ABSTRACT
BACKGROUND:
This study was conducted to identify the factors associated with acute gastrointestinal (GI) toxicity during pelvic chemoradiotherapy (PCRT) in patients with rectal cancer.
METHODS:
We analyzed 177 patients with rectal cancer treated from 2007 through 2010. Clinical information, including weekly diarrhea and proctitis toxicity grade during PCRT, was recorded. GI structures including bowel and anal canal were contoured. The associations between toxicity and clinical and dosimetric predictors were tested.
RESULTS:
The median age was 60; 76 patients were women; 98 were treated with intensity-modulated radiotherapy (IMRT) and 79 with 3D conformal RT (3DCRT). A higher rate of grade 2+ diarrhea was observed in the women, starting at week 4 (24% women vs. 11% men, P = .01; week 5: 33% vs. 12%, P = .002), as well as in all the patients treated with 3DCRT (22% vs. 12% IMRT, P = .03; week 5: 32% vs. 11%, P = .001). On multivariate analysis, the normal tissue complication probability (NTCP) model including bowel V45 (bowel volume receiving ≥45 Gy) showed that being female, and use of 3DCRT, was most predictive of grade 2+ diarrhea (area under the curve [AUC] = 0.76; RS = 0.35; P < .001). A higher rate of grade 2+ proctitis was seen in patients <60 years of age starting at week 3 (21% vs. 9%, P = .02; week 4: 35% vs. 16%, P = .003). The NTCP model including anal canal V15 and younger age was most predictive of grade 2+ proctitis (AUC = 0.67; RS = 0.25; P < .001).
CONCLUSIONS:
Women and all patients who were treated with 3DCRT had higher rates of grade 2+ diarrhea, and the younger patients had a higher rate of grade 2+ proctitis during PCRT. The use of more stringent dosimetric constraints in higher risk patients is a strategy for minimizing toxicity.
Pelvic chemoradiotherapy (PCRT) is an integral part of multidisciplinary care for patients with locally advanced rectal cancer. The use of preoperative pelvic radiotherapy with 5-fluorouracil (5-FU)-based chemotherapy has been shown to improve locoregional control in several randomized trials.1–5 Acute gastrointestinal (GI) toxicity during pelvic irradiation for rectal cancer is common and has been reported in 30–40% of patients who undergo RT with concurrent administration of 5-FU.6–8 Prior dosimetric studies have demonstrated a correlation between the volume of small bowel exposed to at least 15 Gy and grade 3+ diarrhea.9–12 Further studies have shown that intensity-modulated radiation therapy (IMRT) is effective in reducing bowel dose and acute GI toxicity during pelvic RT when compared with 3-dimensional conformal radiotherapy (3DCRT).13 Although the results did not reach statistical significance, in a phase II trial conducted by the Radiation Therapy Oncology Group (RTOG 0822), IMRT-based chemoradiotherapy showed a reduction in grade 2+ GI toxicity, compared with the results in a prior trial in which 3DCRT was used.14 Nevertheless, there is a paucity of data showing which patients are at the highest risk of acute GI toxicity. IMRT treatment planning is considerably more complex, and it is therefore important for physicians to select patients who would benefit most from it, to reduce the risk of acute diarrhea and proctitis based on clinical characteristics. Moreover, with the use of IMRT for pelvic RT, it is critical to define the dose constraints that are associated with lower rates of GI toxicity using patient outcomes data.
Identifying predictors for treatment-related GI toxicity is important in reducing complications. In this study, we investigated predictors for clinically significant diarrhea and proctitis in patients with rectal cancer who received concurrent RT and chemotherapy. We tested the hypothesis that on the basis of their clinical characteristics, some patients have a higher probability of experiencing acute GI toxicity during treatment and that each clinical characteristic leads to a unique toxicity trajectory during PCRT. We report potential dosimetric constraints that can be used for pelvic RT planning, to reduce treatment-related acute complications in high-risk patients.
PATIENTS AND METHODS
Patients and radiation planning and delivery
After obtaining a waiver of authorization from the Institutional Review Board at Memorial Sloan-Kettering Cancer Center, we retrospectively analyzed 177 consecutive patients with rectal cancer treated with RT with concurrent chemotherapy at our institution from 2007 through 2010. The patients' clinical information and weekly acute toxicity grades, describing fatigue, dermatitis, mucositis, nausea, vomiting, diarrhea, proctitis, and cystitis, were obtained through chart review. All toxicity was scored according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. The median age was 60 years. Seventy-six patients (43%) were women. Table 1 provides an overview of the patients' clinical characteristics.
Table 1.
Patient characteristics
| Patients (n) | 177 |
| Age at RT initiation (y) | |
| Mean, median (range) | 59, 60 (19∼94) |
| Sex (n) | |
| Female | 76 |
| Male | 101 |
| Clinical stage (n) | |
| I | 14 |
| II | 35 |
| III | 115 |
| IV | 13 |
| Tumor state (n) | |
| T0* | 1 |
| T1 | 5 |
| T2 | 26 |
| T3 | 125 |
| T4 | 20 |
| Node Stage (n) | |
| N0 | 52 |
| N1 | 92 |
| N2 | 33 |
| Metastasis stage M1 (n) | 14 |
| Tumor location (n) | |
| Distal (0-5 cm from AV) | 71 |
| Mid (6-10 cm from AV) | 80 |
| Proximal (>10 cm from AV) | 26 |
| FOLFOX induction chemotherapy (n) | |
| Yes | 43 |
| No | 134 |
| Chemoradiotherapy sequence (n) | |
| Neoadjuvant | 163 |
| Adjuvant | 14 |
| Chemotherapy agent used (n) | |
| 5-FU | 165 |
| Capecitabine | 12 |
| Mode of radiation therapy (n) | |
| IMRT | 98 |
| 3DCRT | 79 |
| Radiation therapy dose (Gy) | |
| Median, mean (range) | 50, 50 (30–56) |
One patient had nodal recurrence without clinical evidence of recurrent primary tumor.
AV=anal verge.
All patients underwent a pretreatment computed tomography (CT) simulation in the prone position with a full bladder and a marker placed at the anal verge. The gross tumor volume (GTV) consisted of the primary tumor and enlarged regional lymph nodes in patients who underwent neoadjuvant pelvic RT and the tumor bed for patients who underwent adjuvant pelvic RT. For the standard pelvic fields, the clinical target volume (CTV) consisted of the GTV plus the rectum and lymph node regions, including the mesorectum and the presacral, internal iliac, and inferior rectal nodes.15 For T4 disease or anal canal involvement, the external iliac and inguinal nodal regions were included in the CTV. The initial planning target volume (PTV) was a 0.5-cm expansion of the CTV. For the boost fields, CTV_boost consisted of the GTV, adjacent mesorectum, and presacral space, and PTV_boost was a 0.5-cm expansion on the CTV_boost. Normal tissues contoured at the time of RT planning included the bladder, bowel, anal canal (anal verge to anorectal ring), rectum (anorectal ring to rectosigmoid flexure), femoral heads, external genitalia, and, in female patients, the vagina. The bowel contour included the small bowel and sigmoid colon as individual loops, excluding the rectum and anal canal, extending to 1 cm above the PTV.
The patients underwent either 3DCRT or IMRT treatment planning with in-house software, according to the treating physician's preference. The median RT dose of the study cohort was 50 Gy in 25 fractions, and IMRT was the RT delivery method for the majority of patients (55% IMRT vs. 45% 3DCRT). Coverage of the PTV by at least 95% of the prescribed dose was required in all plans. The 3DCRT plans consisted of 3 or 4 orthogonal beams for the pelvic fields and 2 lateral beams and 1 posterior–anterior beam for the boost fields. PTV was treated to 45 Gy in 1.8-Gy fractions followed by a 5.4 Gy boost to the PTV_boost volume. IMRT plans consisted of 7 equally spaced coplanar fields. Dose homogeneity was assessed to minimize volume receiving more than 5% of the prescribed dose. The majority of patients treated with IMRT received 45 Gy in 1.8-Gy fractions to PTV and 50 Gy in 2-Gy fractions to the PTV_boost as an integrated boost.
Chemotherapy
The majority of patients were treated with neoadjuvant as opposed to adjuvant chemotherapy (92% vs. 8%, respectively). Concurrent chemotherapy, used in 93% of the patients, was continuous infusion of 5-FU (225 mg/m2 per day); 7% received concurrent capecitabine (875 mg/m2 twice daily). Forty-eight (27%) patients also received 2 to 4 cycles of induction chemotherapy consisting of folinic acid, 5-FU, and oxaliplatin (FOLFOX) before neoadjuvant chemotherapy.
Statistical analysis
For overall GI toxicity during the course of PCRT, univariate logistic regression analyses were used to identify significant clinical and dosimetric variables and to build predictive models associated with GI toxicities. The dosimetric variables included the bowel volume and anal canal volume receiving a specified dose in Gy (Table 2). Variables with Spearman's rank correlation coefficient (RS) > 0.15 were further analyzed with multivariate logistic regression, for normal tissue complication probability (NTCP) modeling of incidences of diarrhea and proctitis. The models most frequently observed were chosen as NTCP models for GI toxicities, by using leave-one-out cross validation (LOOCV) in the model-selection stage. The predictive models were evaluated by using the receiver operating characteristic (ROC) area under the curve (AUC) and Spearman's rank correlation coefficient metrics. In addition, the goodness of fit of these models was evaluated by comparing the predicted incidence of GI toxicity, calculated from the predictive models, with the actuarial incidence in the cohort. To do so, the patients were binned according to the predicted probability of toxicity, with an equal number of patients at each bin. Fisher's exact test was used to analyze the effect of clinical factors on weekly grade 2+ toxicity scores. Statistical significance was defined by P ≤ .05.
Table 2.
Correlation between bowel and anal canal dosimetric parameters and Grade 2+ GI symptoms
| Parameters | RS | P |
|---|---|---|
| Bowel: grade 2+ diarrhea | ||
| V10 | 0.099 | 0.243 |
| V15 | 0.086 | 0.312 |
| V20 | 0.097 | 0.255 |
| V25 | 0.140 | 0.097 |
| V30 | 0.182 | 0.031 |
| V35 | 0.203 | 0.016 |
| V40 | 0.238 | 0.004 |
| V45 | 0.318* | <0.001 |
| V50 | 0.195 | 0.021 |
| Anal Canal: grade 2+ proctitis | ||
| V10 | 0.151 | 0.073 |
| V15 | 0.169* | 0.041 |
| V20 | 0.161 | 0.055 |
| V25 | 0.167 | 0.048 |
| V30 | 0.163 | 0.065 |
| V35 | 0.150 | 0.076 |
| V40 | 0.146 | 0.064 |
| V45 | 0.109 | 0.197 |
| V50 | 0.027 | 0.754 |
The values in bold represent statistically significant results.
The finding with the highest correlation.
RESULTS
Diarrhea
During the course of PCRT, grade 2+ and 3+ diarrhea was recorded in 44 (25%) and 8 (5%) patients, respectively. The clinical variables of female sex (RS = 0.28; P = .001) and the use of 3DCRT (RS = 0.27; P = .001) correlated significantly with grade 2+ diarrhea on univariate analysis, whereas age, stage at initial presentation, tumor location (distal, mid, or proximal), type of chemotherapy, sequence of RT (neoadjuvant vs. adjuvant), and induction chemotherapy status did not predict clinically significant diarrhea. The incidence of grade 2+ diarrhea did not differ significantly between the patients who received induction chemotherapy and those who did not (30% vs. 22%, P = .082). Based on the analysis of dosimetric variables, bowel volume receiving 45 Gy (V45) correlated most significantly with grade 2+ diarrhea (RS = 0.31; P = <0.001), followed by V40 (Rs = 0.24, P = .005). Table 2 shows the dosimetric parameters used in assessing significant correlation with grade 2+ diarrhea.
On multivariate regression analysis combining dosimetric and clinical variables, the NTCP model combining bowel V45, being female, and the use of 3DCRT was found to be highly predictive of acute grade 2+ diarrhea (AUC = 0.76; RS = 0.35; P < .001). The optimal NTCP modeling for diarrhea was as follows:
where Y = (1.24 × bowel V45) − (1.42 × RT type) + (1.21 × gender) − 0.31. The coding values applied were female = 1, male = 0, 3DCRT = 1, and IMRT = 2.
A comparison of the predicted incidence of grade 2+ toxicity, determined according to the above NTCP model, and the actual incidence of grade 2+ toxicity in our study population is shown in Figure 1. The patients were binned into 6 categories, based on the predicted and actual toxicities, with 1 being the lowest toxicity group and 6 being the highest. Good correlation was shown between the NCTP model using V45 and actual toxicity. Patients with a V45 of <3% had no grade 2+ diarrhea while those with a V45 of 35% or higher had a >50% risk of grade 2+ diarrhea. The risk of grade 2+ diarrhea was significantly increased in patients with a V45 of ≥9%, from an incidence of ≤10% (groups 1–3) to ≥20% (groups 4–6).
Figure 1.
Comparison between the incidences of predicted grade 2+ toxicity according to the NTCP model and the actual incidence of grade 2+ toxicity experienced by the study population for diarrhea (A) and proctitis (B). The patients were binned into 6 categories on the basis of predicted toxicity and actual toxicity, with 1 being the lowest toxicity group and 6 being the highest. The ratio above each category in the figure represents the observed number of patients who experienced grade 2+ toxicity and the total number of patients.
Dose–volume analysis showed that the women consistently had a significantly larger volume of bowel receiving low- and high-dose irradiation (10–45 Gy) compared with that of the men receiving the same dose range. Patients treated with IMRT had significantly less bowel volume that received ≥45 Gy than those receiving 3DCRT (10.9% vs. 21.7%), resulting in a 50% reduction in mean V45 (P < .001) and V50 (2.7% vs. 6.4%; P = .02). Table 3 shows the differences in bowel dose–volume parameters between women and men, and between all patients who underwent IMRT and 3DCRT in the study population.
Table 3.
Mean bowel dose–volume parameters
| Volume Receiving Specified Dose | 3DCRT | IMRT | P |
|---|---|---|---|
| V10 | 70.2% | 79.4% | 0.003 |
| V20 | 47.0% | 62.6% | <0.001 |
| V30 | 31.1% | 38.6% | 0.016 |
| V35 | 27.8% | 31.0% | 0.259 |
| V40 | 25.4% | 23.9% | 0.555 |
| V45 | 21.7% | 10.9% | <0.001 |
| V50 | 6.4% | 2.7% | 0.021 |
| Women | Men | P | |
|---|---|---|---|
| V10 | 82.0% | 70.5% | <0.001 |
| V20 | 64.1% | 49.6% | <0.001 |
| V30 | 42.7% | 30.0% | <0.001 |
| V35 | 36.4% | 24.6% | <0.001 |
| V40 | 30.8% | 20.0% | <0.001 |
| V45 | 20.2% | 12.3% | 0.001 |
| V50 | 6.0% | 3.1% | 0.073 |
| Women Treated With 3DCRT | Women Treated With IMRT | P | |
|---|---|---|---|
| V10 | 81.5% | 82.4% | 0.832 |
| V20 | 62.4% | 65.3% | 0.548 |
| V30 | 43.5% | 42.1% | 0.783 |
| V35 | 39.3% | 34.4% | 0.290 |
| V40 | 36.5% | 26.8% | 0.025 |
| V45 | 31.6% | 12.4% | <0.001 |
| V50 | 9.6% | 3.4% | 0.041 |
| Men Treated With 3DCRT | Men Treated With IMRT | P | |
|---|---|---|---|
| V10 | 62.9% | 76.9% | <0.001 |
| V20 | 37.1% | 60.3% | <0.001 |
| V30 | 23.2% | 35.7% | <0.001 |
| V35 | 20.3% | 28.1% | 0.019 |
| V40 | 18.3% | 21.4% | 0.310 |
| V45 | 15.4% | 9.7% | 0.229 |
| V50 | 4.3% | 2.0% | 0.170 |
The values in bold represent statistically significant results.
A comparison of irradiated bowel volume between women who underwent 3DCRT and women who underwent IMRT was performed. Although no significant difference was observed between the 2 groups in the range of V10–V30, there were statistically significant reductions in patients with mean bowel volumes receiving greater than 35 Gy (V35–V50) (Table 3). Of note, while significantly higher mean bowel volume receiving lower dose RT (V10–V35 Gy) was observed in men who were treated with IMRT, IMRT did not provide significant reduction in high dose bowel volume (V35–V50).
Based on weekly toxicity assessments, a significantly higher rate of grade 2+ diarrhea was observed in women than in men during weeks 4 and 5 of pelvic RT (week 4: 24.2% women vs. 10.8% men, P = .01; week 5: 33.3% vs. 12.2%, P = .002), as well as in all patients treated with 3DCRT vs. IMRT (week 4: 22.2% vs. 11.5%, P = .03; week 5: 32.3% vs. 10.8%, P = .001). Table 4 details the weekly rates of reported grade 2+ diarrhea separated by clinical variables.
Table 4.
Weekly toxicities
| Grade 2+ diarrhea | Women | Men | P |
|---|---|---|---|
| Week 1 | 1.4% | 0% | 0.437 |
| Week 2 | 7.4% | 2.3% | 0.100 |
| Week 3 | 15.7% | 8.6% | 0.074 |
| Week 4 | 24.2% | 10.8% | 0.014 |
| Week 5 | 33.3% | 12.2% | 0.002 |
| Grade 2+ diarrhea | 3DCRT | IMRT | P |
|---|---|---|---|
| Week 1 | 1.4% | 0% | 0.437 |
| Week 2 | 5.6% | 3.5% | 0.250 |
| Week 3 | 14.5% | 9.2% | 0.113 |
| Week 4 | 22.2% | 11.5% | 0.034 |
| Week 5 | 32.3% | 10.8% | 0.001 |
| Grade 2+ proctitis | Age <60 | Age ≥60 | P |
|---|---|---|---|
| Week 1 | 3.4% | 0% | 0.144 |
| Week 2 | 10.0% | 4.0% | 0.087 |
| Week 3 | 21.1% | 8.5% | 0.015 |
| Week 4 | 34.9% | 15.8% | 0.003 |
| Week 5 | 38.2% | 23.4% | 0.025 |
The values in bold represent the statistically significant results.
Proctitis
Grade 2+ proctitis was seen in 57 (32%) patients. No patient experienced grade 3+ proctitis. On univariate analysis, the only clinical variable that significantly correlated with grade 2+ proctitis was age (RS = −0.22; P = .009). Although younger patients are at a higher risk of clinically significant proctitis, other clinical factors did not predict proctitis on univariate analysis. Among the tested dosimetric variables, the volume of anal canal receiving 15 Gy (V15) correlated significantly with grade 2+ proctitis (RS = 0.17; P = .04), followed by V25 (RS = 0.17; P = .05). These results signify that clinically significant proctitis may be a low-dose effect.
On multivariate regression analysis combining dosimetric and clinical variables, the NTCP model including anal canal V15 Gy and age was most predictive of acute grade 2+ proctitis (AUC = 0.67; RS = 0.25; P < .001) in our patients. The optimal NCTP model for proctitis is as follows:
with Y = (1.2 × anal canal V15) − (0.03 × age) − 0.13.
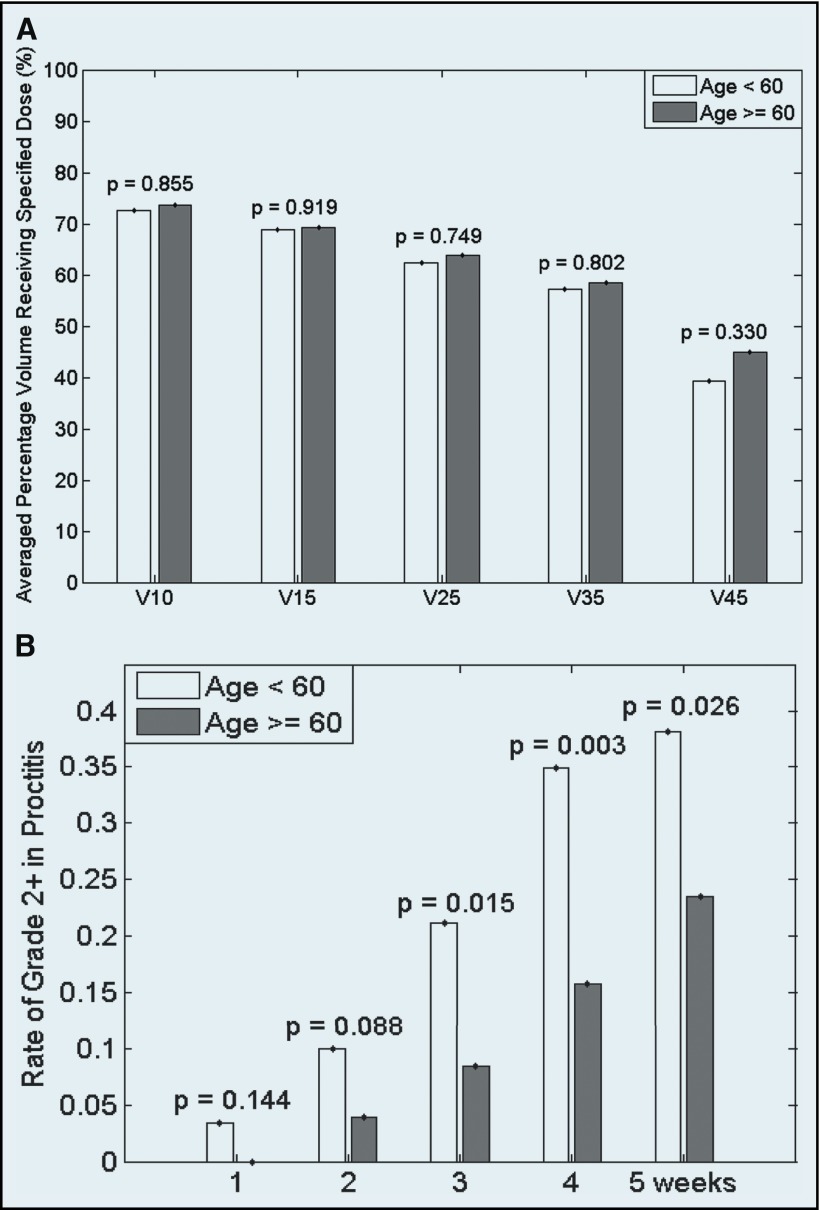
In dose–volume analysis, no difference in the volume of anal canal receiving low- and high-dose RT (V10–V50) was found between patients <60 years of age and those ≥60. However, based on weekly toxicity assessments, starting at week 3, patients <60 had a significantly higher rate of grade 2+ proctitis than did those ≥60, (21.1% vs. 8.5%; P = .02; Table 4). Figure 2 shows the dose–volume and weekly reported proctitis comparisons between patients <60 and those ≥60.
Figure 2.
Anal canal dose–volume (A) and weekly proctitis toxicity (B) comparison between patients <60 years of age and those ≥60.
DISCUSSION
Although pelvic RT with concurrent chemotherapy is an integral part of disease management for patients with locally advanced rectal cancer, clinically significant acute gastrointestinal toxicities, such as diarrhea and proctitis, are common. In prior studies, researchers have looked at the dose–volume relationship of irradiated bowel and GI toxicity9,10,13,16,17; however, the need to identify high-risk clinical characteristics associated with acute GI side effects during PCRT persists. In this study, we investigated clinical and dosimetric variables individually and simultaneously using NTCP modeling to determine the clinical and dosimetric factors that are most predictive for GI toxicity.
Diarrhea
Grade 2+ diarrhea occurred in 25% of our patients, and it correlated significantly with the use of 3DCRT, female sex, and bowel volume receiving ≥45 Gy (V45). In recent studies, Robertson et al10 reported 17% grade 2 and 21% grade 3 diarrhea during PCRT for rectal cancer in 152 patients, and Samuelian et al13 reported 40% grade 2+ diarrhea in 96 patients. It is possible to attribute the higher incidence of grade 3+ diarrhea found by Robertson et al to the 3DRT treatment of the majority (94%) of the patients. Our results showed a reduction in clinically significant diarrhea in those treated with IMRT (10.8% IMRT vs. 32.3% 3DCRT). In RTOG 0822, the use of IMRT showed a reduction in acute grade 2+ toxicity compared with the use of 3DCRT in RTOG 0247 (51% IMRT vs. 58% 3DCRT; P = .31).14 Although it did not reach statistical significance, a benefit was observed. Furthermore, oxaliplatin was used along with capecitabine in RTOG 0822 during pelvic RT, which can explain the elevated GI toxicity rate.
The association between IMRT and lower GI toxicity was also shown by Samuelian et al.13 Similar to our findings, they reported a 25% reduction in grade 2+ diarrhea in patients treated with IMRT. Given that acute diarrhea has been correlated with the volume of small bowel receiving ≥15 Gy,9,11,17 the authors stated that the clinical improvement in diarrhea observed with IMRT is a function of dose reduction in the 15–40-Gy dose range. Our results differed from these prior studies and suggest that clinically significant diarrhea is most likely due to a high-dose effect, with V45 as the most significant predictor of grade 2+ diarrhea. Given that IMRT achieves target conformity with multiple coplanar beams, it often causes a larger volume of normal tissue to be exposed to lower dose RT and simultaneously reducing the volume of normal tissue exposed to high dose RT, compared with that exposed in 3DCRT. In rectal cancer, an approximately 33% reduction in bowel V40 has been reported with the use of IMRT.18 In this study, we found a relative reduction of 50% in bowel V45 with IMRT compared with 3DCRT. Furthermore, we showed that a significantly larger bowel volume received lower dose RT (V10–V30) with IMRT (Table 3). This finding indicates that acute diarrhea during pelvic RT may not be a low-dose effect, but a high-dose effect similar to that shown in patients undergoing pelvic RT for cervical19,20 or anal21 cancer. Although we reported bowel V45 to be the most predictive of grade 2+ diarrhea in our patients, statistically significant correlations between the dose–volume parameter and acute toxicity were observed between V30 and V50. Radiation-induced diarrhea is a multifactorial event and has been linked to small-bowel bacterial overgrowth, malabsorption, and changes in motility.14 Data on the dose effect in these physiologic changes are scarce. Both preclinical and clinical work is needed to better delineate the biologic process after radiation exposure.
It is important to consider how the bowel structure was contoured when comparing results of studies of the dose–volume relationship to toxicity, as was recently indicated by Kavanagh et al.22 In our study, of the large bowel loops, only the sigmoid colon was contoured; therefore, most of the bowel structure was composed of small bowel loops. Although we did not make a distinction between the small bowel and sigmoid colon, investigators in prior studies looked at the dose–volume effect on the small bowel,9,11,17 which may have caused the difference in results. Nevertheless, we speculate that IMRT would result in a larger volume of bowel receiving lower dose RT for the reasons described earlier, regardless of whether the structure is defined by small bowel loops only or is a combination of both small and large bowel loops.
Besides the use of 3DCRT, we also found that the women were at higher risk of developing clinically significant diarrhea than were the men. Because of the wider pelvic inlet, women tend to have a larger volume of bowel in the pelvis. A larger irradiated bowel volume in the women than in the men was seen throughout the entire RT dose spectrum (Table 3). We did not find other clinical characteristics predictive of grade 2+ diarrhea. We included 43 (25%) patients who received FOLFOX induction chemotherapy before chemoradiation; nevertheless, no higher incidence of grade 2+ toxicity was found in those patients. Although pelvic RT in the adjuvant setting has been associated with higher GI toxicity,23 we did not find RT sequence to be a predictor of acute toxicity. However, because only 8% of our patients received pelvic RT in the adjuvant setting, the analysis may be underpowered.
In addition to investigating the predictors of maximum grade 2+ toxicity, we studied the trajectory of clinically significant diarrhea during PCRT. In women and in all patients treated with 3DCRT, grade 2+ diarrhea started at week 1, whereas in the men and in all patients treated with IMRT, diarrhea started at week 2. No significant difference in the rate of grade 2+ diarrhea between women vs. men and between all patients treated with 3DCRT vs. IMRT was found until week 4. This result supports that clinically significant diarrhea is a high-dose RT effect, as the adverse consequence of larger irradiated bowel volume in women and the benefit of high-dose bowel volume sparing of IMRT was not observed until later in the PCRT course.
Our results showed that bowel V45 <3% resulted in no incidence grade 2+ diarrhea, and V45 ≥27% correlated with ≥20% risk in grade 2+ diarrhea in our patients (Figure 1). These dose constraints can be considered in RT treatment planning, but it is important to recognize that the dose–volume relationship in this study is specific to our definition of bowel. It is also important to validate this dose constraint in other populations undergoing pelvic RT, including those with other pelvic malignancies, such as anal cancer; validation studies are under way at our institution. Furthermore, our data suggest that to reduce GI toxicity, IMRT should be considered in women undergoing PCRT for rectal cancer in both neoadjuvant and adjuvant settings.
Proctitis
Grade 2+ proctitis occurred in 32% of our patients, and it significantly correlated with younger age and anal canal volume receiving ≥15 Gy (V15). There are limited data in the rectal cancer literature on the incidence and clinical predictors of chemoradiation-induced acute proctitis. Sameulian et al13 reported 15% grade 2+ proctitis in patients undergoing RT for rectal cancer, with no significant difference in incidence between the patients treated with IMRT or 3DCRT. We also did not find RT technique to be a significant predictor for clinically significant proctitis. In a study examining patient-reported outcomes, Chen et al12 described frequent or very frequent pain in 36%, urgency in 41%, tenesmus in 31%, and mucus discharge in 23% of patients. However, it is unclear how the patient-reported outcomes correlated with CTCEA grading. No clinical predictor of the reported toxicities was described. In our study, age was the only clinical characteristic found to be associated with grade 2+ proctitis. In patients younger than the median age, clinically significant proctitis was observed in 38.2% of the patients vs. 23.4% in patients ≥60 years of age.
Although rectal dose constraints are used in the treatment of prostate cancer,24,25 in the setting of rectal cancer PCRT, a rectal dose constraint is not usually clinically achievable. As in patients with anal cancer, those treated for rectal cancer with PCRT can experience symptoms of anorectal pain, rectal urgency, and tenesmus, which are coded as proctitis in CTCAE and may be due, in part, to anal canal inflammation. As the anal canal can be outside the clinical target, we tested dosimetric variables associated with grade 2+ proctitis and found that anal canal V15 was most predictive of toxicity, followed by V25. A trend toward statistical significance was also seen in V10, V20, V30, V35, and V40 (Table 2), indicating that, when anal canal dose–volume parameters are used, clinically significant proctitis occurs at a lower RT dose.
Although a higher incidence of grade 2+ proctitis was observed in patients <60 years of age, there were no significant differences in anal canal dose–volume parameters between the younger and older patients. This result suggests that there was no dose effect in the development of proctitis with regard to age, unlike the effect of RT technique and the sex of the patient and their associations with clinically significant diarrhea. A possible explanation for younger patients' experiencing higher incidence of grade 2+ proctitis is that they are more likely to report this subjective symptom or that there may be some difference in RT-induced inflammatory response in the younger patients. With these results, it is theoretically feasible to consider a dose constraint for the anal canal in younger patients in an attempt to reduce proctitis symptoms. However, given that proctitis is a low-dose RT effect in the anal canal, it has to be considered extremely carefully, on an individual-by-individual basis, as clinical target volume and tumor control cannot be compromised, especially in patients with low-lying rectal cancer. Furthermore, this dose constraint should be validated before clinical implementation, as anal canal dose constraint has not been routinely used.
A limitation to the current study is that we did not incorporate patient-reported outcomes. Although most studies to date used clinician-reported toxicities, Flores et al26 suggested that there are discrepancies between clinically recorded and patient-reported toxicities, especially proctitis, in patients undergoing PCRT for rectal cancer. They showed that while diarrhea scoring showed moderate agreement between physicians and patients, proctitis scoring showed only slight agreement, with patients reporting incidences of proctitis significantly higher than clinicians. Correlating dose–volume constraint directly with patient-reported outcome is important in validating its accuracy. We plan to study the application of this correlation prospectively.
In conclusion, grade 2+ diarrhea and proctitis are common side effects among patients undergoing pelvic RT with 5FU-based chemotherapy for locally advanced rectal cancer. Our study demonstrated that women were at higher risk for grade 2+ diarrhea and that IMRT reduced the risk of grade 2+ diarrhea. A dose constraint on the bowel using V45 should be considered for patients undergoing pelvic RT for rectal cancer, and IMRT planning should be considered in women, particularly in those with more small bowel evident in the pelvis on pretreatment imaging. Prospective data that incorporate patient-reported outcomes are necessary to validate these findings.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Stockholm Rectal Cancer Study Group: Preoperative short-term radiation therapy in operable rectal carcinoma: a prospective randomized trial. Cancer 66:49–55, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Cedermark B, Johansson H, Rutqvist LE, et al. : The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma: a prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 75:2269–2275, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Medical Research Council Rectal Cancer Working Party: Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 348:1605–1610, 1996 [PubMed] [Google Scholar]
- 4. Swedish Rectal Cancer Trial: Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 336:980–987, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Sauer R, Becker H, Hohenberger W, et al. : Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Roh MS, Colangelo LH, O'Connell MJ, et al. : Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27:5124–5130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bosset JF, Collette L, Calais G, et al. : Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Smalley SR, Benedetti JK, Williamson SK, et al. : Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol 24:3542–3547, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Robertson JM, Lockman D, Yan D, et al. : The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 70:413–418, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Robertson JM, Sohn M, Yan D: Predicting grade 3 acute diarrhea during radiation therapy for rectal cancer using a cutoff-dose logistic regression normal tissue complication probability model. Int J Radiat Oncol Biol Phys 77:66–72, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Gunnlaugsson A, Kjellen E, Nilsson P, et al. : Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol 46:937–944, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Chen RC, Mamon HJ, Ancukiewicz M, et al. : Dose–volume effects on patient-reported acute gastrointestinal symptoms during chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 83:e513–e517, 2012 [DOI] [PubMed] [Google Scholar]
- 13. Samuelian JM, Callister MD, Ashman JB, et al. : Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 82:1981–1987, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Garofalo M, Moughan J, Hong T, et al. : RTOG 0822: a phase H study of preoperative (PREOP) chemoradiotherapy (CRT) utilizing IMRT in combination with capecitabine (C) and oxaliplatin (O) for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 81:S3–S4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myerson RJ, Garofalo MC, El Naqa I, et al. : elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 74:824–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baglan KL, Frazier RC, Yan D, et al. : The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 52:176–183, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Tho LM, Glegg M, Paterson J, et al. : Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys 66:505–513, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Arbea L, Ramos LI, Martinez-Monge R, et al. : Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol 5:17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simpson DR, Song WY, Moiseenko V, et al. : Normal tissue complication probability analysis of acute gastrointestinal toxicity in cervical cancer patients undergoing intensity modulated radiation therapy and concurrent cisplatin. Int J Radiat Oncol Biol Phys 83:e81–e86, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Roeske JC, Bonta D, Mell LK, et al. : A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol 69:201–207, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Devisetty K, Mell LK, Salama JK, et al. : A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. Radiother Oncol 93:298–301, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Kavanagh BD, Pan CC, Dawson LA, et al. : Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 76:S101–S107, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Huang EY, Sung CC, Ko SF, et al. : The different volume effects of small-bowel toxicity during pelvic irradiation between gynecologic patients with and without abdominal surgery: a prospective study with computed tomography-based dosimetry. Int J Radiat Oncol Biol Phys 69:732–739, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Chan LW, Xia P, Gottschalk AR, et al. : Proposed rectal dose constraints for patients undergoing definitive whole pelvic radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 72:69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Swanson GP, Stathakis S: Rectal dose constraints for intensity modulated radiation therapy of the prostate. Am J Clin Oncol 34:188–195, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Flores LT, Bennett AV, Law EB, et al. : Patient-reported outcomes vs. clinician symptom reporting during chemoradiation for rectal cancer. Gastrointest Cancer Res 5:119–124, 2012 [PMC free article] [PubMed] [Google Scholar]