Abstract
The efficacy of many of pain-relieving drugs is based on mechanisms by which the drugs interfere with the body’s natural pain-mediating pathways. By contrast, although it is less popular, other drugs including opioids exert more powerful analgesic actions by augmenting endogenous inhibitory neural circuits for pain mediation. Recently, a novel endogenous pain-inhibitory principle was suggested and is now attracting both scientific and clinical attentions. The central players for the actions are particular body lipids: resolvins. Although research is in the preclinical phase, multiple hypotheses have actively been matured regarding the potency and molecular and neural processes of the analgesic effects of these substances. Consistently, accumulating experimental evidence has been demonstrating that treatment with these lipid substances is strongly effective at controlling diverse types of pain. Treatment of resolvins does not appear to disturb the body homeostasis as severely as many other therapeutic agents that interrupt the body’s natural signaling flow, which enables us to predict their fewer adverse effects. This paper serves as a review of currently documented painkilling actions of resolvins, summarizes the potential cellular and receptor-mediated mechanisms to date, and discusses the many clinical uses for these therapeutic lipids that have not yet been tested. Future scientific efforts will more concentrate to unveil such aspects of the substances and to construct clear proofs of concept for pain relief.
Keywords: Pain, inflammation, resolvin, analgesics, GPRs, TRP channels.
INTRODUCTION
A variety of analgesics are clinically available for relieving pathological pain. The analgesic category typically includes opioids, non-steroidal anti-inflammatory drugs (NSAIDs) including cyclooxygenase (COX) inhibitors, and other drugs with somewhat recently discovered molecular targets including voltage-gated channels and NMDA receptors. In addition, atypical analgesics often include drugs that were originally developed for other neurological purposes but that extended their indications to the pain field, such as some of anticonvulsants, tricyclic anti-depressants, cannabinoids, toxins, etc. Although there are existing drugs with numerous kinds of chemical genuses and diverse functions, there is still a strong demand for novel painkillers. This is partly because, to a certain extent, all those analgesic drugs have some disadvantage in terms of efficacy, safety or adverse effects. Well known examples are relatively low efficacies and gastro-intestinal or cardiovascular adverse effects of NSAIDs. Opioids and cannabinoids carry risks of addiction and resistance. Toxins have a narrow safety window. Therefore, numerous approaches are currently being attempted in academy and industry to unearth novel painkilling target genes and proteins in the human body and to screen the prospective chemical leads [1].
In many pathologic conditions, inflammation is tightly connected to generation of pathologic pain. One of the four cardinal signs of inflammation is ‘dolor’ (pain), which indicates that most inflammatory diseases are accompanied by pain. Indeed, this was proven by the fact that NSAIDs are among essential parts of pain medications. The concept of the anti-inflammatories is well known and simple: in order to eliminate pain, inflammation, the cause of pain should be suppressed or removed. Accordingly, studies in the 20th century focused on resisting or blocking ‘pro-inflammatory’ processes to dampen inflammation. This approach was born from the concept that inflammation actively develops in response to injuries, malfunctions, or infections and then passively disappears once the pathological insults are removed. However, during the 1980’s and 1990’s, a new proof of principle began to appear: ‘active resolution’ of inflammation [2]. Mounting experimental evidence shows that the resolution of inflammation is a dynamic process in which a number of endogenous cellular and molecular components play their different ‘pro-resolving’ roles.
Among the molecular drivers for inflammation resolution, omega-3-derived resolvins and neuroprotectins have drawn attention due to their potent pro-resolving and anti-inflammatory capacities in concentrations as low as nanomoles [3]. Understandably, evidence is quickly growing for its pain-relieving potential, too [4]. Although pro-resolving lipids primarily resolve inflammation by acting on the vascular and immune systems, the peripheral and central nervous systems also express target receptors for these lipids and react robustly with the substances. This indicates that pain attenuation may be accomplished not only via the resolution of inflammation but also via direct ‘resolution of pain’ in parallel by acting on the pain-mediating neural circuits. Details of such action mechanisms and possible pain indications for the resolving lipids are summarized here. Both anti-inflammatory and pro-resolving strategies have the same purpose (elimination of inflammation and pain). However, resolvin treatments are of particular interest not only because they are novel and potent. Curiosity also lies on the fact that, unlike ‘anti’-inflammatories, the resolvin strategy does not purely depend on blocking a natural event but is instead oriented toward promoting a natural defense pathway. Thus, such a mechanism might lead to joining rare cases in pain controlling approaches, after a long interval, following opioid or endocannabinoid concepts, in which endogenous pain-suppressing neural circuits are utilized and augmented. Would it be as potent as such traditional challenges but free from serious adverse effects or rapid tolerance? Is it practically avoiding some of NSAIDs side effects resulting from the ‘anti’-approach such as cardiovascular defects, delayed resolution, and increased vulnerability of infections due to unbalanced beneficial prostaglandins? It remains to be shown.
INFLAMMATION AND ITS RESOLUTION
The pain-relieving properties for resolvin genus started to be understood only several years ago [5,6]. Resolvins were initially identified in the inflammatory exudates and were shown to actively resolve the inflammation [3], and mechanistic information accounting for their pro-resolution efficacies and molecular theories were constructed based on repeated lines of evidence. Because pain-relief is among important final outcomes of inflammation resolution, first the general consensus for the mechanism is introduced briefly, and then direct pain-relieving processes by which changes in neuronal excitation occur is discussed.
Inflammation and Inflammatory Pain
Inflammation is a protective mechanism in response to physical or chemical tissue damage, or infections. In the early phase of inflammation, the vascular system elevates blood flow to the damaged area by locally regulating arteriolar and capillary functions. Mediators such as bradykinin increase the vascular permeability. These vascular changes result in leukocyte recruitment (primarily, neutrophils) in the tissue. In addition, the molecular machinery of neutrophil-adhesion becomes active and pro-inflammatory cytokines and tissue chemoattractants are released. The resultant fluid retention (tumor), and heat (color) from the increased local blood (rubor) flow in the damaged tissue cause the cardinal signs of inflammation.
Other cardinal signs are dolor (pain) and functio laesa (loss of function). The nociceptor nerve terminal innervating the damaged region may receive large quantities of afore mentioned mediators or cytokines from the tissue, microbial invaders, and leukocytes. Bradykinin and bacterial content such as bacterial endotoxins directly evoke nerve excitation [7, unpublished observation]. Prostaglandins, interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α), which stimulate polymorphonuclear neutrophils, also sensitize nociceptors. Leukotrienes and oxygen free radicals from neutrophils may behave like damage signals on nociceptors although those are essential for leukocyte communication and invader clearance [8]. Pain caused by such nociceptor activation and tissue dysfunction due to damage itself prevents normal macroscopic function of the tissue or organ. Disturbance of the biochemical environment by damage and by defense cascades may also perturb the microscopic tissue function.
Pain could be more exacerbated in the effector phase, where cell-specific responses occur. Nociceptor nerve terminals not only propagate their signals afferently, leading to pain perception, but also secrete peptidergic transmitters including calcitonin gene-related peptide (CGRP) and substance P. Those mediators are paracrine pro-inflammatory signals that stimulate tissues and leukocytes. Evidence of autocrine actions of CGRP and substance P is also increased. Accordingly, the inflammation gets deteriorated (neurogenic inflammation) and a subsequent increase in pro-inflammatory mediators again excites nociceptors [9]. Excessive nociceptive signals conveyed to higher synapses cause their plastic changes, namely central sensitization. Such abnormal synaptic facilitation can occurs in the spinal synapses between nociceptors and spinal dorsal horn neurons and also in higher brain regions [10]. The facilitated releases of peptidergic (here again, CGRP and substance P) and non-peptidergic (glutamate and aspartate) neurotransmitters and the elevated responsiveness of receptors (NMDA and AMPA) result in hyperalgesia, allodynia, and spontaneous pain. In this process, functionally heightened microglia surrounding the nociceptive synapses instigates and maintains the plastic changes by communicating with the neuronal components using chemokine and purinergic signalings [11,12]. Once this vicious plasticity circuit is fixed, it may become independent of whether the original tissue damages or inflammation are resolved, which indicates that chronic and pathological pain is settled. If inflammation is not resolved, and instead becomes chronic, of course it causes sustained stimulation of nociceptors, which ultimately leads to chronic pain and its aggravation.
Resolution of Inflammation
For a long time resolution has been thought to be passively achieved through the termination of pro-inflammatory processes. Over the past decade researchers began to build up a new scenario that the resolution is a self-limiting active process carried by endogenous ‘resolvents’ [see review: 13]. Pro-resolving mediators already start to be released in the early phases of inflammation. As the resolving substances exert their influence over those of pro-inflammatory mediators in the late phase of inflammation, the tissue enters the resolution phase. Pro-resolving mediators consist of divergent molecules released from neutrophils and inflamed tissues. By and large, these molecules augment recruitment of macrophages and wound closing. Corticosteroids, IL-10, adenosine, secretory leukocyte protease inhibitor (SLPI), transforming growth factor-β (TGF-β), lipoxins, resolvins, neuroprotectins, and prostaglandin D2 are all examples of pro-resolving mediators. The critical step appears to be the lipid mediator switch. Pro-inflammatory prostaglandins were reported to induce expression of genes that encode enzymes including 15-lipoxygenase (15-LOX), which produce resolving lipids, resolvins and neuroprotectins [14]. The pro-resolving lipids are now collectively termed as ‘specialized pro-resolving mediators (SPMs)’.
SPMs: Resolvins and Neuroprotectins
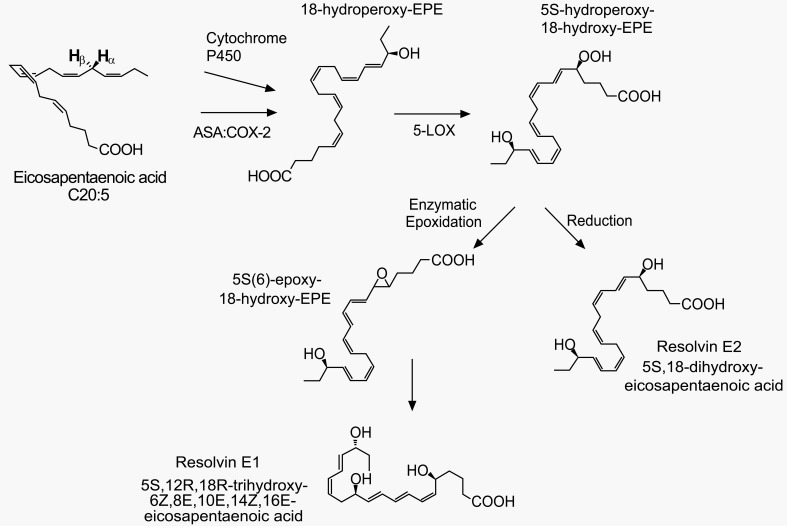
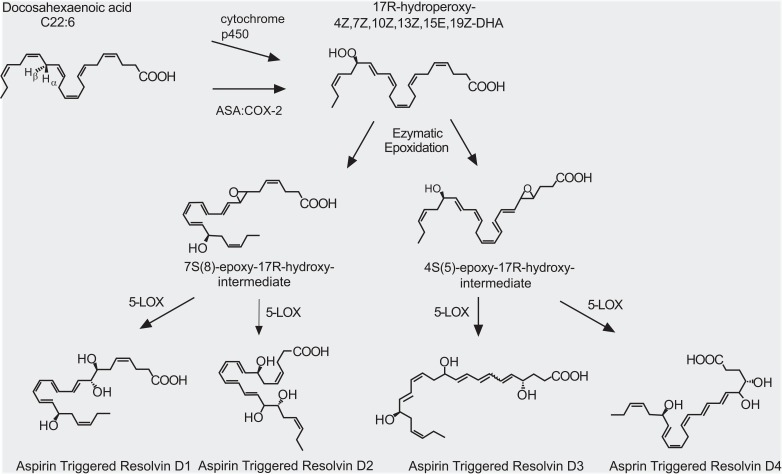
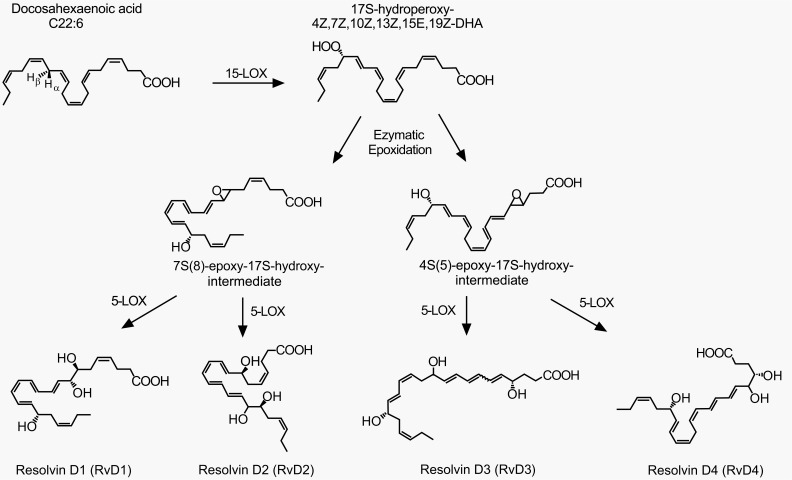
To date, totally approximately 30 kinds of SPMs have been found in inflammatory exudates: D-series and E-series resolvins and their various isomers, neuroprotectin D1, and maresin 1 [3,15,16, lipidomics gateway: http://www.lipidmaps.org/]. They are biosynthesized from omega-3 docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Figs. 1-3) [3]. Resolvin productions require multistep enzyme conversions, namely a transcellular biosynthesis process involving endothelial cell-neutrophil interactions. The source lipids and intermediates are transcellularly transferred to become final products [15,17]. The final places are often neutrophils and macrophages and those cells eventually release the resolvins. 5-lipoxygenase (5-LOX) is commonly important for all resolvin productions and other enzymes are necessary for intermediate-specific oxidations. Synthesis of D series (D stands for DHA) requires 5-LOX and 15-LOX (Fig. 2). The biosyntheses of E series (E stands for EPA) and of 17(R)-resolvin Ds require cytochrome P450 (or instead, aspirin-triggered COX-2) in the upstream of the 5-LOX actions (Figs. 1 and 3) [18]. Because the aspirin-acetylated form of COX-2 contributes to the resolvin generation, this may be another therapeutic mechanism for aspirin. Under non-inflamed conditions, enzyme expression levels are low. When acutely inflamed, enzyme expressions are strongly induced by a signaling cascade of pro-inflammatory mediators such as prostaglandins, IL-1β, etc. [14]. For example, COX-2 and 15-LOX are increased in peripheral inflamed tissues, nociceptor sensory neurons and the spinal cord [19,20,21]. Resolvins and neuroprotectins generated from such de novo pathways begin to resolve inflammation as described below.
Fig. (1).
E-series resolvins and their biosynthetic pathways. A series of enzyme actions (cytochrome p450 or aspirin (ASA)-acetylated cyclooxygenase-2 (COX-2), 5-LOX, etc.) generate E-series resolvins from eicosapentaenoic acid (EPA). The figure is reproduced with permission from Kohli and Levy [105].
Fig. (3).
AT (aspirin-triggered) resolvin Ds and their biosynthetic pathways. A series of enzyme actions (cytochrome p450 or aspirin (ASA)-acetylated cyclooxygenase-2 (COX-2), 5-LOX, etc.) generate AT-resolvin Ds from docosahexaenoic acid (DHA). The figure is reproduced with permission from Kohli and Levy [105].
Fig. (2).
D-series resolvins and their biosynthetic pathways. A series of enzyme actions (15-LOX, 5-LOX, etc.) generate D-series resolvins from docosahexaenoic acid (DHA). The figure is reproduced with permission from Kohli and Levy [105].
Mechanisms of E Series Resolvins’ Actions
It has been confirmed in a variety of experimental inflammatory conditions that SPMs exert ‘dual-actions’: pro-resolving and anti-inflammatory actions. Anti-inflammatory actions of resolvins are attained largely by down-regulating recruitment and function of the polymorphonuclear neutrophils, and by decreasing pro-inflammatory mediator production and secretion. In terms of pro-resolving actions, resolvins promote recruitment of monocyte/macrophage, their non-phlogistic phagocytosis, and IL-10 secretory function to clear exogenous and endogenous pro-inflammatory components.
The anti-inflammatory and pro-resolving effects of resolvin E1 (RvE1) seem to be via specific G-protein coupled receptor (GPR)-mediated actions. RvE1 antagonizes type 1 leukotriene B4 receptor (BLT1) in neutrophils and of dendritic cells, and activates chemR23 receptors expressed in monocytes. BLT1 antagonism leads to suppression of infiltration and nuclear factor Kappa B (NFêB) or mitogen-activated kinase (MAP kinase) intracellular signaling, which causes a decrease in pro-inflammatory mediator production and secretion. ChemR23 is expressed largely in monocyte cell types such as macrophages, microglia, and dendritic cells [17,22,23,24]. Activation of ChemR23 promotes phagocytic clearance of apoptotic neutrophils. Chemerin, a specific ligand for chemR23, has displayed similar immunological effects as RvE1 in inflammation for both in vivo and in vitro [23,25].
Based on these processes, detailed aspects of resolution can be observed in various inflammatory models. In peritonitis, RvE1-induced resolution is confirmed in terms of neutrophil recruitment, dendritic cell migration, and expression of inflammatory cytokines and chemokines at nanomolar levels [26,27]. In 2,4-dinitrofluorobenzene-induced atopic dermatitis, RvE1 attenuated inflammatory reactions such as skin swelling, production of inflammatory cytokines (IFN-r, IL-4) and infiltration of CD4(+), CD8(+) T cells, mast cells, and eosinophils [28]. In allergic airway inflammation, RvE1 promoted the migration and cytotoxic function of natural killer cells and down-regulated Thelper-17 cells by suppressing the production of pro-inflammatory IL-23 and IL-6 [29,30]. RvE1 exhibited a protective effect in colitis by decreasing leukocyte infiltration [31]. In acute lung inflammation, RvE1 attenuated anti-apoptosis signal and lead to phagocytosis-induced neutrophil apoptosis [32]. Treatment of RvE1 diminished neutrophil accumulation and promoted Escherichia coli clearance in the lung in a murine model of aspiration pneumonia [33]. Topically administered RvE1 dampened local inflammation and osteoclast-mediated bone loss in rabbit periodontis [34]. Further, in localized aggressive periodontitis of human, RvE1 reversed the defected phagocytic function of macrophages [35]. In stromal keratitis caused by herpes simplex virus, treatment of RvE1 significantly suppressed the level of angiogenesis and lesions [36]. RvE1, RvD1 and neuroprotectin D1 all strongly protected against neovascularization in a murine model of oxygen-induced retinopathy [22]. Recently, RvE2 was also shown to play a resolving role similar to that for RvE1 in a receptor-mediated mechanism [37,38].
Mechanisms of D Series Resolvins’ Actions
In a murine dorsal-skin air pouch model, RvD1 was first shown to block neutrophil recruitment [3]. RvDs utilize different GPR pathways than those for RvEs. Lipoxin A4, another resolving lipid, shares a receptor for resolution signals. Through lipoxin A(4)/Annexin-A1 receptor/formyl-peptide receptor 2 (ALX/FPR2) and also through GPR32, RvD1 limits neutrophil recruitment [39]. It is unclear whether other RvDs and their epimers share the same GPR-interaction mode. Recently 17(R)-RvD1 (also known as aspirin-triggered RvD1 and AT-RvD1) was shown to activate the same GPRs [40].
There is mounting evidence indicating that RvDs are powerful resolvents. RvD1 was detected in mouse kidney upon bilateral ischemic insults. Treatment of RvD1 ameliorated interstitial fibrosis and leukocyte infiltration, and blocked Toll-like receptor (TLR)-mediated macrophage activation, which improved the function and morphology of the kidney [41]. In peritonitis, RvD1 regulated microRNA (miRNA) expression [42]. Such overexpressed miRNA then up-regulated IL-10 in macrophages. In ALX/FPR2 knockout mice, RvD1 failed to reduce leukocyte infiltration and to affect the levels of miR-208 and IL-10 [40]. In inflamed obese adipose tissues, RvD1 reduced secretion of IFN-γ/LPS-induced Thelper1 cytokines (IL-6, monocyte chemotactic protein-1 (MCP-1: also known as chemokine (C-C motif) ligand 2 (CCL2)), and TNF-α). RvD1 also lead to nonphlogistic phagocytosis by macrophages of the stromal vascular fraction. [43]. Further in inflamed obese adipose tissues, RvD1 and RvD2 rescued expression and secretion of adiponectin and decreased pro-inflammatory adipokines such as TNF-α, IL-6, and IL-1β [44]. In diabetic mice, RvD1 treatment disturbed accumulation of apoptotic thymocytes and promoted resolution of peritonitis, wound closing, and diabetic macrophage phagocytosis [45].
RvD1 also dampens inflammation driven by oxidative stress [46]. Oxidative stress generates hyperoxygenated lipids including 4-hydroxy-2-nonenal (HNE) and 4-oxo-2-nonenal (ONE). These lipidergic products are known to be acute pain-producing agents via transient receptor potential ankyrin type 1 (TRPA1) activation but are more extensively known as inflammation-inducers via conjugation with endogenous glutathione. RvD1 prevented infiltration of Gr-1(+) leukocytes into the region inflamed by a glutathione-conjugated HNE injection.
RvD1 reversed inflammation in lipopolysaccharide-induced acute lung injury [47]. RvD1 and AT-RvD1 resolved allergic inflammatory responses in airways [48]. RvD1 showed decreased IL-6 and TNF-α production in amyotrophic lateral sclerosis with 1,100 times greater potency than its precursor lipid DHA [49]. Treatment of D series resolvins improved disease parameters including body weight loss, colonic damage and polymorphonuclear infiltration in dextran sulfate sodium or 2,4,6-trinitrobenzene sulfonic acid-induced colitis [50]. A recent finding suggested that the actions of D-series resolvins are more beneficial than predicted in bacterial infection [51]. In infection-induced inflammation, RvD1, RvD5 and neuroprotectin D1 all were able to directly enhance bacterial clearance without evoking pro-inflammatory signals. Those RvDs even down-regulated NF-κB and TNF-α gene expression. More interestingly, the endogenously dominant resolvent was RvD5 and it also utilized the RvD1 receptor, GPR32, for resolution. Treatment of these RvDs even greatly improved antibiotic effectiveness for bacterial clearance. These results emphasize the practical usefulness of D series resolvin treatment in infection-mediated diseases.
EFFECTS OF RESOLVINS ON PAIN
Inflammatory pain mechanisms account for many aspects of pathological and chronic pain [52]. Most simply, pro-inflammatory mediators from inflamed tissues and leukocytes directly stimulate nerve terminals. Prostaglandins lower the nociceptive thresholds of damage-sensing ion channels including TRP ion channels via protein kinase C and protein kinase A phosphorylation cascades by activating their EP or DP type receptors expressed in the sensory nerves [53]. IL-1β also increases sensory neuronal excitabilities via p38 mitogen-activated protein kinase (p38 MAP kinase)-dependent pathway, which leads to relief of slow inactivation of tetrodotoxin-resistant voltage-gated sodium channels and enhancement of persistent TTX-resistant current [54]. In a similar MAP kinase-dependent manner, TNF-α positively modulates tetrodotoxin-resistant sodium channels leading to mechanical hyperalgesia [55]. It also up-regulates TRPV1 expression and affects central synaptic strength [56,57,58]. Bradykinin is able to directly evoke nociceptor excitation [7,59,60]. Its B1 or B2 receptor activation cascade produces phospholipase C and phospholipase A2 lipid products which open TRPV1 and TRPA1. Nerve growth factor released from inflamed tissues acts on the nociceptors in a manner similar to those for bradykinin (direct excitation) and TNF (sensitization) [59,61,62]. Moreover, a group of Toll-like receptors (types 3, 4, 7, and 9) are expressed in nociceptors and can detect pathogen-associated molecular patterns (PAMPs) of infected bacteria or viruses [63,64,65]. This induces sensory neuronal excitation and sensitization, resulting in pain or itching. In addition, increased nerve firings due to such constant excitation or sensitization result in exacerbation of inflammation through the neurogenic inflammation cycle as described above. Such inflammatory molecular mechanisms are often suggested to locally contribute to development, maintenance, and deterioration of neuropathic pain caused by damage of the nerve itself in recent studies [52].
From extensive anti-inflammatory and pro-resolving potentials of resolvins and related lipids as mentioned in the above section, their accompanying painkilling effects are easily predictable. Again, this is the reason that one major therapeutic purpose of known anti-inflammatories is pain-relief. Even for a popular analgesic aspirin, an increase in RvD levels might be a mechanism of action since COX-2 becomes able to produce RvD when it is acetylated by aspirin. Surprisingly, a series of recent findings from 2010, have demonstrated that resolvins also act directly on sensory neuronal pathways including nociceptor terminals, dorsal horn synapses, and neighboring microglia. As well as their GPR resolvin receptors expressed in the neuronal components, they are also able to modify functions of sensory ion channels which play roles of detecting or processing environmental damage inputs. The details are summarized in the below.
Resolvins D1 and E1 on the Peripheral Pain Mechanism
Now the focus is turning to the direct actions of resolvins on pain and nerves [see review: 4]. Pioneering research has been conducted by the Ji lab [5]. First of all, powerful analgesic efficacies of RvE1 at nanograms were demonstrated in extensive forms of pain animal models which are mostly inflammatory ones (formalin, complete Freund’s adjuvant (CFA), carrageenan, and TNF-α. cf. for TRPV1-mediated acute pain, capsaicin is used). Mechanisms other than anti-inflammatory and pro-resolving actions on leukocytes may be involved and theses differential mechanisms largely consist of peripheral and central neural hypotheses. Peripherally, RvE1 injected intraplantarlily prevented neutrophil infiltration and paw edema, and lowered expression of pro-inflammatory cytokines and chemokines including TNF-α, IL-1β, IL-6, MCP-1, and macrophage inflammatory protein-1α (MIP-1α) in carrageenan-inflamed paw tissues, which may reflect a typical resolution [5]. Direct actions on nociceptors were also observed. Sensory TRP ion channels such as TRPV1 and TRPA1 in nociceptors play crucial roles in the detection of damage signals both in normal and inflammatory conditions and are considered important peripheral analgesic targets. RvE1 blocked capsaicin (a TRPV1-specific agonist)-evoked spontaneous pain and TRPV1-mediated heat pain. It is likely due to the GPR Gαi signaling-mediated modification of TRP functions [5,57]. The dorsal root ganglia where nociceptor cell bodies are clustered express chemR23 GPR. Colocalization of ChemR23 with TRPV1 in DRG was also confirmed [5].
Since both pre- and post-synapses in the spinal cord dorsal horn and neighboring microglia seem to conserve the same GPR signaling cascade, similar inhibitory mechanisms may explain the central region when RvE1 is intrathecally administered (see below). The final post-synaptic effectors should be different from those for peripheral terminals or central presynapses of the DRG nociceptors (TRP channels), which are NMDA receptors in the dorsal horn neurons although they may share extracellular signal–regulated kinase (ERK) inhibition as an intermediate downstream. Attention to the spinal microglia, a non-neuronal component supporting dorsal horn synaptic strength has risen sharply because of its significance in maintaining pathologic plasticity in the nociceptive synapses [66,11]. The monocytic origin of the spinal microglia suggests the presence of resolvin-specific GPRs and whether and if any, how significantly spinal glial functions will be changed upon resolvins are burning questions [67]. Another striking point is that the efficacy of intrathecal RvE1 for formalin-induced second phase pain was greater than the efficacy of morphine and COX-2 inhibitor NS-398. Intrathecal treatment of RvE1 or RvD1 also reduces inflammatory heat and mechanical hypersensitivity. Chemerin, the ChemR23-specific GPR ligand showed a similar effect on RvE1 in this pain model [5].
The Ji group suggested that, as in leukocytes, the GPR signaling paradigm also dominates the principle for such a versatility of painkilling actions of resolvins on the neuronal circuit in multiple pain phenotypes. This appears more obvious particularly when examining the central mechanism by which synaptic strength is modified. In the second phase of formalin pain, RvE1 effects were disturbed by an intrathecal injection of Gαi-coupled GPR inhibitor pertussis toxin and were prevented by spinal ChemR23 knockdown. Moreover, chemerin mimicked resolvin’s spinal effect as mentioned above. The relationship between RvD1, AT-RvD1, GPR32, and ALX/FPR2 on central inhibitory actions has yet to be explored. ALX/FPR2 expression in spinal astrocytes has been reported in a different study [68].
The Ji group constantly suggests that peripheral mechanisms also depend on GPR signaling. RvD2 inhibited both TRPV1 and TRPA1, and Maresin 1 inhibited TRPV1 activation in DRG neurons [57,69]. Pretreatment of pertussis toxin or of GDPβS abrogated the inhibitory effects, which indicates that the TRP channel blockade is mediated by Gai-coupled signaling despite the unknown identities of the RvD2 and maresin receptors. These GPR intracellular signaling cascades may be coupled with certain specific TRP channels, because the inhibitory effects are limited to particular TRP subtypes. For example, RvE1 effect is only obvious for TRPV1 inhibition but not for TRPA1 [5,57].
With other reslovins, specificity issues lead our group to a different hypothesis. Our group focused on the effects of RvD1 and AT-RvD1 on peripheral sensory modality and TRP specificity [6,70]. In terms of in vitro channel inhibition RvD1 showed sharp specificity to subtypes of sensory TRPs, which were TRPA1, TRPV3, and TRPV4. Other nociceptive sensory TRPs such as TRPV1, TRPV2, and TRPM8 were resistant to RvD1 [6]. On the relevant in vivo sensory phenotypes for TRPA1, V3 and V4 modalities, their agonist-specific acute pain behaviors were well blocked by RvD1 peripheral treatments. TRPA1 and TRPV4 mediate mechanical hyperalgesia and allodynia. These mechanical hyper-sensitivities under CFA inflammation as well as hypotonicity-induced pain under prostaglandin priming, were all suppressed by acute local RvD1 treatments. TRPV3 and TRPV4 are heat sensors and CFA-inflammatory heat hyperalgesia was also blocked. Formalin is a standard pain-producing reagent for observing peripheral (phase I) and central (phase 2) pain mechanisms. Phase I action is caused due to acute nociception via TRPA1-specific activation by formalin [71]. Phase I formalin-induced pain was significantly dampened, suggesting that RvD1 inhibits TRPA1.
The inhibitory actions of AT-RvD1 were only specific to TRPV3, which well corresponds with inhibitory outcomes observed in the behavioral phenotype assays using an TRPV3 agonist, and heat insult as the physical stimulus sensed by TRPV3 under CFA or carrageenan-inflammation [70]. Mechanical phenotypes were not affected by peripheral applications of AT-RvD1, the time course of which was so immediate that a general resolving mechanism could not occur [70]. Interestingly, these two resolvins appeared to be rather direct. Cathelicidin LL-37 and Trp-Lys-Tyr-Met-Val-Met, the agonists of RvD receptor ALX/FPR2 failed to repeat the TRP inhibitory actions [6]. Gallein, a Gβγ inhibitor, also failed to modify TRPV3 inhibition by 17R-RvD1, indicating that GPR signaling unlikely mediates this inhibition. The potency of RvD actions on leukocytes that engage GPR signaling was different from the potency of RvD actions on TRP channels, which implicates that different receptors with different affinities are involved [72]. A number of polyunsaturated fatty acids have been found as TRP ligands, suggesting that other unknown lipids might act on TRP channels [73]. Since the Ji lab and our lab demonstrated the effects of different resolvins, the GPR-mechanism and the TRP-directly mechanism might be differentially important for each tested resolvin. Otherwise, both of the molecular mechanisms may work for resolvin actions, but the contribution indices of each mechanism could depend on neuronal subpopulations or pathologic conditions that control the receptor expression and sensitivity. Different availabilities of inactivating metabolisms for each SPMs in different tissues may also be responsible for this action spectrum. Further studies including the use of binding assays remains to dissect the details of signalings.
Regardless of which molecular mechanism is dominant, it is clear that resolvins have a direct effect on the nociceptive neural pathway and that they produce beneficial outcomes for pathological pain. Resolvins also have another advantage. Ji group and Our group have commonly shown that those resolvent lipids do not change normal pain thresholds despite their powerful potency [5,6,57,70]. Central synaptic strength was also checked in this regards in the Ji’s lab. RvE1 treatment did not alter basal spontaneous excitatory postsynaptic currents (sEPSC) although it reversed the elevated sEPSC frequency associated with capsaicin or TNF-α. Thus, it is likely that exaggerated synaptic strength caused by certain inflammatory pathologies reverts to normal activity with resolvins [5, 57].
Challenges on Various Pain Types
The analgesic parameters are being accumulated in multiple forms of chronic pain and also with different resolvin species. Resolvins have analgesic effects in postoperative pain models. Intraplantarly pre-injected RvE1 and RvD1 prevented incision-induced mechanical and heat hyperalgesia in hind paws [5]. The Strichartz lab found that intrathecal RvD1 prevented or rescued postoperative mechanical allodynia and hyperalgesia in a skin-muscle retraction rat model [74]. They also found that the efficacy of intrathecal RvD1 injections are time-dependent, which suggests that relatively early injection procedures might be more effective [74]. In a rodent chronic pancreatitis model using trinitrobenzene sulfonic acid, intrathecal RvD1 treatment lead to suppression of mechanical allodynia [75]. Regarding the molecular mechanism, RvD1 attenuated the phosphorylation of NMDA receptor subunits NR1 and NR2B, and down-regulated the expression of cytokines such as TNF-α, IL-1β and IL-6 in the spinal cord dorsal horns [75].
Nerve injuries can be caused by accidents, viral infections, diabetic neuropathy, surgeries, and chemo-therapy. These conditions may induce a form of chronically uncontrollable pain called neuropathic pain, which is now a serious issue in terms of individual and public health. Neuropathic pain is also mediated by chronic plastic changes in central synaptic strength on the neural pain pathway, where microglial activation is an important step in the painful synaptic plasticity mentioned above. Consensus for the molecular mechanism is that microglial activation causes p38 MAP kinase activation, which results in the releases of pro-inflammatory cytokines including IL-1β, TNF-α, and growth factors like brain-derived neurotrophic factor (BDNF). Those substances strengthen spinal synaptic transmission and sensitization. This cascade reminds of a typical paradigm of pro-inflammatory pathway that occurs in leukocytes and suggests that the therapeutic hypothesis using resolvins can possibly be developed. Indeed, the Ji lab demonstrated that neuropathic pain is alleviated by either intrethecal pretreatment or post-treatment of RvE1 and suggested that the most probable mechanism is inhibition of microglial activation [67].
Effects of other SPMs on Pain
Systemic administrations of AT-RvD1 or its precursor substance 17(R)-hydroxy-docosahexaenoic acid (17(R)-HDoHE) prevented inflammatory pain in a CFA-induced arthritis model [20]. 17(R)-HDoHE prevented joint stiffness but not paw and joint edema. Those treatments diminished the expression of TNF-α and IL-1β in CFA-inflamed paw tissues and expression of NF-κB and COX-2, particularly in the DRG and spinal cord of the inflamed animals [20]. 17(R)-HDoHE can be converted to other RvD substances (AT-RvD2, AT-RvD3, AT-RvD4 and AT-RvD5), and it might be interesting whether or not these other D series also have therapeutic potentials for treating arthritic pain [76].
Maresin 1 (MaR1) is a DHA-derived lipid which is shown to be produced by human macrophages and has a strong resolution potential [69]. Serhan et al. showed that MaR1 potently blocked TRPV1 activation, but not TRPA1 activation, in cultured DRG sensory neurons and that this effect was mediated via Gαi pathway. Further they demonstrated that the analgesic effect of MaR1 in an acute pain model using intraplantarly capsaicin-injection and in a neuropathic pain model (mechanical allodynia) using intraperitoneal vincrisitin injection.
Taken together, a number of resolvin series are now thought to relieve inflammatory and neuropathic pain by controlling neural components in peripheral and central circuits. The next step is to determine whether the therapeutic effects of resolvins can be extended to different types of pathologic pain and which practical indications are most promising.
PROSPECTS
Recent studies have defined the analgesic actions of resolvins, and are evolving into research dealing with more specific types of pain. However, many detailed properties and other possibilities of these potentially beneficial compounds still remains to be explored, from the fundamental basis of action mechanisms and to clinical utilities.
Kinetic and Spatial Analyses
Benefits of resolvins are well-established through animal assays with extra-administrations as mentioned and through preclinical and clinical studies on synthetic derivatives (see below). On the other hand, quantitative indices describing the natural oscillation of their endogenous forms need to be more clarified. For example, kinetic events regarding the temporal productions of specific intermediates or final resolvins as well as enzyme activities and expression levels are still blurred in many types of inflammatory pain. It should also be more precisely determined whether a common timeline dominates its fate regardless of disease types.
There are still some questions about spatial quantitation and contributions. During inflammation, it is unclear which types of cells near the damaged site provide a majority of DHA and EPA, and whether the major sources are temporarily variable and dependent on inflammation progress. Circulating DHA and EPA, mostly in their albumin-bound forms in the normal plasma, may become important since they can penetrate when tissues are inflamed and the local vascular permeability is increased [77]. Among neutrophils and monocyte/macrophages, it would be interesting which (or both?) the major participant in ‘transcellular’ resolvin production is and whether this is dependent on damage types (e.g., physical injury or infection) considering that targeting a specific cell type is also possibly among therapeutic strategies. Along the same line, we should consider whether we can add the nerve and microglia as major donators or just stable ones since they are spatially and numerically fixed compared to diffusible leukocytes. Nonetheless, the parameters generated by neural components might also be dynamic because they might be functionally exaggerated due to pro-inflammatory signals under damaged conditions. Because of this fixed localization, it is possibly that the neural components might be long-term contributors for chronic pain, which remains unclear. Current observations in the central nervous system on the fate of synaptamide, a non-resolvin DHA derivative, might give a hint for such aspect of mediator actions [78,79].
More microscopically, kinetic analyses of contribution indices for individual receptor subtypes (chemR23, GPR32, ALX/FPR2, and sensory TRP channels) and their mutual interactions are still largely lacking. Such quantitative receptor information may help to narrow down practically more valuable target receptors. At the tissue level, a question might be raised whether resolvins, as acetylcholine or corticosteroids do, contributes more globally to resolution in tissues, otherwise to a certain distinct regions with relatively specific potencies [80,81]. It might be related not only to resolvin receptor expression but also to its counterpart condition, how much aggressive and tissue-specific, the pro-inflammatory flow or pro-inflammatory pain flow is. This could also reflect some tendencies like which tissue type is prone to generate inflammation filled with pus, bloody or serous fluid, respectively [see review: 13]. For such multiple interrogations, transgenic animals with specific resolvin depletions (maybe using enzyme knockout in the production metabolism) or without GPRs may be useful and some of those are already available [82,83]. Again, this approach might help filter more valuable target receptors.
Resolvin is likely able to reduce the duration of inflammation and help complete termination of the inflammation. This seems obviously beneficial as it should prevent conversion of a tissue state to chronic inflammation and chronic inflammatory pain, and chronic states are difficult to completely reverse. However, inflammation is an essential protection mechanism in the body and it might need to be carefully observed whether premature termination by an extra-resolvin treatment is less helpful for clearing initial damage signals or invading microorganisms or for wound repair. In this respect, several promising results have been reported. RvDs and RvE1 were found to significantly contribute to bacterial clearance [33,51]. In addition, resolvins were shown to promote injury repair via increased phosphoinositide 3 kinase-dependent migration and decreased accumulation of apoptotic cells [45,84]. This suggests that resolvin rapidly and ultimately help successful completion of rescue of injuries, rather than lead to incomplete defense.
Precursors and other LOX Products
In terms of de novo resolvin production, substrate (DHA or EPA) availability might be important in addition to elevated enzyme activity and expression levels. Thus the maintaining a diet high in omega-3s could be helpful because these essential fatty acids are not biosynthesized in human body. However, it is not yet possible to conclude whether substrate availability is a critical factor for elevating resolvin production under inflammatory states. For pain, immediate administration of the omega-3 precursor lipids DHA and EPA does not provide dramatic benefits when compared to the effect of resolvins on formalin-induced second phase pain, although the precursor lipids showed marginal rescues [5]. In fact, for anti-inflammation, the omega-3 lipids seem to have differential possibilities. DHA is known to suppress inflammation likely via its own GPR targets including GPR40 and GPR120 [85,86,87]. Oh et al., suggest that contribution by resolvins is limited or negligible for the DHA effect on the obesity/insulin resistance which differs from what González-Périz et al. suggested [88]. Because only a few inflammatory diseases have been examined and the DHA-GPR120 hypothesis is even unexplored in the sensory neural circuit so far, future comparisons might be more important for the eventual conclusion both for lipids and for GPRs in pain-related regards.
Historically, a different aspect has been more highlighted in the lipoxygenase-pain axis. For example, 12-LOX or 5-LOX products (hypdroperoxyeicosateteraenoic acids or hydroxyoctadecadienoic acids) have constantly been shown to evoke nociceptive firing via direct sensory TRP channel activation [89,90]. These interactions with nociceptive TRPs frequently account for downstream signaling cascades of inflammatory mediator bradykinin-induced pain and noxious heat-evoked pain (see above). Such a role as lipid messengers of other LOX metabolites in pain induction has lead to a hypothesis that LOX inhibition might result in pain-relief [91,73]. Quantitative information on the counterbalance between resolvins and other LOX products is still largely lacking at an angle of pain induction and relief. By broadening target pools throughout the metabolites via these enzymatic pathways, approaches including lipidomic kinetics may cover this question in the future.
Stable Analogue Development
One potential issue on the way of the development of resolvins as drugs may originate from its chemical nature itself: those are lipids. Moreover, those are highly unstable lipids compared to their precursors such as DHA and EPA. Resolvins are created by body’s sophisticated metabolic cascades. The metabolic modifications (oxidation, hyperoxydation, epoxidation, etc.) are largely labile and vulnerable to further metabolism. Indeed, RvE1 had a shorter analgesic duration than synthetically modified one [5], which indicates that natural forms are likely prone to degradation or metabolism. Therefore, minor chemical modifications that retain resolvin’s critical backbone for its biological function (more accurately, for receptor binding affinity) while preventing its quick degradation, might be important when finding chemical leads. In fact, such approaches seem to have been rarely applied to lipids compared to other biological macromolecules. Successful examples have been produced in protein drugs and carbohydrate drugs (glycomimetics). There are so many cases in protein preparations for vaccines and biosimilars that it is difficult to pinpoint which are representative [92]. In terms of glycomimetics, oseltamivir and zanamivir had a tremendous impact on influenza therapies and its associated drug markets [93]. By contrast, when it comes to lipidomimetics, the primary focus has been only on the delivery carriers such as liposomes. In this regard, it appears to be impressive that total synthesis of resolvins becomes active [94]. One meta-bolically stable synthetic analog (19-para-fluoro-phenoxy-RvE1) was reported publicly and shown to retain biological activity [95,96]. As mentioned above, the pioneering works of the Ji group on this synthetic analog appear to be important steps in the field of lipidergic resolvents on pain, and possibly for lipid therapeutic approaches as a whole [5].
Potential Advantages and Disadvantages to Resolvins
NSAIDs are mostly synthetic small molecules that do not naturally exist, and that disturb the natural signaling flow in our body. In contrast, resolvins are naturally occurring molecules. And the known action mechanisms are to boost natural resolution pathways. In this view, even with exogenous administrations, the possibility of a safety concern might be less than in synthetic drugs. However, one should remember that opioid drugs are another example of a potent imitator of an endogenous mechanism. Opioids mimic endorphin or enkephalin and promote the pain-inhibitory neural circuit by activating GPR opioid receptors, resulting in profound analgesia. However, the body’s ability to quickly build a tolerance to opioids (by receptor down-regulation via a feedback mechanism) and the adverse effects of opioids (through stimulating off-target regions) reduce their therapeutic windows. Thus, these days, taking up those challenges, clinical fields keep developing delicate and strict administrative regimens to minimize the side effects and those control methods are being evolved. These are important lessons to keep in mind when using a potent endogenous modulator to obtain a therapeutic outcome by exaggerating its effect. One advantage that resolvins have over opioids is that resolvin treatment only affects pathologic pain but not normal pain [5,20,74]. In mice and rats, regardless of injection routes (intrathecal, intraplantarly or, systemic), the resolvins did not modify normal thermal or mechanical pain. Opioids are known to reduce normal pain sensitivity [97]. This might be not only a clinical benefit but also a clue as to how a pathologic pain circuit might be anatomically and pharmacologically differentiated from a normal pain circuit.
As new target receptors, GPRs and TRPs for resolvins may create a number of other opportunities. These molecular targets do not compete with other traditional targets in terms of cellular and molecular mechanisms. Therefore, although resolvins may be less potent in the form of a single treatment, they may have a place in the market when combined with other NSAIDs or anti-inflammatories. Furthermore, although NSAIDs have known their painkilling effects, they tend to delay resolution because their pain relief mechanism lead to the reduction of pro-resolving prostaglandins [98,99]. By contrast, the primary effect of resolvins is pro-resolution. In addition, NSAIDs do not significantly contribute to injury repair, but resolvins are shown to actively help with injury repair [45,69,84,100]. Moreover, the resolving action of resolvins includes elevation of innate immunity, for example, by augmenting macrophage functions. A dramatic example is that RvDs have shown great efficacy against bacterial infection [51]. Such versatility may result in multiple combining points to realize cooperative or synergistic actions when co-administered with existing anti-inflammatories or analgesics.
Clinical Relevance
At the moment, it is difficult to anticipate which types of pain will best respond to analgesic effects of resolvin treatment. Considering the importance of natural inflammatory resolution, somewhere in NSAID territory might be tried in the near future. For inflammation itself, it is announced that a synthetic resolvin analog RX-10045 completed phase II for eye dryness. RX-10001 (which is RvE1) by oral administration completed phase I and appears to be considering clinical applications for asthma, rheumatoid arthritis, and colitis, all of which are diseases potentially accompanied by serious pain or sensory hypersensitivity. Numerous factors such as efficacy, safety, cost, and market share, affect delicate target disease setting in the industry. Neuropathic pain may become one of the most intriguing challenges because of the low availability of efficacious painkillers and uncontrollable nature of the disease. Only a few studies focused on neuropathic pain models but they demonstrated promising results [67,69]. Inflammation is now known to be an important component of one of the mechanisms behind neuropathic pain. A critical administration point that maximize efficacy must be determined before its chronic state begins.
Tailored medicine may also be derived in this field. The discovery of resolvins leads to an attention to their production pathways. Genetic variance or a differential expression profile of COX-2 and lipoxygenases from individual to individual may be observed. Patients with relatively delayed resolution due to low enzyme activity (slow resolvers) might be more sensitive to resolvin treatment [101]. For other pain-related genes, for example, GTP cyclohydrolase 1, such patient-oriented approaches turn out to be promising [102].
CONCLUSION
This paper reviewed a series of findings showing that resolvin species are potent anti-inflammatory and pro-resolving molecules and that their extraneous administrations successfully mimic and even greatly promote the resolution mechanism. This review also described the latest promising experimental results showing that these powerful lipids produce neural component modifications and effectively suppress various types of pain by a paradigm similar to that observed in inflammation.
As mentioned above, potent painkilling strategies that mimic or augment an endogenous mechanism have been generated and successful. Resolvin and its mechanism may possibly be among most recently discovered ones. The highly beneficial outcomes coupled with a surprising potency and limited or negligible adverse effects are raising possibility to generate practically useful therapeutics. As resolvin drug treatments are developed, lessons learned from previous experiences with traditional painkillers may minimize the trial and error process. Interestingly, opioid and cannabinoid practices originated from the discoveries of the exogenous natural compounds. Even for pain-producing mechanism, capsaicin opened sensory TRP ion channel research field. Although it is too hypothetical, an inverse concept might also be possible that unnoticed ‘natural resolvents’ mimicking resolvins may already be available in the nature. The identities of resolvin receptors are being discovered. This information may accelerate the development of more stable, efficacious, and cost-effective synthetic leads.
Resolution of inflammation and resolution of pain seem to be tightly linked, but more evidence is required to know whether those are completely synchronized. For example, prostaglandin D2 and its receptors were shown to act as a pro-nociceptive signal in peripheral and spinal levels [103,104]. Thus, a hypothesis that resolution contributors are necessarily engaged in dampening pain should be carefully built up. Also, how and where such a resolvent that modifies pain state negatively (or positively, too) communicates with resolvin series at viewpoint of pain exacerbation might be an interesting question to delicately define resolvin’s action. In summary, there are still a number of questions to be answered despite consistently promising results showing that resolvins are powerful analgesic candidates. As resolvins continue to achieve significant attention in basic and clinical fields, endeavors and efforts in the near future will provide the answers and suggest more detailed directions.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea (2012000540) and Korea Health technology R&D Project of Ministry of Health & Welfare (A111373).
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Max MB, Stewart WF. The molecular epidemiology of pain a new discipline for drug discovery. Nat. Rev. Drug Discov. 2008;7:647–658. doi: 10.1038/nrd2595. [DOI] [PubMed] [Google Scholar]
- 2.Serhan CN, Savill J. Resolution of inflammation the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple antinociception. Br. J. Pharmacol. 2010;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo CH, Yoo MH, You HJ, Cho SH, Mun YC, Seong CM, Kim JH. Transepithelial migration of neutrophils in response to leukotriene B4 is mediated by a reactive oxygen species-extracellular signal-regulated kinase-linked cascade. J. Immunol. 2003;170:6273–6279. doi: 10.4049/jimmunol.170.12.6273. [DOI] [PubMed] [Google Scholar]
- 9.Chiu IM, vonHehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP do pain and emory share similar mechanisms. Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia a big problem from molecules in small glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain. Neuron Glia Biol. 2007;3:255–268. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. Viewpoint The resolution of inflammation. Nat. Rev. Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 14.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain human blood and glial cells.Autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 17.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment anti-inflammatory properties and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okubo M, Yamanaka H, Kobayashi K, Fukuoka T, Dai Y, Noguchi K. Expression of leukotriene receptors in the rat dorsal root ganglion and the effects on pain behaviors. Mol. Pain. 2010;6:57. doi: 10.1186/1744-8069-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima-Garcia JF, Dutra RC, daSilva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 22.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem NJr, Serhan CN, Smith LE. Increased dietary intake of omega-3- polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, LePoul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 26.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer.Stereochemical assignments anti-inflammatory properties and enzymatic inactivation. J. Biol. Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 27.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 28.Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator.Resolvin E1 ameliorates 2-4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2012;14:384–391. doi: 10.1016/j.intimp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23 interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin e1 in the timely resolution of allergic airway inflammation. J. Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1 an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid protects against 2-4-6- trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ElKebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. U.S A. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, VanDyke TE. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 35.Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, VanDyke TE. Impaired phagocytosis in localized aggressive periodontitis rescue by Resolvin E1. PLoS One. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J. Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2 identification and anti-inflammatory actions pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler. Thromb. Vasc. Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and prokk microRNAs. Am. J. Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 42.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, Arroyo V, Clària J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 44.Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution Therapy for the Treatment of Delayed Healing of Diabetic Wounds. Diabetes. 2013; 62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br. J. Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir. Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, LaCava A, Wiedau-Pazos M. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord inhibition of inflammation by resolvin D1. Am. J. Neurodegener. Dis. 2012;1:60–74. [PMC free article] [PubMed] [Google Scholar]
- 50.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J. Immunol. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- 51.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol. 2011;24:400–407. doi: 10.1097/ACO.0b013e32834871df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin X, Gereau RW4th. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaible HG, vonBanchet GS, Boettger MK, Bräuer R, Gajda M, Richter F, Hensellek S, Brenn D, Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann. N. Y. Acad. Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 57.Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1 inflammatory pain and spinal cord synaptic plasticity in mice distinct roles of resolvin D1 D2 and E1. J. Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization distinct and overlapping role of interleukin-1beta interleukin-6 and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4-5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 60.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 61.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 62.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 63.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res. 2011;90:759–764. doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 65.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J. Immunol. 2011;186:6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji RR, Suter MR. p38 MAPK microglial signaling, and neuropathic pain. Mol. Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu ZZ, Berta T, Ji RR. Resolvin E1 Inhibits Neuropathic Pain and Spinal Cord Microglial Activation Following Peripheral Nerve Injury. J. Neuroimmune Pharmacol. 2012;8(1):37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-Resolvin D1 specifically inhibits TRPV3 leading to peripheral antinociception. Br. J. Pharmacol. 2012;165:683–692. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J. Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bang S, Yoo S, Oh U, Hwang SW. Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch. Pharm. Res. 2010;33:1509–1520. doi: 10.1007/s12272-010-1004-9. [DOI] [PubMed] [Google Scholar]
- 74.Huang L, Wang CF, Serhan CN, Strichartz G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng QX, Feng F, Feng XY, Li SJ, Wang SQ, Liu ZX, Zhang XJ, Zhao QC, Wang W. Resolvin D1 reverses chronic pancreatitis-induced mechanical allodynia phosphorylation of NMDA receptors and cytokines expression in the thoracic spinal dorsal horn. BMC Gastroenterol. 2012;12:148. doi: 10.1186/1471-230X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu ZZ, Ji RR. Resolvins are potent analgesics for arthritic pain. Br. J. Pharmacol. 2011;162:274–277. doi: 10.1111/j.1476-5381.2011.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates novel mechanisms in resolution. J. Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim HY, Spector AA, Xiong ZM. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat. 2011;96:114–120. doi: 10.1016/j.prostaglandins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HY, Spector AA. Synaptamide endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88:121–125. doi: 10.1016/j.plefa.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.vanMaanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis aggravation of arthritis in nicotinic acetylcholine receptor a7 subunit gene knockout mice. Ann. Rheum. Dis. 2010;69:1717–1723. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 81.Cutolo M, Foppiani L, Minuto F. Hypothalamic-pituitary-adrenal axis impairment in the pathogenesis of rheumatoid arthritis and polymyalgia rheumatica. J. Endocrinol. Invest. 2002;25:19–23. [PubMed] [Google Scholar]
- 82.Luangsay S, Wittamer V, Bondue B, DeHenau O, Rouger L, Brait M, Franssen JD, deNadai P, Huaux F, Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009;183:6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- 83.Hartt JK, Barish G, Murphy PM, Gao JL. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes.Molecular characterization of FPR2 a second mouse neutrophil FPR. J. Exp. Med. 1999;190:741–747. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation. Am. J. Physiol. Cell. Physiol. 2012;302:C1331–1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 86.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamoto K, Nishinaka T, Matsumoto K, Kasuya F, Mankura M, Koyama Y, Tokuyama S. Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res. 2012;1432:74–83. doi: 10.1016/j.brainres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 88.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids a role for resolvins and protectins. FASEB. J. 2009; 23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoo S, Han S, Park YS, Lee JH, Oh U, Hwang SW. Lipoxygenase inhibitors suppressed carrageenan-induced Fos-expression and inflammatory pain responses in the rat. Mol. Cells. 2009;27:417–422. doi: 10.1007/s10059-009-0059-2. [DOI] [PubMed] [Google Scholar]
- 92.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 93.Ernst B, Magnani JL. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winkler JW, Uddin J, Serhan CN, Petasis NA. Stereocontrolled total synthesis of the potent anti-inflammatory and pro-resolving lipid mediator resolvin D3 and its aspirin-triggered 17R-epimer. Org. Lett. 2013;15:1424–1427. doi: 10.1021/ol400484u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 96.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 97.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 98.Chan MMY, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase 2 inhibition and restored by prostaglandin E2 mediated lipoxin A4 production. J. Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 100.Yanes O, Clark J, Wong DM, Patti GJ, Sánchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 2010; 6: 411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, deSouza PM, Gilroy DW. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. U S A. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Costigan M, Latremoliere A, Woolf CJ. Analgesia by inhibiting tetrahydrobiopterin synthesis. Curr. Opin. Pharmacol. 2012;12:92–99. doi: 10.1016/j.coph.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ebersberger A, Natura G, Eitner A, Halbhuber KJ, Rost R, Schaible HG. Effects of prostaglandin D2 on tetrodotoxin-resistant Na+ currents in DRG neurons of adult rat. Pain. 2011;152:1114–1126. doi: 10.1016/j.pain.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 104.Kanda H, Kobayashi K, Yamanaka H, Noguchi K. COX-1-dependent prostaglandin D2 in microglia contributes to neuropathic pain via DP2 receptor in spinal neurons. Glia. 2013;61:943–956. doi: 10.1002/glia.22487. [DOI] [PubMed] [Google Scholar]
- 105.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br. J. Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]