Abstract
Chronic infantile neurological cutaneous and articular (CINCA) syndrome is a severe chronic inflammatory disease of early onset, characterized by cutaneous symptoms, central-nervous-system involvement, and arthropathy. In the present study, we report, in seven unrelated patients with CINCA syndrome, distinct missense mutations within the nucleotide-binding site of CIAS1, a gene encoding cryopyrin and previously shown to cause Muckle-Wells syndrome and familial cold urticaria. Because of the severe cartilage overgrowth observed in some patients with CINCA syndrome and the implications of polymorphonuclear cell infiltration in the cutaneous and neurological manifestations of this syndrome, the tissue-specific expression of CIAS1 was evaluated. A high level of expression of CIAS1 was found to be restricted to polymorphonuclear cells and chondrocytes. These findings demonstrate that CIAS1 missense mutations can result in distinct phenotypes with only a few overlapping symptoms and suggest that this gene may function as a potential inducer of apoptosis.
Chronic infantile neurological cutaneous and articular (CINCA) syndrome, also known as “neonatal onset multisystemic inflammatory disease,” is a rare congenital inflammatory disorder characterized by a triad of (1) neonatal onset of cutaneous symptoms, (2) chronic meningitis, and (3) joint manifestations with recurrent fever and inflammation (Prieur and Griscelli 1981; Hassink and Goldsmith 1983; Prieur et al. 1987; Torbiak et al. 1989; Prieur 2001). Persistent and migratory skin rash associated with skin perivascular polymorphonuclear infiltrates is present in patients, starting at birth. A progressive neurological impairment results from chronic meningitis caused by polymorphonuclear cell (PMNC) infiltration. A progressive visual defect and perceptive deafness frequently occurs with increasing age. Joint symptoms manifest as recurrent joint flares with or without severe radiologically evident modifications involving the growth cartilage or bone epiphysis (fig. 1). In addition, common morphological features (fig. 1), a shortening of distal limbs, and growth retardation give a sibling-like resemblance between unrelated patients. Intrafamilial recurrence, although much rarer than sporadic cases, is suggestive of an autosomal dominant inheritance pattern. However, the small number of familial cases have until now impeded a genomewide linkage approach. Recently, several genes involved in recurrent inflammatory syndromes, which also are associated with intermittent episodes of cutaneous rash, arthralgia, and fever, have been localized and identified. These include the TNFRSF1A gene on chromosome 12p13, associated with familial Hibernian fever (also called “TNF-receptor–associated periodic syndrome” [MIM 142680]) (McDermott et al. 1999); the MEFV gene on chromosome 16p13.3, associated with familial Mediterranean fever (FMF [MIM 249100]) (The French FMF Consortium 1997; The International FMF Consortium 1997); the MVK gene on chromosome 12q24, associated with hyper-IgD syndrome (MIM 260920) (Drenth et al. 1999; Houten et al. 1999); and the CIAS1 gene on chromosome 1q44, associated with the Muckle-Wells syndrome (MWS [MIM 191900]) and familial cold autoinflammatory syndrome (FCAS [MIM 120100]) (Hoffman et al. 2001).
Figure 1.
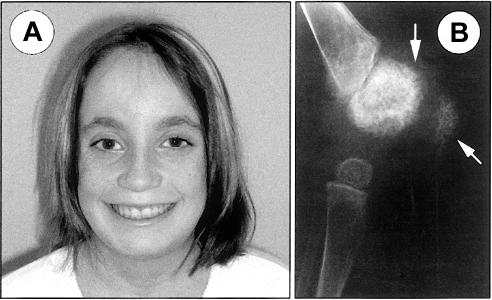
Typical CINCA syndrome features. A, Facial appearance of patient 6 at age 12 years, with characteristic frontal bossing and protruding eyes. B, Radiological bone and joint modifications. Bilateral severe bone deformities of the knees of patient 7 at age 2.5 years, resulting in hard bony enlargement without any suggestion of synovial thickening on palpation. Arrows show the growth cartilage burst and increased irregular patellar opacity.
In the familial cases of CINCA syndrome that we have investigated, disease segregation was compatible with linkage to the CIAS1-containing genetic region on chromosome 1 (data not shown) (Cuisset et al. 1999), a finding that prompted us to search for mutations of CIAS1 in patients with CINCA. We have therefore studied seven unrelated white families with classic features of CINCA (table 1) and have sequenced the CIAS1 coding sequence (GenBank accession number AF427617), to search for the presence of mutations. Both strands of the CIAS1 coding sequence, as well as each exon/intron flanking sequence, was screened by direct sequencing of PCR fragments, through use of combinations of primer pairs described elsewhere (Hoffman et al. 2001). In these families, mutation analysis identified distinct missense mutations, all located in CIAS1 exon 3 (table 1 and fig. 2). In family 1, a T1718C de novo transition appeared in the proband, changing a phenylalanine into a serine codon (F573S). A C916A transition (leading to Q306L) was identified in the proband from family 2. In family 3, both patients and their affected father display a C1307A transition, leading to a threonine-to-asparagine codon change (T436N). A G907A transition changes an aspartic acid (a negative acidic residue highly conserved at this position among members of the NACHT subfamily [Koonin and Aravind 2000]) into an asparagine codon (D303N; fig. 2B) in patient 6 and in her affected father. A nucleotide transition was also found in patient 4 (A1073G, leading to H358R), in patient 5 (T1985C, leading to M662T), and in patient 7 (T926C, leading to F309S); these appeared as de novo mutations in these three probands. These mutations were not present in any of the parents of patients with sporadic cases, in any unaffected individual in these families (fig. 2A), or in >70 ethnically related healthy control individuals, which excludes the possibility that these mutations are common polymorphisms. No mutation could be identified in an additional patient with a diagnosis of CINCA syndrome who presented with neonatal onset of skin lesions, joint inflammation, and radiologically severe arthropathy but without detectable chronic meningitis at the time of the study. Although this result does not rule out the possibility that there is a mutation elsewhere in the sequence that affects mRNA translation, this may alternatively suggest either genetic heterogeneity of this disease or the occurrence of an acquired form in this sporadic case.
Table 1.
CINCA Cases and Results of Mutations Analysis of CIAS1
| Probandin Familya | Clinical Characteristics | DNA Base Changeb | Amino Acid Change |
| 1 | Neonatal onset, skin lesion, chronic meningitis, joint inflammation, sensory organ impairment, dysmorphy | T1718C | F573S |
| 2 | Neonatal onset, skin lesion, chronic meningitis, joint inflammation, sensory organ impairment, dysmorphy | C916A | Q306L |
| 3a | Onset at age 6 mo, skin lesion, chronic meningitis, joint inflammation, sensory organ impairment, dysmorphy | C1307A | T436N |
| 3b | Onset at age 3 years, joint inflammation, sensory organ impairment, dysmorphy | C1307A | T436N |
| 4 | Neonatal onset, skin lesion, joint inflammation, sensory organ impairment, dysmorphy | A1073G | H358R |
| 5 | Onset during the first year of life, skin lesion, chronic meningitis, joint inflammation, sensory organ impairment, dysmorphy | T1985C | M662T |
| 6 | Neonatal onset, skin lesion, chronic meningitis, joint inflammation, sensory organ impairment, dysmorphy | G907A | D303N |
| 7 | Neonatal onset, skin lesion, chronic meningitis, joint inflammation, radiological severe arthropathy, sensory organ impairment, dysmorphy | T926C | F309S |
Precise clinical information was not available for the two patients from family 3 during the neonatal period or for the affected fathers in families 3 and 6.
Numbers represent the base location in the cDNA sequences, where base 1 is the first base of the second ATG codon.
Figure 2.
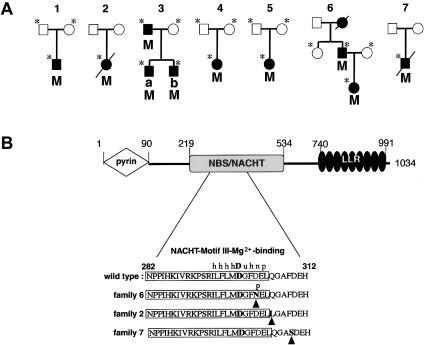
A, Mutation segregation in pedigrees of families with CINCA. Square symbols denote males; circles denote females; affected individuals are denoted by blackened symbols, and segregating CIAS1 mutations are denoted by the capital letter M. In each pedigree, family members from whom DNA samples were available for analysis are marked by an asterisk. Autosomal dominant inheritance of CINCA is suggested by the segregation observed in families 3 and 6. B, Mutation locations on CIAS1 in three patients and domain structure of CIAS1. Residues 1–90 form the N-terminal pyrin domain, residues 219–534 form the NBS/NACHT domain, and residues 740–991 form the C-terminal leucine rich repeats. Shown at the bottom is the sequence of the NBS domain around the consensus motif-III Mg2+-binding site (boxed) found in the NACHT subfamily of NTPases (following Koonin and Aravind 2000) with the mutations (arrowheads) found in three families with CINCA. h = hydrophobic residues; u = tiny residues; n = negatively charged residues; p = polar residues.
CIAS1 encodes cryopyrin, a protein composed of two protein-protein interaction domains, an amino-terminal pyrin-like domain, and a carboxy-terminal domain containing leucine-rich repeats (“LLR” in fig. 2B) (Hoffman et al. 2001). In addition, cryopyrin includes a central nucleotide-binding site (NBS) domain that belongs to the NACHT subfamily of NTPases, which is characterized by the presence of seven distinct characteristic conserved motifs, including the ATP/GTPase-specific P loop and the Mg2+-binding site (Koonin and Aravind 2000). Several other proteins from the CED4/Apaf1 family of proteins (Zou et al. 1997), including members of the caspase activation and recruitment domain family, share the same domain architecture (Hlaing et al. 2001; Martinon et al. 2001; Miceli-Richard et al. 2001). This family of proteins coordinates the assembly of signaling complexes that regulate the activation of NF-κB, cytokine processing, and apoptosis (Martin 2001). They are thought to occupy a crucial position in stress-associated signaling pathways that culminate in cell death or inflammatory responses. Accordingly, the pyrin domain of CIAS1 (also known as “pyrin-containing Apaf1-like protein 1” [PYPAF1]) was recently shown to interact selectively with the pyrin domain of the apoptosis protein ASC, leading to the activation of NF-κB (Manji et al. 2002), which suggests a function of CIAS1 related to inflammation.
All of the observed mutations in patients with CINCA fall within exon 3 of CIAS1; five of them modify the region encoding the NBS/NACHT domain, with a cluster of three mutations very close to the Mg2+-coordinating aspartate (D300) (fig. 2B) (Koonin and Aravind 2000; Falcon-Perez et al. 2001) and thus potentially disrupting nucleotide binding-site function. Since this region is thought to contain binding sites for upstream regulators, mutations in this region may affect the control of NF-κB activation.
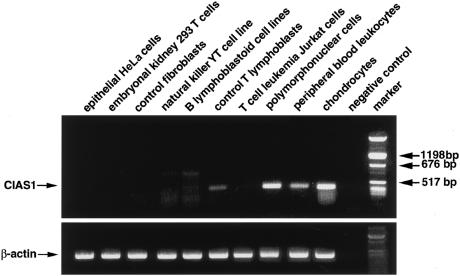
A previous study has shown that expression of CIAS1 is restricted to peripheral blood leukocytes (Hoffman et al. 2001). Tissue expression of CIAS1 was examined, and CIAS1 was found to be strongly expressed in the PMNC, whereas it was undetectable or expressed at a lower magnitude in B and T lymphoblasts, respectively (fig. 3). Interestingly, high-level expression was also detected in chondrocytes (fig. 3)—a provocative finding, given the phenotype of patients with CINCA syndrome, since most of the clinical features result from the deleterious effect of PMNC infiltration of various organs and from manifestations such as growth retardation, cartilage burst, epiphyseal anomalies, late closure of the fontanel, and morphological anomalies (frontal bossing, saddle-back nose), which suggest a cartilage target. These data suggest that CIAS1 may function as an inducer of apoptosis in these cells. Thus, in addition to its role in NF-κB activation, CIAS1 may also be connected to the apoptosis pathway. Of great interest are the recent studies that highlight a potential dual role of NF-κB, as an inducer both of proinflammatory mediators during the onset of inflammation and of anti-inflammatory mediators and apoptosis during the resolution of inflammation (Lawrence et al. 2001; Karin and Lin 2002). An attractive hypothesis would be that mutations in the regulatory nucleotide-binding domain of CIAS1 affect the NF-κB activation pathway in a way that leads to a defective switching of NF-κB between these two functions. This hypothesis would be in agreement with the in vivo model of rat carageenin-induced acute inflammation, in which inhibition of NK-κB during the resolution of inflammation protracts inflammatory responses and prevents apoptosis (Lawrence et al. 2001).
Figure 3.
Semiquantitative RT-PCR of CIAS1 mRNA expression in various tissues with β-actin levels shown below. RNAs (50ng) from each tissue was reverse transcribed and PCR amplified by a one-step RT-PCR using the following oligonucleotides: 5′-TTGAAGAGGAGTGGATGGGTT-3′ (sense on exon 2) and 5′-GATGGGTGGGTTTGGGTC-3′ (antisense on exon 3), which predict a 507-bp product of CIAS1. β-actin oligonucleotides, 5′- TACCACTGGCATCGTGATGGACT-3′ (sense on exon 2) and 5′-TCCTTCTGCATCCTGTCGGCAAT-3′ (antisense on exon 3), were used in the same conditions as a control for RNA integrity and RT efficiency. A negative control containing no input RNA was also included.
The association of chronic inflammation with recurrent fever, cutaneous rash, and joint symptoms is a common characteristic of severe disorders within the group of autoinflammatory diseases. However, CINCA syndrome is an original entity defined by neonatal onset and a unique cluster of manifestations, including chronic PMNC meningitis, as well as the severe and unique joint manifestations, characterized by symmetrical epiphyseal and metaphyseal cartilage anomalies. These features differentiate CINCA from FCAS (Zip et al. 1993) and MWS (Muckle 1979), recently shown to result from missense mutations in the same exon of CIAS1 (Hoffman et al. 2001). None of the mutations identified in the patients with CINCA reported in the present study have so far been associated with FCAS or MWS. The mutations occur de novo in five of the seven patients with CINCA syndrome, a higher proportion than that observed in the families with FCAS and MWS that have previously been reported (2/4). Although these data are not statistically significant, the early onset and severity of CINCA syndrome, which can be fatal in some patients, in comparison with the delayed and milder phenotype occurring in FCU/FCAS, may favor the occurrence of de novo mutations. The analysis of a greater number of patients with each condition is required to validate this preliminary observation and to determine whether disease expression strictly depends on the nature and localization of the CIAS1 mutation, or whether it is also influenced by still-undefined environmental or genetic factors.
In conclusion, we show that CIAS1 mutations can lead to very distinct phenotypes among the autoinflammatory diseases, including CINCA syndrome. Given the peculiar phenotype observed in patients with CINCA, in terms of PMNC activation and cartilage overgrowth, the high level of expression of CIAS1 that we show to be restricted to these two types of cells strongly supports the hypothesis of a role of CIAS1 in apoptosis. Since CIAS1 was recently shown in vitro to activate NF-κB, we speculate that mutations in CIAS1 may disturb the physiological balance between the proinflammatory and proapoptotic function of NK-κB, by favoring the former. Further studies will be necessary to better identify this anti-inflammatory pathway. Its elucidation may provide a potential new target for the treatment of inflammatory disease.
Acknowledgments
We thank the families involved in the study, for their cooperation. We are grateful to Drs. Laurence Mallet and Maité Corvol, for providing us with chondrocyte RNAs, and to Cécile Dumont, for excellent technical assistance. This work was supported by grants from INSERM, l’Association de Recherche sur le Cancer, and l’Association Vaincre les Maladies Lysosomales.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CIAS1 [accession number AF427617])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FMF [MIM 249100], TNF-receptor–associated periodic syndrome [MIM 142680], HID [MIM 260920], MWS [MIM 191900], and FCAS [MIM 120100])
References
- Cuisset L, Drenth JP, Berthelot JM, Meyrier A, Vaudour G, Watts RA, Scott DG, Nicholls A, Pavek S, Vasseur C, Beckmann JS, Delpech M, Grateau G (1999) Genetic linkage of the Muckle-Wells syndrome to chromosome 1q44. Am J Hum Genet 65:1054–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth JP, Cuisset L, Grateau G, Vasseur C, van de Velde-Visser SD, de Jong JG, Beckmann JS, van der Meer JW, Delpech M (1999) Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat Genet 22:178–181 [DOI] [PubMed] [Google Scholar]
- Falcon-Perez JM, Martinez-Burgos M, Molano J, Mazon MJ, Eraso P (2001) Domain interactions in the yeast ATP binding cassette transporter Ycf1p: intragenic suppressor analysis of mutations in the nucleotide binding domains. J Bacteriol 183:4761–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French FMF Consortium, The (1997) A candidate gene for familial Mediterranean fever. Nat Genet 17:25–31 [DOI] [PubMed] [Google Scholar]
- Hassink SG, Goldsmith DP (1983) Neonatal onset multisystem inflammatory disease. Arthritis Rheum 26:668–673 [DOI] [PubMed] [Google Scholar]
- Hlaing T, Guo RF, Dilley KA, Loussia JM, Morrish TA, Shi MM, Vincenz C, Ward PA (2001) Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J Biol Chem 276:9230–9238 [DOI] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 29:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ, Frenkel J, Dorland L, de Barse MM, Huijbers WA, Rijkers GT, Waterham HR, Wanders RJ, Poll-The BT (1999) Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet 22:175-177 [DOI] [PubMed] [Google Scholar]
- International FMF Consortium, The (1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 90:797–807 [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A (2002) NF-κB at the crossroads of life and death. Nat Immunol 3:221–227 [DOI] [PubMed] [Google Scholar]
- Koonin EV, Aravind L (2000) The NACHT family—a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem Sci 25:223–224 [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA (2001) Possible new role for NF-κB in the resolution of inflammation. Nat Med 7:1291–1297 [DOI] [PubMed] [Google Scholar]
- Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, Mak S, Lora JM, Briskin M, Jurman M, Cao J, DiStefano PS, Bertin J (2002) PYPAF1: A PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-κB. J Biol Chem 277:11570–11575 [DOI] [PubMed] [Google Scholar]
- Martin SJ (2001) Dealing the CARDs between life and death. Trends Cell Biol 11:188–189 [DOI] [PubMed] [Google Scholar]
- Martinon F, Hofmann K, Tschopp J (2001) The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr Biol 11:R118–R120 [DOI] [PubMed] [Google Scholar]
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, et al (1999) Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97:133–144 [DOI] [PubMed] [Google Scholar]
- Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, Chamaillard M, Zouali H, Thomas G, Hugot JP (2001) CARD15 mutations in Blau syndrome. Nat Genet 29:19–20 [DOI] [PubMed] [Google Scholar]
- Muckle TJ (1979) The “Muckle-Wells” syndrome. Br J Dermatol 100:87–92 [DOI] [PubMed] [Google Scholar]
- Prieur AM (2001) A recently recognised chronic inflammatory disease of early onset characterised by the triad of rash, central nervous system involvement and arthropathy. Clin Exp Rheumatol 19:103–106 [PubMed] [Google Scholar]
- Prieur AM, Griscelli C (1981) Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. J Pediatr 99:79–83 [DOI] [PubMed] [Google Scholar]
- Prieur AM, Griscelli C, Lampert F, Truckenbrodt H, Guggenheim MA, Lovell DJ, Pelkonnen P, Chevrant-Breton J, Ansell BM (1987) A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome. A specific entity analysed in 30 patients. Scand J Rheumatol Suppl 66:57–68 [DOI] [PubMed] [Google Scholar]
- Torbiak RP, Dent PB, Cockshott WP (1989) NOMID—a neonatal syndrome of multisystem inflammation. Skeletal Radiol 18:359–364 [DOI] [PubMed] [Google Scholar]
- Zip CM, Ross JB, Greaves MW, Scriver CR, Mitchell JJ, Zoar S (1993) Familial cold urticaria. Clin Exp Dermatol 18:338–341 [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413 [DOI] [PubMed] [Google Scholar]