Abstract
Chronic pain is a major therapeutic problem as the current treatment options are unsatisfactory with low efficacy and deleterious side effects. Voltage-gated Ca2+ channels (VGCCs), which are multi-complex proteins consisting of α1, β, γ, and α2δ subunits, play an important role in pain signaling. These channels are involved in neurogenic inflammation, excitability, and neurotransmitter release in nociceptors. It has been previously shown that N-type VGCCs (Cav2.2) are a major pain target. U.S. FDA approval of three Cav2.2 antagonists, gabapentin, pregabalin, and ziconotide, for chronic pain underlies the importance of this channel subtype. Also, there has been increasing evidence that L-type (Cav1.2) or T-type (Cav3.2) VGCCs may be involved in pain signaling and chronic pain. In order to develop novel pain therapeutics and to understand the role of VGCC subtypes, discovering subtype selective VGCC inhibitors or methods that selectively target the inhibitor into nociceptors would be essential. This review describes the various VGCC subtype inhibitors and the potential of utilizing VGCC subtypes as targets of chronic pain. Development of VGCC subtype inhibitors and targeting them into nociceptors will contribute to a better understanding of the roles of VGCC subtypes in pain at a spinal level as well as development of a novel class of analgesics for chronic pain.
Keywords: α2δ subunit, Inhibition, L-type Ca2+ channels, N-type Ca2+ channels, Pain, Targeting, T-type Ca2+ channels, Voltage-gated Ca2+ channels
1. INTRODUCTION
Pain is the somatosensory detection of noxious stimuli, helping our body be aware of damaging stimuli. These signals are transmitted into the central nervous system through a subset of peripheral nerve fibers, which are called nociceptors. These consist of thinly myelinated Aδ and unmyelinated C fiber primary sensory neurons [1]. These neurons are pseudobipolar as they have two peripheral and central branches arising from a single axon coming from the cell body. These cell bodies are clustered in dorsal root ganglia (DRG). The sizes of the cell bodies of nociceptors range from small (100-400 µm2) to medium (400-1200 µm2). Generally, large DRG cells are not considered to be nociceptive neurons. The presynaptic terminals, which are the central branches of nociceptors, form synapses with second order neurons in specific locations of the spinal cord. For example, Aδ fibers terminate in lamina I and V, while C fibers project to Lamina I and II. Neurotransmitter release in the synapse is the primary mode of transmission to the central nervous system from nociceptors. Substance P, CGRP, and glutamate are representative examples of these neurotransmitters. The peripheral nerve endings innervate the skin and viscera that receive either the high threshold internal (inflammatory soups) or external (heat, acid, and pressure) signals. These nerve endings also release substance P and CGRP, which may contribute to neurogenic inflammation [2].
Nociceptors are specialized high-threshold neurons that respond to noxious stimuli. The peripheral nerve endings of nociceptors express specialized high threshold receptors such as ligand gated ion channels (TRP channels, P2X channels, and ASIC channels, etc) and G-protein coupled receptors (Mas-related G-protein class, etc). These proteins convert high threshold external or internal stimuli into electrical signaling such as action potentials, which are propagated through primary sensory neurons. Here, voltage-gated Na+ channels (Nav1.3, Nav1.7, and Nav1.8), as relay stations, are in charge of this action potential propagation. As a final step in the peripheral nervous system, the electrical signaling ends in presynaptic terminals and transmits to second order neurons in the spinal cord. In the presynaptic terminals, neuronal voltage-gated Ca2+ channels (VGCCs, in particular, Cav2.2) play an important role in pain neurotransmitter release such as CGRP, Substance P, and glutamate. Taken together, this series of events (transduction, propagation, and transmission) in peripheral nociceptors comprise pain signaling.
While the pain signaling works as a physiological defense system to protect ourselves from harmful conditions, persistent tissue and nerve injury cause chronic inflammatory and neuropathic pain, which comprise major medical problems. Inflammatory pain is caused by tissue injury, which release inflammatory mediators containing cytokines and neuropeptides. Neuropathic pain is caused by neural damage by HIV infection, cancer, mechanical trauma, and spinal cord injury. Dysfunctional pain is defined by pain with no identifiable sources. Fibromyalgia, irritable bowel syndrome, interstitial cystitis, erythromelalgia, paroxysmal extreme pain disorder, and complex regional pain syndrome (CRPS) are examples of neuropathic or dysfunctional pain. Nociceptive or inflammatory pain can be called adaptive because the pain usually disappears after avoidance of stimuli or resolution of injury. The neuropathic pain and dysfunctional pain are maladaptive, in which pain occurs without identifiable and detectable stimuli (spontaneous pain) and also with hyperalgesia and allodynia [3]. These symptoms are a result of the neuron’s increased sensitivity to painful- and normal-threshold stimuli, and hyperexcitability. Modulations of ion channels and GPCRs by chronic pathological pain are involved in high sensitivity or hyperexcitability.
A significant amount of progress in understanding chronic pain mechanisms has been achieved, although it is still not complete due to its complexity and multiple mechanisms. Many proteins have been suggested as targets for pain therapeutics; however, actual therapeutic targets are few in number. The nociceptive and inflammatory pain currently are mainly addressed with NSAIDs and opioids that inhibit COX enzymes and activate opioids receptors, respectively. However, current therapeutics for chronic and maladaptive pain are not satisfactory. The major problems of current chronic pain medicines are side effects and low efficacy [4]. For example, opioids and NSAIDs (e.g. codeine and ibuprofen) have several side effects such as tolerance and gastric problems, respectively. More importantly, they are not considered effective especially against neuropathic chronic pain. Number needed to treat (NNT; an epidemiological measure of effectiveness) of these drugs is around 4 (NNT, 1 is ideal and 2.5 is satisfactory). Other than these medications, antidepressants, antiepileptics, topical capsaicin, and local anesthetics have been used for chronic neuropathic pain, but they still have the same limitations as opioids and NSAIDs [5]. With an unmet need for pain therapeutics, it is important to pursue the development of novel therapeutics with high efficacy and low side effects.
Among the targets of chronic pain, inhibiting VGCCs in nociceptors is an effective way for reducing pain. As mentioned above, VGCCs are directly involved in neurotransmitter release at synapses in the spinal cord, as well as neurogenic inflammation in the peripheral nerve endings. This means that VGCCs for neurons of the peripheral nerve system are end points for both transmitting pain signals into central nervous system and for evoking neurogenic pain in the periphery. In addition, many endogenous factors up-regulated by neuropathic pain modulate VGCCs to induce hypersensitivity [6]. Currently, three drugs targeting VGCCs are commercially available for neuropathic pain medication; pregabalin, gabapentin, and ziconotide. However, pregabalin and gabapentin have serious side effects and low efficacy, and also their role in inhibiting VGCCs is controversial. Ziconotide still has severe side effects and requires intrathecal administration through a catheter and pump. On the positive side, it doesn’t lead to tolerance and dependence and is more effective for refractory pain than even opioids [7]. In this regard, discovering more selective and potent drugs for VGCCs and targeting them into nociceptors is beneficial for chronic pain therapeutics. The following will describe VGCC subtypes as targets of chronic pain, VGCC subtype inhibitors, and the possibility of therapeutic application and strategies based on the characteristics of VGCCs.
2. VOLTAGE-GATED CA2+ CHANNELS
Calcium ions (Ca2+) exist at a higher level of concentration outside the cell membrane (~2 mM) than inside (~50-100 nM). The Ca2+ gradient is maintained until the activation of VGCCs by membrane depolarization, which is evoked by activation of ligand gated ion channels or GPCRs. The resulting Ca2+ influx (10 to 100 times increase of intracellular Ca2+) plays a role in muscle contraction, hormone secretion, synaptic transmission, enzyme activity, and gene expression in different cell types such as muscle, endocrine cells, and neurons [8, 9].
The functional VGCCs are a protein complex of α1, α2δ, β, and γ subunits. The α1 subunits are the key molecule of the channel complex because it is capable of Ca2+ conduction, or generating Ca2+ influx. The α2δ, β, and γ subunits are auxiliary. They increase the expression of α1 subunits in the plasma membrane as well as modulate the function of α1 subunits, resulting in functional diversity in different cell types. The α2δ subunits increase maximum currents by trafficking of the α1 subunits. Also, they increase the inactivation rate as well as shift steady state inactivation into hyperpolarization. The β subunits located in the cytosol also increase maximum currents by enhancing trafficking of α1 subunits, shifting activation to the left, and increasing open probability. However, the role of γ subunits as auxiliary subunits is controversial. Rather, they are more important in modulating AMPA receptors. A recent review described other functions of auxiliary subunits of VGCCs [10].
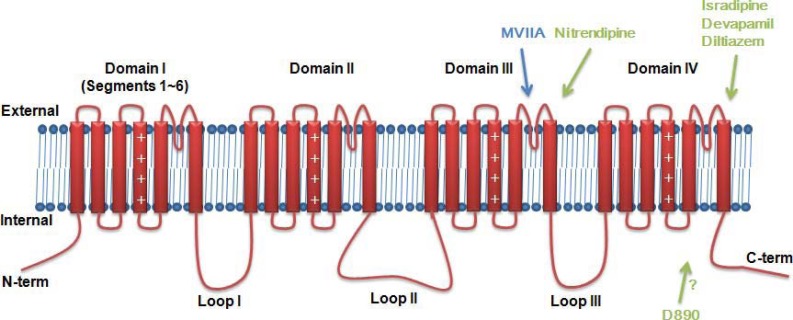
The α1 subunit consists of ~2000 amino acids and four repeated domains, each of which is homologous with voltage-gated potassium channels (VGPCs). These domains are connected with three intracellular loops. In each domain, first four transmembrane segments (S1-S4) form the voltage sensing domain, while S5, P-loop, and S6 form the pore domain (Fig. 1). Based on sequence differences, the mammalian α1 subunits are currently classified into 10 subtypes. These subtypes have different biophysical properties (activation, inactivation, conductance, and deactivation), expression patterns, and pharmacology (Table 1).
Fig. (1).
Primary structure of α1 subunits of VGCCs.
Table 1.
Classification of Voltage-gated Ca2+ Channels
| Current Name | Channel Name | Gene Name | Half Activation Voltage (Reference [23]) | |
|---|---|---|---|---|
| High-voltage activated (HVA) | L-type | Cav1.1 | CACNA1S | 8~14mV |
| Cav1.2 | CACNA1C | -4~-18.8mV | ||
| Cav1.3 | CACNA1D | -15~-37mV | ||
| Cav1.4 | CACNA1F | -2.5~-12mV | ||
| P/Q-type | Cav2.1 | CACNA1A | -5~10mV | |
| N-type | Cav2.2 | CACNA1B | 7.8~9.7mV | |
| R-type | Cav2.3 | CACNA1E | -29.1~3.5mV | |
| Low-voltage activated (LVA) | T-type | Cav3.1 | CACNA1G | -46mV |
| Cav3.2 | CACNA1H | -46mV | ||
| Cav3.3 | CACNA1I | -44mV |
L-type VGCCs, first characterized from cardiac muscle, include four genes (Cav1.1~1.4). These channels are activated at high voltage, thus classified as HVA (high voltage activated) Ca2+ channels. Their half-maximal activations are around -20 to 0mV, except for Cav1.3, which is activated by low voltages (-30 ~ -20mV). These channels are mainly expressed in cardiac and skeletal muscle cells and responsible for muscle contraction, hormonal secretion, and gene transcription. Dihydropyridine (DHP) antagonized L-type VGCCs and had been useful for isolating and characterizing these channels. Also, phenylalkylamine (PAA) and benzothiazepine (BTZ) have antagonizing effects against the channels.
P/Q-type VGCCs (Cav2.1) were found to be expressed in purkinje cells and cerebellar granule neurons, and N-type VGCCs (Cav2.2) were characterized from the brain [11]. These channels are expressed in presynaptic nerve terminals and play an important role in neurotransmitter release. These two types have been known to play a complementary role. These channels are antagonized by peptide neurotoxins ω-Agatoxin IVA and ω-conotoxin MVIIA, respectively. Residual current of R-type VGCCs (Cav2.3) still remains after blocking L-, N-, P/Q-type VGCC subtypes. Another peptide neurotoxin SNS-482, isolated from the tarantula Hysterocrates gigas, inhibits R-type VGCCs. The expression of P/Q-type, N-type, and R-type Cav channels are restricted to neurons. R-type VGCCs as well as L-type VGCCs (Cav1.3 only) are activated at rather lower voltage than other channels, but still are classified as HVA channels.
The other class of VGCCs (Cav3.1~3.3) are LVA (low voltage activated) T-type VGCCs. These channels are activated with half voltage activation of -46 ~ -44mV, thus open at resting membrane potentials. These channels regulate pacemaking, subthreshold activation, and repetitive firing in cardiac or neuronal cells. Interestingly, T-type VGCCs are not modulated by auxiliary subunits such as α2δ and β subunits [12, 13].
In addition to subunit associations and subtype differences, alternative splicing gives more diversity in the function of VGCCs. For example, N-type VGCCs have a number of splice variants, which depend on developmental stage and cell types [14, 15]. The splicing events are regulated by a neuronal splicing factor such as Nova [16]. For example, Nova-2 increases inclusion of e24a and decreases inclusion of e31a of N-type VGCCs in brain [17]. These variants showed distinct gating properties such as channel activation, inactivation and drug responsiveness. It has been shown that e37a of N-type VGCCs are expressed in capsaicin sensitive neurons, which are involved in pain behaviors. Further, in order to see the specific role of e37a of N-type VGCCs, e37a was replaced with e37b in mice. These mice showed no differences in pain behaviors, but less responsiveness to morphine. This fact suggested that splice variants can affect the pharmacology of N-type VGCCs and alternative splicing by splicing factors are important for pain signaling [18-20].
In generating action potentials, voltage-gated Na+ channels (VGSCs) and voltage-gated K+ channels (VGPCs) are involved in rising and falling phase. Thus, VGSC blocker abolishes action potentials. However, in contrast to VGSC blockers, VGCC blockers have shown small effects on action potentials. For example, L-type VGCC blockers abolish contractile force without a major change in the action potentials. Generally, VGCC blockers broaden the falling phase of action potentials [21]. However, it has been argued that less contribution of L-type VGCC currents in action potentials result from slow and use-dependent inhibition of L-type VGCC blockers [22]. Notably, T-type VGCCs are activated at near resting membrane potentials; therefore, it will amplify weak signals and cause low-amplitude intrinsic neuronal oscillations. T-type VGCC knockout (Cav3.1-/-) shows reduced burst firing in thalamocortical neurons, suggesting its contribution to repetitive firing of action potentials. Two VGCC subtypes, Cav1.3 and Cav2.3, are activated at relatively low voltage so that they may have distinct roles with other HVA Ca2+ channels such as spontaneous firing or rhythmic burst discharges [23, 24]. These diverse characteristics of each subtype orchestrate neuronal spikes in sensory nervous system.
3. VGCC SUBTYPES AND PAIN
The role and importance of each VGCC subtype in pain signaling would be firstly glimpsed by where they are expressed in pain processing in the DRG and spinal cord. Further, the effect of genetic ablation of VGCC subtypes in mice on pain behaviors gives invaluable information about their function in pain. Also, their roles in chronic pain mechanisms, which are related to peripheral and central sensitization, would be determined by up- or down- regulation by neuropathic and inflammatory pain. It has been shown that various subtypes of VGCCs are expressed in dorsal root ganglia (DRG) according to their sizes [25]. In situ hybridization using RNA probes showed that all subtypes of VGCCs are expressed in rat DRG neurons except Cav1.4 and Cav3.1. It also has been shown that α2δ1 and α2δ2 subtypes are expressed in small and medium neurons (possibly nociceptors), while α2δ3 subtype is expressed in large neurons [26]. VGCC subtypes are also expressed in the spinal cord, suggesting critical roles in pain signaling through second order neurons in the spinal cord [27, 28]. In presynaptic nerve terminal of DRG and postsynaptic cells in spinal cord, VGCCs play different roles. VGCC subtypes in presynaptic terminals are involved in neurotransmitter release and excitability; those in the spinal cord are involved in excitability and central sensitization [29]. In peripheral nerve endings, it has been suggested that VGCC subtypes play an important role in peptide release, which might be involved in neurogenic inflammation [2]. Several reviews have already described the importance of VGCC subtypes in terms of pain signaling [30, 31]; this review focuses on the particular roles of VGCC subtypes based on recent evidence on their localization, genetic ablation, and regulation upon chronic pain.
3.1. L-type Ca2+ Channels
L-type VGCCs are broadly expressed in skeletal and cardiac muscle, neurons, auditory hair cells, pancreatic cells, and retina [32]. Several lines of evidence have shown that DRG and spinal cord neurons express Cav1.2 and Cav1.3 [33, 34]. Electrophysiology in DRG showed that L-type VGCCs are present largely in small and large neurons [25]. Whether these channels are regulated in chronic pain state is not conclusive. One study using RT-PCR showed that L-type VGCCs are down-regulated in DRG upon chronic constriction injury (CCI) and sciatic nerve axotomy in rats [35]. They suggested that decreases of Cav1.2 and Cav1.3 in DRG contribute to hyperexcitability of neuropathic pain by modulating Ca2+-dependent inactivation or facilitation as a negative feedback [36]. Inversely, it has been shown that Cav1.2 is up-regulated in the spinal cord in the sciatic nerve ligation (SNL) model. In this study, it has also been shown that knockdown of Cav1.2 using siRNA and peptide nucleic acids (PNA) reversed the neuropathic pain, while that of Cav1.3 did not [37]. This fact suggests that spinal Cav1.2 is important in pain signaling. It was supported by miR-103 decreasing translational levels of Cav1.2. Neuropathic pain decreases miR-103 and thus increases Cav1.2, resulting in hyperexcitability and allodynia [38]. Cav1.3 rather is involved in other sensory modalities or motor neurons according to its expression in lamina IX in spinal cord [39]. Knockout mice of Cav1.3 did not display atypical pain behaviors. The lack of effects in Cav1.3-/- mice might result from compensation effects, but the fact that RNAi knockdown of Cav1.3 did not affect pain behavior supports diminished role of Cav1.3 to pain signaling [40, 34]. Overall, although Cav1.3 is expressed in the spinal cord and brain stem, it seems to have fewer effects on pain signaling. However, it could not be ruled out because rather low voltage activation of Cav1.3 might contribute to spontaneous neuronal firing similar with T-type VGCCs. Another interesting aspect of L-type VGCCs in pain processing is their expression in peripheral nerve endings. Ca2+ influxes in corneal nerve terminals branching Aδ- and C-fibers are inhibited by nifedipine and diltiazem [2]. This result suggests that L-type VGCCs also are involved in neuropeptide release in peripheral nerve endings, which contribute to neurogenic inflammation.
3.2. P/Q-type Ca2+ Channels
P/Q-type VGCCs are more centrally expressed than peripherally, thus being more associated with migraine. Missense mutations of P/Q-type VGCCs cause familial hemiplegic migraine (FHM) [41]. It has been known that neuronal P/Q-type VGCCs are complementary to N-type VGCCs in the central nervous system. For example, these two channels mediate neurotransmitter release in distinct hippocampal inhibitory neurons [42]. Also, in DRG neurons, Cav2.1 is not localized with substance P or CGRP, which showed similar localization with Cav2.2. ω-agatoxin IVA, a P/Q-type VGCC blocker, did not affect the release of substance P or CGRP [27, 43]. In the spinal cord, they are located in presynaptic nerve terminals of lamina II-VI, while Cav2.2 is in the presynapse of lamina I and II. Therefore, it is likely that P/Q-type VGCCs are more involved in polysynaptic transmission rather than monosynaptic transmission between nociceptors and lamina I and II [29]. ω-agatoxin TK, a P/Q-type VGCC blocker, had no effect on postsynaptic Ca2+ influx in lamina I [29]. However, we cannot rule out the possibility of relevance to chronic pain, because a recent report showed that induction of spinal LTP, a marker of chronic pain, was strongly suppressed with ω-agatoxin IVA [44].
In accordance with its expression, the effects of Cav2.1 in pain signaling are rather complicated. In SNL neuropathic pain model, ω-agatoxin IVA has little effect on pain behaviors [45-47]. In knockout mice for Cav2.1, mice show enhanced sensitivity to thermal stimuli. Also, two P/Q-type VGCC mutant mice Leaner mice (tgla/tgla) and Roller mice (tgrol/tgrol) have been examined for pain behaviors. Leaner mice (tgla/tgla) showed reduced mechanical pain but enhanced heat pain like knockout mice. However, Roller mice (tgrol/tgrol) showed reduced sensitivity to heat pain in a gene concentration-dependent manner [48-50]. These facts suggest that the role of P/Q-type VGCCs in pain is complicated and is involved in different sensory modalities compared to N-type VGCCs. Therefore, the role of P/Q-type VGCCs in pain should be interpreted in specific contexts, as another review suggested [30].
3.3. N-type Ca2+ Channels
Cav2.2 is expressed in the central and peripheral nervous systems including the brain, spinal cord, and primary sensory neurons [51, 27]. It has been reported that Cav2.2 and substance P expression overlapped in DRG and the spinal cord. In accordance with their location, Cav2.2 antagonists ω-conotoxins MVIIA and GVIA block neurotransmitter release in the spinal cord [43, 52-54]. Autoradiography of ω-conotoxins also show that Cav2.2 is rich in laminae I and II [55, 56]. However, contrary to suggested specific localization of Cav2.2 with substance P, electrophysiology in DRG shows that N-type VGCC current are present evenly regardless of the size [25, 57]. In terms of regulation, it has been shown that the expressions of Cav2.2 are modulated by inflammatory and neuropathic pain. For example, inflammation by intraplantar CFA increases N-type VGCC protein expression but not mRNA [58]. CCI model, a neuropathic pain model, showed increased immunoreactivity against Cav2.2 in lamina II in spinal dorsal horn [59]. However, partial sciatic nerve injury (PSNL) did not regulate Cav2.2 but rather up-regulate the α2δ subunit [60]. Inflammation by intraplantar CFA reduced the contribution of N-type VGCCs in NK1 positive neuron in lamina I [61]. Therefore, up-regulation of α1 subunit of Cav2.2 by inflammation and nerve injury is ambiguous. Rather, increase of Cav2.2 depends on α2δ subunit, which traffic Cav2.2 into cell membranes because mRNA of Cav2.2 has not been changed upon inflammatory pain [58]. Also, it is possible that α2δ subunit cause synaptogenesis so that increase of synaptic area affects upregulation of Cav2.2 [62].
Genetic ablation of Cav2.2 also confirmed its importance on chronic pain. Three groups independently generated Cav2.2-/- mice and examined for pain behaviors [63-65]. Their results in acute pain are not consistent with each other. Kim et al. showed a reduction in mechanical acute pain but not thermal acute pain [64]. However, in two other groups, knockout mice only showed a reduction in thermal acute pain. In accordance with this inconsistence on acute pain, ω-conotoxins’ effect on acute pain are conflicting [66-69]. However, in formalin test, all knockout mice showed delayed responses in phase II, suggesting N-type VGCCs are involved in inflammatory pain. Also, intrathecal ω-conotoxins potently block pain behaviors by neuropathic pain [45]. These results suggest antagonizing Cav2.2 is more beneficial in reducing pathological pain than acute pain, although the molecular basis of these differential effects remains elusive.
3.4. R-type Ca2+ Channels
HVA Ca2+ currents resistant to L-, P/Q-, and N-type VGCC blockers are R-type VGCC currents, which are generated by Cav2.3. The channel subtype is also expressed in DRG and the spinal cord. Like Cav2.2, there has been a report that Cav2.3 has several, at least six, isoforms. Among them, Cav2.3(e) is expressed in small, IB4 negative, and capsaicin positive DRG cells, suggesting this isoform is potentially involved in pain signaling [70, 71]. In fact, SNX-482, an antagonist for R-type VGCCs, reduced hyperexcitability of C- and Aδ- fibers in SNL neuropathic pain models [72, 73]. In terms of regulation, it has been shown that R-type VGCC currents was reduced by neuropathic pain models such as PSNL, while N-type VGCC currents was increased, suggesting adaptive changes into N-type VGCCs. However, interestingly, Cav2.3-/- mice showed the adaptive changes into L-type VGCCs, suggesting a role of R-type VGCCs in adaptive mechanisms by neuropathic pain [74]. This might explain why Cav2.3-/- mice showed normal behavior against acute pain [75, 76]. Also, there is a report that R-type VGCCs are expressed in PAG (periaqueductal gray) and has an anti-nociceptive role. These results show that, like P/Q-type VGCCs, the contribution of R-type VGCCs to pain is rather complicated.
3.5. T-type Ca2+ Channels
T-type VGCCs are broadly expressed in heart, muscle, brain, and peripheral nerve, suggesting its contribution to cardiac function, epileptics, sleep, and pain. In the pain signaling, T-type VGCCs (Cav3.2 and Cav3.3) also are expressed in small and medium DRG and spinal cord, being involved in excitability, neurotransmitter release, and pain sensitization. It has been reported that presynaptic Cav3.2 was involved in glutamate release in synapse of lamina I and II [77, 78]. In particular, spinal T-type VGCC currents was important in hyperalgesia in NK receptors positive lamina I neurons, emphasizing their role in central sensitization [79]. In terms of regulation, it has been shown that T-type VGCC currents are down-regulated by neuropathic pain condition such as the CCI model. Control mice showed 25% T-type VGCC current in medium sized DRG cells but these were abolished in the neuropathic pain model [80, 81]. However, more recently, it has been shown that T-type VGCC current is increased in small neurons of CCI neuropathy model and also that Cav3.2 and Cav3.3 mRNA is increased in rat chronic compression of DRG (CCD) model [82, 83]. The different regulations of T-type channels in small and medium sized DRG cells and their physiological effects should be further investigated. Possibly, medium sized DRG cells do not participate in pain circuitry. Shin et al. showed that Cav3.2 is involved in D-hair receptors. Cav3.2-/- mice showed no effects on mechanistic nociceptive C-fibers [84]. Despite the promiscuous role of T-type channels, other evidences indicated that Cav3.2 is involved in specific contexts of pain such as visceral pain and diabetic neuropathic pain. For example, butyrate, which is increased by irritable bowel syndrome, up-regulated T-type VGCC currents and antisense knockdown of Cav3.2 reversed the hypersensitivity [85]. Selective T-type VGCC blocker, (3β,5α,17β)-17-hydroxyestrane-3-carbonitrile, reduced thermal and mechanical allodynia from diabetic neuropathy in leptin deficient mice [86]. Silencing of Cav3.2 using antisense oligodeoxynucleotides also reduced hyperalgesia and allodynia of rat CCI model [87]. Further, antisense for Cav3.2 and Cav3.3 attenuated pain in CCD model [88]. In accordance with these knock down experiment, Cav3.2 knockout mice showed attenuation in pain behaviors [89]. Interestingly, there was a report that genetic ablation of Cav3.1 causes hypersensitivity to noxious visceral stimuli, which is involved in a lack of thalamic burst firing [90]. However, we cannot exclude the possibility of Cav 3.1 involvement in neuropathic pain such as the SNL model [91]. Taken together, among LVA VGCCs, peripheral Cav3.2 is an extremely prominent target for analgesic development.
3.6. α2δ Subunits
α2δ subunits increase Ca2+ current amplitude by increasing functional α1 subunits at membrane site [92]. Therefore, α2δ subunits have been used to increase Ca2+ currents in functional assay for VGCCs [93]. Also, these subunits modulate gating kinetics and pharmacology of α1 subunits. These biophysical characteristics might affect hyperexcitability by neuropathic pain [94]. The importance of α2δ subunits in chronic pain has been verified that the subunits are up-regulated by neuropathic and inflammatory pain [95]. For example, inflammation by intraplantar CFA increases mRNA and protein expression of α2δ subunits [58]. It has been shown that mRNA levels of α2δ1 are upregulated particularly in small DRG of PNSL models [96]. In addition, the relevance of α2δ subunits in pain is supported by interesting evidence that α2δ subunit homolog straightjacket is also responsible for thermal pain in Drosophila [97]. In accordance with this result, α2δ3 mutant mice showed increased latency on thermal pain [97]. Recently, another mechanism of α2δ has been revealed that the α2δ subunit is responsible for synaptogenesis by interaction with thrombospondins [62]. This fact suggests that the synaptogenesis by α2δ subunit might affect hyperexcitability and chronic pain.
It has been known that gabapentinoids such as gabapentin and pregabalin alleviate neuropathic pain by reducing Ca2+ current. However, the direct inhibiting effects for VGCCs are controversial. Several report in the literature reported that gabapentin inhibited high-threshold Ca2+ current by acting on α2δ subunits [98-100]. However, other evidences showed that these drugs did not show VGCC current blocking effects [92]. More likely, gabapentin may reduce excitability by inhibiting synaptogenesis. The exact mechanism of α2δ in chronic pain remains elusive.
4. VGCC INHIBITORS
Three VGCC subtypes (Cav1.2, Cav2.2, and Cav3.2) play an important role in either DRG or the spinal cord in pain processing and chronic pain development. Given that these α1 subunits are 24-multiple transmembrane domains, it is no wonder that various extracellular ligands (either exogenous or endogenous) and intracellular modulators interact with VGCCs and modulate functions of VGCC subtypes, thus resulting in regulation of chronic pain phenotypes. These regulators can be used for therapeutic developments for pathological pain. For example, ω-conotoxin MVIIA, which extracellularly interacts with N-type VGCCs, has been approved by U.S. FDA for refractory pain. A tat-associated peptide CRMP-2, which intracellularly interferes with the interaction between N-type VGCCs and collapsin (a synaptic protein enhancing the function of N-type Cav currents), showed analgesic effects [101]. These inhibitors were quite useful for pain therapeutic developments and strategy for targeting these into nociceptors.
4.1. L-type Ca2+ Channel Inhibitors
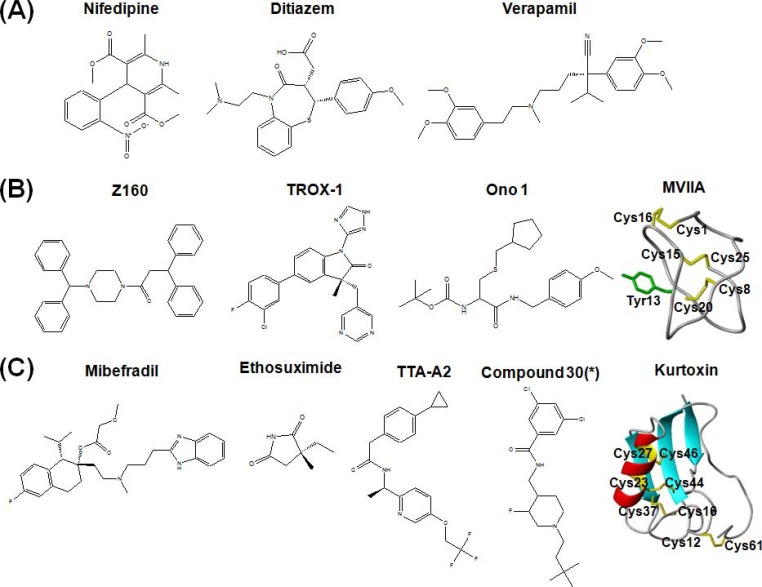
It has been well established that classic calcium channel blockers DHP, PAA, and BTZ mainly target L-type VGCCs. Nifedipine, verapamil, and diltiazem are representative calcium channel blockers, respectively (Fig. 2A) [102]. These has been called “Ca2+ antagonist” because they are neutralized with elevated Ca2+ concentration [103]. These drugs have been known to bind to pore forming regions (S5 or S6 in domain III and IV, Fig. 1). For example, isradipine (DHP) and devapamil (PAA) interact with S6 in domain IV [104]. Nitrendipine (DHP) binds to S5-S6 of domain III [105]. Diltiazem (BTZ) also binds to S5 and S6 in domain III and IV [106]. It has been known that the binding site of DHP is close to the extracellular surface based on the blocking effects of extracellular and intracellular application of charged DHP [107]. DTZ323, a quaternary 1,5-benzothiazepine analog, inhibited current extracelluarly, but not intracellulary, showing that BTZ also interacts with the extracellular portion of Ca2+ channels. However, D890, quaternary form of methoxyverapamil, only inhibited Ca2+ current intracellularly, showing that binding site of PAA can be accessed inside of the cells. It should be noted that voltage-gated ion channels are moving targets which show conformational changes between closed, open, inactivated states. Some drugs showed different affinities against different state of channels. This phenomenon is called “state-dependency” of a drug, which results in “use dependency” or “frequency dependency” [108]. The state or use dependency of the drug is useful in terms of therapeutic developments because neuropathic pain is usually related to hyper-excitability. It has been shown that nitrendipine binds the inactivate state of cardiac calcium channels with subnanomolar affinity but the resting state with ~700nM affinity [109]. Other than these small molecules, conotoxin TxVII blocks L-type VGCCs. Interestingly, TxVII inhibits DHP sensitive currents in cultured Lymnaea RPeD1 neurons, but not in PC12 cells, suggesting that how it has subtype selectivity [110]. However, there is still no information which subtype is involved in the action of TxVII and it might modulate pain signaling.
Fig. (2).
Molecular structures of VGCC subtype blockers (A) L-type VGCC blockers (B) N-type VGCC blockers (C) T-type VGCC blockers. * indicates that Compound 30 is a label used in [168].
As many authors mentioned, analgesic effects of L-type Cav blockers are ambiguous. Ca2+ channel blockers, including nifedipine, verapamil, and diltiazem had no effects on pain behaviors when injected intrathecally [111, 73]. However, intraperitoneal injection of verapamil reduced mechanical and thermal pain in rats. Also, intrathecal verapamil and flunarizine reduced thermal pain in an opioid receptor dependent manner [112, 113]. In the neuropathic pain model of SNL, nicardipine reduced pain behaviors [37]. It is probable that these blockers act on L-type VGCCs in both excitatory and inhibitory neurons in the spinal cord, showing sum effects of the blockers. Also, different affinities of blockers against Cav1.2 and Cav1.3 might explain the inconsistency of in vivo analgesic activities. Therefore, subtype specific blockers against Cav1.2 or the specific targeting of drugs into nociceptors might improve the analgesic effect of L-type VGCC blockers because of the preferential expression of Cav1.2 in nociceptor and its pain modulation.
4.2. N-type Ca2+ Channel Inhibitors
In contrast to the organic blockers for L-type VGCCs, peptide neurotoxins from cone snails have been used for isolation and characterization of N-type VGCCs. ω-conotoxins GVIA and MVIIA isolated from the venom of Conus geographus and Conus magus, respectively, block N-type VGCCs with nanomolar affinity (Fig. 2B). Electro-physiology using chimeric channels has shown that ω-conotoxin GVIA bind to S5 and H5 in domain III [114]. Binding sites of ω-conotoxins GVIA and MVIIA are partially overlapped, which suggest they have similar binding mechanisms [115]. Due to their high specificity and nanomolar range affinity, they have been useful to characterize the function of N-type VGCCs in physiology. Eventually, synthetic ω-conotoxin MVIIA was approved by U.S. FDA in 2004 for refractory pain. Since confirming N-type VGCCs as a pain therapeutic target, many academics and companies have developed other peptide neurotoxins and active small molecules. The main reason of this is that the use of ziconotide has been limited due to uncomfortable intrathecal administration, severe side effects, and cost of production [116]. A good example is ω-conotoxin CVID isolated from Conus catus, which now is in clinical trials as Leconotide. This conotoxin has been improved in terms of selectivity for N-type over P/Q-type VGCCs. In behavioral tests, intrathecal ω-conotoxin CVID has a better therapeutic index in pain animal studies [69]. Surprisingly, a recent report showed that intravenously administered Leconotide has an analgesic effect without side effects [117]. This could circumvent the need for the uncomfortable intrathecal administration of Ziconotide. Another ω-conotoxin FVIA, isolated from Conus fulmen, has a better reversibility compared to ω-conotoxin MVIIA, which might improve side effects and managements of ziconotide administration. Several spider toxins also showed improvements in terms of their effects on motor coordination. The examples are huwentoxin-I and PnTx3-6, which showed low motor deficits [118, 119]. However, all these peptide neurotoxins are still expensive to produce due to limited peptide synthesis and then low cysteine folding yield.
As for small molecules against Cav2.2, Takashi et al. described current developments of N-type VGCC blockers [120]. Several groups tried to develop small molecules based on a structural analysis of ω-conotoxin MVIIA or GVIA. Other groups tried to design and synthesize blockers based on pre-existing organic blockers. Currently, the developed small molecules have several moieties such as piperazine, 4-aminopiperidine, aryl sulfonamide, and aryl heterocycle moiety. The snutch group designed and synthesized two N-type VGCC blockers NP078585 and NP118809 to favor selectivity over L-type VGCCs. The chemical structures are based on a structure-activity relationship of flunarizine and lomerizine. NP118809 has been favored for further development due to low off-target activity to hERG Kv channels that mediate cardiac action potentials. Currently, it has been developed as Z160 (previously, NMED-160 or MK-6721) and is in phase IIa study by Zalicus (Fig. 2A). Methoxy groups adjacent to benzoyl linker of NP078585, which has trimethoxybenzyl piperazine moiety from flunarizine and lomerizine, are substituted with S-Me group, resulting in a high potency for N-type VGCCs [121, 122]. Merck designed and synthesized aminopiperidine sulfonamide inhibitors, which are state-dependent blockers. These compounds inhibit CFA-induced inflammatory pain and SNL neuropathic pain in a Cav2.2 expression-dependent manner [123]. TROX-1 was also discovered by Merck (Fig. 2A). An IC50 of this compound in a depolarized state is 400 nM in rat DRG, but in a hyperpolarized state is 2.6 μM, showing a characteristic of state dependent blockers. Oral 10mg/kg TROX-1 showed analgesic effects in inflammatory and neuropathic pain similarly with NSAIDs and pregabalin [124, 125]. Ono Pharmaceutical designed and synthesized L-cysteine based N-type VGCC blockers (Fig. 2A). Oral administration of this compound (30mg/kg) showed analgesic effects in the rat formalin test and CCI neuropathic pain, but high dose (100mg/kg) did not have an effect on cardiac or motor function [126, 127].
4.3. T-type Ca2+ Channel Inhibitors
Mibefradil (Ro 40-5967), a tetralol derivative, was developed as an anti-anginal and anti-ischemic agent (Fig. 2C) [128, 129]. However, it was withdrawn by Roche due to its interaction with liver enzyme [130]. In spite of the withdrawal, it has been used for research due to its selectivity for T-type VGCCs. Intraperitoneal injection of mibefradil reduced mechanical and thermal pain [131]. In rat neuropathic pain model (SNL), intraplanar and intraperitoneal mibefradil showed dose dependent effects on mechanical and thermal allodynia, but intrathecal injection did not show any effect [132]. In contrast to this, intrathecal mibefradil decreased neuropathic pain by CCD models [83]. These data suggested the involvement of T-type VGCCs in acute and neuropathic pain. Interestingly, intraperitoneal mibefradil enhances antinociceptive effects of opioids and blocks its tolerance but not dependence, although its mechanism is not clear [133]. Intraperitoneal ethosuximide, another T-type VGCC blocker, showed lower efficacy than mibefradil [132]. Intrathecal ethosuximide also showed dose dependent inhibition of neuropathic pain by SNL and spinal hyperexcitability [134]. Intraperitoneal ethosuximide had analgesic effects on chemotherapy induced pain, which is resistant to opioids and MK801, an NMDA antagonist [135].
Inspired by analgesic effects with mibefradil and ethosuximide, many academic labs and companies have tried to find selective and potent T-type VGCC blockers [136]. One achievement is TTA-A2 synthesized by Merck (Fig. 2C). This compound blocked recombinant T-type VGCCs with an IC50 of 10~90 nM at more depolarized voltages (-75~-80mV, large fraction of channels in inactivated state), while with an IC50 of 4~22 μM at hyperpolarized voltages (-100~-110mV) in heterologous T-type VGCC expressed in HEK cells and native T-type VGCCs [137, 138]. This evidence showed use dependency of TTA-A2, which is useful for reducing hyperexcitability related to chronic pain [137, 138]. The analgesic effects of oral TTA-A2 were examined on acute pain and an irritable bowel syndrome model, which is butyrate-induced hypersensitivity. TTA-A2 inhibited both type of pain but did not reduce acute pain in Cav3.2 knockout mice. However, oral administration of TTA-A2 disturbs sleep in normal mice but not double knockout of Cav3.1 and Cav3.3, indicating possible side effects. TTA-A2 inhibited presynaptic T-type VGCCs in the superficial laminae of dorsal horn, only affecting excitatory synaptic transmission [77]. Merck also developed quinazolinone T-type VGCC blocker TTA-Q4, which showed synergetic effects with TTA-A2 [139]. A novel compound has been identified through high throughput screening to block Cav3.1 and Cav3.3 with 20~60 nM affinities. Their developments focused on epilepsy, but targeting this into the peripheral nervous system would be beneficial (Fig. 2C) [140, 141]. Recently, A803467, a blocker of TTX resistant Nav1.8, has been examined on T-type VGCCs due to structural similarity of Nav1.8 and Cav3. It also inhibit T-type VGCCs with IC50 of ~5μM at a holding potential of -110mV and interestingly stabilized slow inactivated state of hCav3.2 (not Cav3.1 and Cav3.3) [142]. Therefore, analgesic mechanism of A803467 might partially result from T-type VGCC inhibition.
Zonisamide, 1,2-benzisoxazole-3-methanesulfonamide, is known as an anticonvulsant and inhibitor of repetitive firing, thus it is proposed as a T-type VGCC blocker [143]. It also was involved in central poststroke pain [144]. In a recent electrophysiological study, a high dose (2mM) of zonisamide partially inhibited Cav3.2 current with 30% block and did not show use-dependent block [145]. Trazodone, a treatment for insomnia and depression, also blocked heterogeneous T-type VGCC current with an IC50 of 23~45μM. Butamben, a local anesthetic, also inhibits T-type VGCC currents with an IC50 of 200μM in addition to N-type VGCCs. Interestingly, it accelerates T-type VGCC kinetics [146]. It has been shown that trimethadione, as a T-type VGCC blocker, inhibited hyperalgesia in a dose dependent manner. However, it does not inhibit LVA current in somatosensory thalamic neurons [147, 148].
It should be noted that many endogenous molecules modulate T-type VGCCs in addition to the small molecules described above. For example, it has been reported that the endogenous lipid NAGly (N-arachidonoyl glycine) or NAGABA-OH inhibited T-type VGCC currents with a preference to Cav3.2 by stabilizing inactivated state based on the observation of hyperpolarized shift in the steady-state inactivation [149]. These lipids blocked recombinant Cav3.2 with EC50 of ~600nM and ~200nM, respectively, and showed selectivity over the cannabinoid receptor or TRPV1. In behavior tests using Cav3.2-/- mice, these lipoamino acids showed thermal analgesia in a Cav3.2-dependent manner. Other endogenous modulators of T-type VGCCs are 5β reduced neuroactive steroids [150, 151]. These endogenous molecules have potential to be developed into analgesics.
Another family of inhibitors modulating T-type VGCCs is peptides or protein neurotoxins produced by the venom organ of venomous animals such as spiders, scorpions, sea anemones, and cone snails. The founding member of T-type VGCC blocking toxin is kurtoxin, isolated from the venom of the scorpion Parabuthus transvaalicus [152]. It was reported that kurtoxin blocks Cav3.1. However, it turned out to block other HVA channels as well as Cav3.1 and Cav3.3 [153]. More recently, it has been reported that ProTx-I preferentially block Cav3.1 over Cav3.2 by a interaction with the putative voltage sensing domain (S3-S4 linker in domain IV) of hCav3.1 [154]. Three-dimensional structures of kurtoxin and ProTx-1 have been determined by nuclear magnetic resonance. Ensuing structural studies will provide invaluable pharmacopore information similar to what was shown for the N-type VGCC blocker ziconotide [155].
5. PROSPECTS
VGCC subtype inhibitors including ziconotide (ω-conotoxin MVIIA) showed both potential and limitations as chronic pain therapeutics. Several strategies for overcoming the limitations have been implemented to enhance the inhibitor specificity for voltage-gated ion channels (either small molecule or peptide), some of which were already applied to VGCC subtypes. These include silencing nociceptors, a development of use-dependent blockers, and expression of tethered peptide neurotoxins.
5.1. Silencing Nociceptor
Binshtok et al. reported an interesting approach to overcome low specificity of a local anesthetic lidocaine [156]. Although the strategy targeted VGSCs, it deserves consideration as a potential application to VGCCs. Lidocaine (pKa 7.8) has more portions of lipid soluble forms, which can penetrate the cell membrane to reach its binding site. QX-314 (low pKa), first synthesized by the Astra Phamaceutical Company in the early 1970s, is a quaternary ammonium derivative of lidocaine. It is a permanently charged form and cannot permeate the membrane. Thus, it has been known to have a binding sidedness that only by internal application of QX-314 it reach its binding site. Binshtok et al. recognized that large molecule such as QX-314 can pass through the pore of activated TRPV1, which is expressed specifically in nociceptors. Therefore, nociceptor-specific internalization of QX-314 has been achieved through co-application of QX-314 and capsaicin, a ligand for TRPV1. Internalized QX-314 specifically blocked animal pain behaviors for several hours without any sensory or motor deficits. However, external QX-314 alone did not block sodium current. Internalization of QX-314 by capsaicin in the cells has been verified by tandem mass spectroscopy and electrophysiology [157, 158]. It is very meaningful that the specificity of VGSC blocker QX-314 is achieved by the localization of TRPV1, although previously QX-314 was only considered a research tool. In the same way, if small molecules for VGCCs have internal binding sidedness, this strategy should be considered. While targeting VGSCs result in inhibiting action potential generation for propagation of pain signaling, targeting VGCCs in nociceptors may be even more effective by blocking both synaptic transmission in presynaptic nerve terminals as well as peptide release in peripheral nerve ending.
There have been several studies that characterize the sidedness of permanently charged organic calcium antagonists. For example, the effect of charged DHP (amlodipine and SDZ 207-180) on L-type VGCC currents has been examined internally by electrophysiology. However, the internally applied charged DHP did not show any blocking effect, meaning DHP is not appropriate for this strategy [107, 159]. However, D-890 (N-methyl-verapamil) is active only when inside the cells so it might be a good option for this silencing strategy using TRPV1 channel [160]. It would be interesting if the recently discovered N-type and T-type VGCC blockers can be used in this strategy.
5.2. High Throughput Screening for Searching use Dependent Blockers
As described above, VGCCs are moving targets and thus several blockers can preferentially bind to the inactivated state; a common characteristics of hyperexcitable cells [161]. It has been suggested that ziconotide is a state-independent drug, which might influence side effects. Several academic and industry labs have tried to develop the use dependent drugs for VGCCs. One way to discover use dependent drugs for VGCCs is via high throughput Ca2+ imaging experiments. Detection of intracellular Ca2+ influx using fluorescence dyes is a well-established readout of the function of VGCC subtypes [162]. Therefore, screening VGCC subtypes are relatively easy compared to other VGSC or VGPC subtypes. For high throughput screening, cell lines heterologously expressing VGCC subtypes is frequently used. However, typical cell lines (e.g. HEK cells) for screening have rather high membrane potentials. For example, HEK293 cell lines have a resting membrane potentials at -12mV, which inactivates most of the VGCC subtypes [163]. To overcome this, inward rectifier K+ channels are co-transfected with VGCC subtypes. After transfecting the K+ channels, the membrane potentials can be controlled with potassium concentrations for a use-dependent assay. One group used 4 mM and 14 mM KCl as baseline buffers, which represent resting and inactivated states of VGCC subtypes, respectively. They then used a high concentration of KCl (e.g. 80mM) to induce depolarization [125]. This high throughput assay was complemented by an automated patch clamp assay, which achieves accuracy before they conducted manual patch clamp [164]. Differently from HVA VGCC subtypes, screening for T-type VGCCs seems rather difficult because of the existence of a large portion of inactivated channels even at resting membrane potentials. Two groups already reported a T-type VGCC assay with co-transfection of T-type VGCCs and Kir2.2 [165, 166]. Another group used an interesting approach to use gramicidin to remove inactivation [167]. These improvements of methodologies for high throughput screening will help the identification of VGCC subtype blockers, some of which will show use-dependent activity.
5.3. Tethered Peptide Neurotoxin and Gene Therapy
Peptide neurotoxins are another large class of ion channel blockers. These toxins have a high proportion of cysteines in their sequences, which form multiple disulfide bonds and thus contribute to rigid structural integrity. These toxins have been isolated from the venom of cone snails, spiders, scorpions, snakes, and sea anemones. This means that peptide neurotoxins are encoded by genes and modifiable by genetic engineering. Interestingly, several endogenous cysteine-rich peptides or proteins also modulate voltage-gated ion channels. For example, lynx1 is a glycosyl-phosphatidylinositol (GPI) anchored protein which has 10 cysteines and modulates nicotinic acetylcholine receptors [168]. CKAMP-44 also has 8 cysteines and modulates AMPA receptors [169]. This protein has a transmembrane domain so that they are also membrane-tethered. Inspired by GPI anchored lynx1, Ibañez-Tallon et al. showed that peptide neurotoxins tethered by GPI has a blocking effect on α7 nicotinic acetylcholine receptor in Zebrafish, which could overcome for the problems of genetic ablation of ion channels such as compensation [170]. Tethered spider toxins targeting VGSCs are also expressed in Drosophila to affect circadian rhythm. Peptide neurotoxins blocking VGCC subtypes also have been tested in mice. Tethered fluorescent peptide neurotoxins, ω-conotoxin MVIIA and ω-Aga IVA inhibiting Cav2.2 and Cav2.1, respectively, have been delivered through lenti virus to show a reduction in chronic pain [171].
Tethered peptide neurotoxins can be used for two purposes. One is for novel peptide neurotoxin screenings to identify their function. A large set of peptide neurotoxin genes have been characterized without identification of their respective functions. It is difficult to characterize their functions one by one through peptide neurotoxin synthesis and functional assay. It is relatively easier to use model animals such as Drosophila and C. elegans, which have well documented behaviors. This approach will accelerate identifying peptide blockers for VGCC subtypes. The other purpose is to use tethered peptide neurotoxins for gene therapy. Potentially, the tethered ω-conotoxin MVIIA can be targeted into specific cells (eventually nociceptors) by virus targeting [172]. Although safety concerns still remain to be considered, as gene therapy has only recently been approved in Europe, gene therapy using tethered peptides can be a promising way to treat neuropathic pain [173].
6. CONCLUSION
Pain therapeutics for chronic pain still have unmet need because current therapy is unsatisfactory mainly due to low efficacy or high side effects. Considering the important roles of VGCC subtypes in neurotransmitter release, excitability, and neurogenic inflammation, VGCC subtypes (Cav1.2, Cav2.2, Cav3.2, and α2δ) in nociceptors are promising targets for chronic pain. Many VGCC subtype blockers that have been developed have contributed to chronic pain therapeutics and understanding the role of VGCC subtypes. Known and novel VGCC blockers (or identified by improved high throughput screening methods) can be targeted into nociceptor improve medication for chronic pain in terms of high efficacy and safety.
ACKNOWLEDGEMENTS
I thank A. Yekkirala and J. Sprague for useful comments. This work was partially supported by the National Research Foundation of Korea Grant funded by the Korean Government (Ministry of Education, Science and Technology) (NRF-2011-357-C00125).
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Woolf CJ, Ma Q. Nociceptors noxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Gover TD, Kao JP, Weinreich D. Calcium signaling in single peripheral sensory nerve terminals. J. Neurosci. 2003;23(12):4793–4797. doi: 10.1523/JNEUROSCI.23-12-04793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costigan M, Scholz J, Woolf CJ. Neuropathic pain a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf CJ. Overcoming obstacles to developing new analgesics. Nat. Med. 2010;16(11):1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 5.Smith HS, editor. Philadelphia.: Saunders/Elsevier.; 2009. Current therapy in pain. [Google Scholar]
- 6.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59(6):882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Pexton T, Moeller-Bertram T, Schilling JM, Wallace MS. Targeting voltage-gated calcium channels for the treatment of neuropathic pain: a review of drug development. Expert. Opin. Investig. Drugs. 2011;20(9):1277–1284. doi: 10.1517/13543784.2011.600686. [DOI] [PubMed] [Google Scholar]
- 8.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect. Biol. 2011;3(8):a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology XLVIII.Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57(4):411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 10.Dolphin AC. Calcium channel auxiliary alpha2delta and beta subunits trafficking and one step beyond. Nat. Rev. Neurosci. 2012;13(8):542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 11.Dubel SJ, Starr TV, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP, editors. 11. Vol. 89. U.S.A.: Proc. Natl. Acad. Sci.; 1992. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. pp. 5058–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacinova L, Klugbauer N, Hofmann F. Absence of modulation of the expressed calcium channel alpha1G subunit by alpha2delta subunits. J. Physiol. 1999;516(Pt 3):639–645. doi: 10.1111/j.1469-7793.1999.0639u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert RC, Maulet Y, Mouton J, Beattie R, Volsen S, DeWaard M, Feltz A. T-type Ca2+ current properties are not modified by Ca2+ channel beta subunit depletion in nodosus ganglion neurons. J. Neurosci. 1997; 17(17): 6621–6628. doi: 10.1523/JNEUROSCI.17-17-06621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipscombe D, Andrade A, Allen SE. Alternative splicing: Functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim. Biophys. Acta. 2012 doi: 10.1016/j.bbamem.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipscombe D, Raingo J. Alternative splicing matters.N-type calcium channels in nociceptors. Channels (Austin). 2007;1(4):225–227. doi: 10.4161/chan.4809. [DOI] [PubMed] [Google Scholar]
- 16.Allen SE, Darnell RB, Lipscombe D. The neuronal splicing factor Nova controls alternative splicing in N-type and P-type CaV2 calcium channels. Channels (Austin). 2010;4(6):483–489. doi: 10.4161/chan.4.6.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, McDonough SI, Lipscombe D. Alternative splicing in the voltage-sensing region of N-Type CaV2. channels modulates channel kinetics. . J. Neurophysiol. 2004;92(5):2820–2830. doi: 10.1152/jn.00048.2004. [DOI] [PubMed] [Google Scholar]
- 18.Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41(1):127–138. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 19.Raingo J, Castiglioni AJ, Lipscombe D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 2007;10(3):285–292. doi: 10.1038/nn1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade A, Denome S, Jiang YQ, Marangoudakis S, Lipscombe D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat. Neurosci. 2010;13(10):1249–1256. doi: 10.1038/nn.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bean BP. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8(6):451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 22.Helton TD, Xu W, Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J. Neurosci. 2005;25(44):10247–10251. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcantoni A, Vandael DH, Mahapatra S, Carabelli V, Sinnegger-Brauns MJ, Striessnig J, Carbone E. Loss of Cav1. channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J. Neurosci. 2010;30(2):491–504. doi: 10.1523/JNEUROSCI.4961-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaman T, Lee K, Park C, Paydar A, Choi JH, Cheong E, Lee CJ, Shin HS. Cav2. channels are critical for oscillatory burst discharges in the reticular thalamus and absence epilepsy. Neuron. 2011;70(1):95–108. doi: 10.1016/j.neuron.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol. 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yusaf SP, Goodman J, Pinnock RD, Dixon AK, Lee K. Expression of voltage-gated calcium channel subunits in rat dorsal root ganglion neurons. Neurosci. Lett. 2001;311(2):137–141. doi: 10.1016/s0304-3940(01)02038-9. [DOI] [PubMed] [Google Scholar]
- 27.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons interneurons and nerve terminals. J. Neurosci. 1998;18(16):6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu PD, Randic M. Low and high-voltage-activated calcium currents in rat spinal dorsal horn neurons. J. Neurophysiol. 1990;63(2):273–285. doi: 10.1152/jn.1990.63.2.273. [DOI] [PubMed] [Google Scholar]
- 29.Heinke B, Balzer E, Sandkuhler J. Pre and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur. J. Neurosci. 2004;19(1):103–111. doi: 10.1046/j.1460-9568.2003.03083.x. [DOI] [PubMed] [Google Scholar]
- 30.Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J. Pain. 2006;7(1 ) Suppl 1 :S13–30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin). 2010;4(6):510–517. doi: 10.4161/chan.4.6.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS. Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J. Clin. Invest. 2001;108(7):1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobremez E, Bouali-Benazzouz R, Fossat P, Monteils L, Dulluc J, Nagy F, Landry M. Distribution and regulation of L-type calcium channels in deep dorsal horn neurons after sciatic nerve injury in rats. Eur. J. Neurosci. 2005;21(12):3321–3333. doi: 10.1111/j.1460-9568.2005.04177.x. [DOI] [PubMed] [Google Scholar]
- 34.Sukiasyan N, Hultborn H, Zhang M. Distribution of calcium channel Ca(V)1. immunoreactivity in the rat spinal cord and brain stem. Neuroscience. 2009;159(1):217–235. doi: 10.1016/j.neuroscience.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Kim DS, Yoon CH, Lee SJ, Park SY, Yoo HJ, Cho HJ. Changes in voltage-gated calcium channel alpha(1) gene expression in rat dorsal root ganglia following peripheral nerve injury. Brain Res. Mol. Brain Res. 2001;96(1-2):151–156. doi: 10.1016/s0169-328x(01)00285-6. [DOI] [PubMed] [Google Scholar]
- 36.Tang Q, Bangaru ML, Kostic S, Pan B, Wu HE, Koopmeiners AS, Yu H, Fischer GJ, McCallum JB, Kwok WM, Hudmon A, Hogan QH. Ca(2)(+)-dependent regulation of Ca(2)(+) currents in rat primary afferent neurons role of CaMKII and the effect of injury. J. Neurosci. 2012;32(34):11737–11749. doi: 10.1523/JNEUROSCI.0983-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fossat P, Dobremez E, Bouali-Benazzouz R, Favereaux A, Bertrand SS, Kilk K, Leger C, Cazalets JR, Langel U, Landry M, Nagy F. Knockdown of L calcium channel subtypes differential effects in neuropathic pain. J. Neurosci. 2010;30(3):1073–1085. doi: 10.1523/JNEUROSCI.3145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favereaux A, Thoumine O, Bouali-Benazzouz R, Roques V, Papon MA, Salam SA, Drutel G, Leger C, Calas A, Nagy F, Landry M. Bidirectional integrative regulation of Cav1. calcium channel by microRNA miR-103 role in pain. EMBO J. 2011;30(18):3830–3841. doi: 10.1038/emboj.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Moller M, Broman J, Sukiasyan N, Wienecke J, Hultborn H. Expression of calcium channel CaV1. in cat spinal cord light and electron microscopic immunohistochemical study. J. Comp. Neurol. 2008;507(1):1109–1127. doi: 10.1002/cne.21595. [DOI] [PubMed] [Google Scholar]
- 40.Clark NC, Nagano N, Kuenzi FM, Jarolimek W, Huber I, Walter D, Wietzorrek G, Boyce S, Kullmann DM, Striessnig J, Seabrook GR. Neurological phenotype and synaptic function in mice lacking the CaV1. alpha subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience. 2003;120(2):435–442. doi: 10.1016/s0306-4522(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 41.Uchitel OD, Inchauspe CG, Urbano FJ, DiGuilmi MN. CaV2. voltage activated calcium channels and synaptic transmission in familial hemiplegic migraine pathogenesis. J. Physiol Paris. 2012;106(1-2):12–22. doi: 10.1016/j.jphysparis.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Either N or P type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron. 1997;18(3):463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 43.Evans AR, Nicol GD, Vasko MR. Differential regulation of evoked peptide release by voltage-sensitive calcium channels in rat sensory neurons. Brain Res. 1996;712(2):265–273. doi: 10.1016/0006-8993(95)01447-0. [DOI] [PubMed] [Google Scholar]
- 44.Ohnami S, Tanabe M, Shinohara S, Takasu K, Kato A, Ono H. Role of voltage-dependent calcium channel subtypes in spinal long-term potentiation of C-fiber-evoked field potentials. Pain. 2011;152(3):623–631. doi: 10.1016/j.pain.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Matthews EA, Dickenson AH. Effects of spinally delivered N and P type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain. 2001;92(1-2):235–246. doi: 10.1016/s0304-3959(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 46.Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J. Pharmacol. Exp. Ther. 1994;269(3):1117–1123. [PubMed] [Google Scholar]
- 47.Yamamoto T, Sakashita Y. Differential effects of intrathecally administered N and P type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain Res. 1998;794(2):329–332. doi: 10.1016/s0006-8993(98)00306-0. [DOI] [PubMed] [Google Scholar]
- 48.Fukumoto N, Obama Y, Kitamura N, Niimi K, Takahashi E, Itakura C, Shibuya I. Hypoalgesic behaviors of P/Q-type voltage-gated Ca2+ channel mutant mouse rolling mouse Nagoya. Neuroscience. 2009;160(1):165–173. doi: 10.1016/j.neuroscience.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Luvisetto S, Marinelli S, Panasiti MS, D'Amato FR, Fletcher CF, Pavone F, Pietrobon D. Pain sensitivity in mice lacking the Ca(v)2.alpha1 subunit of P/Q-type Ca2+ channels. Neuroscience. 2006;142(3):823–832. doi: 10.1016/j.neuroscience.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 50.Ogasawara M, Kurihara T, Hu Q, Tanabe T. Characterization of acute somatosensory pain transmission in P/Q-type Ca(2+) channel mutant mice leaner. FEBS Lett. 2001;508(2):181–186. doi: 10.1016/s0014-5793(01)03052-6. [DOI] [PubMed] [Google Scholar]
- 51.Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9(6):1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 52.Santicioli P, DelBianco E, Tramontana M, Geppetti P, Maggi CA. Release of calcitonin gene-related peptide like-immunoreactivity induced by electrical field stimulation from rat spinal afferents is mediated by conotoxin-sensitive calcium channels. Neurosci. Lett. 1992;136(2):161–164. doi: 10.1016/0304-3940(92)90039-a. [DOI] [PubMed] [Google Scholar]
- 53.Gruner W, Silva LR. Omega-conotoxin sensitivity and presynaptic inhibition of glutamatergic sensory neurotransmission in vitro. J. Neurosci. 1994;14(5 Pt 1):2800–2808. doi: 10.1523/JNEUROSCI.14-05-02800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holz GGt, Dunlap K, Kream RM. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J. Neurosci. 1988;8(2):463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerr LM, Filloux F, Olivera BM, Jackson H, Wamsley JK. Autoradiographic localization of calcium channels with [125I]omega-conotoxin in rat brain. Eur. J. Pharmacol. 1988;146(1):181–183. doi: 10.1016/0014-2999(88)90501-8. [DOI] [PubMed] [Google Scholar]
- 56.Gohil K, Bell JR, Ramachandran J, Miljanich GP. Neuro-anatomical distribution of receptors for a novel voltage-sensitive calcium-channel antagonist SNX-230 (omega-conopeptide MVIIC). Brain Res. 1994;653(1-2):258–266. doi: 10.1016/0006-8993(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 57.Scroggs RS, Fox AP. Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J. Neurosci. 1992;12(5):1789–1801. doi: 10.1523/JNEUROSCI.12-05-01789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu SG, Zhang XL, Luo ZD, Gold MS. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151(3):633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cizkova D, Marsala J, Lukacova N, Marsala M, Jergova S, Orendacova J, Yaksh TL. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp. Brain Res. 2002;147(4):456–463. doi: 10.1007/s00221-002-1217-3. [DOI] [PubMed] [Google Scholar]
- 60.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 2001;21(6):1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rycroft BK, Vikman KS, Christie MJ. Inflammation reduces the contribution of N-type calcium channels to primary afferent synaptic transmission onto NK1 receptor-positive lamina I neurons in the rat dorsal horn. J. Physiol. 2007;580(Pt.3):883–894. doi: 10.1111/j.1469-7793.2000.t01-1-02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saegusa H, Kurihara T, Zong S, Kazuno A, Matsuda Y, Nonaka T, Han W, Toriyama H, Tanabe T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20(10):2349–2356. doi: 10.1093/emboj/20.10.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim C, Jun K, Lee T, Kim SS, McEnery MW, Chin H, Kim HL, Park JM, Kim DK, Jung SJ, Kim J, Shin HS. Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol. Cell Neurosci. 2001;18(2):235–245. doi: 10.1006/mcne.2001.1013. [DOI] [PubMed] [Google Scholar]
- 65.Hatakeyama S, Wakamori M, Ino M, Miyamoto N, Takahashi E, Yoshinaga T, Sawada K, Imoto K, Tanaka I, Yoshizawa T, Nishizawa Y, Mori Y, Niidome T, Shoji S. Differential nociceptive responses in mice lacking the alpha(1B) subunit of N-type Ca(2+) channels. Neuroreport. 2001;12(11):2423–2427. doi: 10.1097/00001756-200108080-00027. [DOI] [PubMed] [Google Scholar]
- 66.Sluka KA. Blockade of calcium channels can prevent the onset of secondary hyperalgesia and allodynia induced by intradermal injection of capsaicin in rats. Pain. 1997;71(2):157–164. doi: 10.1016/s0304-3959(97)03354-x. [DOI] [PubMed] [Google Scholar]
- 67.Sluka KA. Blockade of N and P/Q type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J Pharmacol. Exp. Ther. 1998;287(1):232–237. [PubMed] [Google Scholar]
- 68.McGivern JG. Ziconotide a review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007;3(1):69–85. doi: 10.2147/nedt.2007.3.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott DA, Wright CE, Angus JA. Actions of intrathecal omega-conotoxins CVID GVIA MVIIA and morphine in acute and neuropathic pain in the rat. Eur. J. Pharmacol. 2002;451(3):279–286. doi: 10.1016/s0014-2999(02)02247-1. [DOI] [PubMed] [Google Scholar]
- 70.Fang Z, Park CK, Li HY, Kim HY, Park SH, Jung SJ, Kim JS, Monteil A, Oh SB, Miller RJ. Molecular basis of Ca(v)2. calcium channels in rat nociceptive neurons. . J. Biol. Chem. 2007;282(7):4757–4764. doi: 10.1074/jbc.M605248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang Z, Hwang JH, Kim JS, Jung SJ, Oh SB. R-type Calcium Channel Isoform in Rat Dorsal Root Ganglion Neurons. Korean J. Physiol. Pharmacol. 2010;14(1):45–49. doi: 10.4196/kjpp.2010.14.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matthews EA, Bee LA, Stephens GJ, Dickenson AH. The Cav2. calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur. J. Neurosci. 2007;25(12):3561–3569. doi: 10.1111/j.1460-9568.2007.05605.x. [DOI] [PubMed] [Google Scholar]
- 73.Murakami M, Nakagawasai O, Suzuki T, Mobarakeh II, Sakurada Y, Murata A, Yamadera F, Miyoshi I, Yanai K, Tan-No K, Sasano H, Tadano T, Iijima T. Antinociceptive effect of different types of calcium channel inhibitors and the distribution of various calcium channel alpha 1 subunits in the dorsal horn of spinal cord in mice. Brain Res. 2004;1024(1-2):122–129. doi: 10.1016/j.brainres.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 74.Yang L, Stephens GJ. Effects of neuropathy on high-voltage-activated Ca(2+) current in sensory neurones. Cell Calcium. 2009;46(4):248–256. doi: 10.1016/j.ceca.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T, editors. 11. Vol. 97. U.S.A.: Proc. Natl. Acad. Sci.; 2000. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. pp. 6132–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saegusa H, Matsuda Y, Tanabe T. Effects of ablation of N and R type Ca(2+) channels on pain transmission. Neurosci. Res. 2002;43(1):1–7. doi: 10.1016/s0168-0102(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 77.Jacus MO, Uebele VN, Renger JJ, Todorovic SM. Presynaptic Cav3. channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 2012;32(27):9374–9382. doi: 10.1523/JNEUROSCI.0068-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiss N, Zamponi GW. Control of low-threshold exocytosis by T-type calcium channels. Biochim. Biophys. Acta. 2012;1828 (7):1579–86. doi: 10.1016/j.bbamem.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 79.Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299(5610):1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 80.Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86(1-2):43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- 81.McCallum JB, Kwok WM, Mynlieff M, Bosnjak ZJ, Hogan QH. Loss of T-type calcium current in sensory neurons of rats with neuropathic pain. Anesthesiology. 2003;98(1):209–216. doi: 10.1097/00000542-200301000-00032. [DOI] [PubMed] [Google Scholar]
- 82.Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J. Neurophysiol. 2008;99(6):3151–3156. doi: 10.1152/jn.01031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen XJ, Xu SY, Chen ZX, Yang CX, Liang H, Li H. The roles of T-type calcium channel in the development of neuropathic pain following chronic compression of rat dorsal root ganglia. Pharmacology. 2010;85(5):295–300. doi: 10.1159/000276981. [DOI] [PubMed] [Google Scholar]
- 84.Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR. A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat. Neurosci. 2003;6(7):724–730. doi: 10.1038/nn1076. [DOI] [PubMed] [Google Scholar]
- 85.Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrere C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, Eschalier A, Bourinet E, Ardid D. 27. Vol. 108. U.S.A.: Proc. Natl. Acad. Sci.; 2011. T type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. pp. 11268–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Latham JR, Pathirathna S, Jagodic MM, Choe WJ, Levin ME, Nelson MT, Lee WY, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes. 2009;58(11):2656–2665. doi: 10.2337/db08-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3. T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24(2):315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen XJ, Li ZJ, Chen ZX, Fang ZY, Yang CX, Li H, Zeng YM. Intrathecal administration of Cav3. and Cav3.3 antisense oligonucleotide reverses tactile allodynia and thermal hyperalgesia in rats following chronic compression of dorsal root of ganglion. Acta Pharmacol. Sin. 2006;27(12):1547–1552. doi: 10.1111/j.1745-7254.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 89.Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Attenuated pain responses in mice lacking Ca(V)3. T-type channels. Genes Brain Behav. 2007;6(5):425–431. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 90.Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin HS. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302(5642):117–119. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- 91.Na HS, Choi S, Kim J, Park J, Shin HS. Attenuated neuropathic pain in Cav3. null mice. Mol. Cells. 2008;25(2):242–246. [PubMed] [Google Scholar]
- 92.Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486(7401):122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McNaughton NC, Bleakman D, Randall AD. Electro-physiological characterisation of the human N-type Ca2+ channel II: activation and inactivation by physiological patterns of activity. Neuropharmacology. 1998;37(1):67–81. doi: 10.1016/s0028-3908(97)00153-6. [DOI] [PubMed] [Google Scholar]
- 94.Mould J, Yasuda T, Schroeder CI, Beedle AM, Doering CJ, Zamponi GW, Adams DJ, Lewis RJ. The alpha2delta auxiliary subunit reduces affinity of omega-conotoxins for recombinant N-type (Cav2.) calcium channels. J. Biol. Chem. 2004;279(33):34705–34714. doi: 10.1074/jbc.M310848200. [DOI] [PubMed] [Google Scholar]
- 95.Bauer CS, Rahman W, Tran-van-Minh A, Lujan R, Dickenson AH, Dolphin AC. The anti-allodynic alpha(2)delta ligand pregabalin inhibits the trafficking of the calcium channel alpha (2) delta 1 subunit to presynaptic terminals in vivo. Biochem. Soc. Trans. 2010;38(2):525–528. doi: 10.1042/BST0380525. [DOI] [PubMed] [Google Scholar]
- 96.Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res. Mol. Brain Res. 2001;95(1-2):1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 97.Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, Diatchenko L, Gupta V, Xia CP, Amann S, Kreitz S, Heindl-Erdmann C, Wolz S, Ly CV, Arora S, Sarangi R, Dan D, Novatchkova M, Rosenzweig M, Gibson DG, Truong D, Schramek D, Zoranovic T, Cronin SJ, Angjeli B, Brune K, Dietzl G, Maixner W, Meixner A, Thomas W, Pospisilik JA, Alenius M, Kress M, Subramaniam S, Garrity PA, Bellen HJ, Woolf CJ, Penninger JM. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143(4):628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hendrich J, VanMinh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. 9. Vol. 105. U. S. A.: Proc. Natl. Acad. Sci.; 2008. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. pp. 3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br. J. Pharmacol. 2002;135(1):257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37(1):83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 101.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat. Med. 2011;17(7):822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Snutch TP, Sutton KG, Zamponi GW. Voltage-dependent calcium channels--beyond dihydropyridine antagonists. Curr. Opin. Pharmacol. 2001;1(1):11–16. doi: 10.1016/s1471-4892(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 103.Fleckenstein A. History of calcium antagonists. Circ. Res. 1983;52(2 Pt 2):I3–16. [PubMed] [Google Scholar]
- 104.Schuster A, Lacinova L, Klugbauer N, Ito H, Birnbaumer L, Hofmann F. The IVS6 segment of the L-type calcium channel is critical for the action of dihydropyridines and phenylalkylamines. EMBO J. 1996;15(10):2365–2370. [PMC free article] [PubMed] [Google Scholar]
- 105.Yamaguchi S, Okamura Y, Nagao T, Adachi-Akahane S. Serine residue in the IIIS5-S6 linker of the L-type Ca2+ channel alpha 1C subunit is the critical determinant of the action of dihydropyridine Ca2+ channel agonists. J. Biol. Chem. 2000;275(52):41504–41511. doi: 10.1074/jbc.M007165200. [DOI] [PubMed] [Google Scholar]
- 106.Hockerman GH, Dilmac N, Scheuer T, Catterall WA. Molecular determinants of diltiazem block in domains IIIS6 and IVS6 of L-type Ca(2+) channels. Mol. Pharmacol. 2000;58(6):1264–1270. doi: 10.1124/mol.58.6.1264. [DOI] [PubMed] [Google Scholar]
- 107.Kass RS, Arena JP, Chin S. Block of L-type calcium channels by charged dihydropyridines.Sensitivity to side of application and calcium. J. Gen. Physiol. 1991;98(1):63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sohail Z. Voltage-gated ion channels as drug targets. Wiley-VCH. Weinheim. 2006 [Google Scholar]
- 109.Bean BP, editor. 20. Vol. 81. USA.: Proc. Natl. Acad. Sci.; 1984. Nitrendipine block of cardiac calcium channels high-affinity binding to the inactivated state. pp. 6388–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sasaki T, Feng ZP, Scott R, Grigoriev N, Syed NI, Fainzilber M, Sato K. Synthesis bioactivity and cloning of the L-type calcium channel blocker omega-conotoxin TxVII. Biochemistry. 1999;38(39):12876–12884. doi: 10.1021/bi990731f. [DOI] [PubMed] [Google Scholar]
- 111.Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing blockade of N and P type channels inhibits formalin-induced nociception. J. Neurosci. 1994;14(8):4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Todorovic SM, Pathirathna S, Meyenburg A, Jevtovic-Todorovic V. Mechanical and thermal anti-nociception in rats after systemic administration of verapamil. Neurosci. Lett. 2004;360(1-2):57–60. doi: 10.1016/j.neulet.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 113.Weizman R, Getslev V, Pankova IA, Schrieber S, Pick CG. Pharmacological interaction of the calcium channel blockers verapamil and flunarizine with the opioid system. Brain Res. 1999;818(2):187–195. doi: 10.1016/s0006-8993(98)01175-5. [DOI] [PubMed] [Google Scholar]
- 114.Ellinor PT, Zhang JF, Horne WA, Tsien RW. Structural determinants of the blockade of N-type calcium channels by a peptide neurotoxin. Nature. 1994;372(6503):272–275. doi: 10.1038/372272a0. [DOI] [PubMed] [Google Scholar]
- 115.Feng ZP, Doering CJ, Winkfein RJ, Beedle AM, Spafford JD, Zamponi GW. Determinants of inhibition of transiently expressed voltage-gated calcium channels by omega-conotoxins GVIA and MVIIA. J. Biol. Chem. 2003;278(22):20171–20178. doi: 10.1074/jbc.M300581200. [DOI] [PubMed] [Google Scholar]
- 116.Penn RD, Paice JA. Adverse effects associated with the intrathecal administration of ziconotide. Pain. 2000;85(1-2):291–296. doi: 10.1016/s0304-3959(99)00254-7. [DOI] [PubMed] [Google Scholar]
- 117.Kolosov A, Aurini L, Williams ED, Cooke I, Goodchild CS. Intravenous injection of leconotide, an omega conotoxin synergistic antihyperalgesic effects with morphine in a rat model of bone cancer pain. Pain Med. 2011;12(6):923–941. doi: 10.1111/j.1526-4637.2011.01118.x. [DOI] [PubMed] [Google Scholar]
- 118.Chen JQ, Zhang YQ, Dai J, Luo ZM, Liang SP. Antinociceptive effects of intrathecally administered huwentoxin-I a selective N-type calcium channel blocker in the formalin test in conscious rats. Toxicon. 2005;45(1):15–20. doi: 10.1016/j.toxicon.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 119.deSouza AH, Lima MC, Drewes CC, daSilva JF, Torres KC, Pereira EM, deCastroJunior CJ, Vieira LB, Cordeiro MN, Richardson M, Gomez RS, Romano-Silva MA, Ferreira J, Gomez MV. Antiallodynic effect and side effects of Phalpha1beta, a neurotoxin from the spider Phoneutria nigriventer comparison with omega-conotoxin MVIIA and morphine. Toxicon. 2011;58(8):626–633. doi: 10.1016/j.toxicon.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 120.Yamamoto T, Takahara A. Recent updates of N-type calcium channel blockers with therapeutic potential for neuropathic pain and stroke. Curr. Top Med. Chem. 2009;9(4):377–395. doi: 10.2174/156802609788317838. [DOI] [PubMed] [Google Scholar]
- 121.Zamponi GW, Feng ZP, Zhang L, Pajouhesh H, Ding Y, Belardetti F, Dolphin D, Mitscher LA, Snutch TP. Scaffold-based design and synthesis of potent N-type calcium channel blockers. Bioorg. Med. Chem. Lett. 2009;19(22):6467–6472. doi: 10.1016/j.bmcl.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 122.Pajouhesh H, Feng ZP, Zhang L, Jiang X, Hendricson A, Dong H, Tringham E, Ding Y, Vanderah TW, Porreca F, Belardetti F, Zamponi GW, Mitscher LA, Snutch TP. Structure-activity relationships of trimethoxybenzyl piperazine N-type calcium channel inhibitors. Bioorg. Med. Chem. Lett. 2012;22(12):4153–4158. doi: 10.1016/j.bmcl.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 123.Shao PP, Ye F, Chakravarty PK, Varughese DJ, Herrington JB, Dai G, Bugianesi RM, Haedo RJ, Swensen AM, Warren VA, Smith MM, Garcia ML, McManus OB, Lyons KA, Li X, Green M, Jochnowitz N, McGowan E, Mistry S, Sun SY, Abbadie C, Kaczorowski GJ, Duffy JL. Aminopiperidine sulfonamide Cav2. channel inhibitors for the treatment of chronic pain. J. Med. Chem. 2012;55(22):9847–9855. doi: 10.1021/jm301056k. [DOI] [PubMed] [Google Scholar]
- 124.Abbadie C, McManus OB, Sun SY, Bugianesi RM, Dai G, Haedo RJ, Herrington JB, Kaczorowski GJ, Smith MM, Swensen AM, Warren VA, Williams B, Arneric SP, Eduljee C, Snutch TP, Tringham EW, Jochnowitz N, Liang A, EuanMacIntyre D, McGowan E, Mistry S, White VV, Hoyt SB, London C, Lyons KA, Bunting PB, Volksdorf S, Duffy JL. Analgesic effects of a substituted N-triazole oxindole (TROX-1) a state-dependent voltage-gated calcium channel 2 blocker. J. Pharmacol. Exp. Ther. 2010;334(2):545–555. doi: 10.1124/jpet.110.166363. [DOI] [PubMed] [Google Scholar]
- 125.Swensen AM, Herrington J, Bugianesi RM, Dai G, Haedo RJ, Ratliff KS, Smith MM, Warren VA, Arneric SP, Eduljee C, Parker D, Snutch TP, Hoyt SB, London C, Duffy JL, Kaczorowski GJ, McManus OB. Characterization of the substituted N-triazole oxindole TROX-1 a small-molecule state-dependent inhibitor of Ca(V)2 calcium channels. Mol. Pharmacol. 2012;81(3):488–497. doi: 10.1124/mol.111.075226. [DOI] [PubMed] [Google Scholar]
- 126.Seko T, Kato M, Kohno H, Ono S, Hashimura K, Takenobu Y, Takimizu H, Nakai K, Maegawa H, Katsube N, Toda M. L-Cysteine based N-type calcium channel blockers structure-activity relationships of the C-terminal lipophilic moiety and oral analgesic efficacy in rat pain models. Bioorg. Med. Chem. Lett. 2002;12(17):2267–2269. doi: 10.1016/s0960-894x(02)00456-0. [DOI] [PubMed] [Google Scholar]
- 127.Seko T, Kato M, Kohno H, Ono S, Hashimura K, Takimizu H, Nakai K, Maegawa H, Katsube N, Toda M. Structure-activity study of Lamino acid-based N-type calcium channel blockers. Bioorg. Med. Chem. 2003;11(8):1901–1913. doi: 10.1016/s0968-0896(02)00558-8. [DOI] [PubMed] [Google Scholar]
- 128.Pitt B. Diversity of calcium antagonists. Clin. Ther. 1997;19 ( Suppl A):3–17. doi: 10.1016/s0149-2918(97)80033-1. [DOI] [PubMed] [Google Scholar]
- 129.Bakx AL, vanderWall EE, Braun S, Emanuelsson H, Bruschke AV, Kobrin I. Effects of the new calcium antagonist mibefradil (Ro 40-5967) on exercise duration in patients with chronic stable angina pectoris a multicenter placebo-controlled study.Ro 40-5967 International Study Group. . Am. Heart J. 1995;130(4):748–757. doi: 10.1016/0002-8703(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 130.Veronese ML, Gillen LP, Dorval EP, Hauck WW, Waldman SA, Greenberg HE. Effect of mibefradil on CYP3A4 in vivo. J. Clin. Pharmacol. 2003;43(10):1091–1100. doi: 10.1177/0091270003256687. [DOI] [PubMed] [Google Scholar]
- 131.Todorovic SM, Meyenburg A, Jevtovic-Todorovic V. Mechanical and thermal antinociception in rats following systemic administration of mibefradil a T-type calcium channel blocker. Brain Res. 2002;951(2):336–340. doi: 10.1016/s0006-8993(02)03350-4. [DOI] [PubMed] [Google Scholar]
- 132.Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105(1-2):159–168. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- 133.Dogrul A, Zagli U, Tulunay FC. The role of T-type calcium channels in morphine analgesia development of antinociceptive tolerance and dependence to morphine and morphine abstinence syndrome. Life Sci. 2002;71(6):725–734. doi: 10.1016/s0024-3205(02)01736-8. [DOI] [PubMed] [Google Scholar]
- 134.Matthews EA, Dickenson AH. Effects of ethosuximide, a T-type Ca(2+) channel blocker on dorsal horn neuronal responses in rats. Eur. J. Pharmacol. 2001;415(2-3):141–149. doi: 10.1016/s0014-2999(01)00812-3. [DOI] [PubMed] [Google Scholar]
- 135.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1-2):150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 136.Reger TS, Yang ZQ, Schlegel KA, Shu Y, Mattern C, Cube R, Rittle KE, McGaughey GB, Hartman GD, Tang C, Ballard J, Kuo Y, Prueksaritanont T, Nuss CE, Doran SM, Fox SV, Garson SL, Li Y, Kraus RL, Uebele VN, Renger JJ, Barrow JC. Pyridyl amides as potent inhibitors of T-type calcium channels. Bioorg. Med. Chem. Lett. 2011;21(6):1692–1696. doi: 10.1016/j.bmcl.2011.01.089. [DOI] [PubMed] [Google Scholar]
- 137.Francois A, Kerckhove N, Meleine M, Alloui A, Barrere C, Gelot A, Uebele VN, Renger JJ, Eschalier A, Ardid D, Bourinet E. State-dependent properties of a new T type calcium channel blocker enhance Ca(V)3. selectivity and support analgesic effects. Pain. 2013;154(2):283–293. doi: 10.1016/j.pain.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 138.Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, Doran SM, Barrow JC, Yang ZQ, Reger TS, Koblan KS, Renger JJ. In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. J. Pharmacol. Exp. Ther. 2010;335(2):409–417. doi: 10.1124/jpet.110.171058. [DOI] [PubMed] [Google Scholar]
- 139.Uebele VN, Nuss CE, Fox SV, Garson SL, Cristescu R, Doran SM, Kraus RL, Santarelli VP, Li Y, Barrow JC, Yang ZQ, Schlegel KA, Rittle KE, Reger TS, Bednar RA, Lemaire W, Mullen FA, Ballard JE, Tang C, Dai G, McManus OB, Koblan KS, Renger JJ. Positive allosteric interaction of structurally diverse T-type calcium channel antagonists. Cell Biochem. Biophys. 2009;55(2):81–93. doi: 10.1007/s12013-009-9057-4. [DOI] [PubMed] [Google Scholar]
- 140.Shipe WD, Barrow JC, Yang ZQ, Lindsley CW, Yang FV, Schlegel KA, Shu Y, Rittle KE, Bock MG, Hartman GD, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Uebele VN, Nuss CE, Connolly TM, Doran SM, Fox SV, Kraus RL, Marino MJ, Graufelds VK, Vargas HM, Bunting PB, Hasbun-Manning M, Evans RM, Koblan KS, Renger JJ. Design synthesis and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. J. Med. Chem. 2008;51(13):3692–3695. doi: 10.1021/jm800419w. [DOI] [PubMed] [Google Scholar]
- 141.Yang ZQ, Barrow JC, Shipe WD, Schlegel KA, Shu Y, Yang FV, Lindsley CW, Rittle KE, Bock MG, Hartman GD, Uebele VN, Nuss CE, Fox SV, Kraus RL, Doran SM, Connolly TM, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Marino MJ, Graufelds VK, DiLella AG, Reynolds IJ, Vargas HM, Bunting PB, Woltmann RF, Magee MM, Koblan KS, Renger JJ. Discovery of 14-substituted piperidines as potent and selective inhibitors of T-type calcium channels. J. Med. Chem. 2008;51(20):6471–6477. doi: 10.1021/jm800830n. [DOI] [PubMed] [Google Scholar]
- 142.Bladen C, Zamponi GW. Common mechanisms of drug interactions with sodium and T-type calcium channels. Mol. Pharmacol. 2012;82(3):481–487. doi: 10.1124/mol.112.079715. [DOI] [PubMed] [Google Scholar]
- 143.Leppik IE. Zonisamide chemistry mechanism of action and pharmacokinetics. Seizure. 2004;13 ( Suppl 1):S5–9. doi: 10.1016/j.seizure.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 144.Takahashi Y, Hashimoto K, Tsuji S. Successful use of zonisamide for central poststroke pain. J. Pain. 2004;5(3):192–194. doi: 10.1016/j.jpain.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Matar N, Jin W, Wrubel H, Hescheler J, Schneider T, Weiergraber M. Zonisamide block of cloned human T-type voltage-gated calcium channels. Epilepsy Res. 2009;83(2-3):224–234. doi: 10.1016/j.eplepsyres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 146.Beekwilder JP, vanKempen GT, vandenBerg RJ, Ypey DL. The local anesthetic butamben inhibits and accelerates low-voltage activated T-type currents in small sensory neurons. Anesth. Analg. 2006;102(1):141–145. doi: 10.1213/01.ane.0000189599.79451.34. [DOI] [PubMed] [Google Scholar]
- 147.Barton ME, Eberle EL, Shannon HE. The antihyperalgesic effects of the T-type calcium channel blockers ethosuximide trimethadione and mibefradil. Eur. J. Pharmacol. 2005;521(1-3):79–85. doi: 10.1016/j.ejphar.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 148.Zhang YF, Gibbs JW, 3rdCoulter DA. Anticonvulsant drug effects on spontaneous thalamocortical rhythms in vitro ethosuximide trimethadione and dimethadione. Epilepsy Res. 1996;23(1):15–36. doi: 10.1016/0920-1211(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 149.Barbara G, Alloui A, Nargeot J, Lory P, Eschalier A, Bourinet E, Chemin J. T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. J. Neurosci. 2009;29(42):13106–13114. doi: 10.1523/JNEUROSCI.2919-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, Nilsson KR, Zorumski CF, Covey DF, Jevtovic-Todorovic V. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol. Pharmacol. 2004;66(5):1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- 151.Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids. Pain. 2005;114(3):429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Chuang RS, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat. Neurosci. 1998;1(8):668–674. doi: 10.1038/3669. [DOI] [PubMed] [Google Scholar]
- 153.Sidach SS, Mintz IM. Kurtoxin a gating modifier of neuronal high and low threshold ca channels. J. Neurosci. 2002;22(6):2023–2034. doi: 10.1523/JNEUROSCI.22-06-02023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ohkubo T, Yamazaki J, Kitamura K. Tarantula toxin ProTx-I differentiates between human T-type voltage-gated Ca2+ Channels Cav3. and Cav3.2. J. Pharmacol. Sci. 2010;112(4):452–458. doi: 10.1254/jphs.09356fp. [DOI] [PubMed] [Google Scholar]
- 155.Lee CW, Bae C, Lee J, Ryu JH, Kim HH, Kohno T, Swartz KJ, Kim JI. Solution structure of kurtoxin a gating modifier selective for Cav3 voltage-gated Ca(2+) channels. Biochemistry. 2012;51(9):1862–1873. doi: 10.1021/bi201633j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449(7162):607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 157.Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreiros N, Strominger A, Cobos EJ, Ghasemlou N, Geisslinger G, Reeh PW, Bean BP, Woolf CJ. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J. Neurosci. 2013;33(1):315–326. doi: 10.1523/JNEUROSCI.2804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Puopolo M, Binshtok AM, Yao GL, Oh SB, Woolf CJ, Bean BP. Permeation and block of TRPV1 channels by the cationic lidocaine derivative QX-314. J. Neurophysiol. 2013;109(7):1704–12. doi: 10.1152/jn.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kwan YW, Bangalore R, Lakitsh M, Glossmann H, Kass RS. Inhibition of cardiac L-type calcium channels by quaternary amlodipine implications for pharmacokinetics and access to dihydropyridine binding site. J. Mol. Cell Cardiol. 1995;27(1):253–262. doi: 10.1016/s0022-2828(08)80024-7. [DOI] [PubMed] [Google Scholar]
- 160.Leblanc N, Hume JR. D 600 block of L-type Ca2+ channel in vascular smooth muscle cells comparison with permanently charged derivative D 890. Am. J. Physiol. 1989;257(4 Pt 1):C689–695. doi: 10.1152/ajpcell.1989.257.4.C689. [DOI] [PubMed] [Google Scholar]
- 161.Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2005;2(4):662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73(5):862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 163.Sogaard R, Ljungstrom T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells functional characteristics and pharmacology. Am. J. Physiol. Cell Physiol. 2001;280(4):C859–866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- 164.Hermann D, Mezler M, Swensen AM, Bruehl C, ObergruBerger A, Wicke K, Schoemaker H, Gross G, Draguhn A, Nimmrich V. Establishment of a secondary screening assay for p/q-type calcium channel blockers. Comb. Chem. High Throughput Screen. 2013;16(3):233–243. doi: 10.2174/1386207311316030008. [DOI] [PubMed] [Google Scholar]
- 165.Kim Y, Kang S, Lee JY, Rhim H. High throughput screening assay of alpha(1G) T-type Ca2+ channels and comparison with patch-clamp studies. Comb. Chem. High Throughput Screen. 2009;12(3):296–302. doi: 10.2174/138620709787581710. [DOI] [PubMed] [Google Scholar]
- 166.Xie X, VanDeusen AL, Vitko I, Babu DA, Davies LA, Huynh N, Cheng H, Yang N, Barrett PQ, Perez-Reyes E. Validation of high throughput screening assays against three subtypes of Ca(v)3 T-type channels using molecular and pharma-cologic approaches. Assay Drug Dev. Technol. 2007;5(2):191–203. doi: 10.1089/adt.2006.054. [DOI] [PubMed] [Google Scholar]
- 167.Belardetti F, Tringham E, Eduljee C, Jiang X, Dong H, Hendricson A, Shimizu Y, Janke DL, Parker D, Mezeyova J, Khawaja A, Pajouhesh H, Fraser RA, Arneric SP, Snutch TP. A fluorescence-based high-throughput screening assay for the identification of T-type calcium channel blockers. Assay Drug Dev. Technol. 2009;7(3):266–280. doi: 10.1089/adt.2009.191. [DOI] [PubMed] [Google Scholar]
- 168.Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N. lynx1 an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23(1):105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 169.vonEngelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H. CKAMP44 a brain-specific protein attenuating short term synaptic plasticity in the dentate gyrus. Science. 2010;327(5972):1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- 170.Ibanez-Tallon I, Wen H, Miwa JM, Xing J, Tekinay AB, Ono F, Brehm P, Heintz N. Tethering naturally occurring peptide toxins for cell-autonomous modulation of ion channels and receptors in vivo. Neuron. 2004;43(3):305–311. doi: 10.1016/j.neuron.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 171.Auer S, Sturzebecher AS, Juttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibanez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat. Methods. 2010;7(3):229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- 172.Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J, Schneider IC, Munch RC, Petznek H, Kontermann RE, Koehl U, Johnston IC, Keinanen K, Muller UC, Hohenadl C, Monyer H, Cichutek K, Buchholz CJ. Specific gene transfer to neurons endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat. Methods. 2010;7(11):929–935. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- 173.Moran N. First gene therapy nears landmark European market authorization. Nat. Biotechnol. 2012;30(9):807–809. doi: 10.1038/nbt0912-807. [DOI] [PubMed] [Google Scholar]