Abstract
Some deleterious X-linked mutations may result in a growth disadvantage for those cells in which the mutation, when on the active X chromosome, affects cell proliferation or viability. To explore the relationship between skewed X-chromosome inactivation and X-linked mental retardation (XLMR) disorders, we used the androgen receptor X-inactivation assay to determine X-inactivation patterns in 155 female subjects from 24 families segregating 20 distinct XLMR disorders. Among XLMR carriers, ∼50% demonstrate markedly skewed X inactivation (i.e., patterns ⩾80:20), compared with only ∼10% of female control subjects (P<.001). Thus, skewed X inactivation is a relatively common feature of XLMR disorders. Of the 20 distinct XLMR disorders, 4 demonstrate a strong association with skewed X inactivation, since all carriers of these mutations demonstrate X-inactivation patterns ⩾80:20. The XLMR mutations are present on the preferentially inactive X chromosome in all 20 informative female subjects from these families, indicating that skewing is due to selection against those cells in which the XLMR mutation is on the active X chromosome.
As a result of X-chromosome inactivation (Lyon 1961), heterozygous females are mosaic for X-linked gene expression, with one population of cells expressing genes from the maternal X chromosome and the other population expressing genes from the paternal X chromosome (Nance 1964). The relative ratio of these two cell populations in a given female is frequently referred to as the “X-inactivation pattern.” For female carriers of an X-linked mutation or structural abnormality, one cell population may be at a selective growth disadvantage, resulting in clonal outgrowth of cells with one or the other parental X chromosome active (Belmont 1996; Puck and Willard 1998; Willard 2000). Because the choice of one or the other X chromosome early in the process of X inactivation is generally random (Lyon 1961), significant deviation or skewing from an expected mean X-inactivation pattern (i.e., 50:50) in a specific population of female carriers suggests that the X-linked mutation alters in vivo cell viability or proliferation (Lyon 1968; Nyhan et al. 1970).
Mental retardation is a phenotypic component common to several of the disorders associated with skewed X inactivation (Willard 2000). Because of this anecdotal association, we sought to explore the possibility that a general defect in cell viability or proliferation, as measured by skewed X inactivation in peripheral blood cells, is commonly associated with X-linked mental retardation (XLMR). XLMR represents a diverse class of genetic mutations. There are ∼150 XLMR disorders, which fall into three classes: X-linked recessive and partly dominant disorders (including syndromes, neuromuscular disorders, and metabolic disorders), X-linked dominant lethal disorders, and nonspecific XLMR disorders (Cabezas et al. 1999; Stevenson et al. 1999; Hamel et al. 2000; Chelly and Mandel 2001). Although >30 XLMR genes have been cloned to date (Chelly and Mandel 2001), the commonality of defects leading to mental retardation is not understood at the cellular or molecular level. Our data demonstrate that skewed X inactivation is a consistent feature of at least half of all families with XLMR, suggesting that XLMR mutations represent a unique class of X-linked mutations characterized by a general defect in cell viability or proliferation. The data further suggest that, despite the most prominent clinical feature being restricted to the central nervous system, the responsible genes are likely to be expressed in peripheral blood cells, where they will be more accessible to experimental study.
To investigate a possible association between skewed X inactivation and XLMR, we studied 24 families segregating 20 distinct XLMR disorders (table 1). These families were selected solely on the basis of the clinical presentation in affected males and therefore represent an apparently unbiased collection of carrier female subjects. Using the androgen receptor (AR) X-inactivation assay (Allen et al. 1992), we determined the X-inactivation patterns of all available female subjects, both carriers and noncarriers, from the families with XLMR (n=155 female subjects).
Table 1.
Families with XLMR: Association with Skewed X Inactivation
|
No. of Carriersb |
||||||
| Familya | XLMR Disorder | Region ofLinkage | Total | With InactivationPattern ⩾80:20 | Xid | Descriptionc |
| Strong association (n=4): | ||||||
| K8135 | XLMR, short stature, tremor | q22-q24 | 5 | 5 | 1/1 | Short stature, tremor, behavioral abnormalities |
| K8210 | Williams | q28 | 4 | 4 | 3/3 | Muscle hypoplasia, hypotonia, frontal bossing, death at early age |
| K8300 | Pai | q28 | 7 | 7 | 3/3 | Profound mental retardation, death at early age, nonambulatory |
| K8435 | Mulvenna-Trotter-Fisher | q27 | 7 | 7 | 4/4 | Seizures, IgE deficiency, large head, moderate mental retardation |
| Incomplete association (n=14): | ||||||
| K8005 | Allan-Herndon Dudley | q13-q21 | 3 | 1 | Severe mental retardation, severe hypotonia, ataxia, abnormal facies | |
| K8090 | Allan-Herndon Dudley | q13-q21 | 2 | 0 | Severe mental retardation, severe hypotonia, ataxia, abnormal facies | |
| K8225 | Allan-Herndon Dudley | q13-q21 | 2 | 1 | Severe mental retardation, severe hypotonia, ataxia, abnormal facies | |
| K8020 | Aarskog-Scott | q11.21 | 3 | 0 | Short stature, facial, skeletal, and urogenital anomalies | |
| K8250 | Aarskog-Scott | q11.21 | 2 | 2 | 2/2 | Short stature, facial, skeletal, and urogenital anomalies |
| K8285 | Aarskog-Scott | q11.21 | 3 | 0 | Short stature, facial, skeletal, and urogenital anomalies | |
| K8765 | Agenesis corpus collosum | q28 | 7 | 2 | 1/1 | Neuromuscular spasticity, unsteady gait |
| K8065 | MRX7 | p11-p14 | 6 | 3 | 2/2 | Nonspecific XLMR |
| K8355 | XLMR, seizures, ataxia | p21-p11.2 | 6 | 2 | Seizures, ataxia, aphasia, autism | |
| K8070 | Miles-Carpenter MRSX4 | q13-q22 | 5 | 3 | 3/3 | Arched fingertips, microcephaly |
| K8240 | XLMR with cleft lip/palate | q12-q21 | 5 | 3 | 1/1 | Sloped forehead, short stature, small testicular volume |
| K8615 | XLMR, spastic paraplegia | p12-q12 | 3 | 2 | Spastic paraplegia, club feet, dystonia | |
| K8075 | Wieacker-Wolff | Proximal X q arm | 2 | 1 | Neuromuscular, muscle atrophy | |
| K8610 | FG syndrome | q13-q21 | 2 | 1 | Macrocephaly, imperforate anus, and congenital hypotonia | |
| No apparent association (n=6): | ||||||
| K8035 | XLMR, arched fingerprints | q13-q21 | 3 | 0 | Arched fingerprints, hypotonia, areflexia | |
| K8045 | Arena | q22-q25 | 2 | 0 | Severe spastic paraplegia, ataxia | |
| K8100 | Armfield | q28 | 3 | 0 | Short stature, cleft palate, seizures, glaucoma, severe mental retardation | |
| K8295 | Lujan | None | 4 | 0 | Marfanoid, triangular facies, narrow palate, hypernasal voice | |
| K8395 | XLMR, spastic paraplegia | Proximal X q arm | 2 | 0 | Spastic paraplegia, nystagmus; carriers have gait abnormalities | |
| K8450 | MRX32 | p21-p22.2 | 6 |
0 |
Nonspecific and variable mental retardation | |
| Total | 20 distinct disorders | 94 | 44 | 20/20 | ||
Most of the families have been described elsewhere (Lubs et al. 1996).
Carrier or noncarrier status was determined by linkage analysis, by pedigree analysis (in the case of obligate carriers) and, where possible, by direct mutation screening.
Specific clinical features are provided in the Miami XLMR database (Cabezas et al. 1999).
Xi = inactive X chromosome; data are number of informative carriers in whom the mutation was present on the preferentially inactive X chromosome/total number of informative carriers.
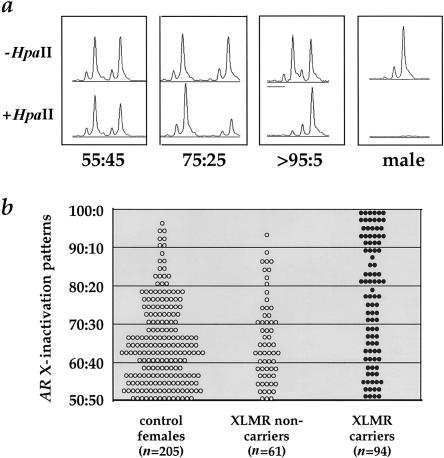
Illustrative examples of AR X-inactivation tracings are shown in figure 1a. The distribution of X-inactivation patterns for XLMR carriers (n=94) and noncarriers (n=61) is shown in figure 1b. Approximately 9% of female control subjects (n=205) demonstrate skewed X-inactivation patterns ⩾80:20, which is generally consistent with previous estimates (Nance 1964; Gale et al. 1994; Naumova et al. 1996; Plenge et al. 1997, 1999). In contrast, ∼50% of the XLMR carriers demonstrate X-inactivation patterns that are ⩾80:20 (tables 1 and 2; P<.001). Analysis of the XLMR carrier distribution at other thresholds of skewed X inactivation are also statistically highly significant (table 2). The effect is most dramatic at patterns of X inactivation ⩾90:10; nearly a third of XLMR carriers show such skewing, compared with only a few percent of female control subjects. Thus, these data establish that, in peripheral blood cells, skewed X inactivation is a common feature of XLMR carriers.
Figure 1.
X-inactivation patterns in XLMR disorders. a, AR X-inactivation–pattern tracings. The top tracings represent the undigested DNA (−HpaII) from three female control subjects and from a male control subject; the bottom tracings represent DNA digested with HpaII prior to PCR (+HpaII). The relative intensity of the two alleles after digestion represents the AR X-inactivation pattern for each individual (expressed as a ratio and normalized to the undigested samples). The tracing in males disappears, representing complete digestion of the unmethylated allele on the active X chromosome. Details of the AR X-inactivation assay have been described elsewhere, including methods for correcting for unequal peak heights owing to preferential allele amplification (Allen et al. 1992; Naumova et al. 1996; Plenge et al. 1997, 1999). b, Distribution of AR X-inactivation patterns in families with XLMR and control subjects. The AR X-inactivation patterns are shown for two control populations (unblackened circles) and for the XLMR carrier population (blackened circles).
Table 2.
Skewed X-Inactivation Patterns in XLMR Carriers
|
Frequency of Skewed X Inactivationa(%) |
|||
| X-InactivationPattern | FemaleControlSubjects | XLMRNoncarriersb | XLMRCarriersc |
| ⩾90:10 | 3 | 2 | 30 |
| ⩾80:20 | 9 | 15 | 48 |
| ⩾70:30 | 30 | 41 | 63 |
To assess statistically the distribution of X-inactivation patterns, a χ2 test was used to compare the number of female carriers above and below a particular threshold value (⩾90:10, ⩾80:20, and ⩾70:30) to that of the control population, as described by Plenge et al. (1999). To control for multiple hypothesis testing, a Bonferroni correction was applied, and the significance value was set at P<.01.
Results were not statistically significant.
All results were significant (P<.001).
To address whether the increased incidence of skewed inactivation was due to an association with skewing in only a subset of families, we examined separately each family with XLMR in which there were at least three female carriers (fig. 2 and table 1). Of the 20 distinct XLMR disorders examined in this way, 4 show a strong association with skewed X inactivation, in that all female carriers within each family demonstrate an X-inactivation pattern ⩾80:20. This is particularly striking for families K8435, K8300, and K8135, in which either all seven or all five carriers show such extreme skewing (P≪.0001 for each family) (table 1). An additional seven families show an incomplete association, with at least two—but not all—carriers demonstrating highly skewed patterns of inactivation. Given the rarity of highly skewed patterns in the general female population, however, each of these patterns is statistically significant (P<.01). Of the remaining families, only two (K8450 and K8295) had a large number of carriers with no apparent association between carrier status and skewing.
Figure 2.
Pedigrees of illustrative families with XLMR. AR X-inactivation patterns are shown near each informative female subject. Blackened symbols denote affected individuals, unblackened symbols denote unaffected individuals, and symbols with a black dot denote carriers.
For certain X-linked conditions, secondary cell selection is believed to occur after an initially random X-inactivation pattern has been established (Belmont 1996; Puck and Willard 1998; Willard 2000). For X-linked disorders associated with skewing, cell selection most likely occurs against those cells in which the mutation is on the active X chromosome; the mutation is therefore predicted to be associated with the preferentially inactive X chromosome. To determine whether the XLMR mutations in our families reside on the preferentially active or inactive X chromosome, we followed the cosegregation of the XLMR mutation and the differentially methylated AR allele (i.e., the allele associated with the inactive X chromosome). Of the XLMR carriers with X-inactivation patterns ⩾80:20, the XLMR mutation was on the preferentially inactive X chromosome in all 20 informative carriers (table 1). These data provide strong evidence that the differential growth advantage does, in fact, occur in favor of cells in which the XLMR mutation is on the active X chromosome.
The possibility of cell selection against certain X-linked mutations has been appreciated by geneticists for some time (Lyon 1968; Nyhan et al. 1970). However, previous studies have focused on either a specific X-linked disorder or a specific family, with emphasis on the clinical presentation of carrier females, and thus demonstrate a potentially significant ascertainment bias. In the present study, we have ascertained families with XLMR through a male index patient, without regard to the phenotype of female carriers.
The most important question raised by our study is whether skewed X inactivation is specific to particular classes of X-linked mutation (such as XLMR and the immune-deficiency syndromes [Belmont 1995]) or whether this phenomenon applies more generally to mutations in all X-linked genes. Although limited, available studies favor the hypothesis that skewing is restricted to certain classes of X-linked disorders (Belmont 1996; Willard 2000). If skewing were common to X-linked mutations generally, one would predict a diversity of phenotypes in disorders associated with skewed inactivation. However, of approximately a dozen X-linked disorders demonstrating either a complete or partial association with skewing (Willard 2000), only one—focal dermal hypoplasia (Gorski 1991)—does not have a mental-retardation or immune-deficiency phenotype.
If X-linked mutations were commonly associated with variable or incompletely penetrant skewed inactivation, as was observed for ∼50% of the families with XLMR in the present study, one would predict the detection of skewing for carriers of many (and perhaps all) X-linked disorders. In contrast to this prediction, however, several studies have demonstrated apparently random X inactivation in many female carriers, through use of a variety of assays (Willard 2000). As part of the present study, we examined X-inactivation patterns in our collection of carriers of Duchenne muscular dystrophy; these patterns did not differ significantly from those of female control individuals (data not shown). Notwithstanding the detection of occasional (usually symptomatic) carriers with demonstrated skewed X inactivation (Puck and Willard 1998), it is clear that skewing is not frequently observed in a high proportion of carriers of most X-linked conditions. Thus, these results also favor the hypothesis that skewing is specific to certain classes of X-linked mutations.
It may appear surprising that a group of disorders affecting the central nervous system would have a negative effect on cell proliferation in an apparently unrelated tissue (peripheral blood cells). One possible explanation is that peripheral blood cells serve as a phenotypic surrogate for cells in the central nervous system. Accordingly, XLMR genes, as a class, might affect in vivo cell viability or proliferation in many tissue types, and a number of examples are consistent with this suggestion. For example, genes responsible for syndromic XLMR are widely expressed and have demonstrated general roles in transcriptional regulation, cell proliferation, and/or development (Chelly and Mandel 2001). Some nonspecific XLMR genes also appear to be involved in cell proliferation and/or global transcriptional regulation (Allen et al. 1998; D'Adamo et al. 1998; Kutsche et al. 2000; Couvert et al. 2001). Thus, the apparent functions of at least some XLMR genes provide support for the hypothesis that these genes affect cell viability or proliferation generally. This finding also has practical significance, since it suggests that, for a substantial subset of XLMR disorders, the relevant loci are likely expressed in peripheral blood and thus are potentially accessible for experimentation (i.e., expression arrays).
In addition to providing potential insight into XLMR pathogenesis, the finding of skewed X inactivation may assist in the mapping of XLMR genes. Other studies have used the phenotype of skewed X inactivation to both establish X linkage (Zoghbi et al. 1990; Krepischi et al. 1998; Amir et al. 1999) and narrow the critical region for mutant genes (Gibbons et al. 1992; Sirianni et al. 1998). In XLMR, this approach would be especially useful in small families in which there are few affected males and in which carrier status is critical to achieving a meaningful LOD score (Lubs et al. 1999).
Assignment of carrier status may also be important for establishing a diagnosis and for genetic counseling in XLMR conditions. In pedigrees with nonspecific mental retardation that are so small that it is not possible to distinguish between X-linked and autosomal patterns of inheritance, the detection of multiple females with highly skewed X inactivation (i.e., patterns ⩾90:10)—a decidedly unlikely occurrence for autosomal or non-XLMR mutations—would greatly raise the suspicion that the disorder in question is XLMR.
Acknowledgments
We thank Amy Cottle, for assistance with the AR X-inactivation assay, and Jim Amos-Landgraf and Laura Carrel, for helpful discussions. This work was supported by National Institutes of Health research grants GM45441 (to H.F.W.) and HD26202 (to H.L., C.S., and H.F.W.).
References
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA (1998) PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet 20:25–30 [DOI] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW (1992) Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239 [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188 [DOI] [PubMed] [Google Scholar]
- Belmont JW (1995) Insights into lymphocyte development from X-linked immune deficiencies. Trends Genet 11:112–116 [DOI] [PubMed] [Google Scholar]
- ——— (1996) Genetic control of X inactivation and processes leading to X-inactivation skewing. Am J Hum Genet 58:1101–1108 [PMC free article] [PubMed] [Google Scholar]
- Cabezas DA, Arena JF, Stevenson RE, Schwartz C, Goldberg S, Morales A, Lubs HA (1999) XLMR database. Am J Med Genet 85:202–205 [DOI] [PubMed] [Google Scholar]
- Chelly J, Mandel JL (2001) Monogenic causes of X-linked mental retardation. Nat Rev Genet 2:669–680 [DOI] [PubMed] [Google Scholar]
- Couvert P, Bienvenu T, Aquaviva C, Poirier K, Moraine C, Gendrot C, Verloes A, Andres C, Le Fevre AC, Souville I, Steffann J, des Portes V, Ropers HH, Yntema HG, Fryns JP, Briault S, Chelly J, Cherif B (2001) MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet 10:941–946 [DOI] [PubMed] [Google Scholar]
- D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D (1998) Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet 19:134–139 [DOI] [PubMed] [Google Scholar]
- Gale RE, Wheadon H, Boulos P, Linch D (1994) Tissue specificity of X chromosome inactivation patterns. Blood 83:2899–2905 [PubMed] [Google Scholar]
- Gibbons RJ, Suthers GK, Wilkie OM, Buckle VJ, Higgs DR (1992) X-linked alpha-thalessemia/mental retardation (ATR-X) syndrome: localization to Xq12-q21.31 by X inactivation and linkage analysis. Am J Hum Genet 51:1136–1149 [PMC free article] [PubMed] [Google Scholar]
- Gorski JL (1991) Father-to-daughter transmission of focal dermal hypoplasia associated with nonrandom X-inactivation: support for X-linked inheritance and paternal X chromosome mosaicism. Am J Med Genet 40:332–337 [DOI] [PubMed] [Google Scholar]
- Hamel BC, Chiurazzi P, Lubs HA (2000) Syndromic XLMR genes (MRXS): update 2000. Am J Med Genet 94:361–363 [DOI] [PubMed] [Google Scholar]
- Krepischi AC, Kok F, Otto PG (1998) X chromosome inactivation patterns in patients with Rett syndrome. Hum Genet 102:319–321 [DOI] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, Boavida MG, David D, Chelly J, Fryns JP, Moraine C, Ropers HH, Hamel BC, van Bokhoven H, Gal A (2000) Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet 26:247–250 [DOI] [PubMed] [Google Scholar]
- Lubs H, Chiurazzi P, Arena J, Schwartz C, Tranebjaerg L, Neri G (1999) XLMR genes: update 1998. Am J Med Genet 83:237–247 [PubMed] [Google Scholar]
- Lubs HA, Schwartz CE, Stevenson RE, Arena JF (1996) Study of X-linked mental retardation (XLMR): summary of 61 families in the Miami/Greenwood Study. Am J Med Genet 64:169–175 [DOI] [PubMed] [Google Scholar]
- Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190:372–373 [DOI] [PubMed] [Google Scholar]
- ——— (1968) Chromosomal and subchromosomal inactivation. Ann Rev Genet 2:31–52 [Google Scholar]
- Nance WE (1964) Genetic tests with a sex-linked marker: glucose-6-phosphate dehydrogenase. Cold Spring Harbor Symp Quant Biol 29:415–424 [DOI] [PubMed] [Google Scholar]
- Naumova AK, Plenge RM, Bird LM, Leppert M, Morgan K, Willard HF, Sapienza C (1996) Heritability of X chromosome inactivation phenotype in a large family. Am J Hum Genet 58:1111–1119 [PMC free article] [PubMed] [Google Scholar]
- Nyhan WL, Bakay B, Connor JD, Marks JF, Keele DK (1970) Hemizygous expression of glucose-6-phosphate dehydrogenase in erythrocytes of heterozygotes for the Lesch-Nyhan syndrome. Proc Natl Acad Sci USA 65:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge RM, Hendrich BD, Schwartz C, Arena JF, Naumova A, Sapienza C, Winter RM, et al (1997) A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet 17:353–356 [DOI] [PubMed] [Google Scholar]
- Plenge RM, Tranebjaerg L, Jensen PK, Schwartz C, Willard HF (1999) Evidence that mutations in the X-linked DDP gene cause incompletely penetrant and variable skewed X inactivation. Am J Hum Genet 64:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck JM, Willard HF (1998) X inactivation in females with X-linked disease. New Engl J Med 338:325–328 [DOI] [PubMed] [Google Scholar]
- Sirianni N, Naidu S, Pereira J, Pillotto RF, Hoffman EP (1998) Rett syndrome: confirmation of X-linked dominant inheritance and localization of the gene to Xq28. Am J Hum Genet 63:1552–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Schwartz CE, Schroer RJ (1999) X-linked mental retardation. Oxford University Press, New York. [Google Scholar]
- Willard HF (2000) The sex chromosomes and X chromosome inactivation. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B (eds) The metabolic and molecular bases of inherited Disease, 8th ed. McGraw-Hill, New York, pp 1191–1221 [Google Scholar]
- Zoghbi HY, Percy AK, Schultz RJ, Fill C (1990) Patterns of X chromosome inactivation in the Rett syndrome. Brain Dev 12:131–135 [DOI] [PubMed] [Google Scholar]