SUMMARY
Precise spatiotemporal regulation of signaling activators and inhibitors can help limit developmental crosstalk between neighboring tissues during morphogenesis, homeostasis, and regeneration. Here, we find that the secreted Wnt inhibitor Dkk1b is abundantly produced by dense regions of androgen-regulated epidermal tubercles (ET) on the surfaces of adult male zebrafish pectoral fins. High-speed videos and amputation experiments reveal that pectoral fins and their ET are employed for male spawning. Formation and vigorous turnover of ET involve Dkk1b induction and maintenance, whereas Dkk1b is typically restricted from the regeneration blastema after amputation injury. When amputation occurs through an ET-containing region, a Dkk1b-enriched wound epidermis forms and blastema formation is disrupted, compromising regeneration. Thus, homeostatic signaling by key breeding ornaments can interfere with injury-activated tissue regeneration. Our findings help explain sexually dimorphic fin regeneration in zebrafish, and have implications for how regenerative potential might decline as development progresses or during species evolution.
Keywords: zebrafish, regeneration, blastema, evolution, sexual ornament, reproduction, epidermal appendage, stem cells, androgen, Wnt, Dkk1
INTRODUCTION
Certain salamanders and fish possess a remarkable ability to regenerate lost or damaged adult tissues, including appendages, spinal cord, brain, heart, retina, lens, jaws, and intestinal portions. By contrast, most higher species lose regenerative potential as they develop. For instance, capacities for limb or heart regeneration diminish during developmental progression in frogs and mice, respectively (Dent, 1962; Muneoka et al., 1986; Porrello et al., 2011). Little is known about the molecular basis for stage-dependent loss of regenerative capacity, which likely involves changes in both cell-intrinsic and -extrinsic factors (Lin et al., 2013).
Wnt ligands have been implicated in myriad developmental events like embryonic patterning, organogenesis, tissue homeostasis, and regeneration (Clevers and Nusse, 2012; Logan and Nusse, 2004). To limit their range of biological activity, there exist many inhibitors that block events leading to GSK3β inactivation and β-catenin stabilization. These factors typically act by binding to Wnts or LRP co-receptors, and include secreted Dickkopfs (Dkks), Frizzled-related proteins, Wnt inhibitory protein, and Sclerostin (Cheng et al., 2011; Hsieh et al., 1999; Leyns et al., 1997; Li et al., 2005). For example, this counterbalance is observed in embryonic stem cells during fate decisions: localized Wnt signals direct pluripotency, whereas Wnt inhibition leads to differentiation (Habib et al., 2013; ten Berge et al., 2011). Moreover, reduced or hyperactivated Wnt/β-catenin signaling are common causes of developmental defects and disease (Logan and Nusse, 2004; Zimmerman et al., 2012). Thus, control of the presence of Wnt activators and inhibitors can be critical in multiple biological contexts.
Zebrafish can regenerate amputated fins throughout life. However, we recently found that males of several laboratory strains display defects in regeneration of pectoral fins, appendages that regenerate efficiently in females. Male regenerative problems arise during sexual maturation and hinge on androgen signaling, thus representing a model for age- and sex-dependent loss of regenerative potential (Nachtrab et al., 2011). Wnt/β-catenin signaling is required for regeneration of zebrafish caudal fins (Kawakami et al., 2006; Stoick-Cooper et al., 2007), and transcriptional profiling comparisons between male and female fins indicated elevated levels of the inhibitor Dkk1b in male pectoral fins (Nachtrab et al., 2011). Moreover, inhibition of GSK3 activity could partially rescue regeneration of male pectoral fins, suggesting a functional consequence of high Dkk1b levels. Yet, the cellular and molecular basis for the presence of Dkk1b in male pectoral fins, and how these levels influence regeneration, are unknown.
Here, we have used transgenesis to identify sexually dimorphic expression domains of dkk1b in adult zebrafish, including a primary site of Dkk1b synthesis within fields of small structures called epidermal tubercles (ET) present on the surfaces of male zebrafish pectoral fins. We find that male zebrafish strategically employ ET to grasp the female during spawning. ET formation is stimulated de novo by androgens, and ET are continually renewed in males through proliferation and differentiation events that spatiotemporally control epidermal Dkk1b levels and Wnt target gene activation. We also find that this homeostatic process short-circuits the regeneration of amputated male pectoral fins. Regenerating fins typically decrease dkk1b expression during blastema formation, coincident with mesenchymal Wnt target gene activation. However, if amputation occurs through an ET-containing region, Dkk1b levels remain high, and Wnt target activation and regeneration are impeded. Together, our results indicate that the capacity for fin regeneration in male zebrafish is compromised by simultaneous use of a conserved signaling pathway to maintain key sexual features. This type of signaling interference is likely to be broadly relevant to tissue regenerative potential.
RESULTS
Dkk1b Production Is Sexually Dimorphic and Enriched in Male Epidermal Tubercles
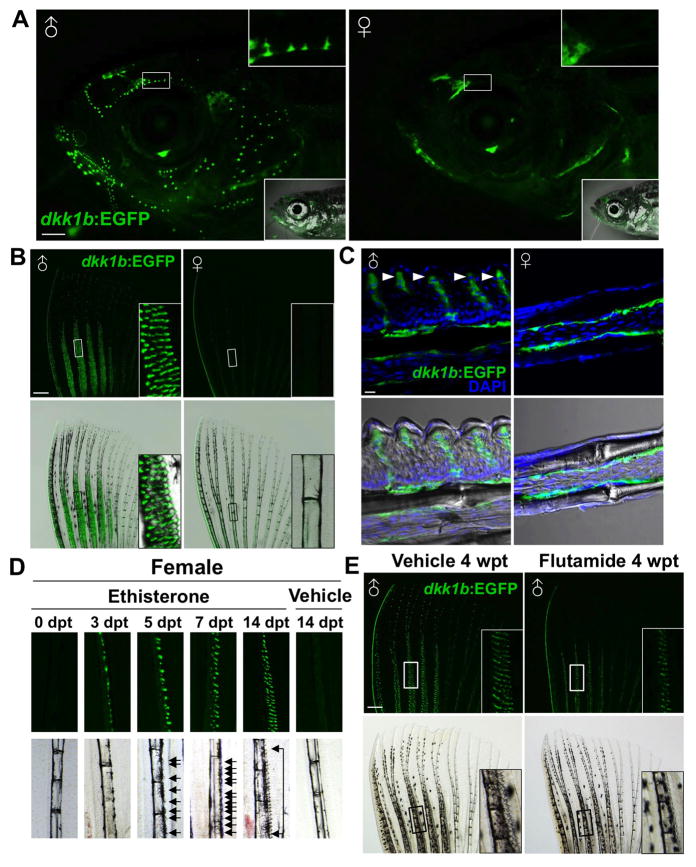
To visualize where Dkk1b is synthesized in adult zebrafish, we generated BAC transgenic animals with EGFP under the control of dkk1b regulatory sequences (Tg(dkk1b:EGFP)pd60; referred to hereafter as dkk1b:EGFP). We identified dkk1b:EGFP expression in several adult tissues of both sexes, including the ventral portion of the eyes, the lower jaw, neuromasts of the lateral line, and faint expression in the osteoblast compartments and distal tips of fin rays (Figures 1A–C; data not shown). Additionally, many small dkk1b:EGFP+ epidermal ornaments were present on the surfaces of the head (Figure 1A) and anterior body scales of male, but not female, animals (Figure S1A). Similarly, all male fins except caudal fins contained dkk1b:EGFP-expressing epidermal ornaments, spaced relatively evenly on distal segments (Figures S1B). The most prominent male-specific, dkk1b:EGFP+ features populated dense fields on the dorsal sides of anteromedial rays of pectoral fins (Figure 1B). We refer to these features hereafter as epidermal tubercles (ET), as they resemble similar structures described in other fish species and have been referred to in those cases as tubercles, contact organs, or papillary features (Kinoshita, 2009; Wiley and Collette, 1970). Sections through male pectoral fins visualized these ET as thickened regions of epidermis covered by a rigid keratinized cuticle. Each individual ET contained a multicellular dkk1b:EGFP expression domain underlying a spiked cuticle (Figure 1C). Thus, dkk1b expression in adult zebrafish is sexually dimorphic, with much of this expression found in male-specific epidermal ornaments on the head, trunk, and fins.
Figure 1. The Secreted Wnt Signaling Inhibitor Dkk1b is Produced by Androgen-Dependent Male Epidermal Tubercles.
(A) Sexually dimorphic dkk1b:EGFP expression in the heads of adult male (left) and female (right) zebrafish, present in constellations of epidermal tubercles. Insets in (top) enlarge area in white boxes. Insets in (bottom) indicate bright-field images.
(B) dkk1b:EGFP expression in male and female pectoral fins. Only male fins contain prominent ET on anteromedial rays. Insets in (B) enlarge area in white boxes. Note that the exterior of the anterior fin ray also expresses dkk1b:EGFP.
(C) Confocal images of longitudinal sections through dkk1b:EGFP pectoral fins, indicating male-specific ET domains (arrowheads). Both sexes display faint expression in the osteoblast compartment, although it is difficult to detect in some sections.
(D) Fluorescent (top) and bright-field (bottom) images of adult female pectoral fin rays after Eth treatment for the indicated durations. dkk1b:EGFP is detectable at 3 days post-treatment (dpt), and ET (arrows) are detectable at 5 dpt.
(E) Flutamide treatment decreased the number of ET in male dkk1b:EGFP pectoral fins and reduced their definition. Insets display enlarged areas from white or black boxes. Scale bars = 1 mm (A); 500 μm (B, E); 10 μm (C). See also Figure S1.
Male Zebrafish Employ Pectoral Fins and ET for Spawning
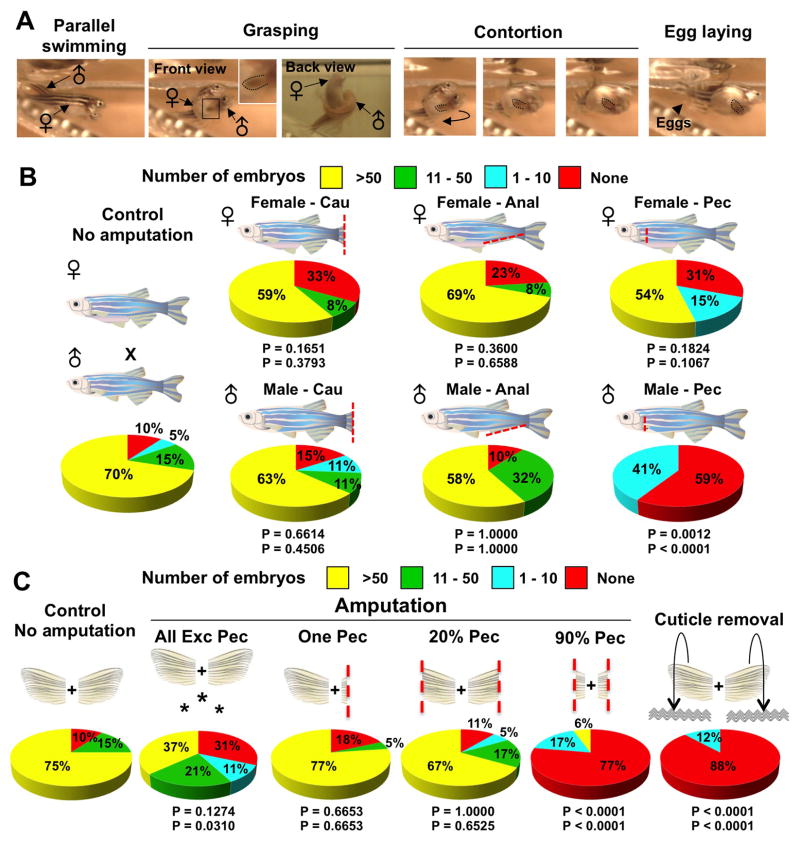
We identified ET on pectoral fins of male zebrafish from multiple different strains, including Ekkwill (used in this study), AB, Wik, and Tuebingen (Figure S1C). However, we could find no scientific reports describing them in zebrafish. In several species like goldfish, the analogous structures are referred to as breeding or nuptial tubercles, as they can appear seasonally and/or are thought to somehow facilitate contact during spawning (Ghadially and Whiteley, 1952; Wiley and Collette, 1970). The distribution of tubercles among fins and on the head and body varies among different species (Wiley and Collette, 1970). To assess how male zebrafish use pectoral fins during reproductive behavior, we filmed spawning pairs with a high-speed camera. We observed that, as male zebrafish pursue females from the rear and side, they position the dorsal, ET-containing side of a single pectoral fin firmly beneath the female abdomen. While contact is maintained, the male places its posterior trunk over the female and then contorts against the female body as eggs are released (Figure 2A; Movie S1). These events are rapid and can occur several times between each mating pair. Thus, our analysis of zebrafish mating behavior suggested a key role for pectoral fins, and their dorsal ET-containing surfaces, in grasping the female to stimulate or force egg laying.
Figure 2. Male Pectoral Fins and ET are Important Breeding Structures.
(A) Still images of zebrafish mating behavior acquired by high-speed video. 1) Parallel swimming. The male chases the female and attempts to align in a parallel position. 2) Grasping. The male positions one of his pectoral fins below the female abdomen, while placing his posterior trunk over that of the female. 3) Contortion. The male bends his body, arching away from the female. 4) Egg laying. These activities by the male stimulate egg release (arrowhead). Inset enlarges male pectoral fin. Doted lines indicate male pectoral fin. See also Movie S1.
(B) Pie charts of mating test after complete fin amputations as indicated in cartoons. ‘Cau’, ‘Anal’, and ‘Pec’ indicate full (>90%) amputation of caudal, anal, and pectoral fins, respectively. n = 12 to 22 animals as described in Table S1. The top P value is calculated from Fisher’s exact test between the ‘no amputation’ control and the experimental group for the percentage of successful matings, with 1 or more embryos considered successful (None vs. >1 embryos). The bottom P value is calculated from Fisher’s exact test between the ‘no amputation’ control and the experimental group for the percentage of successful matings, with 10 or more embryos considered successful (0–10 embryos vs. >10 embryos). As zebrafish typically have 50–200 embryos per mating, a clutch size of 1–10 embryos is unusually small and of borderline success.
(C) Pie charts showing results of mating tests after various fin injuries indicated by the above cartoons were given to male zebrafish. Asterisks denote experiments in which all fins except pectoral fins were amputated. n = 17 to 22 animals as described in Table S2. See also Movie S1. See 2B legend for description of P-values.
To test whether pectoral fins and their ET are required for spawning, we performed several experiments. First, we found that full amputation of female caudal, anal, or pectoral fins did not significantly affect spawning (clutches >10 eggs = 85%, 67%, 77%, and 54%; n = 20, 12, 13, and 13 for no amputation, caudal, anal, and pectoral fin amputees, respectively), nor did full amputation of male caudal or anal fins (clutches >10 eggs = 74% and 90%; n = 19 each for caudal and anal fin amputees, respectively). By contrast, males with fully amputated pectoral fins bred very poorly (clutches >10 eggs = 0%; n = 22; Figure 2B; Table S1). Videos of paired animals indicated that single pectoral fin amputees successfully spawned only when approaching the female from the side with an intact fin (Movie S1). Double amputees pursued females but failed to spawn (Movie S1). In partial amputation experiments, we found that the amount of remaining pectoral fin tissue had graded effects on fecundity, with less severe male amputees breeding effectively (clutches >10 eggs = 90%, 84%, and 6%; n = 20, 18, and 18 for no amputation, 20% pectoral fin amputation, and 90% pectoral fin amputation, respectively). We also amputated all fins in males except pectoral fins, and found that a majority of these animals still stimulated female laying (clutches >10 eggs = 58%; n = 19). On the contrary, surgical removal of male pectoral fin cuticles – a major structural component of ET - abrogated fecundity (clutches >10 eggs = 0%; n = 17; Figure 2C; Table S2). These results (and Figure 7 experiments described below) reveal that, while other fins are dispensable for mating, males require their pectoral fins and physically intact ET to stimulate egg laying.
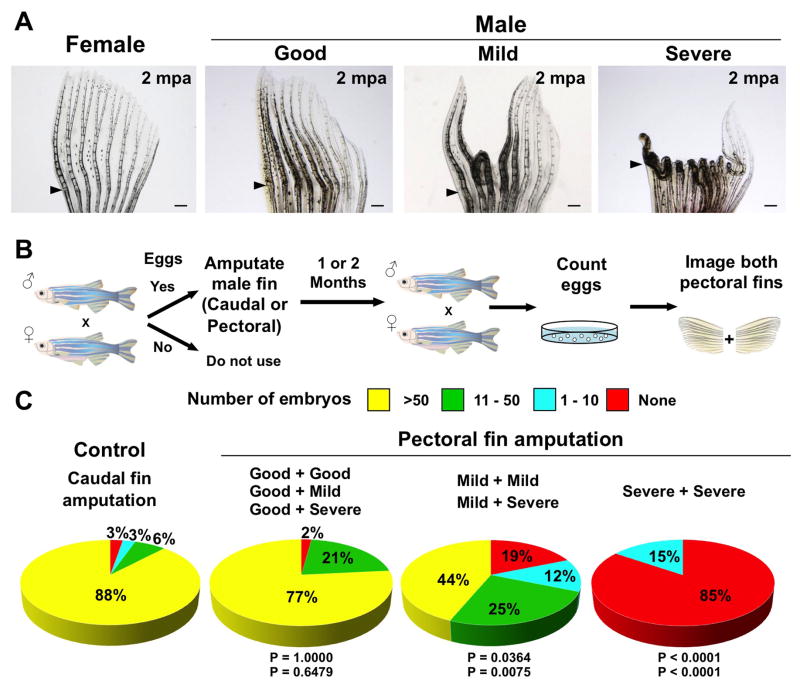
Figure 7. Long-term Regenerative Defects Inhibit Male Spawning.
(A) Whole-mount images of pectoral fins at 2 months post-amputation (mpa). ‘Good’ indicates that all fin rays between the first to sixth fin rays have completely regenerated. ‘Mild’ indicates that 1–3 fin rays between first to sixth fin rays show defective regeneration. ‘Severe’ indicates that at least 4 of 6 fin rays between first to sixth fin rays failed to regenerate normally. Arrowheads indicate amputation planes.
(B) Cartoon summarizing experiments in which mating tests were performed 1–2 months after amputation.
(C) Pie charts with results of mating tests 1–2 months after amputation of caudal or pectoral fins, with each pectoral fin scored after mating as in (B). Males that regenerated more effectively stimulated better laying than those with defective regeneration. n = 13 to 75 animals as described in Table S3. The top P value is calculated from Fisher’s exact test between the ‘no amputation’ control and the experimental group for the percentage of successful matings, with 1 or more embryos considered successful (None vs. >1 embryos). The bottom P value is calculated from Fisher’s exact test between the ‘no amputation’ control and the experimental group for the percentage of successful matings, with 10 or more embryos considered successful (0–10 embryos vs. >10 embryos).
Scale bars = 500 μm (A)
Androgen Induces Wnt Reporter/Dkk1b Expression and ET Formation from Fields
We next examined zebrafish of different ages to determine when ET and dkk1b expression appear in males. We began to observe divergent expression of dkk1b:EGFP in rudimentary pectoral fin ET of juvenile males as early as 6 weeks post-fertilization (wpf), but not at 4 wpf (Figures S1D and S1E). These ET increased markedly in size and density by the adult stage (Figures 1B and 1C). Similarly, Dkk1b-producing epidermal features appeared in the head and trunk at 8 wpf and became more prominent with sexual maturity (Figure S1F).
Male-specific traits in vertebrates such as lion manes, deer antlers, and swordtail fish swords are typically regulated by androgen (Thornton et al., 2001; Williams and Carroll, 2009), as are tubercles on male medaka and goldfish fins (Ghadially and Whiteley, 1952; Uwa, 1968). We treated adult female zebrafish with ethisterone (Eth), known to be androgenic in fish (Uwa, 1968), and examined pectoral fins for dkk1b:EGFP induction and morphologic changes. We found that dkk1b:EGFP was detectable by 2–3 days post-treatment (dpt) in anteromedial regions of pectoral fins. By 5 dpt, we noticed small ET associated with areas of dkk1b:EGFP induction, and with longer Eth treatments, these ET increased in density and definition (Figure 1D). Thus, female pectoral fins also contain fields that are competent to ET induction. In other experiments, we treated males with flutamide (Flu), an androgen signaling inhibitor (Martinovic-Weigelt et al., 2011). Flu treatment significantly decreased the number of ET per segment and reduced their definition (Figure 1E; Figure S1G), while also decreasing dkk1b:EGFP fluorescence (Figure S1H). These findings demonstrate that androgen regulates ET development and the associated production of Dkk1b in pectoral fins.
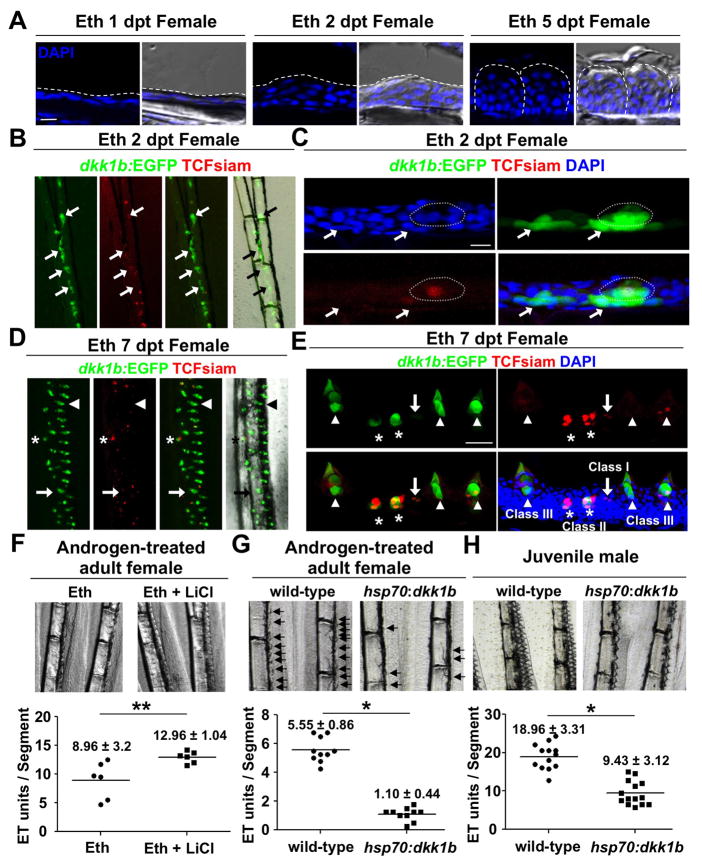
To define stages of ET morphogenesis, we treated adult females with Eth and imaged sections of ET. While the epidermal tissue on the dorsal sides of pectoral fins contained only a few cell layers in untreated animals and those treated for just one day (1 dpt), we observed local thickening of this tissue at 2 dpt, and many ET by 5 dpt (Figures 3A and 3D). As Wnt/β-catenin signaling is critical for formation of mammalian skin appendages from placodes (Blanpain and Fuchs, 2009; Mikkola, 2007), we next examined potential roles for this pathway during ET formation. We crossed a Wnt signaling reporter line (Tg(7xTCF-Xla.Siam:nlsmCherry; hereafter referred to as TCFsiam) (Moro et al., 2012) with dkk1b:EGFP and examined TCFsiam and dkk1b:EGFP expression within female pectoral fins after Eth treatment. By 2 dpt, basal and suprabasal epidermal cells broadly expressed dkk1b:EGFP in areas of future ET formation (arrows in Figures 3B and 3C), while a small subset of these cells expressed TCFsiam (circle in Figure 3C). Within days, dkk1b:EGFP expression localized to discreet ET units.
Figure 3. Induction of de novo ET Formation by Androgen and Wnt Signaling.
(A) Section images of the dorsal side of a female pectoral fin after the indicated duration of androgen treatment. Dashed lines indicate the outer epidermal border (Left and Middle) or individual ET (Right). Note that local epidermal thickening is observed by 2 dpt.
(B, C) Whole-mount (B) and section (C) images of female TCFsiam; dkk1b:EGFP pectoral fins after 2 days of androgen treatment. Arrows indicate dkk1b:EGFP expression in basal layers in forming ET. Dotted line outlines an ET precursor with one middle cell expressing weak TCFsiam.
(D, E) Whole-mount (D) and section (E) images of female TCFsiam;dkk1b:EGFP pectoral fins after 7 days of androgen treatment. ET show stage-specific expression profiles. Putative newly forming ET express TCFsiam (Class I, arrow). Putative immature ET show both TCFsiam and dkk1b:EGFP expressions (Class II, asterisks). Mature ET express only dkk1b:EGFP or both TCFsiam and dkk1b:EGFP (Class III, arrowheads). Note that TCFsiam expression is weak in mature ET.
(F) LiCl treatment increases ET formation in androgen-treated adult females. Data are mean ± standard deviation (s.d.), **P < 0.05 by two-tailed Student’s t-test
(G, H) Ubiquitous overexpression of dkk1b blunts ET formation in androgen-treated adult females (G) and juvenile males (H). Arrows indicate developing ET in (G). Data are mean ± s.d., *P < 0.001 by two-tailed Student’s t-test
Scale bars = 10 μm (A, C); 50 μm (E). See also Figure S2.
As early ET matured and the fields began to increase in density (7 dpt), we found different dkk1b:EGFP and TCFsiam expression profiles that could be distinguished as 3 classes based on their morphologies. Some expression units were TCFsiam+; dkk1b:EGFP− (arrows in Figures 3D and 3E), had few epidermal layers, and showed little evidence of mature ET features (Class I). A second type of structure with local epidermal thickening but no cuticle contained more TCFsiam+ cells, with many of these cells also expressing dkk1b:EGFP (Class II; asterisks in Figures 3D and 3E). Finally, larger structures containing cuticles were composed primarily of dkk1b:EGFP+ cells and few or no TCFsiam+ cells (Class III; arrowheads in Figures 3D and 3E). Class III units had a spiked appearance of mature ET. The presence of these 3 classes of ET suggested a model in which active Wnt signaling predominates in immature cells, Dkk1b presence marks the onset of differentiation, and double-positive cells represent developmental intermediates.
The balance between Wnt activation and inhibition is thought to be critical for induction and/or patterning of epidermal appendages (Sick et al., 2006), evidenced by studies in which Dkk1 was ectopically expressed (Andl et al., 2002; Liu et al., 2008). To examine effects of increased Wnt/β-catenin signaling on ET formation, we treated adult females with Eth and LiCl, which inhibits GSK3 activity (Klein and Melton, 1996). At 7 dpt, females treated with both LiCl and Eth had ~45% more ET that females treated with Eth alone (ET units/segment = 8.96 and 12.96 for Eth and Eth/LiCl treatments, respectively; n = 6 fins each; Figure 3F). LiCl treatment on its own for 7 days did not induce ET formation (n = 6 fins; data not shown). Conversely, to test the effects of inhibiting Wnt/β-catenin signaling on ET formation, we overexpressed Dkk1b using a heat-shock inducible transgenic line (Tg(hsp70l:dkk1b-GFP); hereafter referred to as hsp70:dkk1b). Daily heat-shock activates Dkk1b-GFP fluorescence throughout the animal, including epidermal cells within fins (Figure S2). First, we gave adult female hsp70:dkk1b animals and wild-type clutchmates a daily Eth treatment along with a daily heat-shock over a one-week period. In these experiments, dkk1b induction significantly decreased the number of newly formed ET (ET units/segment = 1.10 and 5.55 for hsp70:dkk1b and wild-types, respectively; n=10 fins each; Figure 3G). We then gave 6-week-old juvenile male hsp70:dkk1b animals and control clutchmates a daily heat-shock for 4 weeks. Ectopic dkk1b expression also inhibited new ET formation in these experiments (ET units/segment = 9.43 and 18.96; n = 14 and 13 fins for hsp70:dkk1b and wild-types, respectively; Figure 3H). Together, these results indicate that Wnt/β-catenin signaling has a positive role in de novo ET formation.
Male ET Undergo Continual Renewal
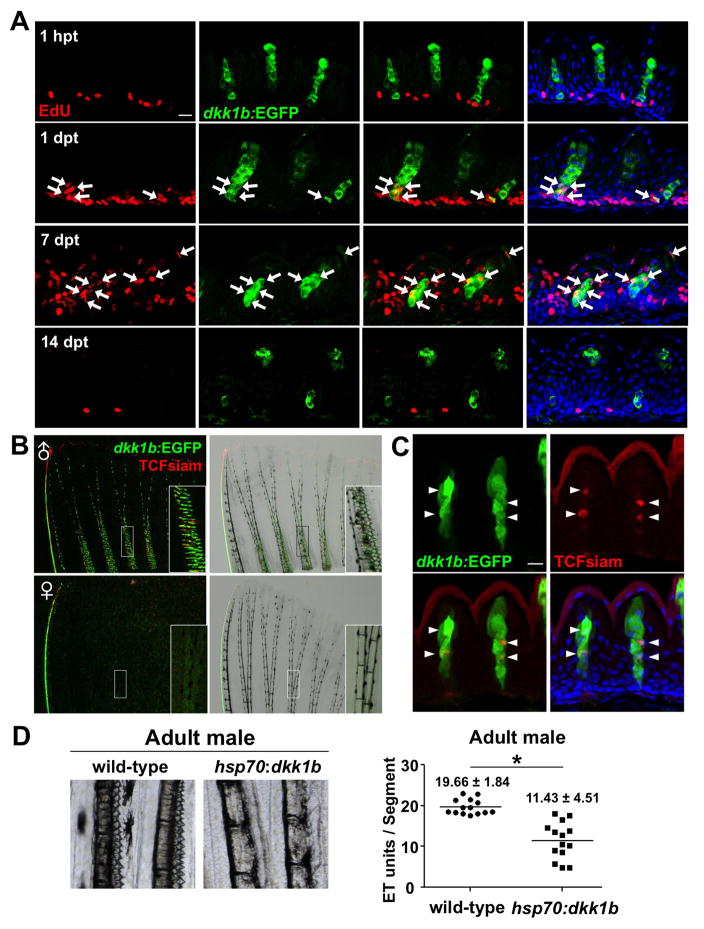
We observed that male ET cuticles were frequently discarded after mating, suggesting that ET cells are routinely replaced (Figures S3A and S3B). To examine proliferation and turnover in ET cells, we gave male dkk1b:EGFP animals a single pulse of the nucleotide analog EdU by intraperitoneal injection and collected pectoral fins at 1 hour post treatment (hpt), and at 1, 7, and 14 dpt. At 1 hpt, some basal epidermal cells between ET units, and occasionally a few cells adjacent to the basal epidermal layer, showed strong EdU signals, but ET units themselves were not EdU+ (zero ET units with cells positive for both EdU and dkk1b:EGFP, of 36 examined ET units; 6 animals were tested; Figure 4A). By 1 dpt, many of the cells located in the basal and suprabasal layers were labeled by EdU, indicating high proliferative activity. Also, some dkk1b:EGFP+ cells at the base of ET were EdU+ (23 ET units with cells positive for both EdU and dkk1b:EGFP, of 43 examined ET units; 6 animals were tested; arrows in Figure 4A), indicating that cells proliferating within the past day contribute to ET maintenance. At 7 dpt, EdU+ cells were detected throughout the ET including the cuticle nuclei (Figure 4A), suggesting that newly divided cells become differentiated cuticle cells within 7 days. At 14 dpt, the majority of ET had weak or undetectable EdU signals, whereas occasional cells in the basal epidermal layer (or adjacent to the basal layer) retained strong EdU labeling (Figure 4A). These results indicate that male pectoral fin ET undergo continual turnover, ostensibly via the proliferative activity of an epidermal cell population(s) located between adjacent ET units.
Figure 4. Male Pectoral Fin ET Undergo Vigorous Renewal.
(A) Section images of male dkk1b:EGFP pectoral fin ET at 1 hour, 1 day, 7 days, or 14 days after EdU injection. Arrows indicate EdU+dkk1b:EGFP-expressing cells. Green: EGFP immunofluorescence; red: EdU immunofluorescence; blue: DAPI.
(B) Whole-mount images of TCFsiam; dkk1b:EGFP pectoral fins. Males expressed both reporters in ET.
(C) Section image of a male TCFsiam; dkk1b:EGFP pectoral fin ET. Arrowheads indicate TCFsiam+ cells. Cuticle shows red autofluorescence in these images.
(D) Ubiquitous overexpression of dkk1b decreased the number of ET in male pectoral fins and reduced their definition. Data are shown as mean ± s.d., *P < 0.001 by two-tailed Student’s t-test.
Scale bars = 10 μm (A, C). See also Figure S3.
To examine Wnt target gene activation during ET homeostasis, we examined the pectoral fins of adult male TCFsiam; dkk1b:EGFP zebrafish. These animals displayed both EGFP and nuclear mCherry expression in pectoral fin ET (Figure 4B), with colocalization evident in some cells (Figure 4C). To test the effects of increasing Dkk1b levels, we gave adult male hsp70:dkk1b animals and wild-type clutchmates a daily heat-shock regimen for 6 weeks before assessing ET morphology. In these experiments, dkk1b overexpression reduced ET density (ET units/segment = 11.43 and 19.66 for hsp70:dkk1b and wild-types, respectively; n = 14 fins each; Figure 4D). Taken together, these results indicate that ET are actively renewed from a basal layer cell population(s), and that this renewal is dependent on local regulation of Dkk1b levels and Wnt target gene activation.
Dkk1b Regulation during Fin Regeneration
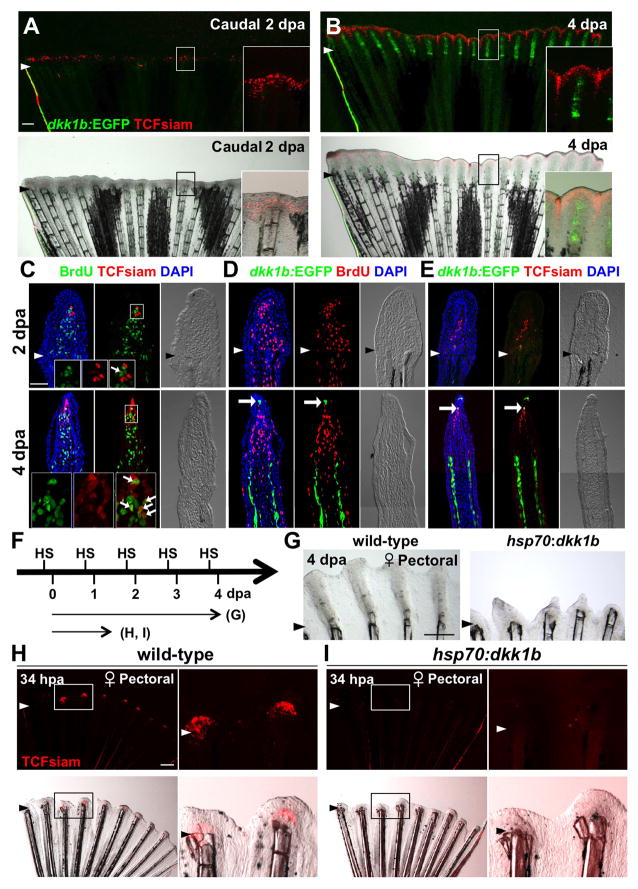
Fin regeneration occurs through formation of a proliferative mass of mesenchymal cells called the blastema, which is subsequently organized during outgrowth into areas of cell proliferation and patterning (Akimenko et al., 2003; Poss, 2010). Previously, it was shown that Wnt/β-catenin signaling is activated and required for zebrafish caudal fin regeneration (Kawakami et al., 2006; Moro et al., 2012; Stoick-Cooper et al., 2007). We examined spatial and temporal expression patterns of TCFsiam and dkk1b:EGFP during fin regeneration. In female caudal fins, TCFsiam expression was detectable in newly formed blastemal cells at 2 dpa, while dkk1b:EGFP was not detectable in the regenerate (Figures 5A and 5C–E). Although a few TCFsiam+ cells were labeled with BrdU (proliferative) after a brief (30 minute) BrdU exposure, the majority were BrdU-negative (non-proliferative) (Figure 5C). Combined with previous results by other groups (Kawakami et al., 2006; Moro et al., 2012; Stoick-Cooper et al., 2007), these data indicate that Wnt/β-catenin signaling is active during blastema formation, and dkk1b expression is diminished during this stage.
Figure 5. Blastema Formation Involves Wnt Target Gene Activation and Dkk1b Attenuation.
(A, B) Female TCFsiam; dkk1b:EGFP caudal fins at 2 and 4 dpa, shown as whole-mount images. TCFsiam is expressed in distal regions during regeneration. dkk1b:EGFP is not detectable at 2 dpa, but is evident in 4 dpa regenerates. Arrowheads indicate amputation plane.
(C) Section images of female TCFsiam caudal fins at 2 (Top) or 4 dpa (Bottom). TCFsiam is expressed in a subset of cells in the distal portion of the blastema, some of which are BrdU-positive 30 minutes after treatment. Arrows indicate BrdU+ and TCFsiam+ cells. Amputation site in 4 dpa is proximal to imaged area.
(D) Section images of female dkk1b:EGFP caudal fins at 2 (Top) or 4 dpa (Bottom). dkk1b:EGFP is not detectable at 2 dpa. At 4 dpa, it is expressed in a very small domain at the distal tip of the regenerate (arrow) and in more proximal areas of osteoblast patterning. Expression domains are adjacent to areas of low BrdU incorporation (red), but not to more proliferative blastemal mesenchyme.
(E) Section images of female TCFsiam; dkk1b:EGFP caudal fins at 2 (Top) or 4 dpa (Bottom). dkk1b:EGFP+ cells at the distal tip of the 4 dpa regenerate colocalize with TCFsiam (arrow).
(F) Experimental design for experiments testing effects of heat-induced overexpression of dkk1b during regeneration.
(G) Whole-mount images of 4 dpa wild-type and hsp70:dkk1b female pectoral fin regenerates, indicating that overexpression of dkk1b inhibits regeneration. n = 6.
(H, I) Whole-mount images of regenerates at 34 hpa, indicating that dkk1b overexpression blocks induction of TCFsiam in the newly formed blastema. n = 6.
Scale bars = 500μm (A, G, H); 50 μm (C–E). See also Figure S4.
After 4 days of regenerative outgrowth, many TCFsiam+ cells were evident in the distal portion of the blastema, which contains BrdU-positive and -negative cell populations (Figure 5C). By contrast, dkk1b:EGFP was restricted to a very small number of cells located within the BrdU-negative distal-most portion of the blastema (arrows in Figure 5D; Figure S4). These dkk1b:EGFP expressing cells typically co-localized with TCFsiam expression (Figure 5E). dkk1b:EGFP was also detectable at 4 dpa in larger areas of osteoblast patterning proximal to the most proliferative parts of the blastema (Figures 5B and 5D; Figure S4B). We observed similar regenerating expression signatures at 2 and 4 dpa in female pectoral fins, male caudal fins, and posterior regions of male pectoral fins (Figure S4 and data not shown). That these regions of dkk1b:EGFP expression were adjacent to non-proliferative mesenchymal cells was suggestive of an inhibitory influence. Consistent with this observation, dkk1b overexpression in hsp70:dkk1b animals blocked the induction of TCFsiam fluorescence at 34 hpa (Figures 5H and 5I). A longer-term dkk1b overexpression regimen inhibited pectoral fin regeneration, as had been shown previously for caudal fins (Figure 5G) (Stoick-Cooper et al., 2007). Thus, the regeneration of amputated fins normally involves the reduction of Dkk1b during blastema formation, and regulation of Dkk1b levels in distinct domains during outgrowth.
Amputated Male Pectoral Fins Develop a Dkk1b-Enriched Wound Epidermis
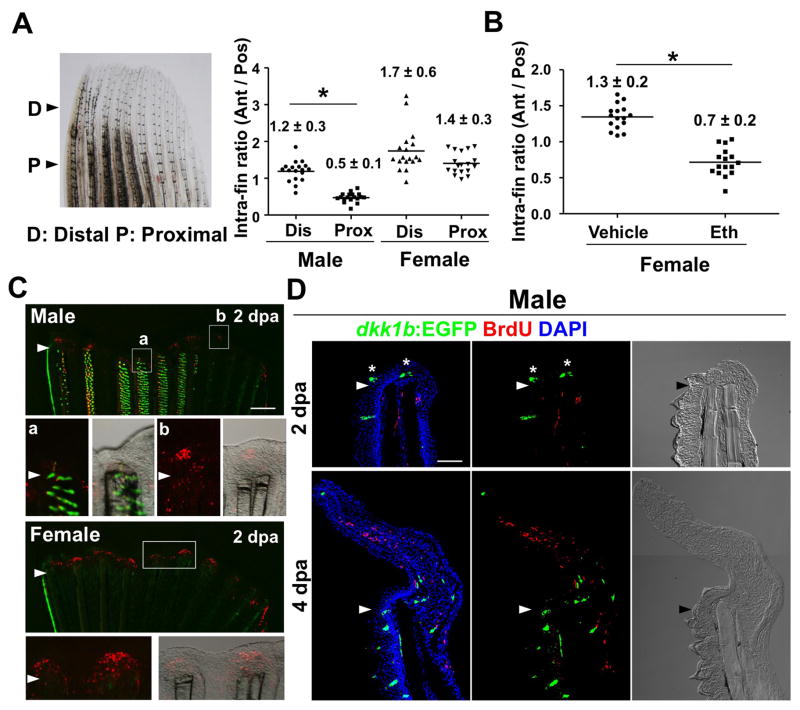
As mentioned earlier, regenerative defects in male pectoral fins were associated with high dkk1b expression and could be partially rescued by GSK3 inhibition (Nachtrab et al., 2011). Several observations from our previous and current study point to ET as the underlying basis. First, as visualized by our reporter strain, male pectoral fin ET are the most prominent Dkk1b source in adult zebrafish fins (Figure 1B). Second, regenerative defects are limited to anteromedial pectoral fin rays, which happen to be the rays with dense ET (Nachtrab et al., 2011). Third, we found that anterior rays amputated within ET fields show defective regeneration, whereas those rays amputated distal to ET areas regenerate with comparable efficacy to posterior rays (Figure 6A; Figures S5A and S5B). Next, we gave females a prolonged treatment with Eth to induce ET formation, prior to amputation. Females treated with Eth for 14 days before a one-day washout period showed anteromedial ray regeneration defects at 4 dpa that were similar to those of males (Figure 6B; Figure S5C).
Figure 6. Evidence that Dkk1b-Producing ET Inhibit Blastema Formation after Fin Amputation.
(A) Quantification of intra-fin ratio of regenerate lengths at 4 days post-amputation (dpa). The ratio of anterior ray length to posterior ray length was calculated separately for each animal as indicated. Regeneration of male anterior regions was inhibited when fins were amputated through proximal ET-containing regions (P and Pro, ‘Proximal’; 50% amputation), but not through distal regions (D and Dis, ‘Distal’; 10–20% amputation). n = 18, mean ± s.d.; *P < 0.001 by one-way analysis of variance (ANOVA) with Bonferroni’s posttest.
(B) Quantification of intra-fin ratio in adult females after 14 days of treatment with Eth, followed by one day of washout. Conditions that stimulated formation of dense dkk1b:EGFP+ ET were sufficient to inhibit regeneration of female anterior fin regions. n = 16. Data are mean ± s.d. *P < 0.001 by two-tailed Student’s t-test.
(C) Whole-mount images of TCFsiam; dkk1b:EGFP pectoral fins at 2 dpa. TCFsiam expression is detectable in female pectoral fin blastemas (Bottom) and male pectoral fins (Top, b). By contrast, TCFsiam expression is low or undetectable in anterior blastemas of male pectoral fins (Top, a).
(D) Section images showing stunted male pectoral fin regeneration at 2 dpa, with dkk1b:EGFP+ ET within and just ventral to the wound epidermis (asterisk). By 4 dpa, regenerates are dysmorphic, typically angling ventrally away from dkk1b:EGFP+ ET before overcorrecting dorsally.
Scale bars = 500μm (B, C); 50 μm (D). See also Figure S5.
To further investigate whether Dkk1b production by ET interferes with regeneration, we examined Wnt target gene activation during blastema formation in male pectoral fins. Although posterior rays of male pectoral fins showed TCFsiam fluorescence at 2 dpa (‘b’ in Figure 6C), TCFsiam expression in anteromedial rays was weak or undetectable at 2 dpa (‘a’ in Figure 6C). Tissue sections indicated that anterior 2 dpa regenerates were capped by a region of dkk1b:EGFP+-containing epidermis, with limited mesenchymal cell proliferation beneath (Figure 6D; Figures S5D). We quantified endogenous dkk1b mRNA present in tissue distal to the amputation plane at 2 dpa, and found 3-fold higher levels in male anteromedial fin regions compared to females (Figure S5F). We also examined expression of axin2, an endogenous Wnt target gene, in male and female pectoral fins. axin2 was clearly detectable in anteromedial ray blastemas of 2 dpa regenerating female pectoral fins, as reported for caudal fins (Stoick-Cooper et al., 2007), but weak or undetectable in the corresponding male structures (Figure S5G). This analysis indicated that early Wnt signaling is deficient in amputated male pectoral fin rays that are covered dorsally with ET.
We similarly examined male pectoral fin regeneration during outgrowth, and found that 4 dpa regenerating anteromedial rays were typically bent at an angle at the amputation plane away from the ET, before correcting in a dorsal direction (Figure 6D; Figure S5E). If allowed 1–2 months for recovery, these animals displayed a spectrum of regenerative outcomes that we could score as ‘good’, ‘mild’, or ‘severe’ (n = 61, 34, and 57 of 152 fins, for good, mild, and severe scores, respectively; Figure 7A). Fins with severe scores had at least 4 defective anteromedial rays that folded back onto the dorsal side, as would be predicted from assessment of 4 dpa regenerates (Figure 7A; also see Figure 6D).
To test whether regenerative efficiency contributed to reproductive ability, we assessed spawning by individual males from this panel. We found that males with a good regeneration score for one or both pectoral fins stimulated females to lay in 98% of pairings (clutches >10 eggs; n = 47), a number comparable to animals that recovered from caudal fin amputation (clutches >10 eggs = 94%; n = 75). By contrast, males with at best a mild score for one or both pectoral fins were successful in only 69% of pairings (clutches >10 eggs; n = 16), and males with severe patterning defects on both fins were never successful at spawning (clutches >10 eggs; n = 13; Figures 7B and 7C; Table S3). These results were consistent with results of our earlier experiments, indicating that pectoral fins with intact, dorsal ET-containing surfaces are required for reproductive success. Moreover, they demonstrate that the sexually dimorphic regeneration defects we have found are in many cases substantial enough to hinder the ability to reproduce.
Together, our results indicate that male-specific defects in zebrafish fin regeneration result from the collateral effects of ET maintenance signals on key responses to amputation injury (Figure S6). We find that Wnt target gene activation is inhibited after amputation, blastema formation is delayed, and early growth becomes aberrantly directed away from the ET/Dkk1b source.
DISCUSSION
Here we have defined a biological benefit of the dense regions of ET that coat the surfaces of male zebrafish pectoral fins. There is relatively little described about the courtship rituals of zebrafish, a model system that has been informative for embryologists for decades. We find that male zebrafish use the dorsal, ET-containing surfaces of these fins to help grasp female zebrafish, a key step in a dynamic mating process and prologue to egg release and fertilization. Pectoral fins, and an exposed ET-containing surface, are required for successful spawning.
Several observations suggest that the developmental biology of ET is analogous to that of other epidermal appendages like hair, feathers, teeth, nails, and mammary glands (Driskell et al., 2011; Mikkola, 2007). First, ET originate from fields of epithelial thickenings that resemble placodes. Second, early and mature ET activate expression of Wnt-responsive gene elements, similar to TOPGAL activation in hair follicles (DasGupta and Fuchs, 1999; Merrill et al., 2001). Third, ectopic dkk1 expression blocks ET formation, resembling the inhibition of hair follicle initiation by ectopic Dkk1 expression in mice (Andl et al., 2002). Fourth, EdU pulse-chase experiments indicated the presence of basal epidermal label-retaining cells between adjacent ET units. This finding is consistent with the idea that they are slow-cycling stem cell sources for ET cell types, as might be predicted from studies of mammalian epidermal stem cells (Cotsarelis et al., 1990; Morris et al., 2004; Nowak et al., 2008; Taylor et al., 2000; Tumbar et al., 2004).
Our findings indicate that ET also present a potential biological disadvantage, due to aspects of their homeostatic regulatory cycle. Wnt target gene activation and Dkk1b synthesis continue vigorously in these tissues. Our findings indicate that an activator/inhibitor balance is likely to be important for ET morphogenesis, and excess Dkk1b shifts this balance to reduce new ET formation and maintain a differentiated morphology. Dkk1b presence in ET is unlikely to have a strong impact on uninjured fin rays; in fact, baseline levels of Dkk1b are present in osteoblasts and at the distal fin tips. However, upon severe injury, our data indicate that the response observed in most fins is to exclude Dkk1b expression from the regenerating area, enabling Wnt target gene activation and the key early step of blastema formation. Later, Dkk1b localization near less proliferative mesenchyme suggests a role in restricting or fine-tuning proliferation. By contrast, when a male pectoral fin is amputated, a Dkk1b-rich region of ET epidermis can cover the injury site, and blastema formation and subsequent regeneration are disrupted to the extent that it can impede spawning behavior. Thus, amputation injury juxtaposes two expression domains of a Wnt signaling inhibitor in a manner that can be unfavorable for regeneration. Much attention in the field of appendage regeneration has focused on the importance of elevating secreted activators after major injury (Antos and Tanaka, 2010; Jazwinska et al., 2007; Kawakami et al., 2006; Lin et al., 2013; Whitehead et al., 2005). Our current study provides an alternative example in which regulation of an inhibitor appears key for regeneration.
Because endogenous dkk1b:EGFP expression was tightly affiliated with ET in all of our experiments, we cannot distinguish whether the effects of ET are purely chemical, or whether the structure of the male epidermis is also inhibitory. The evidence that part or all of its influence is signaling-based includes Dkk1b regulation in ET and the regeneration blastema, blockade of pectoral fin regeneration by transgenic dkk1b overexpression, and partial rescue of male regenerative defects by androgen receptor antagonism or GSK3 inhibitor treatment ((Nachtrab et al., 2011) and this study). A proportion of male zebrafish show virtually normal pectoral fin regeneration after several weeks, a result that might be due to late-stage recovery or to an amputation that positions fewer ET near the wound epidermis. Interestingly, quantification of pectoral fin ET among males of different zebrafish strains indicates that those strains with fewer total ET have a higher frequency of regenerative success (Figure S1C and (Nachtrab et al., 2011)). It is also possible that ET release additional secreted factors that contribute to sexually dimorphic regeneration in zebrafish. One candidate factor for this is Igfbp2a, which shows elevated expression in male pectoral fins (Nachtrab et al., 2011) and negatively regulates Igf signaling, a pathway required for caudal fin regeneration (Chablais and Jazwinska, 2010). Additionally, androgen might have other effects on fin regeneration, indicated by the observation that androgen treatment slows regeneration of female caudal fins (Nachtrab et al., 2011).
The interaction between specialized male sexual ornaments and the niche within amputated male pectoral fins may be representative of a more general regulatory motif controlling regenerative capacity. Complex tissues employ a finite set of signaling activators and inhibitors to locally and systemically maintain their many cell types and structures. Tissue regenerative capacity likely reflects in part the cumulative regulation of these influences, which can change as animals and their organs and appendages mature. Thus, signaling interference between growing or homeostatic structures might help explain the diminished regeneration during developmental progression that has been reported in other contexts.
Additionally, how evolution has led to loss of regenerative potential in many species including humans has been contemplated for over a century, although there is little mechanistic resolution (Bely and Nyberg, 2010; Brockes et al., 2001; Dent, 1962; Dinsmore and American Society of Zoologists., 1991; Weismann, 1892). It is tempting to speculate on how the results we describe here might be relevant to the evolution of regenerative capacity. As essential mating structures, ET functionality in male zebrafish, including their regulation of secreted factors like Dkk1b, is likely to be under powerful natural selection. It is possible that preservation of these features counterbalances certain selective advantages of appendage regeneration, and that such an interaction might contribute to the establishment of reduced regenerative capacity during species evolution.
EXPERIMENTAL PROCEDURES
Zebrafish, Surgeries, and Drug Treatments
Wild-type or transgenic zebrafish of the outbred Ekkwill (EK) strain were used for all experiments unless indicated, with adults ranging in age from 3 to 8 months. Fins were amputated using iridectomy scissors at 50% of their original length unless indicated otherwise. To measure lengths of regenerates, lengths from the amputation plane to the distal tips of the 3rd and 4th pectoral fin rays (anterior) and 7th and 8th rays (posterior) were determined using Leica Application Suite software and averaged for single anterior and posterior values. To calculate intra-fin ratios, the average length of anterior rays was divided by the average length of posterior rays within same fin. To quantify ET units per segment, we collected one pectoral fin from each animal, and regions 4 segments proximal to the bifurcation point of the 3rd and 4th fin rays were assessed. To remove pectoral fin cuticles, animals were placed dorsal side up into a moist sponge. Fine forceps were used to grasp and strip cuticles from pectoral fins in a proximodistal motion. Remaining cuticles were removed with forceps.
Ethisterone (Sigma E1001) was dissolved in DMSO for a stock concentration of 5 mg/ml, and diluted in aquarium water for a working concentration of 100 ng/ml. Flutamide (Sigma F9397) was dissolved in DMSO at a concentration of 10 mg/ml. Final concentration of flutamide is 1 μg/ml. LiCl (Sigma 203637) was dissolved in fish water at a concentration of 3M. Final concentration of LiCl is 500 μM/ml. Fishwere placed in a 1L solution and water was changed daily. The durations of drug treatments are mentioned in the text. Work with zebrafish was performed in accordance with Duke University guidelines.
To generate Tg(dkk1b:EGFP)pd60 animals, the iTol2 cassette (Suster et al., 2011) was integrated into the BAC clone DKEY-31K12 using Red/ET recombineering technology (GeneBridges). Then, the first exon of the dkk1b gene in the BAC clone DKEY-31K12 was replaced with EGFP cassette by Red/ET recombineering. The EGFP cassette was amplified by PCR from pcrii-egfp-frt-kan-frt with the primers 5′-CACCCAGGGAATACATCCTACAATTCAAGGAATAACATGGTGAGCAAGGGCGAGGAGCTG-3′ and 5′-GCTGTCTGACATAGCATATGCATATTTCTATGCTTACGCCCTTGAAGTTCCTATTCTCTA-3′. One nl of 50 ng/μl purified, recombined BAC was injected into one-cell stage zebrafish embryos along with one nl of 30 ng/μl synthetic Tol2 mRNA, and a stable line was isolated from animals raised to breeding age (Suster et al., 2011). The full name of the Wnt reporter line used in this study is Tg(7xTCFXla.Siam:nlsmCherry)ia5 (Moro et al., 2012).
Wild-type or hsp70:dkk1b (full name, Tg(hsp70l:dkk1-gfp)w32) female zebrafish received daily heat-shocks as described previously (Wills et al., 2008). Four to 8 month-old fish and 6 week-old fish were used for adult and juvenile experiments, respectively. For Figure 3G, adult female were heat-shocked daily in the morning and were administered 100ng/ml Ethisterone in 1L aquarium water (3 fish/L) in the afternoon for 16 hours until the next morning.
BrdU/EdU Incorporation Assays
Zebrafish were injected intraperitoneally with 2.5 mg/mL BrdU solution 30 minutes prior to collection of fin regenerates. Tissues were fixed, cryosectioned, and stained as previously described (Wills et al., 2008). In some experiments, zebrafish were injected intraperitoneally with 10 mM EdU (Sigma A10055), and male pectoral fins were collected at 1 hour and at 1, 7 and 14 days post-treatment. EdU staining was done as previously described (Salic and Mitchison, 2008). When assessed along with EdU or BrdU, EGFP or mCherry in tissue sections was stained with antibodies. Otherwise, natural EGFP or mCherry fluorescence was captured. All experiments were repeated at least once with at least 3 animals each time. Primary antibodies used in this study were anti-BrdU (1:200, rat, Accurate chemical), anti-GFP (1:500, rabbit, Invitrogen), and anti-Ds-Red (1:500, rabbit, Clontech). Secondary antibodies usedin this study were Alexa 488 (1:500, Invitrogen), Alexa 594 (1:500, Invitrogen), and Alexa 594 azide (10–20 μM, Sigma).
Imaging
Whole-mount images were acquired using an M205FA stereofluorescence microscope (Leica). Images of tissue sections (10 μm for fin regenerates and 16 μm for ET) were acquired using an LSM 700 confocal microscope (Zeiss). Projection images were generated with ZEN software (Zeiss).
ImageJ was used to quantify dkk1b:EGFP fluorescence in Figure S1H. Regions four segments proximal to the bifurcation point of the 3rd and 4th fin rays were assessed, with 10 EGFP+ET chosen from the most anterior two rows in each ray (total 20 EGFP+ ET). A region of interest (ROI) was drawn around the EGFP+ positive domain in a single ET, and integrated density units (IDU; quantification of light emitted) of the ROI and background were measured. Background IDU was averaged from 3 different EGFP− regions.
Mating Experiments
Only males and females that mated during a pre-test were used for experiments in Figure 2. For pre-tests, one male and one female were placed in a standard breeding tank overnight and examined for embryos at noon the next day. Those mating successfully were maintained in standard laboratory aquaria for at least 10 days until mating experiments. Fins other than pectoral fins were amputated nearly completely (over 90% of their original length), and one or both pectoral fins were amputated at the indicated proximodistal locations. After amputation, fish were placed in laboratory aquaria for one day before placement in a breeding tank overnight with one animal of the opposite sex. On the next morning, embryos from the pair were collected and counted at noon.
Fertile males and females were similarly chosen for mating experiments in Figures 7B and 7C. On the next day, caudal or pectoral fins of males were amputated at ~70% of their original length, and animals were placed in laboratory aquaria for 1 or 2 months. These animals were each paired with one female in a breeding tank overnight, and embryos were collected and counted at noon. Each male was paired overnight with a female just once, and on the next day both pectoral fins of the male were collected, imaged, and scored as described in the Figure 7 legend.
For videos of spawning, male pectoral fins were either left intact or amputated nearly completely. One male and one female were placed in divided compartments in a standard breeding tank overnight and kept in the dark until filming. When mating behavior was recorded, the divider was removed and tank inserts containing the fish were tilted to facilitate breeding. A high-speed camera (200 frames/sec, NR5-S1, Redlake) was used to record some videos, and a motion studio program for processing (IDT). Other videos were recorded with an SLR camera (20 frames/sec).
Supplementary Material
Highlights.
Male zebrafish employ pectoral fin epidermal tubercles (ET) for spawning
Male pectoral fin ET cells vigorously renew through Wnt activation and inhibition
Wnt inhibitor Dkk1 produced by ET interferes with signaling during fin regeneration
A paradigm for the loss of tissue regenerative capacity during species evolution
Acknowledgments
We thank J. Burris, A. Eastes, P. Williams and N. Blake for zebrafish care; A. Dickson for artwork; R. Dorsky, R. Duncan, and F. Argenton for transgenic animals; and I. Kim, B. Hogan, A. Nechiporuk, and Poss lab members for comments on the manuscript. K.D.P. is an Early Career Scientist of the Howard Hughes Medical Institute. This work was supported by grants from the National Institutes of Health (GM074057) and the American Federation for Aging Research to K.D.P.
Footnotes
Supplementary Materials include one movie, three tables, and six figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimenko MA, Mari-Beffa M, Becerra J, Geraudie J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev Dyn. 2003;226:190–201. doi: 10.1002/dvdy.10248. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Antos CL, Tanaka EM. Vertebrates that regenerate as models for guiding stem cels. Adv Exp Med Biol. 2010;695:184–214. doi: 10.1007/978-1-4419-7037-4_13. [DOI] [PubMed] [Google Scholar]
- Bely AE, Nyberg KG. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol. 2010;25:161–170. doi: 10.1016/j.tree.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Kumar A, Velloso CP. Regeneration as an evolutionary variable. J Anat. 2001;199:3–11. doi: 10.1046/j.1469-7580.2001.19910003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137:871–879. doi: 10.1242/dev.043885. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L, Xu W. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol. 1962;110:61–77. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- Dinsmore CE and American Society of Zoologists. A History of regeneration research: milestones in the evolution of a science. 1. Cambridge England, New York: Cambridge University Press; 1991. [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially FN, Whiteley HJ. Hormonally induced epithelial hyperplasia in the goldfish (Carrasius auratus) Br J Cancer. 1952;6:246–248. doi: 10.1038/bjc.1952.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Badakov R, Keating MT. Activin-betaA signaling is required for zebrafish fin regeneration. Curr Biol. 2007;17:1390–1395. doi: 10.1016/j.cub.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I, Izpisua Belmonte JC. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232–3237. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M. Medaka: Biology, management, and experimental protocols. Ames, Iowa: Wiley-Blackwell; 2009. [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Nati Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. The J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Lin G, Chen Y, Slack JM. Imparting regenerative capacity to limbs by progenitor cell transplantation. Dev Cell. 2013;24:41–51. doi: 10.1016/j.devcel.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Martinovic-Weigelt D, Wang RL, Villeneuve DL, Bencic DC, Lazorchak J, Ankley GT. Gene expression profiling of the androgen receptor antagonists flutamide and vinclozolin in zebrafish (Danio rerio) gonads. Aqua Toxicol. 2011;101:447–458. doi: 10.1016/j.aquatox.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML. Genetic basis of skin appendage development. Semin Cell Dev Biol. 2007;18:225–236. doi: 10.1016/j.semcdb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, Young RM, Bournele D, Domenichini A, Valdivia LE, Lum L, et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev Biol. 2012;366:327–340. doi: 10.1016/j.ydbio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Holler-Dinsmore G, Bryant SV. Intrinsic control of regenerative loss in Xenopus laevis limbs. J Exp Zool. 1986;240:47–54. doi: 10.1002/jez.1402400107. [DOI] [PubMed] [Google Scholar]
- Nachtrab G, Czerwinski M, Poss KD. Sexually dimorphic fin regeneration in zebrafish controlled by androgen/GSK3 signaling. Curr Biol. 2011;21:1912–1917. doi: 10.1016/j.cub.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Nati Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Suster ML, Abe G, Schouw A, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish. Nat Protoc. 2011;6:1998–2021. doi: 10.1038/nprot.2011.416. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton MJ, Hibberts NA, Street T, Brinklow BR, Loudon AS, Randall VA. Androgen receptors are only present in mesenchyme-derived dermal papilla cells of red deer (Cervus elaphus) neck follicles when raised androgens induce a mane in the breeding season. J Endocrinol. 2001;168:401–408. doi: 10.1677/joe.0.1680401. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwa H. Hormonal inhibitions of ethisterone-induced process formation in adult females of the medaka, Oryzias latipes. Embryologia. 1968;10:147–154. doi: 10.1111/j.1440-169x.1968.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Weismann A. Das Keimplasma; eine Theorie der Vererbung. Jena: Fischer; 1892. [Google Scholar]
- Whitehead GG, Makino S, Lien CL, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- Wiley ML, Collette BB. Breeding tubercles and contact organs in fishes: Their occurrence, structure, and significance. Bulletin of the American Museum of Natural History. 1970;143:143–216. [Google Scholar]
- Williams TM, Carroll SB. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat Rev Genet. 2009;10:797–804. doi: 10.1038/nrg2687. [DOI] [PubMed] [Google Scholar]
- Wills AA, Kidd AR, 3rd, Lepilina A, Poss KD. Fgfs control homeostatic regeneration in adult zebrafish fins. Development. 2008;135:3063–3070. doi: 10.1242/dev.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt pathways in disease. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.