.
Introduction
The lymphatic system, which serves almost all tissues throughout the body, plays a crucial role in maintaining fluid balance. Given that the lymphatics essentially provide a route for all “large” objects to return to the blood circulation, it is not surprising that they play important roles in immune cell trafficking (1), and the uptake of lipid as well (2). In the periphery, lymphatics drain the adipose tissue bed, providing a route for the removal of adipokines and lipoproteins (3-5). In the intestine most dietary lipid is packaged into chylomicrons by enterocytes, after which they are taken up by intestinal lymphatics, referred to as lacteals, and transported to the blood (6,7). Lymphatics are thought to achieve the role of initial uptake through the specialized morphological features of the initial lymphatic capillaries. Initial vessels are anchored to the tissue space with filaments that, in coordination with the unique characteristics of the initial lymphatic cell-cell junctions, allow large proteins and cells to enter into the vessel, while preventing their backflow out of the vessel (8,9). Whether there are differential mechanisms for the uptake of lipid by lymphatics remains unknown, however recent reports suggest that lymphatics remove cholesterol from the periphery by scavenger receptor class B type I (SR-B1) mediated transport of HDL (10). The extent that similar mechanisms exist in the intestine, where the lipid load on the lymphatics is highest, is less known (6).
Given their versatile role in the trafficking of large molecules to the vasculature, there has been a growing interest in using the lymphatics as a target for delivering a therapeutic payload. These approaches can broadly be divided into two categories: those that target peripheral lymphatic uptake by delivery into the interstitium (usually intradermal) (11-16), and those that target intestinal lymphatics upon oral administration (17-20). Interestingly, almost every approach to date treats lymphatic uptake as a relatively passive process when designing the delivery strategy. For example, there has been significant emphasis when delivering to the interstitium to either administer particles in the right size range so that they are passively swept into lymphatics by interstitial flow (11) or to administer the payload in such a way that immune cells take it up and carry it into the lymphatics (14). Approaches with oral delivery to lymphatics have been fundamentally similar to that of the interstitium, targeting either immune cells residing in gut lymphoid tissue (21) or targeting the incorporation of the drug into chylomicrons, which are then taken up exclusively by lymphatics (17,22). Recent evidence, however, suggests that lymphatic endothelial cells (LECs) themselves possess active mechanisms for facilitating uptake. For example LECs have unique mechanisms to enhance immune trafficking to lymphatics (23) and entry into lymphatics (24), to assist immune cell migration once inside the lymphatic (25), and to modulate actual lymphatic flow (26). Thus drug delivery approaches that exploit these and other mechanisms of active lymphatic uptake could substantially improve the efficacy of lymphatic targeting approaches.
While there is a growing appreciation for these active lymphatic mechanisms in immune cell trafficking, very little direct evidence is available of similar mechanisms in lymphatic lipid transport. Data from transgenic mouse models have highlighted the importance of proper lymphatic structure and function in dietary lipid uptake and transport, and these studies have begun to elucidate genes essential for a functional lymphatic system particularly as it pertains to lipid transport. For example, mice heterozygous for the gene Prox1, known to be highly expressed in the lymphatic system and pivotal in commitment of cells to the lymphatic lineage, develop a leaky lymphatic system that results in abnormal lipid uptake, spillage of lipoproteins into the abdominal cavity, adipogenesis, and obesity (27). In another study it was found that mice lacking the gene for pleiomorphic adenoma gene-like 2 (PlagL2), a gene expressed in enterocytes and thought to play a role in chylomicron production, developed fat deposits in the interstitial space surrounding the intestinal lymphatics. As a result these mice suffered from postnatal starvation due to poor lipid absorption, suggesting that PlagL2 plays a role in modifying chylomicrons to facilitate uptake by the lacteals, as the histological sections of the lacteals were morphologically the same in wild-type and knock-out (28). Clearly, a functional lymphatic endothelium is required for proper lipid uptake, however the extent that active vs. passive mechanisms are responsible for this process is unknown. Knowledge of these molecular details is crucial for the enhancement of lipid formulation strategies that target lymphatics.
The importance of the lymphatic system in the transport of lipids and lipoproteins has been established in humans and in mouse models. However, the mechanisms by which lipid gains access to the lymphatic system is still incompletely understood, and has historically been a matter of debate (6). Given the unique morphological features of the initial lymphatics of the interstitium discussed above, it was initially thought that lipoproteins, and specifically chylomicrons, gained access to the lacteals through the same specialized junctions that are known to be so important in initial lymphatics of other tissue spaces. Images obtained using transmission electron microscopy (TEM) dating back to the early 1960s initially seemed to confirm this idea, as the junctions and anchoring filaments were clearly observed in a section of fixed rat lacteal, and chylomicrons could be seen between the endothelial junctions (29-31). However, subsequent TEM studies in rat and guinea pig models showed that many of these specialized junctions were closed even during substantial lipid absorption, and that lipid vesicles could be observed within, rather than between the cells of the lymphatic endothelium, leading to the hypothesis that transcellular, and presumably active, transport plays a key role in transport of chylomicrons into the lacteals (32,33). However, strong conclusions from TEM morphology, particularly in this context, is difficult since the sample preparation methods could alter the local hydrodynamics essential for opening LEC junctions during lipid uptake.
Given the continued uncertainty behind the paracellular vs. transcellular lacteal uptake hypothesis (6), a tissue-engineered in vitro model of the intestinal lacteal was recently developed in which the permeability of lymphatic endothelial cells (LECs) to intestinal secreted lipoproteins could be quantified (34). This model was found to recapitulate many of the morphological and functional features of the intestinal lacteal. For example, LECs cultured in the transwell system show normal morphology and stain positive for VE-cadherin-positive cell-cell junctions and express the lymphatic marker LYVE-1. TEM images demonstrated similar transport differences to that in vivo. Finally, the in vitro model was functionally similar to its in vivo counterpart, possessing lymphatic-specific uptake of bodipy, polarized transport of lipid, exclusion of uptake of dextran and albumin (due to the barrier function provided by the Caco-2 cells), and enhanced uptake of bodipy that has been incorporated into Caco-2 cell-secreted lipid and lipoproteins.
Given the attractiveness of lipid formulations as a means for targeting lymphatics in both the intestine and the periphery, the objective of this study was to investigate the active component of lymphatic lipid transport and its relative importance on overall uptake using in vitro models of the peripheral lymphatics and the enterocyte-lacteal interface. We hypothesized that an active transport mechanism shuttles lipid across the lymphatic endothelium, so we quantified the transport kinetics of both free fatty acid (FFA) and lipoproteins across LECs after treatment with the ATP inhibitor NaN3 (35).
Materials and Methods
Cell culture
Human neonatal dermal lymphatic endothelial cells (LECs) were originally harvested as described previously (34). LECs were expanded in flasks coated for 1 hour with 50 μg/mL type I rat tail collagen (BD Biosciences, Bedford, MA) in 0.1% acetic acid and were cultured in EBM (Lonza, Walkersville, MD) supplemented with 20% FBS (Atlanta Biologicals, Lawrenceville, GA), 1% penicillin-streptomycin-amphotericin, 1% Glutamax (both from Life Technologies, Grand Island, NY), 25 mg/mL cyclic-AMP, and 1 mg/mL hydrocortisone acetate (both from Sigma, St. Louis, MO). Media was changed every 2-3 days and LECs were used for experiments at passages 9 and 10. Caco-2 cells (LGC Prochem, Middlesex, England) were expanded in high-glucose DMEM (Thermo Scientific, Logan, UT) supplemented with 20% FBS and 1% penicillin-streptomycinamphotericin. Media was changed every 2-3 days and Caco-2 cells that were seeded for production of lipoproteins were between passages 20 and 30. Human dermal fibroblasts (HDFs) were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin-amphotericin. Media was changed every 2-3 days and HDFs were used for experiments between passages 6 and 9.
Total free fatty acid transport
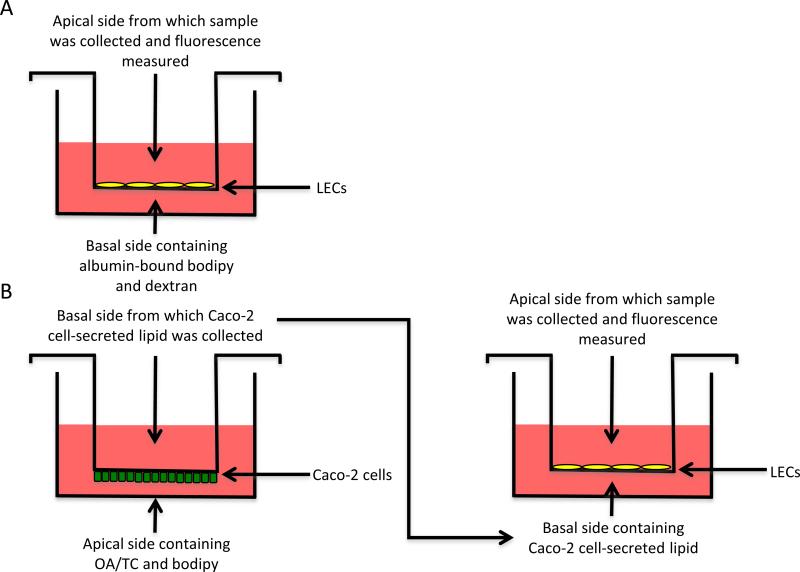
Transwell® permeable membrane supports with 0.4 μm pores (Corning Life Sciences, Corning, NY) were coated for 1 hour with 100 μg/mL type I rat tail collagen in PBS and LECs were seeded at a density of 100,000 cells/cm2 and cultured for 48 hours (Fig. 1A). For a subset of experiments, transwells were coated for 1 hour with 50 μg/mL collagen and HDFs were seeded at a density of 100,000 cells/cm2 and cultured for 48 hours. Prior to transport experiments, cells were incubated for 1 hour with 0, 1, 2, 5, or 10 mM NaN3 (EMD Biosciences, Darmstadt, Germany) in serum-free, phenol red-free DMEM (Lonza). The basal side of the monolayer was incubated with a fluorescent mix containing 140 μg/mL bovine serum albumin (Sigma), 1 μg/mL fluorescent BODIPY® FL C16, 5 μg/mL 3kDa Cascade Blue dextran (both from Life Technologies), and the appropriate concentration of NaN3 for 1 hour. The apical side was treated with the same concentration of NaN3 in serum free media thus. Samples containing albumin-bound complexes (which we refer to as FFA) were collected from the apical side. The addition of additional albumin to the collected samples did not increase the fluorescence, suggesting that all of the bodipy transported across the LECs was bound to albumin (since the addition of albumin to free bodipy increases the quantum yield of the dye). In a subset of experiments, transport time varied from 10 to 30 minutes instead of 1 hour. Fluorescence was measured using a DTX 880 Multimode Detector plate reader (Beckman Coulter, Indianapolis, IN), and fluorescence was used to calculate relative concentration based on a standard curve generated from the fluorescent mix. The effective permeability of bodipy and dextran were calculated using the following equation: , where JS is the flux, ΔC is the concentration gradient, and S is the surface area (34). Transport is represented either as Peff (μm/sec), or as the percentage of Peff of the control condition.
Figure 1. Experimental setup.
A) Experimental setup for FFA transport experiments. B) Experimental setup for Caco-2 cell-secreted lipid experiments. OA/TC, serum, and bodipy are added to the basolateral side of the transwell, and Caco-2 cells secrete lipid on the apical side, which are collected after 18 hours. These Caco-2 cell secretions are added to the basal side of transwells with LECs seeded on the bottom, and fluorescence is measured from the apical side, representing Caco-2 cell-secreted lipid that has been transported across the LECs.
Transcellular free fatty acid transport
After samples were removed from the apical side of the transwell for fluorescence measurement and the calculation of Peff, LECs were rinsed twice with ice-cold PBS and were incubated for another 60 minutes in serum-free, phenol red-free DMEM containing 0, 1, 2, 5, or 10 mM NaN3 after which samples were again collected from the apical side and fluorescence was measured. In a subset of experiments, transport time varied from 10 to 30 minutes instead of 1 hour. Similar calculations were performed, and transport is represented either as flux (μg/sec), or as the percentage of flux compared to the control condition.
Caco-2 cell-secreted lipid transport
Transwell® permeable membrane supports with 0.4 μm pores were inverted and coated for 1 hour with 50 μg/mL type I rat tail collagen in PBS and Caco-2 cells were seeded at a density of 125,000 cells/cm2 on the underside of the membrane. Caco-2 cells were cultured for 21 days to promote development of an enterocyte phenotype, and media was changed every 2-3 days. On day 21, media on the apical Caco-2 cell side of the membrane was replaced with phenol red-free DMEM containing 20% FBS, 1.6 mM oleic acid, 1 mM taurocholic acid, and 5 μg/mL bodipy. Previous work has shown that addition of OA/TC promotes the formation of lipoproteins in the size range of chylomicrons (Luchoomun and Hussain, 1999) and its addition to the Caco-2 cell model can increase the portion of bodipy incorporated into lipoproteins (Dixon et al., 2009). Serum-free, phenol red-free DMEM was added to the basal side of the membrane and Caco-2 cells were incubated for 18 hours to promote formation of chylomicrons (34,36). DMEM containing Caco-2 cell-secreted lipid was collected from the basal side of the cells, pooled and diluted in additional DMEM to bring the total volume to 12 mL. Cascade blue dextran was added (5 μg/mL), and 1 mL of this mixture was placed on the basal side of each transwell membrane containing LECs grown to confluence on the top of the membrane (Fig 1B). Total and transcellular transport experiments were performed as described above, this time measuring the effective permeability and flux of Caco-2 cell-secreted lipid instead of free fatty acid.
Statistical analysis
A one-way ANOVA with the Dunnett's post hoc test was used to compare multiple treatment groups to the control group. A p value of <0.05 was considered statistically significant.
Results
The relationship between ATP and FFA transport across the lymphatic endothelium
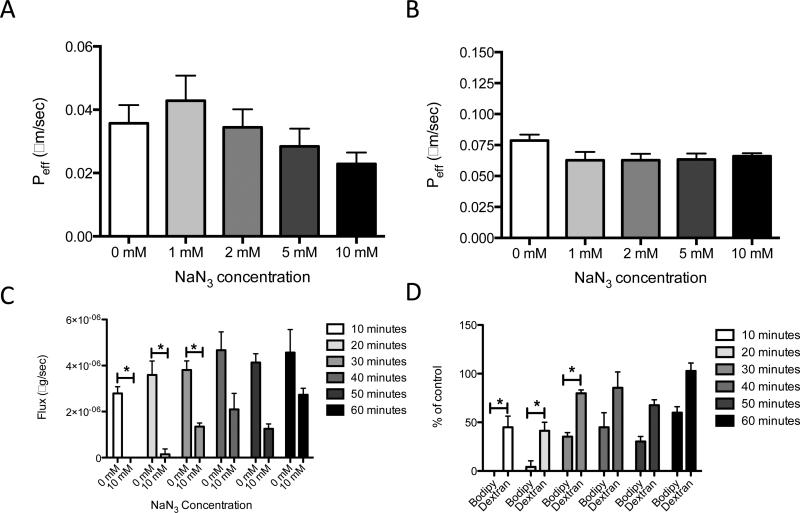
To test the hypothesis that ATP plays a role in active, transcellular transport of lipid across the lymphatic endothelium, the effective permeability (Peff) of the fluorescent fatty acid bodipy bound to albumin was measured after inhibition with NaN3. NaN3 inhibits the production of ATP via its actions on the cytochrome oxidase component of the electron transport chain (35). Since NaN3 is known to be cytotoxic, preliminary experiments were carried out to determine both the optimal and cytotoxic doses of NaN3 and were used to determine the dose range represented in the following experiments (data not shown). When LECs were treated with graded doses of NaN3, there was a non-significant trend toward decreased effective permeability of FFA, with no reduction of transport of dextran after a 60-minute time point (Fig. 2A and 2B). To explore the possibility that inhibition of transport by NaN3 might be time-dependent, experiments were performed at shorter time intervals. In contrast to the data from the 60-minute study, there was a significant decrease in transport upon treatment with 10 mM NaN3 after 10, 20, and 30 minute time points (Fig. 2C). Furthermore, at all time points, bodipy transport was inhibited to a greater extent than dextran transport (Fig. 2D). It is worth noting that at the 10 and 20-minute time points, dextran transport was also reduced, but by 60 minutes no reduction in transport was observed, which is in agreement with previous data (Fig. 2B). These data suggest NaN3 produces its effects quickly, but these effects diminish over time.
Figure 2. Inhibiting ATP reduces total transport of FFA across a LEC monolayer at an early, but not a late time points.
A) Treatment of LECs with graded doses of NaN3 results in a non-significant trend toward decreased transport (Peff) of FFA at a 60-minute time point. B) There is virtually no accompanying change in dextran transport. C) Treatment of LECs with 10 mM NaN3 significantly reduces transport (flux) of bodipy at 10, 20, and 30-minute time points. D) Treatment of LECs with 10 mM NaN3 results in a more drastic reduction in transport of bodipy compared to dextran at all time points *p<0.05.
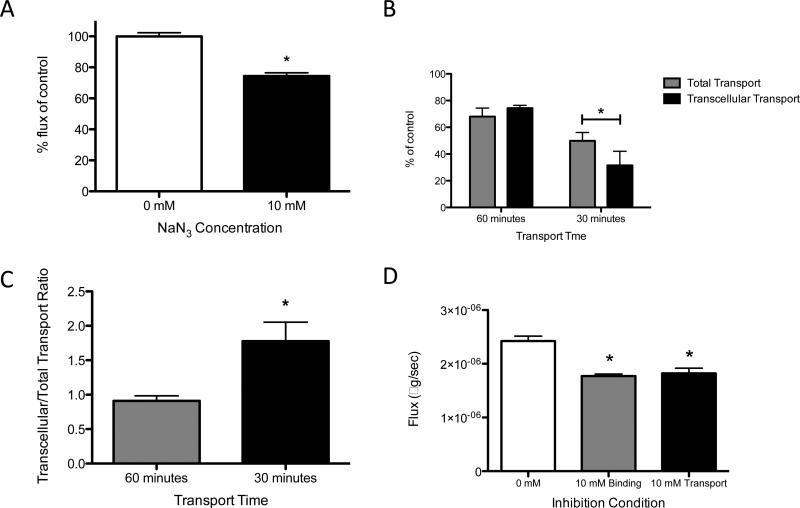
To determine the extent that ATP inhibition alters transcellular transport of FFA across the lymphatic endothelium, experiments were modified as described in the methods section to measure only this component of transport. When LECs were treated with 10 mM NaN3, transcellular transport of FFA was significantly reduced, suggesting that specifically the transcellular component of transport is ATP-dependent (Fig. 3A). Furthermore, when NaN3-mediated inhibition of total versus transcellular transport were compared, no difference was found at the 60-minute time point. However at 30-minutes, transcellular transport was significantly reduced when compared to total transport, suggesting that the ATP-dependency observed in the quickest stages of lipid uptake is driven predominantly by transcellular mechanisms (Fig. 3B and 3C). To determine which stage of lipid transcytosis was ATP-dependent, the inhibitor was added for the entire experiment (i.e. during the uptake and release of lipid), or only during fatty acid release. Both experiments resulted in a significant decrease in fatty acid flux from the cell and no differences were seen between the two inhibition procedures. Thus ATP does not appear to be required for the basal uptake of FFA into the cell, but rather is only needed for the subsequent transcytosis and/or release to the apical side of the cell.
Figure 3. Inhibiting ATP reduces transcellular transport of FFA across a LEC monolayer at both early and late time points.
A) Transcellular transport of FFA, represented as percentage reduction in flux relative to control, is reduced in LECs after treatment with 10 mM NaN3 at a 60-minute time point. B) The percent inhibition of bodipy flux shows no difference between total and transcellular transport conditions at a 60-minutes timepoint. At 30-minutes transcellular transport is reduced more than total transport upon ATP-inhibition. C) The ratio of percent inhibition of transcellular flux to total flux is greater during the first 30-minutes compared with 60 minutes. D) 10 mM NaN3 is used to inhibit both binding and transport of FFA (binding) or just transport of FFA across a LEC monolayer. While both reduce transport relative to control, the addition of the inhibitor during binding has no additive effect on FFA transport *p<0.05.
ATP dependence of FFA transport is a LEC-specific mechanism
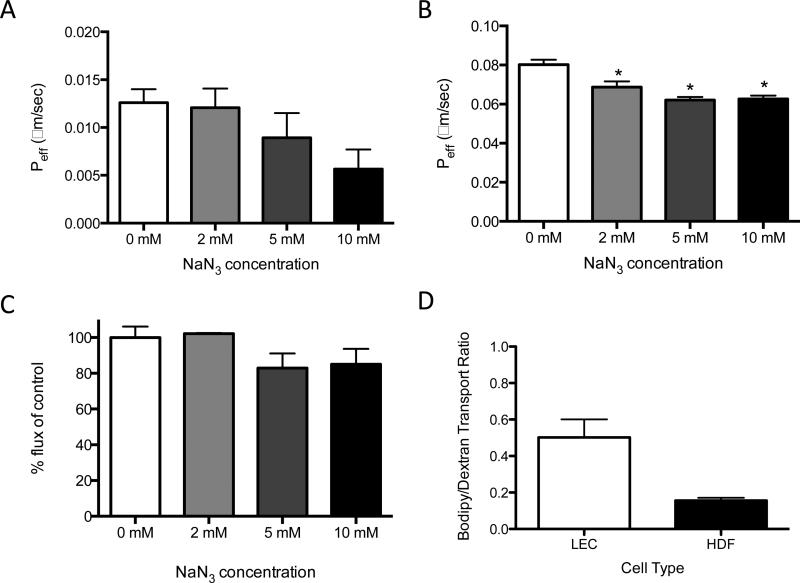
To demonstrate that ATP dependence of lipid transport is specific to LECs, the experiments described above were repeated in a non-lymphatic cell, human dermal fibroblasts (HDFs). In HDFs treated with graded doses of NaN3, there was a non-significant decrease in effective permeability of FFA, which is similar to the pattern observed in LECs (Fig. 4A). Contrary to LECs, there was a small but significant decrease in effective permeability of dextran (Fig. 4B). However, when the specific transcellular component of transport was examined, it was found that there was no difference in transport of FFA in HDFs treated with any of the graded doses of NaN3, suggesting any movement of FFA across a monolayer of HDFs in not dependent upon ATP (Fig 4C). Taken together, the data suggest that the dependence upon ATP of LECs in the transcellular transport of lipid is a mechanism specific to LECs, and not observed in HDFs. Also, it is interesting to note that while LECs and HDFs have a similar effective permeability to 3 kDa dextran, LECs have a much higher effective permeability to FFA than fibroblasts (Fig. 4D).
Figure 4. HDF's are less efficient at transporting lipid when compared to LECs and exhibit no ATP-dependency for transcellular or total transport.
A) Treatment of HDFs with graded doses of NaN3 results in a non-significant trend toward decreased transport (Peff) of FFA at a 60-minute time point, similar to that observed in LECs. B) There is a small but significant accompanying decrease in dextran transport. C) When HDFs are treated with graded doses of NaN3, there is no reduction in transcellular transport of FFA, unlike the decrease observed in LECs. D) LECs exhibit a much higher ratio of bodipy/dextran transport when compared to HDFs. *p<0.05.
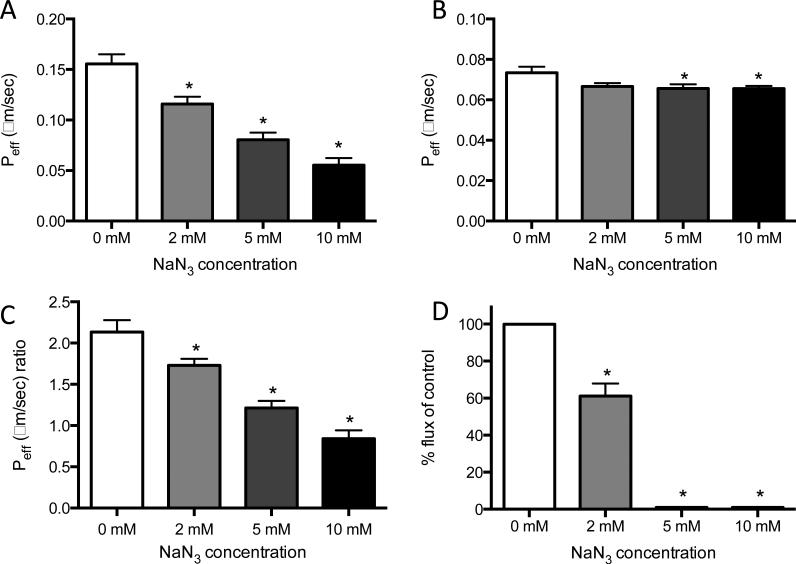
Lymphatic Caco-2 cell-secreted lipid transport is ATP-dependent
Since the most significant role for lymphatics in lipid transport is thought to be the postprandial uptake of chylomicrons in the intestine upon secretion by enterocytes, we investigated the ATP-dependence of lymphatic uptake of chylomicrons using a modified version of our previously validated enterocyte-lacteal interface. Caco-2 cells, which are colon carcinoma cells known to differentiate into an enterocyte phenotype after 21 days in culture, were used to produce chylomicrons in vitro (36). Previous studies have demonstrated that when a mixture of oleic acid and taurocholate are administered to Caco-2 cells in the presence of bodipy, fluorescent lipoproteins consistent in size with chylomicrons are produced (34). When LECs were again treated with graded doses of NaN3, there was a significant and dose-dependent decrease in effective permeability of Caco-2 cell-secreted lipid, indicating that transport of these secretions, which include lipoprotein, is ATP-dependent to a greater extent than was observed with FFA (Fig. 5A). There was a small decrease in effective permeability of dextran (Fig. 5B), but this decrease was much less than that observed for Caco-2 cell-secreted lipid. When the ratio of this secreted lipid to dextran transported was calculated to take into account this small change in dextran transport, it was found once again that treatment with NaN3 produced a significant dose-dependent decrease in transport (Fig. 5C). Transcellular transport of Caco-2 cell-secreted lipid was also reduced in LECs in response to NaN3 treatment, to the point where transport was undetectable at 5 and 10 mM concentrations (Fig. 5D), suggesting that the transcellular transport of Caco-2 cell-secreted lipid carrying Bodipy Fl C16 is significantly more dependent on ATP than when it is bound to albumin as a FFA.
Figure 5. Inhibiting ATP reduces both total and transcellular transport of Caco-2 cell-secreted lipid across a LEC monolayer.
A) Basal to apical total transport of Caco-2 cell-secreted lipid (containing both lipoprotein-incorporated bodipy as well as albumin-associated bodipy) is ATP-dependent and can be inhibited by blocking ATP synthesis in a dose-dependent fashion. B) The transport of dextran is slightly but significantly decreased at higher concentrations of ATP inhibition. C) The ratio of bodipy to dextran transport (Peff) is significantly reduced in a dose-dependent fashion, demonstrating a much greater dependence on ATP for lipoprotein transport when compared to dextran. D) Transcellular transport of Caco-2 cell-secreted lipid is reduced in LECs at 2 nM concentrations of NaN3. At 5 mM and 10 mM concentrations the inhibition reduced the flux of secreted lipid out of LECs to an amount the limit of detection. *p<0.05.
Discussion
Taken together, these data suggest that uptake of lipid is highly ATP dependent. Additionally, this transport of lipid across the lymphatic endothelium is at least partially transcellular, which is an active process that is highly dependent upon ATP, particularly when that lipid is secreted from intestinal epithelial cells. While the non-specific uptake of particulate certainly exists in lymphatics, and is essential to the vessels’ ability to clear the tissue space, the presence of other uptake mechanisms for lipid alluded to here indicate that lipid uptake is not solely the passive process we have historically regarded it to be. The presence of transcellular uptake mechanisms in lymphatics is further supported by a recent paper demonstrating in vivo that HDL is taken up by LECS via transcytosis of HDL by SR-B1 expressed on lymphatic endothelium (10). These emerging evidence are contrary to the conventional process with which lymphatics are thought to remove particulate from the interstitium – indiscriminately draining all molecules within a certain size. In fact the differences in ATP-dependence between Caco-2 secreted lipid and FFA observed in this study suggest that different types of lipid rely on differential modes of transport. On average the size distribution of lipid-carrying particles secreted by Caco-2 cells is much larger than FFA (34,36), thus it is possible that ATP-dependent transcellular transport exists to enhance insufficient paracellular uptake due to large particle sizes.
The role of lymphatics in FFA transport is likely not very significant in the intestine as nearly all absorbed long-chain FFA are hydrolyzed into triglyceride and incorporated into chylomicrons. Additionally, other protein-bound drugs do not show significant lymphatic transport upon oral delivery (37). However, it is likely that lymphatic transport of FFA in the periphery and across the collecting lymphatic wall is of physiologic importance. This is supported by a recent paper which demonstrated that the lymphatics are a key route of drainage from the adipose tissue bed (4). Additionally, findings from the Pond lab demonstrate that lipolysis in perinodal adipocytes influences the uptake of fatty acids into the lymphocytes in the lymph node, providing evidence of fatty acid transfer from adipose into lymph nodes presumably by lymphatics (38). Thus, just as is the case for HDL (10), it is possible that other lipid-based and protein-bound intradermally delivered drug formulations that are known to rely on peripheral lymphatic uptake, could utilize similar transcellular mechanisms. Furthermore, as reviewed by our lab and others (2,39), the lymphatics in the mesentery and periphery are surrounded by adipose tissue, which has been shown to undergo hypertrophy and abnormal lipid metabolism in the setting of metabolic disease. The fact that abnormal adipocyte accumulation and resultant obesity are associated with abnormal lymphatic function suggests there may be a two-way exchange of FFA and lipid between adipocytes surrounding collecting vessels and lymph.
Of course in vitro models are not without limitations, the most obvious in this case being that the three-dimensional in vivo morphology and biophysical environment of the initial lymphatic that enables particulate uptake is not exactly recapitulated in vitro (9). For example, in the intestine, a blood capillary would be present alongside the lacteal, which could theoretically compete for uptake of substances secreted by the enterocytes. Although it is fairly well established that long-chain fatty acids are almost exclusively transported in lymph in the intestine even in the presence of blood capillaries, since the size of the molecules are too large to significantly penetrate the blood endothelial barrier (40). Indeed, near-infrared imaging data from our lab in other tissue beds has demonstrated that particles larger than a few nanometers are preferentially taken up by the lymphatics, not the vasculature (41,42). In vivo the presence of flow in the lacteal through open junctions would produce a convective force that favors paracellular transport. It is particularly challenging to recapitulate hydrodynamic forces in the context of the intestine, as measurements do not exist for these forces at the length-scale of the single lacteal. However, the possibility that hydrodynamic forces, particularly transmural pressure gradients, could impact the relative contribution of active versus passive transport remains a possibility and an area of continued research. While the absence of these hydrodynamic features in the model system here make it difficult to ascertain the exact quantitative significance of ATP-dependent transport in vivo, it is quite clear that they are of great importance in vitro and are specific to lipid. Future work should be done to better incorporate these biophysical details into a more realistic 3D tissue engineered model. Other factors also exist in vivo that may influence the ratio of paracellular to transcellular transport, such as matrix hydration and concentration of lipid, such as the very high concentration of lipid likely to be present after a meal. It is possible that high lipid concentrations may be associated with an increase in paracellular transport. These factors were not investigated but represent interesting directions for continued research.
Next, the cells themselves are likely not biologically identical to their in vivo counterparts. For example, differentiated Caco-2 cells, unlike enterocytes, secrete both the ApoB48 (intestine) and AboB100 (liver) containing lipoproteins. However, given the large amount of evidence for lymphatic transport of the entire range of lipoproteins, it is unlikely that this is of significant importance. It is important to note, as we have shown previously (34), bodipy secreted by lipid fed, differentiated Caco-2 cells, when subjected to size exclusion chromatography, elutes in variety of size fractions with a substantial amount eluting in a fraction of the same size as albumin. However bodipy in this form appears to be functionally different than when it is in its FFA form as suggested in our previous work (34), and confirmed in the study here with the differential effects of ATP depletion on Caco-2 secreted lipid and FFA. While Bodipy has been shown to be metabolized more slowly when added to Caco-2 cells by itself (43), this does not appear to be the case when added in conjunction with other lipids and bile salts. Evidence of reasonable uptake of bodipy in vivo was also recently demonstrated in mice after duodenum infusion of the dye with a lipid cocktail in which the kinetics of bodipy uptake correlated quite closely with total lymph triglyceride (44).
It is important to note that the LECs used here, while a primary cell of low passage number, are taken from human dermis as opposed to the intestine. LECs of a gastrointestinal origin are difficult to isolate and uncommon, and have not been extensively characterized with respect to the degree to which they recapitulate the lacteal in vivo. Nearly all in vitro lymphatic work relies on podoplanin-positive cells of dermal origin, and the extent of the molecular differences between lymphatic cells of different tissues remains unexplored. Previous work by Dixon et al. demonstrated that with respect to cell morphology, transport distance between enterocyte and lymphatics, and uptake of lipid, this model is representative of the important components of lipid uptake in vivo (34). There is also one published in vitro model of LECS that are of intestinal origin (45). It was these cells that were utilized to show the transcytosis and SR-B1 mediated transport of HDL (10). Interestingly the authors demonstrated the same results when using a human LEC line of dermal origin, suggesting that these specialized mechanisms of lipid transport are fundamental to the lymphatic phenotype and that dermal lymphatics are a reasonable in vitro model system for investigating these processes. LECs in the dermis/periphery are exposed to different lipid concentrations and hydrodynamic forces than in the intestine and thus it is currently impossible to speculate on the exact ratio of paracellular to transcellular transport for every anatomical location where lymphatics remove lipid. However, it is likely all LECs use the same general physiological mechanisms for lipid and lipoprotein transport and thus rely at least partially on transcellular transport.
Despite these caveats, we believe the data presented here establishes a useful in vitro model for continued studies on the molecular mechanisms by which lipids and lipoproteins cross the lymphatic endothelium to enter the lymphatic system. Furthermore, the LEC and Caco-2 model represents an interesting model for screening potential drugs of interest in the development of lipid and lipid-based drug formulations, particularly when the designed formulations are targeting lymphatic uptake. Finally, the data here build upon previous qualitative observations suggesting active, transcellular transport of lipid across the lymphatic endothelium. In these studies, we have provided further evidence that the uptake of lipid into lymphatics is driven in large part by lipid-specific, ATP-dependent, transcellular mechanisms. With the knowledge that this transcellular mode of transport contributes significantly to overall lipid transport, we can turn next to elucidating more specific molecular mechanisms of vesicular transport and identify potential receptors and carrier proteins. We can also modify experimental conditions such as lipid content and concentration, tissue hydration, and pressure gradients, to determine in what contexts transcellular transport plays the largest role in lipid transcytosis.
Given the desire to utilize the lymphatics as a route of lipid-based drug delivery systems, whether the administration is oral or intradermal, it seems likely that many of these currently unexplored mechanisms of lymphatic lipid transport would be ideal pathways to inform future drug design. Furthermore, the evidence that albumin-bound lipid and lipoprotein transport occurs via the transcellular route may also have implications for protein-bound drugs and other large macromolecular drugs that extravasate into the interstitial space and are returned to circulation via the lymphatics. In fact, the Caco-2 cell model employed here is one of the most widely used models for in vitro drug screening and development. However, no current in vitro studies with this model consider the secondary barrier to true lymphatic transport, the lymphatics themselves. While overcoming the intestinal barrier and enhancing enterocyte absorption is of utmost important and one of the largest challenges to delivering lipid-based formulations orally, it is highly likely that the lymphatics themselves have a role to play in enhancing or hindering uptake of these drugs. How the drug of interest itself might interact with this biology, either at the level of the enterocyte or the lymphatic endothelium, is currently unknown and warrants further exploration. The model system described here could lend itself well for screening approaches to answer these and other related questions.
Conclusion
In conclusion, we provide here the first evidence that lipid is transported into the lymphatics via an active, transcellular process. This data lends support to TEM images obtained previously that challenged the historical assumption that all lipid transport is passive, and thus further supports the relatively new idea that the lymphatic endothelium plays an active role in passage of lipid rather than merely acting as a passive barrier. We have shown that lymphatic uptake of lipid can be inhibited through the intercellular depletion of ATP, and that this inhibition is largely independent of non-specific paracellular permeability changes through cell junctions, as determined by dextran transport. Future work on these molecular details using an in vitro model system such as this one, as well as the establishment of their roles in vivo, will be crucial if we are to truly optimize drug delivery strategies to target lymphatic transport.
Acknowledgements
This work is supported by the NIH grant R00 HL091133. The Petit Undergraduate Scholars Program also provided generous support for Sydney Rowson.
References
- 1.Randolph G, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature Reviews Immunology. 2005;5(8):617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 2.Dixon B. Lymphatic lipid transport: sewer or subway? Trends in endocrinology and metabolism: TEM. 2010;21(8):480–7. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276(20):5738–46. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller NE, Michel CC, Nanjee MN, Olszewski WL, Miller IP, Hazell M, et al. Secretion of adipokines by human adipose tissue in vivo: partitioning between capillary and lymphatic transport. Am J Physiol Endocrinol Metab. 2011;301(4):E659–67. doi: 10.1152/ajpendo.00058.2011. [DOI] [PubMed] [Google Scholar]
- 5.Nanjee M, Cooke C, Olszewski W, Miller N. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans: associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res. 2000;41(8):1317–27. [PubMed] [Google Scholar]
- 6.Dixon B. Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci. 2010;1207(Suppl 1):E52–7. doi: 10.1111/j.1749-6632.2010.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tso P, Balint J. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–26. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- 8.Trzewik J, Mallipattu S, Artmann G, Delano F, Schmid-Schonbein G. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 2001;15(10):1711–7. doi: 10.1096/fj.01-0067com. [DOI] [PubMed] [Google Scholar]
- 9.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KCY, et al. Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell Metab. 2013;17(5):671–84. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Reddy S, van der Vlies A, Simeoni E, Angeli V, Randolph G, O'Neil C, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology. 2007;25(10):1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 12.Thomas SN, van der Vlies AJ, O'Neil CP, Reddy ST, Yu SS, Giorgio TD, et al. Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials. 2011;32(8):2194–203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–16. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan SP, Koutsonanos DG, del Pilar Martin M, Lee JW, Zarnitsyn V, Choi S-O, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettis RJ, Hirsch L, Kapitza C, Nosek L, Hövelmann U, Kurth H-J, et al. Microneedle-Based Intradermal Versus Subcutaneous Administration of Regular Human Insulin or Insulin Lispro: Pharmacokinetics and Postprandial Glycemic Excursions in Patients with Type 1 Diabetes. Diabetes Technology & Therapeutics. 2011;13(4):443–50. doi: 10.1089/dia.2010.0183. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Gilmore DM, Zubris KAV, Xu X, Catalano PJ, Padera RF, et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials. 2013;34(7):1810–9. doi: 10.1016/j.biomaterials.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trevaskis NL, Charman W, Porter CJH. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60(6):702–16. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trevaskis NL, Shackleford DM, Charman WN, Edwards GA, Gardin A, Appel-Dingemanse S, et al. Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm Res. 2009;26(6):1486–95. doi: 10.1007/s11095-009-9860-z. [DOI] [PubMed] [Google Scholar]
- 19.Yáñez JA, Wang SWJ, Knemeyer IW, Wirth MA, Alton KB. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63(10-11):923–42. doi: 10.1016/j.addr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieux des A, Fievez V, Garinot M, Schneider Y, Pre at V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. Journal of Controlled Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Hussain N, Jaitley V, Florence A. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50(1-2):107–42. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 22.Porter CJH, Trevaskis NL, Charman W. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nature Reviews Drug Discovery. 2007;6(3):231–48. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 23.Teijeira A, Palazón A, Garasa S, Marré D, Aubá C, Rogel A, et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J. 2012;26(8):3380–92. doi: 10.1096/fj.11-201061. [DOI] [PubMed] [Google Scholar]
- 24.Miteva DO, Rutkowski JM, Dixon B, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106(5):920–31. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitschké M, Aebischer D, Abadier M, Haener S, Lucic M, Vigl B, et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood. 2012;120(11):2249–58. doi: 10.1182/blood-2012-03-417923. [DOI] [PubMed] [Google Scholar]
- 26.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA. 2011;108(46):18784–9. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey N, Srinivasan R, Dillard M, Johnson N, Witte M, Boyd K, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37(10):1072–81. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 28.van Dyck F, Braem C, Chen Z, Declercq J, Deckers R, Kim B, et al. Loss of the PlagL2 Transcription Factor Affects Lacteal Uptake of Chylomicrons. Cell Metab. 2007;6(5):406–13. doi: 10.1016/j.cmet.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Casley-Smith J. Identification of chylomicra and lipoproteins in tissue sections and their passage into jejunal lacteals. Journal of Cell Biology. 1962;15(2):259–77. doi: 10.1083/jcb.15.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collan Y, Kalima TV. The lymphatic pump of the intestinal villus of the rat. Scand J Gastroenterol. 1970;5(3):187–96. [PubMed] [Google Scholar]
- 31.PALAY SL, KARLIN LJ. An electron microscopic study of the intestinal villus. II. The pathway of fat absorption. J Biophys Biochem Cytol. 1959;5(3):373–84. doi: 10.1083/jcb.5.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobbins WO, Rollins E. Intestinal mucosal lymphatic permeability - an electron microscopic study of endothelial vesicles and cell junctions. Journal of Ultrastructure Research. 1970;33(1-2):29–59. doi: 10.1016/s0022-5320(70)90117-6. [DOI] [PubMed] [Google Scholar]
- 33.Dobbins WO. Intestinal mucosal lacteal in transport of macromolecules and chylomicrons. Am J Clin Nutr. 1971;24(1):77–90. doi: 10.1093/ajcn/24.1.77. [DOI] [PubMed] [Google Scholar]
- 34.Dixon B, Raghunathan S, Swartz MA. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol Bioeng. 2009;103(6):1224–35. doi: 10.1002/bit.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S, Pedersen PL. ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol. Mol. Biol. Rev. Dec. 2008;72(4):590–641. doi: 10.1128/MMBR.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luchoomun J, Hussain M. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. J Biol Chem. 1999 Jun 30;274(28):19565–72. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- 37.Gershkovich P, Hoffman A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: the importance of degree of association with triglyceride-rich lipoproteins. Eur J Pharm Sci. 2007 Sep;32(1):24–32. doi: 10.1016/j.ejps.2007.05.109. [DOI] [PubMed] [Google Scholar]
- 38.Sadler D, Mattacks CA, Pond CM. Changes in adipocytes and dendritic cells in lymph node containing adipose depots during and after many weeks of mild inflammation. J Anat. 2005;207(6):769–81. doi: 10.1111/j.1469-7580.2005.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty S, Zawieja S, Wang W, Zawieja DC, Muthuchamy M. Lymphatic system: a vital link between metabolic syndrome and inflammation. Ann N Y Acad Sci. 2010;1207(Suppl 1):E94–102. doi: 10.1111/j.1749-6632.2010.05752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kvietys PR, Granger DN. Role of intestinal lymphatics in interstitial volume regulation and transmucosal water transport. Ann N Y Acad Sci. 2010;1207(Suppl 1):E29–43. doi: 10.1111/j.1749-6632.2010.05709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiler M, Kassis T, Dixon B. Sensitivity analysis of near infrared functional lymphatic imaging. J Biomed Opt. 2012;17066019(6):1–11. doi: 10.1117/1.JBO.17.6.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiler M, Dixon B. Differential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front. Physiol. 2013;4(215):1–10. doi: 10.3389/fphys.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thumser A, Storch J. Characterization of a BODIPY-labeled fluorescent fatty acid analogue. Binding to fatty acid-binding proteins, intracellular localization, and metabolism. Molecular and Cellular Biochemistry. 2007;299(1-2):67–73. doi: 10.1007/s11010-005-9041-2. [DOI] [PubMed] [Google Scholar]
- 44.Kassis T, Kohan AB, Weiler MJ, Nipper ME, Cornelius R, Tso P, et al. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. J Biomed Opt. 2012;17(8):086005. doi: 10.1117/1.JBO.17.8.086005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ando T, Jordan P, Joh T, Wang Y, Jennings MH, Houghton J, et al. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol. 2005;3(3):105–15. doi: 10.1089/lrb.2005.3.105. [DOI] [PubMed] [Google Scholar]