Abstract
In germ cells and the early embryo, the mammalian genome undergoes widespread epigenetic reprogramming. Animal studies suggest that this process is vulnerable to external factors. We report two children who were conceived by intracytoplasmic sperm injection (ICSI) and who developed Angelman syndrome. Molecular studies, including DNA methylation and microsatellite and quantitative Southern blot analysis, revealed a sporadic imprinting defect in both patients. We discuss the possibility that ICSI may interfere with the establishment of the maternal imprint in the oocyte or pre-embryo.
Intracytoplasmic sperm injection (ICSI) was introduced in 1992 as a solution for male infertility. Several studies comparing ICSI to traditional in vitro fertilization have established the general safety of ICSI (for review, see Koulischer et al. [1997]). Schieve et al. (2002) reported an excess risk of low and very low birth weight after assisted reproduction, and Hansen et al. (2002) found that ICSI is associated with an increased risk of major birth defects. It has also been reported that there is a slightly higher incidence of sex-chromosome aneuploidy in ICSI, which, however, may be related to the underlying cause of the male infertility (Koulischer et al. 1997). Although most studies have compared the rates of pregnancy and congenital anomalies, only a few have analyzed developmental outcomes. In a case-control study of a relatively small number of children (221 children conceived by ICSI and 208 normally conceived children), Sutcliffe et al. (2001) found no difference in developmental level after 17 mo of follow-up. Here, we report on two unrelated children who were conceived by ICSI and found to have Angelman syndrome (AS [MIM 105830]). In both cases, informed consent was obtained from the parents.
AS is a neurogenetic disorder characterized by severe mental retardation, delayed motor development, poor balance accompanied by jerky movements, absence of speech, and happy disposition. It is caused by loss of function of the maternal allele of the UBE3A gene (MIM 601623) on chromosome 15, resulting from a deletion, a point mutation, a uniparental paternal disomy, or an imprinting defect (for review, see Nicholls and Knepper 2001). The last group of patients have a paternal imprint on the maternal chromosome as a result of a sporadic imprinting error or, less frequently, an inherited imprinting-center (IC) deletion.
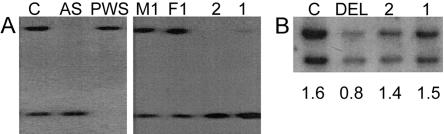
Patient 1 is a 3-year-old girl who was referred for evaluation of global developmental delay, particularly in speech, and hypotonia. She had poor balance, sleep difficulties, an overly happy personality, and no seizures. Her developmental-age equivalent was 18 mo. She was conceived by a 33-year-old mother and a 31-year-old father, who sought ICSI because of oligospermia and decreased sperm motility. The pregnancy was uncomplicated. Previous investigations showed a normal 46,XX karyotype and normal results on fragile X DNA test, metabolic screen, and brain MRI. Results of the girl's physical examination were notable for macrosomia, obesity, microbrachycephaly, AS-like facies, and a flexed-arm posture. Results of chromosome 15 FISH study, with the use of the SNRPN probe (MIM 182279), and uniparental disomy analysis, with the use of microsatellite markers, were normal in blood lymphocytes and cultured skin fibroblasts (data not shown). However, the chromosome 15 methylation pattern obtained by Southern blotting with the use of the SNRPN probe was abnormal, revealing a strong unmethylated band and a faint methylated band, which was 10% of the total intensity by densitometry (fig. 1A). A similar pattern was seen in the patient's fibroblasts (data not shown). Methylation-specific PCR of exon 1 of the SNRPN gene (Zeschnigk et al. 1997) also revealed a strong unmethylated and a weak methylated band (data not shown). Consistent results of the mosaic methylation pattern in this patient were obtained by two independent studies on a second blood sample, as well as on a repeated fibroblast culture, using both methods (data not shown). Results of parental chromosome analyses were normal, as was the mother's chromosome 15 methylation pattern. Quantitative Southern blot analysis (fig. 1B), as well as sequence analysis of the 880-bp AS-IC element did not reveal any IC mutation.
Figure 1.
Molecular analysis. A, DNA was digested with XbaI and NotI and was hybridized with a probe for SNRPN. A normal control subject (C) and the parents of patient 1 (M1, mother; F1, father) have a methylated maternal band (top of lane) and an unmethylated paternal band (bottom of lane) of equal intensity. Patients with AS and Prader-Willi syndrome (PWS) show only a single unmethylated or methylated band, respectively. Patient 2 shows an unmethylated band only. Patient 1 shows a very faint methylated band, in addition to the unmethylated band (increased intensity). Similar results were obtained by methylation-specific PCR (data not shown). B, IC deletion analysis. DNA was digested with EcoRV and was hybridized with a probe for the AS element of the IC (IC1/3; upper band) (Schumacher et al. 1998) and a probe for the RB1 gene on chromosome 13 (lower band). The relative hybridization intensities, as determined by scanning densitometry, are given below each lane. A deletion patient (DEL) has a reduced signal ratio. The two patients described in the present report (1 and 2) have a ratio similar to that of a normal control subject (i.e., no IC deletion). Similar results were obtained in a second, independent experiment (data not shown).
Patient 2 is a 32-mo-old girl who was referred for evaluation of mental retardation and developmental delays, especially in speech. She vomited frequently as an infant and was hyperactive and very friendly. Results of her physical examination were notable for microbrachycephaly, broad mouth, prognathism, normal pigmentation, ataxic gait, and hypotonia of the trunk. Electroencephalogram revealed periods of generalized slow activity and epileptiform activity. Her speech consisted of single sounds and no words. She was conceived by a 31-year-old mother and 32-year-old father, who sought ICSI because of oligospermia secondary to hypogonadotrophic hypogonadism. Pregnancy and birth were uncomplicated. The patient and her parents had normal chromosomes. FISH studies with probes for D15S10 and SNRPN, as well as microsatellite studies, revealed normal chromosomes of biparental origin (data not shown). Methylation analysis showed an unmethylated band only by Southern blotting with the SRNPN probe (fig. 1A) and by methylation-specific PCR (data not shown). As in patient 1, no evidence for an IC mutation was found (fig. 1B).
In normal individuals, the maternal SNRPN allele is methylated, and the paternal allele is unmethylated. Hypomethylation of the SNRPN locus in the two patients indicates that they have a sporadic imprinting defect on the maternal chromosome. Our findings raise the question of whether the imprinting defect in the two children conceived by ICSI is a coincidence or is causally related to the technique of ICSI. We are well aware that we cannot exclude other factors, such as environmental agents or a defect related to male infertility, but the assumption of a causal relationship is not unreasonable for the following reasons:
-
1.
AS is a rare disease that affects ∼1/15,000 newborns. Imprinting defects account for <5% of all cases (i.e., their incidence is 1/300,000 newborns). Although we have found two such patients conceived by ICSI, we have not found any ICSI child who has AS because of a deletion 15q, which is the most frequent cause of AS (70%). This may suggest an overrepresentation of AS imprinting defects among children conceived by ICSI. Although a recent study of 92 ICSI cases found no abnormal methylation of chromosome 15, such a small sample size does not exclude the possibility of a small but increased risk (Manning et al. 2000).
-
2.
As was shown by El-Maarri et al. (2001), the maternal methylation imprint on human chromosome 15 is not established before ovulation but only at or after fertilization. Most, if not all, sporadic AS imprinting defects occur at this time (Buiting et al. 1998). The lack of an IC mutation in the two patients and the detection of a mosaic methylation pattern in patient 1 make an inherited defect unlikely and point to a postzygotic epigenetic defect, although a postzygotic gain of methylation in some cells can not be excluded.
-
3.
ICSI bypasses many of the normal steps involved in fertilization and activation of the oocyte. In addition, ICSI causes other changes (e.g., the introduction of the acrosome and its digestive enzymes into the ooplasm). Furthermore, the oocyte undergoes mechanical stress, which may damage certain intracellular structures. The true impact on fertilization is unknown, but only 50% of ICSI conceptions are successful in humans, and animal studies indicate that other abnormalities may occur, including an alteration in calcium oscillations and delayed decondensation of sperm chromosomes. It is possible that ICSI disrupts the production or function of trans-acting factors or local structures necessary for imprinting of the maternal chromosome 15.
-
4.
Young et al. (2001) have shown that under certain conditions the in vitro culture of sheep pre-embryos that were fertilized in vivo can lead to fetal overgrowth. Those authors found that the overgrowth is associated with hypomethylation of the imprinting control element of the maternal Igf2r allele. Their data demonstrate that maternal imprinting in the pre-embryo is vulnerable to external factors.
In summary, there are some indications that ICSI might interfere with the establishment of the maternal imprint in the oocyte or pre-embryo and increase the risk of imprinting defects. We suggest addressing this question in a long-term follow-up study of a large cohort of children. One may also consider performing a prenatal methylation test for all known imprinted loci in fetuses conceived by ICSI.
Acknowledgments
The authors thank the patients and families, for participation of the studies, K. Buiting, G. Gillessen-Kaesbach, and Neal Lindeman, for discussion, I. T. Bäckert, M. Döbler, and H. Enders, for their contribution, and Ralf Eckhard, Hong Fang, Stuart Hanviriyapunt, Christina Lich, and Julie Nicole, for technical assistance. Part of this work was supported by the Children’s Hospital Boston and the Deutsche Forschungsgemeinschaft.
Electronic-Database Information
Accession numbers and the URL for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for the AS gene [MIM 105830], the UBE3A gene [MIM 601623], and the SNRPN probe [MIM 182279])
References
- Buiting K, Dittrich B, Groß S, Lich C, Färber C, Buchholz T, Smith E, et al (1998). Sporadic imprinting defects in Prader-Willi syndrome and Angelman syndrome: implications for imprint switch models, genetic counseling and prenatal diagnosis. Am J Hum Genet 63:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maarri O, Buiting K, Peery EG, Kroisel PM, Balaban B, Wagner K, Urman B, Brannan CI, Walter J, Horsthemke B (2001) Maternal methylation imprints on human chromosome 15 are established around or after fertilisation. Nat Genet 27:341–344 [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S (2002) The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med 346:725–730 [DOI] [PubMed] [Google Scholar]
- Koulischer L, Verloes A, Lesenfants S, Jamar M, Herens C (1997) Genetic risk in natural and medically assisted procreation. Early Pregnancy 3:164–171 [PubMed] [Google Scholar]
- Manning M, Lissens W, Bonduelle M, Camus M, De Rijcke M, Liebaers I, Van Steirteghem A (2000) Study of DNA-methylation patterns at chromosome 15q11-q13 in children born after ICSI reveals no imprinting defects. Mol Hum Reprod 6:1049–1053 [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL (2001) Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet 2:153–175 [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS (2002) Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med 346:731–737 [DOI] [PubMed] [Google Scholar]
- Schumacher A, Buiting K, Zeschnigk M, Doerfler W, Horsthemke B (1998) Methylation analysis of the PWS/AS region does not support an enhancer-competition model. Nat Genet 19:324–325 [DOI] [PubMed] [Google Scholar]
- Sutcliffe AG, Taylor B, Saunders K, Thornton S, Liebermann BA, Grudzinskas JG (2001) Outcome in the second year of life after in-vitro fertilisation by intracytosplamic sperm injection: a UK case-control study. Lancet 357:2068–2069 [DOI] [PubMed] [Google Scholar]
- Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD (2001). Epigenetic change in IGF2R is associated with fetal overgrowth after sheep culture. Nat Genet 27:153–154 [DOI] [PubMed] [Google Scholar]
- Zeschnigk M, Lich C, Buiting K, Doerfler W, Horsthemke B (1997) A single-tube PCR test for the diagnosis of Angelman and Prader-Willi syndrome based on allelic methylation differences at the SNRPN locus. Eur J Hum Genet 5:94–98 [PubMed] [Google Scholar]