Abstract
Heparan sulfate proteoglycans (HSPGs) are concentrated at neuromuscular synapses in many species, including Drosophila. We have established the physiological and patterning functions of HSPGs at the Drosophila neuromuscular junction by using mutations that block heparan sulfate synthesis or sulfation to compromise HSPG function. The mutant animals showed defects in synaptic physiology and morphology suggesting that HSPGs function both presynaptically and postsynaptically; these defects could be rescued by appropriate transgene expression. Of particular interest were selective disruptions of mitochondrial localization, abnormal distributions of Golgi and endoplasmic reticulum markers in the muscle, and a markedly increased level of stimulus-dependent endocytosis in the motoneuron. Our data support the emerging view that HSPG functions are not limited to the cell surface and matrix environments, but also affect a diverse set of cellular processes including membrane trafficking and organelle distributions.
Introduction
The function of heparan sulfate proteoglycans (HSPGs) has been the subject of intense investigation in the last decade, and it is now clear they play essential roles in development, controlling both responses of cells to secreted growth factors, as well as the distribution of growth factors in the matrix (Lin, 2004; Bülow and Hobert, 2006; Kirkpatrick and Selleck, 2007). HSPGs are also critical for nervous system development, affecting such diverse events as axon guidance and the localization of synaptic components (Hoch et al., 1994; Yamaguchi, 2002; Lee et al., 2004; Rotundo et al., 2005; Jenniskens et al., 2006; Lindwall et al., 2007). A number of growth factors that have a high affinity for heparan sulfate (HS), including neuregulins and hepatocyte growth factor, affect neuromuscular junction (NMJ) development or function (Loeb, 2003; Madhavan and Peng, 2006). The HSPG syndecan-2 participates in ephrin-mediated dendritic spine development (Irie and Yamaguchi, 2004). Thus, HSPGs can potentially affect synapse development in a number of ways, but their contributions to synapse formation and function have not been examined in a genetically accessible system in which heparan sulfate synthesis or modification can be readily manipulated.
In Drosophila, two heparan sulfate-modified core proteins affect the assembly of the neuromuscular junction (Johnson et al., 2006). Syndecan (Sdc), a transmembrane proteoglycan (PG), influences presynaptic terminal growth, whereas Dally-like protein (Dlp), a glycosylphosphatidylinositol (GPI)-linked HSPG, participates in active zone assembly. These two proteoglycans are proposed to play antagonistic roles in regulating the function of the receptor tyrosine phosphatase Dlar.
The analysis of HSPGs to date has mostly focused on their activities at the cell surface and in the extracellular matrix. More recent work has highlighted their cellular functions, demonstrating that HSPGs are not simply growth factor coreceptors, but control growth factor distributions by regulating endocytic trafficking (Marois et al., 2006; Gallet et al., 2008). There are also compelling data suggesting that one or more HSPGs serve to internalize triglyceride-rich lipoprotein particles (MacArthur et al., 2007). Studies of mice bearing mutations in Ndst2, a HS-modifying enzyme critical for mast cell function, suggest that normal HS biosynthesis is required to properly package the components of secretory vesicles, indicating HS has important biosynthetic functions (Forsberg et al., 1999; Humphries et al., 1999).
We are interested in understanding the function of HSPGs in cell–cell communication and chose the Drosophila neuromuscular junction as a model system to explore both their role in the assembly of a defined synapse, as well as their contribution to the well characterized architecture and cellular processes required for normal NMJ activity. We report here a detailed functional investigation using mutants that affect HS chain initiation, polymerization, or sulfation. We find that HS is concentrated at the NMJ, as in vertebrates, and that mutations compromising HS synthesis deplete HS from this cell–cell junction. Loss of HS affects both NMJ structure and function, and causes surprising defects in membrane trafficking and organelle localization in the neuron and in the muscle cell.
Materials and Methods
Drosophila strains.
All flies were cultured at 25°C in vials or bottles containing standard cornmeal, agar, sugar, and yeast medium. Oregon-R (+/+) flies were used as wild-type controls. sfl9B4 FRT2A/TM3 Ser GFP and sfl03844 P[w+, FRT]2A/TM3 Ser GFP were crossed to generate sfl transheterozygous null mutants (Lin and Perrimon, 1999) and sibling controls. yw; FRT[42D] botv510/CyO GFP and yw; FRT[42D] botv423/CyO GFP were crossed to generate botv mutants (Takei et al., 2004) and sibling controls. FRTG13 ttv00681b/CyO ubq::GFP was used as a source of homozygous ttv null mutants (Bellaiche et al., 1998). Heterozygous controls carried TM3 Ser GFP or CyO ubq::GFP balancer chromosomes with wild-type sfl or ttv alleles, except for FM1-43 labeling (see Fig. 7) in which mutant stocks were outcrossed to Oregon-R to generate sfl/+ or ttv/+ heterozygotes. dlp1 st ry/TM6B, Tb and ru h dlp2 st ry e/TM6B, Tb or Df(3L)fz.D21 were crossed to generate dlp mutants. sdc mutants were created by crossing sdc10608/CyO ubq::GFP with Df(2R)48/CyO ubq::GFP. For rescue of ttv mutants, strains bearing a Gal4 driver (elav-Gal4, G14-Gal4, or da-Gal4), ttv00681b, and CyO ubq::GFP were crossed to FRTG13 ttv00681b/CyO ubq::GFP; UAS-ttv-myc. The genotypes of progeny expressing UAS-ttv-myc with a Gal4 driver are shown as elav>ttv-myc, G14>ttv-myc, or da>ttv-myc. Similar crosses were performed to express UAS-mitoGFP or UAS-CD8::GFP. UAS-ttv-myc flies were a generous gift from I. The (Utrecht University, Department of Developmental Biology, Utrecht, The Netherlands); UAS-mitoGFP was obtained from M. O'Connor (Department of Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN). Other fly strains used are described in Flybase and are available from the Bloomington Stock Center.
Figure 7.
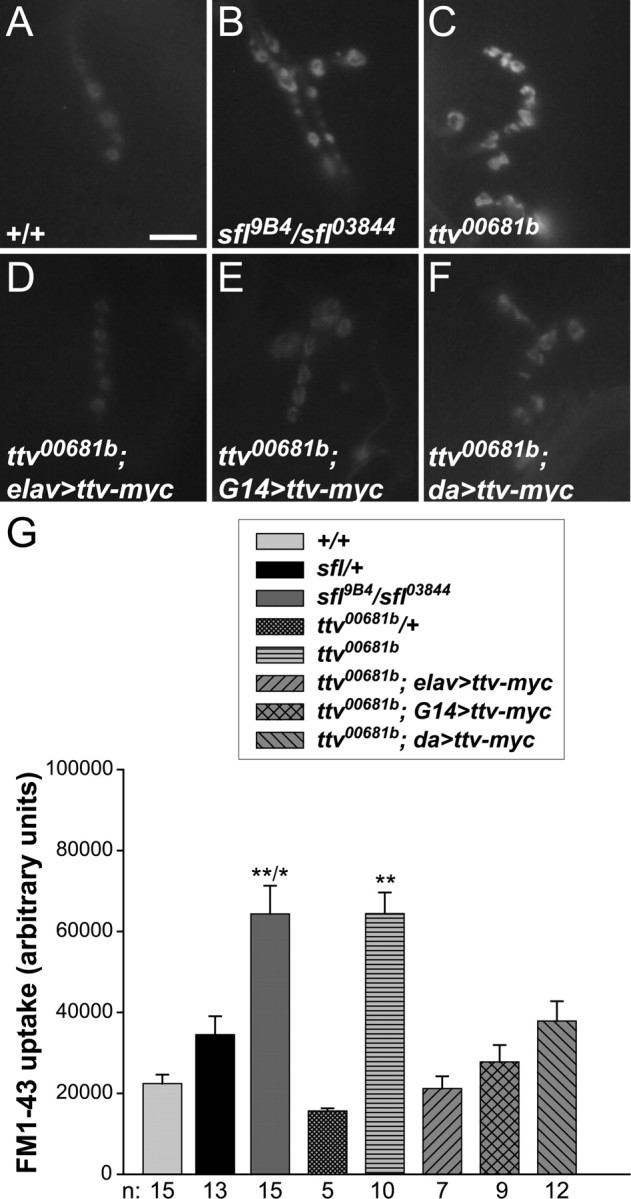
Endocytosis abnormalities in HS biosynthetic mutants. A–F, Images of FM1-43 dye uptake in synapses at muscle 4 of wild type, sfl or ttv mutants, and ttv mutants expressing UAS-ttv-myc with elav-Gal4, G14-Gal4, or da-Gal4. Scale bar, 10 μm. G, Average FM1-43 uptake for the indicated genotypes. Error bars represent SEM. **/*p < 0.001 relative to +/+ and < 0.01 relative to sfl/+; **p < 0.001 compared with +/+ and ttv00681b/+. Sample numbers (n) are shown below. sfl and ttv mutant synapses show substantial increases in vesicle recycling as measured by FM1-43 uptake. This phenotype is rescued by expression of Ttv-myc with elav-Gal4, G14-Gal4, and, to a lesser extent, da-Gal4.
Antibodies and immunohistochemistry.
Third-instar larvae were dissected in ice-cold Ca2+-free HL-3 saline (70 mm NaCl, 5 mm KCl, 20 mm MgCl2, 10 mm NaHCO3, 5 mm trehalose, 100 mm sucrose, 5 mm HEPES, pH 7.2) (Rawson et al., 2003). Larvae were fixed with Bouin's fixative (70% picric acid, 25% 37% formaldehyde, 5% glacial acetic acid) for staining of NMJs with anti-Dally-like (30 min), anti-cytochrome c (5 min), and anti-glutamate receptor IIA (5 min). For staining with other antibodies, larvae were fixed with 4% formaldehyde in PBS for 30 min. Primary antibodies used were mouse anti-cysteine string protein (anti-CSP) (kind gift from K. Zinsmaier, University of Arizona, Arizona Research Laboratories Division of Neurology, Tucson, AZ) at 1:1000, mouse anti-myc (Roche) at 1:100, mouse anti-binding Ig protein (BIP) (Nventa Biopharmaceuticals) at 1:100, mouse anti-p120 (Calbiochem) at 1:200, mouse anti-Dally-like (Developmental Studies Hybridoma Bank) at 1:4, mouse anti-cytochrome c (BD Biosciences Pharmingen) at 1:50, and mouse anti-glutamate receptor IIA (anti-GluRIIA) (Developmental Studies Hybridoma Bank) at 1:100. Staining was visualized with Alexa Fluor-conjugated secondary antibodies (Invitrogen). For heparan sulfate staining, larvae were dissected, fixed with Bouin's fixative for 60 min, and treated with 20 mU of heparitinase I (Seikagaku) in heparitinase I buffer (100 mm sodium acetate, 10 mm calcium acetate, pH 7.0) for 2 h at 37°C. After washing, samples were stained with 3G10 antibody (Seikagaku) at 1:100. Anti-Dally-like, anti-myc, and anti-heparan sulfate signals were amplified with Tyramide Signal Amplification Fluorescence Systems (PerkinElmer). Larvae were stained with fluorescein (FITC)-conjugated goat anti-horseradish peroxidase (anti-HRP) (Jackson ImmunoResearch Laboratories) at 1:50 to show axons and synaptic boutons (Bodmer et al., 1987). Images were captured with a Nikon C1 confocal microscope and analyzed with ImageJ 1.34 s and Adobe Photoshop. Identical confocal settings were used when imaging mutant, rescued, and control samples to allow direct comparisons.
Electrophysiology.
Intracellular recordings were performed as described previously (Rawson et al., 2003) with minor modifications. Thin-walled glass intracellular electrodes were filled with 3 m KCl and had resistances of 10–20 MΩ. Preparations were immersed in room temperature HL-3 saline containing 1.2 mm Ca2+. Miniature excitatory junction potentials (mEJPs) at muscle 6 of the A2 or A3 hemisegment were collected for 1 min to derive an average frequency and strength of spontaneous release. Excitatory junction potentials (EJPs) were recorded by stimulating the nerve with 15 1 ms pulses at a frequency of 1 Hz and at a strength 1.5 times that necessary to evoke the compound response. Only recordings with resting membrane potentials of at least −65 mV were used. Recordings were acquired with an Axonclamp 2B amplifier (Molecular Devices). Mini Analysis program (Synaptosoft) was used to analyze the recordings. The quantal content for a given EJP was calculated by dividing the evoked EJP amplitude by the mEJP amplitude, and then adjusted for nonlinear summation of EJPs (Martin, 1955).
Behavioral assay: muscle contraction assay.
Third-instar larvae were placed on a fresh agar plate. For larvae undergoing continuous forward movements, the numbers of body wall contractions during 1 min were counted.
Electron microscopy.
Third-instar larvae were dissected in Ca2+-free HL-3 saline and processed as described previously (Marqués et al., 2002). Synaptic boutons between muscles 6 and 7 were examined using the JEOL 1200-EXII transmission electron microscope and analyzed with ImageJ 1.34 s. Three sfl03844 mutants and three Oregon-R control animals were analyzed. Between 5 and 45 different sections were used to quantify each phenotype.
Assay for vesicle recycling.
Third-instar larvae were dissected in Ca2+-free normal saline (130 mm NaCl, 5 mm KCl, 2 mm MgCl2, 36 mm sucrose, 5 mm HEPES, pH 7.2). Boutons were stained in 1 μm FM1-43 in high K+ saline (75 mm NaCl, 60 mm KCl, 2 mm MgCl2, 36 mm sucrose, 1.5 mm CaCl2, 5 mm HEPES, pH 7.2) to depolarize the nerve (Ramaswami et al., 1994). Preparations were washed thoroughly in Ca2+-free normal saline to remove noninternalized dye. Images of synaptic boutons at muscle 4 in segment A3 were captured using a Nikon Eclipse E800 fluorescence microscope. Only synaptic boutons in focus were analyzed with Adobe Photoshop and ImageJ 1.34 s.
Statistics.
Numerical data are presented as mean ± SEM. Student's t test was used to compare means between mutant and control groups; significance is indicated as follows: *p < 0.01 and **p < 0.001 compared with +/+ (Oregon-R) and the relevant heterozygous control.
Results
HS is localized at the Drosophila NMJ
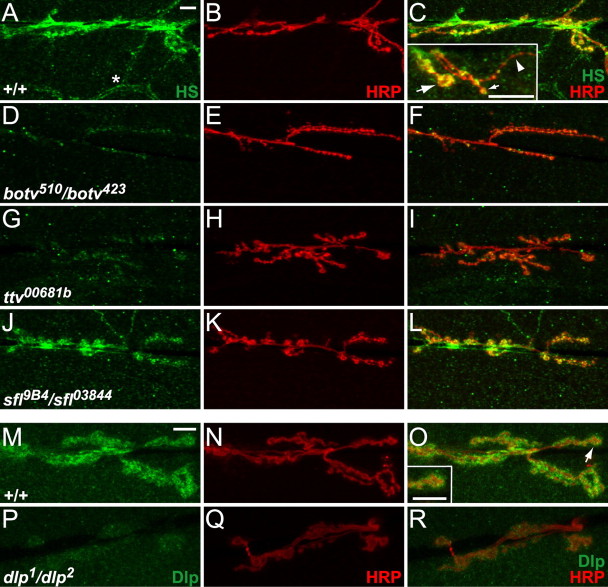
A variety of HSPGs are concentrated at the synapse of the vertebrate NMJ (Jenniskens et al., 2006). These include agrin, which is critical for clustering acetylcholine receptors in the postsynaptic membrane (Hoch et al., 1994), and perlecan, a protein that binds the collagen-tailed form of acetylcholinesterase (Arikawa-Hirasawa et al., 2002). Two HSPGs have been localized to the NMJ in Drosophila, Sdc and Dlp, a transmembrane and a GPI-linked PG, respectively (Johnson et al., 2006). To evaluate the distribution of HS at the Drosophila NMJ, we treated larval preparations with heparitinase, which digests HS chains leaving desaturated uronate epitopes that are recognized by the monoclonal antibody 3G10 (David et al., 1992). At body wall muscles 6 and 7 of third-instar larvae, substantial 3G10 antibody staining is found on axons and synaptic boutons of the NMJ (also labeled with anti-HRP) (Fig. 1A–C) as well as on tracheal branches, the respiratory conducting elements (Fig. 1A, asterisk). Muscles 6 and 7 are innervated by two motoneurons that both use glutamate as the neurotransmitter (Johansen et al., 1989). One motoneuron forms larger type Ib boutons on muscles 6 and 7, whereas the other makes smaller type Is boutons on these and several adjacent muscles (Hoang and Chiba, 2001). Both the type Ib and type Is-derived boutons are stained by 3G10 antibody. We examined 3G10 staining at muscle 13 to evaluate HS localization at type II boutons, which arise from a third type of motoneuron and use multiple neurotransmitters (Hoang and Chiba, 2001). HS is concentrated at type Ib and Is boutons of muscle 13 (Fig. 1C, inset, arrows), but there is little or no staining of type II boutons (Fig. 1C, inset, arrowhead), demonstrating that HS preferentially localizes to specific bouton types.
Figure 1.
HSPGs are localized at the Drosophila NMJ. A–L, NMJs at muscles 6 and 7 were stained with 3G10 antibody (green) to display the localization of HSPGs and anti-HRP (red) to label motoneuron processes. Scale bars, 10 μm. A–C, In wild-type animals, HS is highly enriched at the NMJ, including both type Ib and Is boutons, but it is also enriched on trachea (A, asterisk). At muscle 13 (C, inset), HS is found at type Ib boutons (large arrow) and Is boutons (small arrow), but not at type II boutons (arrowhead). D–I, 3G10 anti-HS staining is nearly eliminated in botv510/botv423 and ttv00681b mutant animals. J–L, sfl9B4/sfl03844 mutants show normal levels of 3G10 antibody staining. M–R, NMJs at muscles 6 and 7 stained with anti-Dlp antibody (green) and anti-HRP (red). Scale bars, 10 μm. M–O, Anti-Dlp staining is enriched at the NMJ and has a somewhat punctate appearance. It overlaps with anti-HRP staining, but has a broader distribution. The inset in O shows a type Ib bouton (O, arrow) in which anti-Dlp staining mostly surrounds anti-HRP staining. P–R, There is no anti-Dlp staining in dlp1/dlp2 mutants, demonstrating specificity of the anti-Dlp antibody.
We used 3G10 antibody staining to evaluate the level of HS remaining at the NMJ in third-instar larvae bearing mutations in HS biosynthetic genes. These animals survive until the larval or pupal stage by relying on maternally provided gene products. The enzyme encoded by brother of tout velu (botv) initiates an HS chain by adding N-acetylglucosamine (GlcNAc) to a tetrasaccharide linker at specific sites on HSPG core proteins (Izumikawa et al., 2006). The HS chain is extended by a copolymerase produced from the tout velu (ttv) and sister of tout velu (sotv) genes (Izumikawa et al., 2006). Next, N-deacetylase/N-sulfotransferase (encoded by sfl) modifies the chain by deacetylating GlcNAc and introducing N-sulfate groups (Toyoda et al., 2000). We found no 3G10 staining at the NMJ in botv or ttv mutants (Fig. 1D–I), as expected for animals that cannot initiate or extend an HS chain. In contrast, sfl mutants exhibit normal 3G10 staining (Fig. 1J–L), showing that HS remains localized at these synapses even though sulfation is dramatically reduced in these animals (Toyoda et al., 2000).
We examined the localization of a particular HSPG, the glypican Dlp, at the NMJ. Anti-Dlp antibody staining showed that Dlp is concentrated in the region of the NMJ (Fig. 1M–O). Anti-Dlp staining appears to surround the synaptic boutons detected by anti-HRP staining, suggesting that Dlp may be present at higher levels on the surface of the muscle cell at the NMJ. This staining is lost in dlp mutant larvae (Fig. 1P–R).
The postsynaptic localization of Dlp protein contrasts with the mostly presynaptic staining seen with 3G10 antibody, suggesting that other presynaptic HSPGs are present at the NMJ. The other HSPG known to localize to the NMJ, Sdc, is also reported to show mostly postsynaptic staining (Johnson et al., 2006), again begging the question of which PG is present at significant levels presynaptically. To evaluate this issue, we used two approaches. First, we used an antibody staining protocol that provided greater penetration of the 3G10 antibody (0.3% Triton X-100) to improve sensitivity, and second, we examined 3G10 staining in both dlp and sdc mutant larvae. With this change in protocol, additional 3G10 staining is observed surrounding the synaptic boutons in wild-type NMJs (supplemental Fig. S1A–F, available at www.jneurosci.org as supplemental material). A significant portion of this signal is lost in dlp mutants (supplemental Fig. S1G–L, available at www.jneurosci.org as supplemental material), although substantial presynaptic staining remains. In addition, dlp mutants have almost no staining on the rest of the muscle cell surface, suggesting that a low level of Dlp is normally present there. In sdc mutants (supplemental Fig. S1M–R, available at www.jneurosci.org as supplemental material), 3G10 staining at and around the synaptic boutons was reduced, but not eliminated; staining elsewhere in the muscle was unaffected. These results demonstrate that Dlp and Sdc are both significant contributors to the HS found at the NMJ, but suggest the possibility that other presynaptic HSPGs may also be present. Alternatively, Dlp and Sdc may have significant presynaptic localization in addition to their described levels postsynaptically.
HS biosynthetic mutants show defects in NMJ physiology
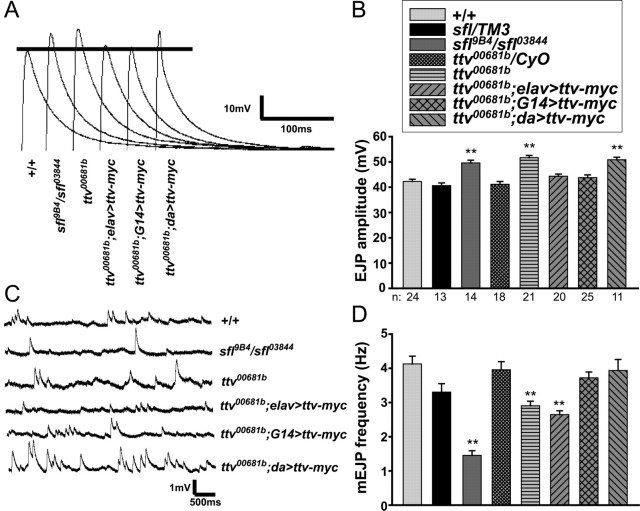
To assess the role of HS biosynthesis in synapse function, we analyzed spontaneous and evoked neurotransmission in third-instar larvae with mutations in HS biosynthetic genes. In response to nerve stimulation, both ttv and sfl mutants exhibited significantly increased EJPs compared with wild-type or heterozygous control larvae (20–25% increase over controls) (Fig. 2A,B). Spontaneous release events (mEJPs) were also affected in these mutants (Fig. 2C,D): mEJP frequency was substantially reduced in sfl and ttv mutants compared with heterozygous or wild-type controls, suggesting that loss of HS biosynthesis affected the spontaneous vesicle release probability in the motoneuron. We also found evidence for changes in postsynaptic responses in sfl and ttv mutants (Fig. 2C). mEJP amplitudes were modestly increased in sfl mutants relative to heterozygous and wild-type controls (sfl mutants, 1.18, compared with sfl/TM3 heterozygotes, 0.79; p < 0.001; compared with +/+, 0.98; p < 0.05; TM3 is a balancer chromosome bearing a wild-type sfl allele). mEJP amplitudes in ttv mutants were significantly elevated compared with ttv/CyO controls (ttv mutants, 1.05; ttv/CyO heterozygotes, 0.82; p < 0.001; CyO is a balancer chromosome bearing a wild-type ttv allele); this phenotype was rescued by neuronal or muscle-directed expression of a wild-type transgene (p < 0.001 and 0.01, respectively) (data not shown). Together, these electrophysiological phenotypes indicate that HSPGs affect both presynaptic and postsynaptic function.
Figure 2.
Functional analysis of sfl and ttv mutant synapses. A, Representative traces of evoked neurotransmission at abdominal muscle 6 of wild type (+/+), sfl9B4/sfl03844 mutants, ttv00681b mutants, and ttv00681b mutant larvae expressing a UAS-ttv-myc transgene driven by elav-Gal4, G14-Gal4, or da-Gal4. B, Average EJP amplitudes evoked in sfl or ttv heterozygous and mutant animals, and in ttv mutant animals expressing UAS-ttv-myc as in A. In all graphs, error bars represent SEM. **p < 0.001 relative to +/+ and the relevant heterozygous control. Sample numbers (n) shown below the graph also apply to D. Both sfl and ttv mutants display increased EJP amplitudes compared with heterozygous controls. Expression of Ttv-myc with elav-Gal4 or G14-Gal4, but not da-Gal4, rescues the EJP amplitude of ttv mutants. C, Representative traces of spontaneous neurotransmission in abdominal muscle 6 of animals with the same genotypes as in A. mEJP amplitudes are increased in sfl and ttv mutants, and rescued by neuronal (elav-Gal4) or, to some extent, muscle (G14-Gal4) expression of Ttv-myc. D, Average mEJP frequency for the indicated genotypes (as in B). sfl and ttv mutants have decreased mEJP frequencies relative to +/+ and heterozygous controls; this phenotype is rescued by UAS-ttv-myc expression with G14-Gal4 or da-Gal4, but not elav-Gal4 (Table 1).
The phenotypes of ttv and sfl mutants differ from those of dlp mutants (Johnson et al., 2006) in that spontaneous neurotransmitter release is affected as well as evoked responses. Thus, loss of HS biosynthesis has broader effects on both presynaptic and postsynaptic function at the NMJ than loss of a single HSPG. Loss of sfl function had more severe effects on mEJP frequency and amplitude than mutation of ttv, suggesting that lack of proper HS modification can be more deleterious than loss of HS chains (see below).
HS biosynthetic mutants have larval locomotion deficits
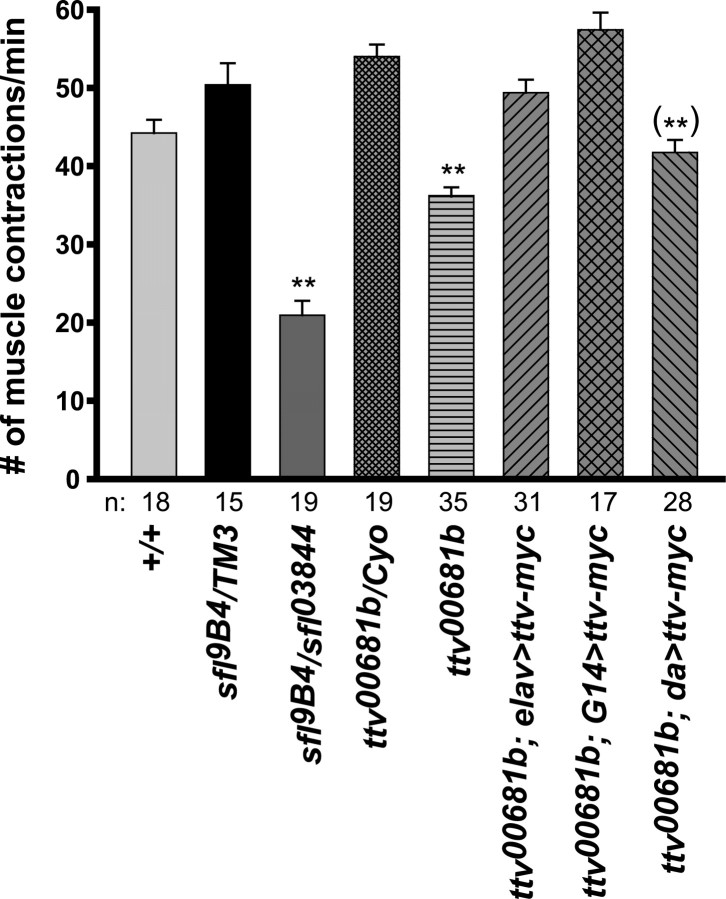
In addition to their electrophysiological phenotypes, we found that ttv and sfl mutant larvae have defects in crawling behavior. Larval crawling consists of repeated “strides” in which muscular contractions propagate from one end of the body to the other. To quantify the locomotion deficit, we counted the number of these peristaltic contractions while larvae crawled on a flat surface. Both sfl and ttv mutants had significantly reduced numbers of contractions per minute in this assay (Fig. 3). As with some of the physiological measures, crawling behavior was more severely affected in sfl than ttv mutants.
Figure 3.
Locomotion defects in HS biosynthetic mutants. Locomotion was quantified as the number of muscular body contractions per minute during larval crawling. Error bars represent SEM. **p < 0.001 relative to +/+ and the relevant heterozygotes. (**)p < 0.001 compared with ttv heterozygotes. Sample numbers (n) are shown below the graph. Both sfl and ttv mutants show a crawling defect. This defect is rescued in ttv mutants by Ttv-myc expression with elav-Gal4 or G14-Gal4, but is not significantly rescued with da-Gal4.
Rescue of NMJ phenotypes by ttv transgene expression
To determine what cells require HS biosynthesis for normal NMJ function, we sought to rescue ttv mutants with a ttv transgene. We characterized the expression patterns of the Gal4 drivers for these experiments using a membrane-tethered green fluorescent protein (GFP) reporter (UAS-CD8::GFP) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). elav-Gal4, a common neuronal driver, drove GFP expression in the CNS and in the motoneuron at the NMJ (supplemental Fig. S2C,D, available at www.jneurosci.org as supplemental material). In addition, elav-Gal4 directed a low level of GFP in muscle tissue (supplemental Fig. S2D, inset, available at www.jneurosci.org as supplemental material). G14-Gal4, which is described as a muscle driver, drove strong expression of GFP at the NMJ and throughout muscles 6 and 7 (supplemental Fig. S2F, available at www.jneurosci.org as supplemental material). However, G14-Gal4 is not solely muscle-specific, because we also observed high levels of neuronal GFP in the mushroom bodies and lower levels in other neurons in the brains of these animals (supplemental Fig. S2E, available at www.jneurosci.org as supplemental material). da-Gal4 is a strong ubiquitous driver, and gave very high levels of GFP in all tissues examined (supplemental Fig. S2G,H, available at www.jneurosci.org as supplemental material).
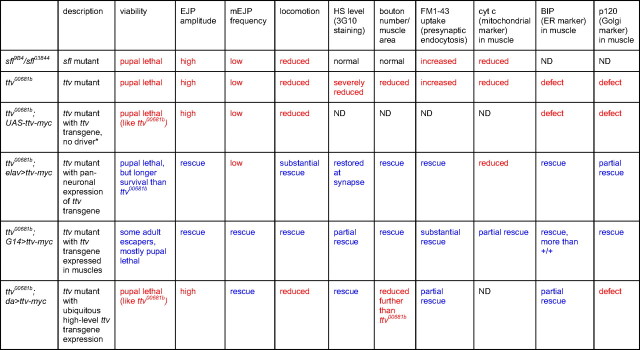
Expression of a myc-tagged form of Ttv with G14-Gal4 was able to rescue ttv mutant animals to adulthood or to a later pupal stage (Table 1). Expression of UAS-ttv-myc with elav-Gal4 also improved viability, but expression with da-Gal4 did not. We evaluated HS biosynthesis in these animals by staining heparitinase-treated NMJ preparations with 3G10 antibody. All three drivers increased 3G10 staining, suggesting that HS synthesis was at least partially restored (Fig. 4A–O). In elav-Gal4>UAS-ttv-myc animals, HS appeared to be localized primarily to the synapse. G14-Gal4 and da-Gal4 gave similar or even more substantial 3G10 staining at the synapse, as well as additional staining in the muscles and tracheal system. da-Gal4>UAS-ttv-myc animals expressed very high levels of Ttv-myc protein relative to those with the G14-Gal4 driver, as judged by anti-myc antibody staining (Fig. 4P–R). The failure to rescue viability suggests that the HS chains in da-Gal4>UAS-ttv-myc animals might be defective in some way or that this level of Ttv protein is somehow deleterious.
Table 1.
Summary of sfl mutant, ttv mutant, and ttv rescue phenotypes
Mutant phenotypes are shown in red; fully or partially rescued phenotypes are shown in blue. ND, Not determined.
*With no Gal4 driver present, there should be no expression of the UAS-ttv-myc transgene.
Figure 4.
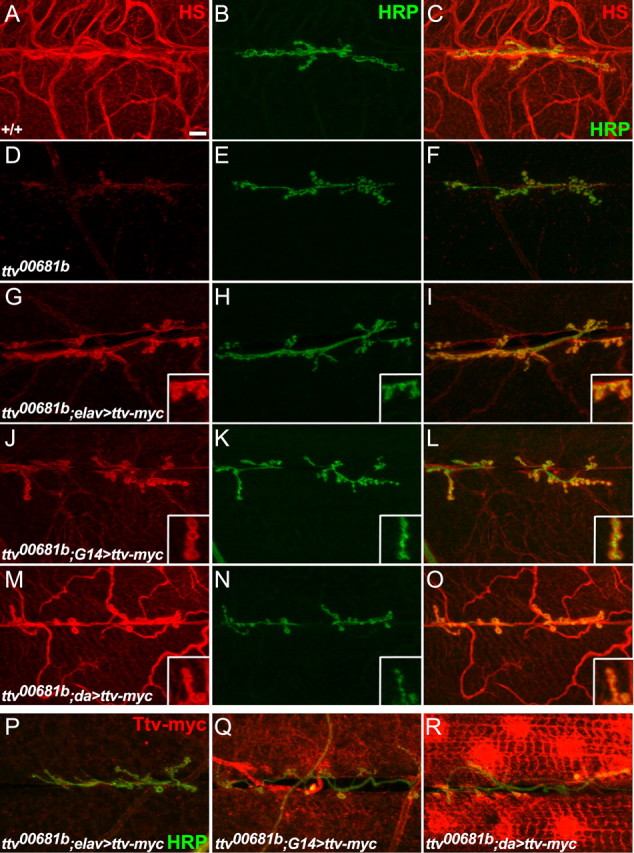
Rescue of HS biosynthesis by Ttv-myc expression. A–O, Anti-HRP (green) and 3G10 anti-HS (red) staining of NMJs at muscles 6 and 7 from wild type, ttv mutants, or ttv mutants expressing Ttv-myc driven by elav-Gal4, G14-Gal4, or da-Gal4. The penetration of the 3G10 antibody was increased by staining in buffer containing 0.3% rather than 0.1% Triton X-100, so the tracheal staining in wild type (A, C) is more apparent than in Figure 1. The insets in G–O show larger images of boutons. Scale bar, 10 μm. D–F, 3G10 staining is lost in ttv00681b mutants. G–I, 3G10 staining is restored to synapses when Ttv-myc is expressed with elav-Gal4. J–L, G14-Gal4 expression of Ttv-myc increases 3G10 staining primarily at synapses, and to a limited extent in the muscles and trachea. Note that 3G10 staining appears to surround the synaptic boutons (insets). M–O, da-Gal4 expression of Ttv-myc almost fully rescues 3G10 staining. P–R, Expression level of Ttv-myc as shown by anti-myc antibody staining (red) of muscle 6 and 7 NMJs from ttv mutants expressing Ttv-myc with elav-Gal4, G14-Gal4, or da-Gal4. Anti-HRP staining is shown in green. da-Gal4 gives extremely high levels of Ttv-myc expression relative to elav-Gal4 or G14-Gal4. Neuronal Ttv-myc (in elav-Gal4>UAS-ttv-myc animals) should localize to the cell bodies, which are not visible in these images.
Expression of UAS-ttv-myc with these same drivers rescued some of the electrophysiological and behavioral phenotypes of ttv mutants (Figs. 2, 3; Table 1), but sometimes with surprising cell type requirements for HS synthesis. For example, the reduced mEJP frequency in ttv mutants suggests a defect in presynaptic function, but this phenotype was rescued by expression of UAS-ttv-myc using the mostly muscle-directed G14-Gal4 driver (Fig. 2D). Pan-neuronal expression with elav-Gal4>UAS-ttv-myc did not increase the mEJP frequency so G14-Gal4-driven expression in neurons cannot be responsible for this rescue. Therefore, we conclude that HS biosynthesis is required specifically in muscles for this motoneuron physiological attribute. The elevated EJP amplitude of ttv mutants was substantially rescued with either G14-Gal4 or elav-Gal4>UAS-ttv-myc (Fig. 2B), suggesting that neuronal expression of Ttv-myc may be sufficient to restore normal muscle responses to motoneuron stimulation. However, since elav-Gal4 gave a low level of UAS-CD8::GFP expression in muscle tissue (supplemental Fig. S1D, available at www.jneurosci.org as supplemental material), we cannot be completely certain about the critical site(s) of HSPG action for these phenotypes. Expression of Ttv-myc using G14-Gal4 also restored normal crawling behavior, whereas elav-Gal4 gave substantial rescue (Fig. 3). The rescue of these multiple electrophysiological and behavioral defects by UAS-ttv-myc expression shows conclusively that these phenotypes result from loss of ttv activity.
Expression of UAS-ttv-myc with the strong ubiquitous da-Gal4 driver did not ameliorate ttv mutant phenotypes to the same extent as the other drivers. da-Gal4-directed expression rescued the mEJP frequency (Fig. 2D), but did not reduce the abnormally elevated EJP amplitude (Fig. 2B), nor did it rescue crawling behavior (Fig. 3). Clearly rescue of ttv mutants cannot be achieved simply by a high level of ubiquitous Ttv expression but depends on careful control of the sites and level of ttv product.
Morphological changes at the synapse of HS biosynthetic mutants
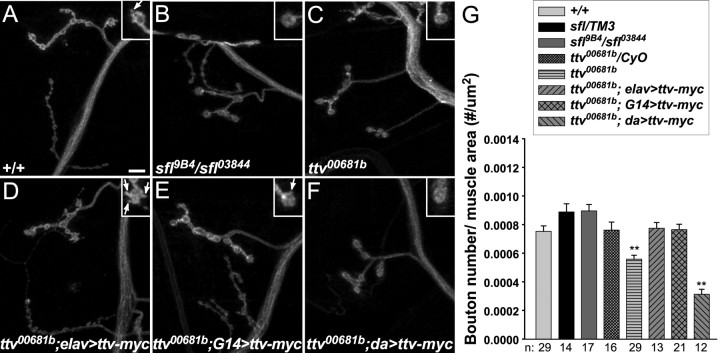
To evaluate the importance of HS biosynthesis for synapse development, we examined the morphology of the NMJ in sfl and ttv mutant larvae (Fig. 5). ttv mutant synapses are reduced in size and have significantly fewer boutons per unit muscle area compared with controls (Fig. 5C,G), whereas sfl synapses are smaller than normal but with no reduction in number of boutons relative to muscle area (Fig. 5B,G). We also noted alterations in bouton morphology: both sfl and ttv motoneuron boutons are bigger than normal, with a reduced number of “buds” (Fig. 5A–C, insets). During larval growth, new boutons can be added to the extending nerve terminals, with the result that larger boutons sometimes display small buds known as satellites. In contrast to normal synapses, we found that sfl and ttv synapses consisted primarily of large boutons without any buds; a similar increase in ttv bouton size was observed previously (Kraut et al., 2001). Our results suggest that these mutants have some defect in initiating new bouton formation, and are consistent with a role for HSPGs in normal synapse growth.
Figure 5.
Synapse morphology in HS biosynthetic mutants. A–F, Anti-HRP staining of muscle 4 NMJs from wild type, sfl or ttv mutants, and ttv mutants expressing UAS-ttv-myc driven by elav-Gal4, G14-Gal4, or da-Gal4. Scale bar, 10 μm. The insets show larger views of terminal type Ib boutons. ttv mutants have fewer, larger boutons than controls, with fewer buds. sfl mutants also have larger boutons with fewer buds. G14-Gal4>UAS-ttv-myc completely rescued ttv synapse and bouton morphology. elav-Gal4>UAS-ttv-myc rescued bouton number and size, but caused increased bouton budding. da-Gal4>UAS-ttv-myc enhanced the ttv mutant phenotypes. G, Average numbers of boutons per unit muscle area for the indicated genotypes. Error bars represent SEM. **p < 0.001 compared with +/+ and the relevant heterozygote control. Sample numbers (n) are shown below the graph. ttv mutant synapses have fewer boutons than controls. This phenotype is rescued by elav-Gal4 or G14-Gal4 driving Ttv-myc expression, and exacerbated by expression of Ttv-myc with da-Gal4 (p < 10−4 compared with ttv00681b mutants).
We rescued the growth defect of ttv mutant synapses by presynaptic and postsynaptic expression of UAS-ttv-myc. Expression with either elav-Gal4 or G14-Gal4 increased the number of boutons per unit muscle area to normal levels (Fig. 5G). Expression with G14-Gal4 also restored a normal level of bouton budding (Fig. 5E). In contrast, expression of UAS-ttv-myc with elav-Gal4 resulted in elevated bouton budding (Fig. 5D, inset), suggesting that presynaptic overexpression of Ttv-myc causes too many new buds to initiate. Ubiquitous overexpression of UAS-ttv-myc with da-Gal4 had the opposite effect on synapse development: the number of boutons relative to muscle area was significantly reduced compared with ttv mutants and we observed no bouton budding (Fig. 5F). Overexpression of UAS-ttv-myc with da-Gal4 in a wild-type animal also reduced bouton number significantly (5.01 × 10−4/μm2 compared with 7.54 × 10−4/μm2 for +/+; p < 0.001); this effect was not seen with elav-Gal4 (8.44 × 10−4 boutons/μm2; p = 0.12). Rather than rescuing the morphology, the HS synthesized in da-Gal4>UAS-ttv-myc animals seems to inhibit synapse growth, showing that appropriate Ttv expression is critical for synapse development.
Abnormal synaptic ultrastructure in sfl mutants
To assess the morphology of HS-deficient synapses in greater detail, we examined sfl mutants by transmission electron microscopy (TEM). The most striking phenotype we observed was a change in the distribution of mitochondria in sfl mutant muscles. In a wild-type synapse, mitochondria are densely packed beneath the postsynaptic membrane near the boutons, but the number of these mitochondria is grossly reduced in sfl mutant NMJs (Fig. 6A,B) (1.11/μm2 compared with 1.82/μm2 for +/+; p < 0.01). The numbers of mitochondria in other parts of the muscle cell, such as between the contractile fibers, were unaffected (Fig. 6C,D) (0.265/μm2 compared with 0.325/μm2 for +/+; p = 0.32), and mitochondrial numbers in motoneurons were unchanged, based on counts of presynaptic mitochondria in TEM sections (data not shown). Thus, the defect may represent a failure to properly localize or retain mitochondria in a specific region of the muscle cell.
Figure 6.
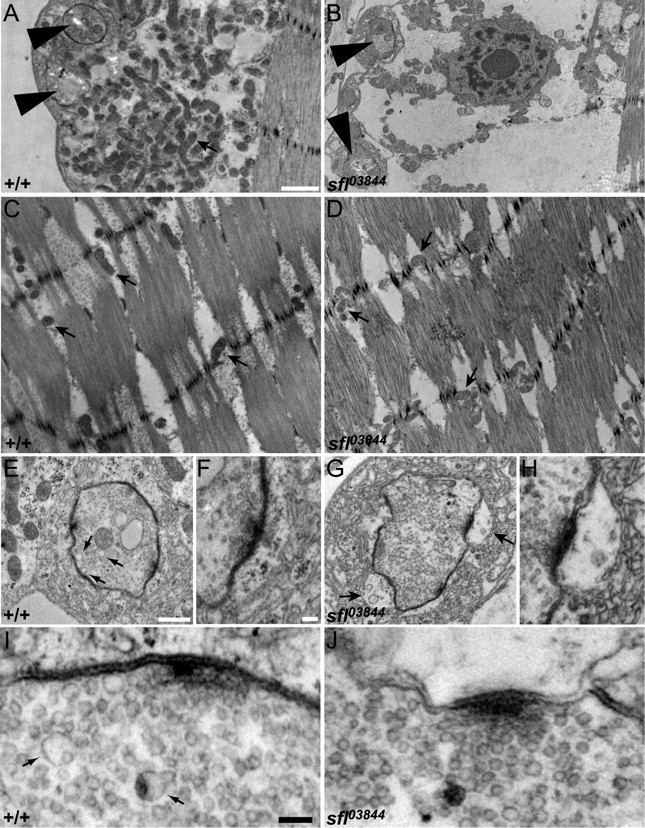
Ultrastructure of sfl mutant synapses. A, B, Electron micrographs showing synaptic boutons (large arrowheads) and the underlying muscle in wild-type and sfl03844 mutant NMJs. Wild-type NMJs have an abundance of mitochondria (arrow) between the boutons and the contractile fibers of the muscle, but sfl mutants have significantly fewer. Scale bar, 1 μm. C, D, Electron micrographs showing the contractile muscle fibers from wild-type and sfl mutant animals (same scale as in A and B). Similar numbers of mitochondria (arrows) are found within the contractile apparatus of sfl mutant muscles as in wild type. E–H, Higher-magnification views of boutons from wild-type and sfl mutant animals. Scale bars: E, G, 0.5 μm; F, H, 0.1 μm. The arrows in E point to 70 nm cisternae, which are more numerous in wild type than in sfl mutants. The arrows in G point to enlarged pockets between the postsynaptic membrane and the subsynaptic reticulum in sfl mutants. I, J, Electron micrographs showing synaptic vesicles near active zones in wild type and sfl mutant NMJs. Scale bar, 0.1 μm. Numerous 35 nm vesicles are seen in both genotypes, but sfl mutants have fewer 70 nm cisternae (arrows) than controls.
sfl mutant synapses appeared to be normal in many respects, with well aligned presynaptic and postsynaptic membranes. We did not detect any changes in the number or morphology of motoneuron active zones (Fig. 6E–J). This finding was confirmed by anti-NC82 staining, a marker for synaptic active zones in Drosophila (our unpublished data). Boutons were surrounded by a normal amount of subsynaptic reticulum, but sfl mutant muscles had gaps in the membranous structures around ∼40% (17 of 44) of the boutons (Fig. 6, compare E, F, with G, H). These enlarged postsynaptic “pockets” are similar to those seen in synapses that developed under partial loss of Wingless (Packard et al., 2002).
We also noted a change in the vesicle population of the presynaptic cells. Wild-type and sfl boutons have similar numbers and distributions of synaptic vesicles (35 nm in diameter), but sfl mutants have reduced numbers of larger 70 nm vesicles, or cisternae (Fig. 6E,I, arrows; compare with G, J). TEM characterization of NMJs in animals with endocytosis defects has demonstrated increased numbers of 70 nm cisternae; these intracellular vesicular structures accumulate when endocytosis is compromised and are presumed to be intermediates in synaptic vesicle recycling (Wucherpfennig et al., 2003; Zhang, 2003). We observed a greater than twofold reduction in the number of cisternae in sfl mutant terminals (0.46/μm2 compared with 1.29/μm2 for +/+; p < 0.001), suggesting that sfl mutants have a vesicle trafficking defect.
sfl and ttv mutants have endocytosis abnormalities
To directly assess membrane trafficking at the Drosophila NMJ, we observed the internalization of a lipophilic dye, FM1-43, after repetitive motoneuron stimulation. Repeated low-frequency depolarization mediated by incubation in high K+ saline results in selective uptake of this fluorescent dye into the recycling pool (also known as the exo/endo pool) of synaptic vesicles (Kidokoro et al., 2004; Rizzoli and Betz, 2005). Using this assay, we found a dramatic increase in FM1-43 fluorescence in the motoneuron terminals of both ttv and sfl mutants compared with wild-type and heterozygous controls (Fig. 7A–C,G). This increase depends on nerve stimulation, because it did not occur when preparations were treated with FM1-43 without high levels of K+ (our unpublished observation). Mutant neurons showed no more fluorescence than controls in the absence of stimulation, demonstrating that mutation of sfl or ttv does not affect membrane affinity of FM1-43 or fluorescence of the dye in the membrane (our unpublished observation). Therefore, we conclude that loss of HS biosynthesis or modification produces an increase in activity-dependent endocytosis at the NMJ. This increase in endocytosis documented by FM1-43 uptake is consistent with the decrease in the number of cisternae observed by TEM: both of these phenotypes are opposite to those observed in endocytic mutants (Wucherpfennig et al., 2003; Zhang, 2003). We did not see a change in the overall number of synaptic vesicles in sfl mutants by TEM; however, the recycling pool labeled by our FM1-43 protocol is reported to represent a modest proportion (14–19%) of all synaptic vesicles (Delgado et al., 2000; Rizzoli and Betz, 2005) so an increase in this portion of the total vesicle pool might not be apparent.
To confirm that the stimulus-dependent increase in FM1-43 uptake is mediated by loss of HS biosynthesis, we demonstrated that the elevated level of FM1-43 internalization found in ttv mutants is restored to normal by elav-Gal4-directed expression of UAS-ttv-myc (Fig. 7D,G). FM1-43 uptake is rescued to somewhat lesser degrees by muscle-directed (G14-Gal4) or ubiquitous expression (da-Gal4) (Fig. 7E–G). These rescue experiments support the conclusion that HSPGs serve to limit synaptic vesicle recycling at the NMJ.
Morphological changes in muscle cells of HS biosynthetic mutants
The TEM analysis of sfl mutants showed that the muscles of these animals have a number of morphological abnormalities, in particular a maldistribution of mitochondria beneath the cell surface in the vicinity of synaptic boutons. To further explore this phenotype, we examined both sfl and ttv mutant NMJs using a mitochondria-specific antibody (anti-cytochrome c) and a mitochondrial-directed GFP marker (mito-GFP). Anti-cytochrome c staining of wild-type NMJs revealed the concentration of mitochondria surrounding the motoneuron boutons, as well as some enrichment around nuclei (Fig. 8A–C). Confirming our TEM analysis, neither sfl nor ttv mutants show a concentration of anti-cytochrome c staining surrounding type Ib synaptic boutons (Fig. 8D–G,J). Muscle-directed (G14-Gal4) expression of UAS-ttv-myc in ttv mutants partially restored the staining around boutons and nuclei, whereas elav-Gal4>UAS-ttv-myc had little effect (Fig. 8G–L). Mitochondrial number in the presynaptic terminals of sfl mutants is unaltered as measured by the levels of Mito-GFP (Fig. 8M,N), a result confirmed by direct counts of mitochondria in TEM sections of sfl nerve terminals (our unpublished data). Thus, HS biosynthetic mutations specifically affect mitochondrial localization in the postsynaptic cell near the motoneuron boutons.
Figure 8.
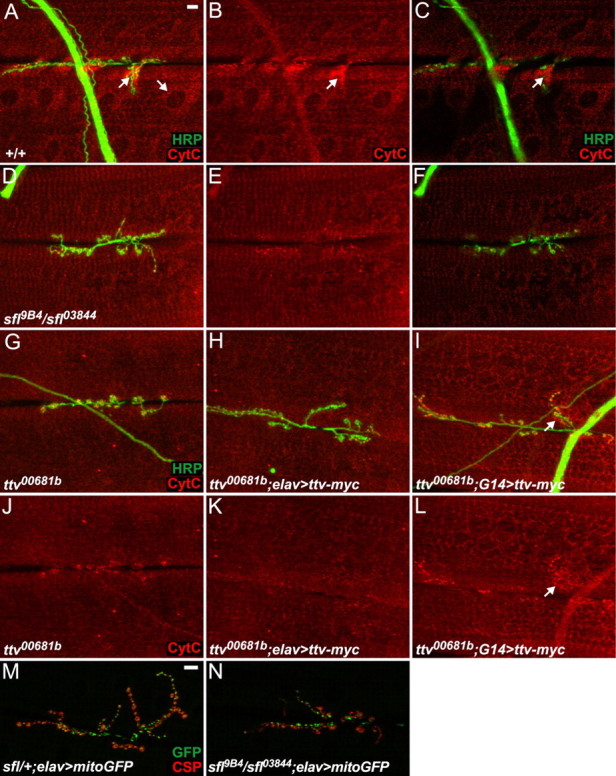
Abnormal mitochondrial localization in muscles of HS biosynthetic mutants. A–L, Muscle 6 and 7 NMJs stained with anti-cytochrome c (red) to visualize mitochondrial localization and anti-HRP (green) to label motoneuron processes. The images in C and F are single confocal images from the superficial region of the NMJ near the boutons; other images are composites showing staining from the surface of the boutons down into the contractile apparatus of the muscle. Scale bar, 10 μm. A–C, In wild-type NMJs, mitochondria are enriched in the vicinity of the boutons and around nuclei (arrows), but are also distributed throughout the muscle. D–G, J, sfl and ttv mutants do not show the normal enrichment of mitochondria around type Ib boutons and nuclei. H, I, K, L, Expression of Ttv-myc with G14-Gal4, but not elav-Gal4, restores significant cytochrome c staining. M, N, NMJs from muscles 6 and 7 of sfl heterozygous or mutant animals expressing a mitochondrial GFP fusion protein (green) driven by elav-Gal4. Anti-CSP staining (red) labels boutons. Scale bar, 10 μm. Mitochondrial localization is unaffected in sfl mutant neurons.
The abnormalities in mitochondrial distributions in the muscles of sfl and ttv mutants prompted us to examine other membrane compartments in muscle cells. We used anti-BIP antibodies to visualize endoplasmic reticulum (ER) and anti-p120 as a Golgi marker (Sanyal et al., 2005; Tsruya et al., 2007). ttv mutants show a marked reduction in the level of anti-BIP immunostaining (Fig. 9A,B); BIP levels in the muscle are rescued by expression of a UAS-ttv-myc transgene in neurons with elav-Gal4, and increased above normal levels by muscle (G14-Gal4) expression (Fig. 9C,D). Golgi organization is also altered in ttv mutant muscles, with dramatically reduced levels of p120 that can be rescued with G14-Gal4-directed expression of UAS-ttv-myc, or partially rescued with elav-Gal4 (Fig. 9E–H). It should be emphasized that these changes in intracellular membrane organization are not a consequence of compromising cell viability: the muscle cells of HS biosynthetic mutants show a normal membrane potential and an increased EJP in response to motoneuron stimulation. These findings demonstrate that the loss of heparan sulfate affects intracellular membrane organization. They suggest that HSPGs participate in intracellular compartment or membrane trafficking, either directly, or indirectly by affecting signaling between the muscle and the motoneuron.
Figure 9.
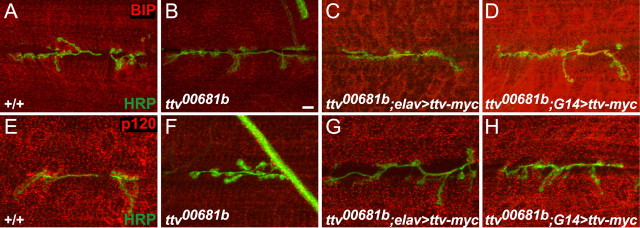
Disruptions of muscle morphology in ttv mutants. A–D, Anti-BIP antibody staining (red) labels the endoplasmic reticulum in NMJs from wild-type, ttv mutant, and ttv mutant animals expressing Ttv-myc with elav-Gal4 or G14-Gal4. Anti-HRP staining is shown in green. Scale bar, 10 μm. ttv mutants have decreased levels of anti-BIP staining in muscles. Expression of Ttv-myc with elav-Gal4 restores normal anti-BIP staining, whereas expression with G14-Gal4 substantially increases anti-BIP staining above the normal level. E–H, Anti-p120 antibody staining (red) labels the Golgi in NMJs of the same genotypes. ttv mutant muscles show reduced levels of anti-p120 staining. Comparatively normal staining is restored by expression of Ttv-myc with G14-Gal4, whereas elav-Gal4 gives partial rescue.
Discussion
Our analyses of Drosophila HS biosynthetic mutants demonstrated that proper HS synthesis is important for many facets of NMJ structure and function. Defects in HS biosynthesis affected synapse growth and the formation of new boutons, disturbed both spontaneous and evoked electrophysiological properties, and produced locomotion defects in mutant animals. Notably, loss of HS also increased synaptic vesicle endocytosis and had profound consequences for intracellular organization in the muscle cell, suggesting that HSPGs normally regulate membrane trafficking and organelle localization both in neurons and muscles.
Mutations affecting different aspects of HSPG biosynthesis cause distinct phenotypes
The consequences of losing HS biosynthetic functions were broader and more substantial than the previously reported defects caused by loss of the individual HSPGs Sdc or Dlp. Johnson et al. (2006) found that both Sdc and Dlp contribute to synaptic development, but Sdc specifically promotes synapse growth, whereas Dlp influences active zone morphology. dlp mutants show defects in synapse physiology as well: muscle responses to nerve stimulation are substantially increased, although spontaneous neurotransmitter release is normal. Although HS biosynthetic mutants had features resembling both sdc and dlp mutants (for example, ttv mutants have a similar reduction in synapse size as sdc mutants, and increased EJP amplitudes like dlp mutants), sfl and ttv also affect spontaneous neurotransmission, unlike dlp. Also, there were no apparent changes in active zone morphology in sfl mutants like those caused by loss of dlp. These differences indicate that sfl and ttv mutants have some defects distinct from those caused by loss of individual HSPGs.
Our analysis of mutants affecting either HS chain synthesis or sulfation shows that their phenotypes are not precisely the same; in fact, loss of sulfation has more severe consequences for spontaneous neurotransmission and locomotion than simple loss of HS chains. Although we cannot know precisely the level of gene function that remains in these animals, either from maternal contributions or some residual gene activity of the mutant alleles [both described as genetic nulls (Bellaiche et al., 1998; Lin and Perrimon, 1999)], we have conducted biochemical and structural analysis of the HS chains in the third-instar larvae of these mutants and showed that the levels of HS were reduced at least 15-fold in ttv and sulfated HS was undetectable in the sfl alleles used (Toyoda et al., 2000). These observations suggest that the unmodified HS chains in sfl mutants have more deleterious biological activities than the few or shortened HS chains that remain in ttv mutants. Proteoglycans carrying unsulfated chains may localize correctly in cells (as reflected in the normal 3G10 staining pattern in sfl mutants), but may fail to bind critical partner proteins or interfere with normal HSPG functions.
Appropriate HS biosynthesis is required for normal NMJ structure and function
We determined the cell type requirement for HS biosynthesis by rescue experiments with ttv mutants. One surprising finding was that widespread high-level expression of ttv+ did not provide full rescue. Indeed, ttv; da-Gal4>UAS-ttv-myc animals showed significantly less rescue than mutants with only neuronal or muscle expression of UAS-ttv-myc, and synapse size was reduced even further in these animals than in ttv mutants. Since overexpression of Ttv-myc with da-Gal4 in an otherwise wild-type animal also reduced synapse size, we conclude that high levels of Ttv protein are detrimental in some way. Ttv is part of a copolymerase with Sotv (Han et al., 2004; Izumikawa et al., 2006); excessive levels of Ttv may enhance HS polymerization (Busse et al., 2007) or interfere with the activity of this protein complex. In addition to potential changes in HS chain length produced by overexpression of Ttv, these polymers may have aberrant levels or patterns of sulfation (Presto et al., 2008).
Our rescue analysis showed some unexpected tissue requirements for HS synthesis. Most strikingly, the reduced mEJP frequency of ttv mutants, a presynaptic defect, was rescued by muscle rather than neuronal expression of ttv+, demonstrating that HS biosynthesis in muscle is critical for a motoneuron property. In addition, G14-Gal4-driven expression rescued other neuronal phenotypes such as synapse growth and activity-dependent endocytosis, which were also ameliorated with elav-Gal4. Conversely, neuronal expression of UAS-ttv-myc restored normal BIP staining and rescued other muscular defects. Although we cannot rule out rescue by the very low level of elav-Gal4-driven expression in muscle cells, neuronal HS synthesis appears to be critical for muscle integrity. These results may reflect the need for HS molecules on the surface of one synaptic partner modulating activities in the other cell (Marqués, 2005).
The organization of intracellular organelles is altered in HS biosynthetic mutants
The localization of mitochondria beneath the postsynaptic membrane was abnormal in HS biosynthetic mutants, as shown both by TEM and anti-cytochrome c staining. The defect was specific to the vicinity of the boutons, because mitochondrial distributions within the muscle contractile fibers were normal, as were levels of mitochondria in the presynaptic terminal. This phenotype was substantially rescued in ttv mutants by expression of a wild-type transgene in muscle, suggesting there is a cell-autonomous requirement for HS production to maintain normal mitochondrial organization in the postsynaptic cell. Presynaptic targeting of mitochondria to axon terminals has been well documented (Stowers et al., 2002; Guo et al., 2005; Glater et al., 2006); anterograde transport depends on kinesin motors and microtubules, whereas anchoring in response to nerve growth factor and phosphoinositide 3-kinase signaling appears to require F-actin (Chada and Hollenbeck, 2004; Hollenbeck and Saxton, 2005; Frederick and Shaw, 2007). Less is known about the importance of postsynaptic mitochondrial localization, although the development and plasticity of dendritic spines depends on mitochondrial distribution (Li et al., 2004). HSPGs could influence mitochondrial organization at the NMJ by affecting extracellular signaling events, such as that described in axon terminals in which nerve growth factor signaling recruits axonal mitochondria to specific sites (Chada and Hollenbeck, 2004; Hollenbeck and Saxton, 2005; Frederick and Shaw, 2007). Alternatively, HSPGs could affect mitochondrial transport within cells, or in some way regulate the fission and fusion events that are a critical feature of mitochondrial biology (Cerveny et al., 2007).
We found that levels of the ER-resident protein BIP and the Golgi protein p120 were reduced in the muscle cells of HS biosynthetic mutants. Expression of a wild-type transgene in either neuron or muscle restored the levels of these ER and Golgi markers, demonstrating that the loss of HS caused these cellular phenotypes. Given that HS-modified proteins are primarily known as molecules of the cell surface and the extracellular matrix, these changes in cellular organization and mitochondrial localization were, on the face of it, surprising. However, there is growing evidence that the activity of HSPGs is not limited to events in the extracellular space or at the plasma membrane. For example, HSPGs, synthesized in the Golgi, may affect the biosynthesis of other protein components destined for secretion or delivery to the cell surface, perhaps serving as a scaffolding molecule within the Golgi. The cellular phenotypes of Ndst2 mutant mice support this model. Ndst2 encodes a sulfotransferase critical for synthesis of heparin, the highly sulfated form of heparan sulfate that is a component of mast cell secretory vesicles. In addition to lacking heparin, mast cells from Ndst2 mice lack a number of proteases that are normally found within secretory granules; this protease defect is posttranslational, suggesting that proper biosynthesis of secretory granule components depends on the ability to make heparin (Forsberg et al., 1999; Humphries et al., 1999). If HSPGs serve as molecular scaffolds for a variety of membrane-associated proteins, loss of HS biosynthesis could affect other cellular compartments as well as secretory granules.
HSPGs may also affect intracellular organization via effects on membrane trafficking, particularly endocytosis. We found that the rate of synaptic vesicle recovery measured by FM1-43 dye internalization was more than doubled in HS biosynthetic mutants compared with controls. This increase in endocytosis was entirely corrected by expression of the wild-type transgene in neurons, and mostly corrected by muscle-driven expression. The number of 70 nm cisternae, presynaptic vesicles known to be affected by rates of endocytosis (Wucherpfennig et al., 2003; Zhang, 2003), found in sfl mutants also support the conclusion that HSPGs limit the recovery of synaptic vesicles. A few previous studies have implicated HSPGs in other endocytic and trafficking processes. For example, Syndecan-2 participates in trafficking of membrane proteins such as FGF receptor and β1-integrin in cultured cells (Zimmermann et al., 2005). Dlp promotes internalization of the lipid-modified morphogens Hedgehog and Wingless (Gallet et al., 2008), perhaps by association with lipoprotein particles (Eugster et al., 2007). HSPGs also play important roles in lipoprotein uptake by the liver: selective loss of Ndst1 in hepatocytes greatly reduces uptake of triglyceride-rich lipoprotein particles, leading to elevated serum triglyceride levels (MacArthur et al., 2007). Clearly, additional studies will be required to elucidate the mechanisms of these complex effects of HSPGs on trafficking and cellular organization.
Footnotes
This work was supported by National Institutes of Health Grants GM054832 and GM067208. We thank M. O'Connor, I. The, K. Zinsmaier, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We are grateful to Alice Ressler for assistance with electron microscopy and to Hiroshi Nakato for helpful discussions.
References
- Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci. 2002;5:119–123. doi: 10.1038/nn801. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, The I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394:85–88. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Barbel S, Sheperd S, Jack JW, Jan LY, Jan YN. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987;51:293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Bülow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- Busse M, Feta A, Presto J, Wilén M, Grønning M, Kjellén L, Kusche-Gullberg M. Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem. 2007;282:32802–32810. doi: 10.1074/jbc.M703560200. [DOI] [PubMed] [Google Scholar]
- Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17:563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Eugster C, Panáková D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellén L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Thérond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Hoang B, Chiba A. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev Biol. 2001;229:55–70. doi: 10.1006/dbio.2000.9983. [DOI] [PubMed] [Google Scholar]
- Hoch W, Campanelli JT, Scheller RH. Agrin-induced clustering of acetylcholine receptors: a cytoskeletal link. J Cell Biol. 1994;126:1–4. doi: 10.1083/jcb.126.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, Sharpe AH, Stevens RL. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EPHB receptor signaling in dendritic spine development. Front Biosci. 2004;9:1365–1373. doi: 10.2741/1325. [DOI] [PubMed] [Google Scholar]
- Izumikawa T, Egusa N, Taniguchi F, Sugahara K, Kitagawa H. Heparan sulfate polymerization in Drosophila. J Biol Chem. 2006;281:1929–1934. doi: 10.1074/jbc.M509138200. [DOI] [PubMed] [Google Scholar]
- Jenniskens GJ, Veerkamp JH, van Kuppevelt TH. Heparan sulfates in skeletal muscle development and physiology. J Cell Physiol. 2006;206:283–294. doi: 10.1002/jcp.20450. [DOI] [PubMed] [Google Scholar]
- Johansen J, Halpern ME, Keshishian H. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J Neurosci. 1989;9:4318–4332. doi: 10.1523/JNEUROSCI.09-12-04318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P. Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res Brain Res Rev. 2004;47:18–32. doi: 10.1016/j.brainresrev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance. J Cell Sci. 2007;120:1829–1832. doi: 10.1242/jcs.03432. [DOI] [PubMed] [Google Scholar]
- Kraut R, Menon K, Zinn K. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr Biol. 2001;11:417–430. doi: 10.1016/s0960-9822(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, Talbot WS, Geisler R, Nüsslein-Volhard C, Selleck SB, Chien CB, Roehl H. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Loeb JA. Neuregulin: an activity-dependent synaptic modulator at the neuromuscular junction. J Neurocytol. 2003;32:649–664. doi: 10.1023/B:NEUR.0000020640.84708.35. [DOI] [PubMed] [Google Scholar]
- MacArthur JM, Bishop JR, Stanford KI, Wang L, Bensadoun A, Witztum JL, Esko JD. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117:153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan R, Peng HB. HGF induction of postsynaptic specializations at the neuromuscular junction. J Neurobiol. 2006;66:134–147. doi: 10.1002/neu.20206. [DOI] [PubMed] [Google Scholar]
- Marois E, Mahmoud A, Eaton S. The endocytic pathway and formation of the Wingless morphogen gradient. Development. 2006;133:307–317. doi: 10.1242/dev.02197. [DOI] [PubMed] [Google Scholar]
- Marqués G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- Marqués G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition of the end-plate potential. J Physiol. 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presto J, Thuveson M, Carlsson P, Busse M, Wilén M, Eriksson I, Kusche-Gullberg M, Kjellén L. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc Natl Acad Sci U S A. 2008;105:4751–4756. doi: 10.1073/pnas.0705807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J Neurobiol. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Rotundo RL, Rossi SG, Kimbell LM, Ruiz C, Marrero E. Targeting acetylcholinesterase to the neuromuscular synapse. Chem Biol Interact. 2005;157–158:15–21. doi: 10.1016/j.cbi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Consoulas C, Kuromi H, Basole A, Mukai L, Kidokoro Y, Krishnan KS, Ramaswami M. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics. 2005;169:737–750. doi: 10.1534/genetics.104.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Fox B, Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J Biol Chem. 2000;275:21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- Tsruya R, Wojtalla A, Carmon S, Yogev S, Reich A, Bibi E, Merdes G, Schejter E, Shilo BZ. Rhomboid cleaves Star to regulate the levels of secreted Spitz. EMBO J. 2007;26:1211–1220. doi: 10.1038/sj.emboj.7601581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Bräuninger M, González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Glycobiology of the synapse: the role of glycans in the formation, maturation, and modulation of synapses. Biochim Biophys Acta. 2002;1573:369–376. doi: 10.1016/s0304-4165(02)00405-1. [DOI] [PubMed] [Google Scholar]
- Zhang B. Genetic and molecular analysis of synaptic vesicle recycling in Drosophila. J Neurocytol. 2003;32:567–589. doi: 10.1023/B:NEUR.0000020611.44254.86. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N′Kuli F, Courtoy PJ, David G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]