ABSTRACT
BACKGROUND:
Preoperative chemoradiotherapy (preopCRT) for locally advanced rectal cancer is associated with grade 3 or higher acute gastrointestinal (GI) toxicity. This study was conducted to determine whether intensity-modulated radiation therapy (IMRT) significantly reduces acute GI toxicity, compared to 3-dimensional conformal RT (3D-CRT) in preopCRT for rectal cancer.
METHODS:
A retrospective analysis was conducted of 48 patients treated between January 2002 and August 2010 with preopCRT for rectal cancer. 3D-CRT or IMRT was administered at a planned dose of 45–50.4 Gy to patients positioned prone on a bowel-displacement device. Data regarding patient and tumor characteristics, treatment, acute toxicity, and tumor response were collected. Comparisons of acute toxicity and treatment response between 3D-CRT and IMRT were performed with the Chi-square or Fisher's exact test.
RESULTS:
There were no significant differences in radiation dose, median age, race, gender, stage, type of concurrent chemotherapy, pathologic complete response (pCR), or type of surgery (lower anterior or abdominal perineal resection) between 3D-CRT and IMRT. There was a significant reduction in grade 2 or higher GI toxicity (3D-CRT, 60.7%; IMRT, 30%; P = .036) and grade 2 or higher diarrhea (3D-CRT, 42.8%; IMRT, 10%; P = .014). Two patients who underwent 3D-CRT required a treatment break (grade 3 diarrhea and grade 3 dehydration). Radiation duration was significantly less (IMRT, 35 days; 3D-CRT, 39 days; P ≤ .0001). pCR rates were 16.7% for 3D-CRT and 21.4% for IMRT (nonsignificant [NS]); pCR+microscopic residual rates were 57.1% for IMRT and 27.8% for 3D-CRT (P = .093).
CONCLUSION:
Maximal bowel displacement with IMRT yields favorable acute GI toxicity and pathologic downstaging profiles, as compared to 3D-CRT in preoperative CRT for rectal cancer and warrants further prospective investigation.
Of the approximately 40,000 new cases of rectal cancer diagnosed in the United States in 2012, approximately one-third were locally advanced.1 For those patients with stage II and III disease, surgery alone is associated with an increased likelihood of local recurrence, and treatment with pelvic radiation (RT) with concurrent fluoropyrimidine chemotherapy has become standard adjuvant therapy.2 Although neoadjuvant chemoradiation followed by total mesorectal excision is advantageous over postoperative adjuvant therapy, in tolerability and local control, acute gastrointestinal (GI) toxicity remains a limiting factor.3,4 For example, 36% of patients in the preoperative arm of the National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03 trial experienced grade 3 or higher diarrhea, whereas Roh et al.3 and Bosset et al5 reported grade 2 or higher in 38% of patients treated with preoperative 5-fluorouracil (5-FU) and pelvic radiation.
These rates of acute GI toxicity are due in part to the large amount of normal small bowel that is in the standard pelvic radiation field. Dose-volume relationships between the amount of small bowel receiving low and intermediate doses of radiation and the rate of severe diarrhea have been reported.6–8 Finding strategies to reduce acute GI toxicity may lead to unplanned chemoradiation treatment breaks, which has been shown to confer untoward local control and survival outcomes.9
One technique for reducing the volume of irradiated small bowel is the use of prone positioning with a bowel-displacement device (belly board).10 More recently, highly conformal treatment approaches have been investigated, such as intensity-modulated radiation therapy (IMRT). In contrast to conventional 2- or 3-dimensional radiation planning methods, IMRT allows discriminatory dose escalation to the target volume, while minimizing radiation exposure to adjacent normal tissues. Improvements in treatment-related morbidity have been described in patients treated with IMRT for other pelvic malignancies, including anal, gynecologic, and prostate.11–13
Although clinical experience with IMRT treatment for rectal cancer remains limited, several dosimetric comparisons of IMRT vs. conventional radiation techniques have shown advantages to using the technique.6,14,15 Our previous preclinical work suggests that IMRT may reduce both the mean dose and volume of small bowel irradiated while maintaining target dose coverage.16 However, determining whether these dosimetric advantages of pelvic IMRT translate into an improved clinical toxicity profile in the preoperative treatment of rectal cancer has not been well studied. Its use with concurrent capecitabine and oxaliplatin has been evaluated in the recently completed phase II protocol, Radiation Therapy Oncology Group (RTOG) 0822.17
At our institution, we retrospectively analyzed and compared acute toxicity and pathologic treatment response in patients receiving pelvic IMRT or 3-dimensional conformal radiation therapy (3D-CRT) with concurrent chemotherapy for rectal cancer, to determine the clinical implications of this highly conformal approach.
PATIENTS AND METHODS
Patient Selection
The study was conducted as a retrospective review approved by the institutional review board of all patients treated preoperatively for rectal cancer between January 2002 and August 2010. Inclusion criteria included histologically confirmed adenocarcinoma of the rectum treated with pelvic radiation with 3D-CRT or IMRT and concurrent 5-FU or capecitabine chemotherapy with a planned RT dose of 45–50.4 Gy and without a prior history of pelvic radiation. Patients with oligometastatic primary rectal adenocarcinoma were also included. During this time, 48 such patients were identified. Data regarding patient and tumor characteristics, treatment, acute toxicity, and tumor response were collected from electronic medical records. The patients were evaluated before therapy with complete history and physical examination; evaluation of laboratory data including baseline carcinogenic embryonic antigen (CEA); and colonoscopy with biopsy. Imaging included endorectal ultrasound (EUS), along with computed tomography (CT) of the chest, abdomen, and pelvis; positron emission tomography/CT (PET/CT); or both.
Treatment Details
All patients were treated with preoperative pelvic 3D-CRT (n = 28) or IMRT (n = 20); 32 received concurrent, continuous 5-FU (prolonged venous infusion of 300 mg/m2, 5 days a week), and 16 received capecitabine (825 mg/m2, twice daily, 5 days a week), at the discretion of the treating medical oncologist. Dose-painted (DP)-IMRT plans were created with a CT-based simulation process. Oral contrast was administered to all patients approximately 30 min before CT simulation, to allow better visualization of the small bowel. The patients were scanned while prone on a bowel-displacement (belly-board) device. A radiopaque marker was placed at the anal verge to assist in target delineation. Axial CT images were then obtained at 2.5-mm intervals from the upper lumbar spine to the mid femur, with a Brilliance large-bore CT (Philips, Andover, MA). Most of the patients received simultaneously administered intravenous contrast. The CT images were then exported to a computer system for IMRT planning (ADAC Pinnacle; Philips) and fused with the PET/CT images, if performed, with MIM Software (MIM Software, Inc., Cleveland, OH).
According to the International Commission on Radiological Units (ICRU) Report 50 guidelines, target and avoidance structures were contoured on each axial CT slice.18 The gross tumor volume (GTV) of the primary rectal cancer was contoured according to the clinical examination, EUS, and radiographic studies. For 3D-CRT planning, a 1.5-cm radial and 2-cm craniocaudad expansion was added to the GTV to delineate the rectal primary's clinical target volume (CTVR). For IMRT planning, a 5-mm automated circumferential expansion was added to the GTV to create the CTVR. Elective nodal CTVs (mesorectum, presacrum, and bilateral internal iliac, with bilateral external iliac inclusion for T4 disease that has invaded adjacent anterior organs and bilateral external iliac and inguinal inclusion for primary involvement of the anal canal), designated CTVN, as well as normal organs, were contoured according to published methods.19 Any gross nodal disease was included in the CTVN. Each preliminary CTV was then manually edited by the treating radiation oncologist, to avoid overlap onto nontarget muscles or bone, which are natural barriers to tumor infiltration. A 1-cm automated circumferential expansion was added to all CTVs, to create the planning target volumes (PTVs, designated as PTVR and PTVN) while accounting for organ motion and patient setup uncertainty. Since 2009, with the implementation of daily image-guided radiation therapy, PTVs have been reduced to 5 mm.
The 3D-CRT prescription was 45 Gy in 25 fractions to the PTVR and PTVN, plus a sequential tumor boost of 5.4 Gy in 3 fractions to the PTVR. A three-field technique (posterior-anterior and laterals) was generally used with mixed photon energy (6 MV posterior-anterior and 16 MV laterals) to 45 Gy, and the boost was administered via a reduced three-field or lateral technique. IMRT was given as 45 Gy/25 fractions to the PTVN, and the PTVR concurrently received 50 Gy in 25 fractions in 7–10 modulated fields with 6-MV photons. Figure 1 displays representative 3D-CRT and IMRT plans in a patient with T4 disease. Planning objectives specified that at least 95% of the PTV receive the prescribed dose or higher. In addition to target coverage, dose homogeneity was carefully assessed with the IMRT plan, to minimize any volume receiving more than 110% of the prescribed dose. After target coverage and homogeneity, IMRT optimization parameters were prioritized for dose reduction to the small bowel region (contoured as individualized loops), followed by the femoral heads, genitalia, and bladder; normal tissue dose objectives have been published.13
Figure 1.
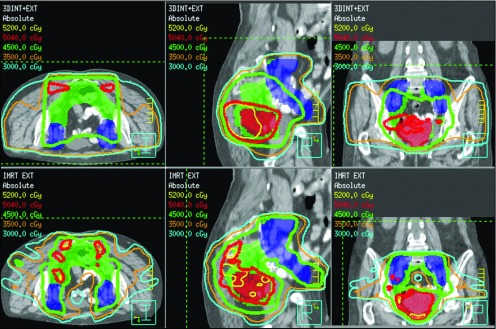
Axial, sagittal, and coronal images for 3D-CRT (top panels) and IMRT (bottom panels) plans in a patient with T4 disease. The primary tumor (shaded red) received 50 Gy, and the internal iliac, mesorectal, and presacral nodes (green) and the external iliac nodes (blue) received 45 Gy. IMRT improved target coverage, while limiting dose to surrounding normal organs, including the small bowel.
Follow-up and Response Assessment
For the monitoring and management of treatment-related morbidity, the treating radiation oncologist saw all patients weekly during the course of treatment and before surgical resection. A complete blood count was obtained every week. Four weeks after the completion of treatment, tumor response was assessed by digital rectal examination, CEA, sigmoidoscope, and PET/CT, and the patients underwent surgical resection approximately 6 weeks after chemoradiation completion. Total mesorectal excision was performed in 27 patients: abdominoperineal resection in 11 and lower anterior resection in 16. Five patients underwent local excision. Pathologic tumor response was determined by a staff histopathologist, who reviewed the surgical specimens. Resection specimens were opened and sectioned on arrival in the surgical pathology laboratory and fixed in 10% formalin before processing. Any visible tumor mass or area of previous tumor was selected for sampling, and sections of 2–3-mm thickness were submitted for processing. The tumors were staged according to American Joint Committee on Cancer (AJCC) 2010 guidelines.
Postresection chemotherapy was administered at the discretion of the medical oncologist, after assessment of pathologic tumor response (5-FU in 8 patients; folinic acid, 5-FU, and oxaliplatin [FOLFOX] in 23; irinotecan plus cetuximab in 1; and capecitabine plus oxaliplatin in 1). Posttreatment follow-up was performed every 3 months for the first 2 years and every 6 months subsequently.
Toxicity Scoring
Acute GI, genitourinary, dermatologic, and hematologic toxicities were assessed by the treating radiation and medical oncology physicians, who used the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (NCI-CTCv3) at each weekly on-treatment visit during chemoradiation therapy, before resection, and in postsurgical follow-up. The incidence of the worst-grade toxicity in a patient up to 90 days after the start of chemoradiation therapy was recorded as an acute-toxicity event.
Statistical Analysis
The incidence of acute toxicity was the primary end point of our analysis. The nonparametric Wilcoxon Mann-Whitney test was used to assess differences in continuous variables between patients undergoing 3D-CRT or IMRT. Differences in proportions and comparisons of the acute toxicities between 3D-CRT and IMRT were performed by using the chi-square or Fisher's exact test when appropriate. A univariate analysis of predictors of acute GI toxicity was performed by logistic regression. All statistical tests were 2-sided, and P < 0.05 was considered statistically significant. The SAS System (Release 9.1; SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
Patient, Tumor, and Treatment Characteristics
A total of 48 patients were treated for rectal cancer from August 2002 through September 2010 (28 with 3D-CRT and 20 with IMRT). Patient, tumor, and treatment characteristics for the 3D-CRT and IMRT treatments are described in Table 1. There were no significant differences in median age, gender, race, tumor size, grade, clinical stage (AJCC 2010), type of chemotherapy used, type of surgery performed, or pathologic complete response. The median total RT dose was similar between the 2 treatment groups; the patients in the 3D-CRT group received a median dose of 50.4 Gy in 28 fractions at 1.8 Gy per fraction, whereas in the IMRT group, the median dose was 50 Gy in 25 fractions at 2 Gy per fraction with the DP technique. Of note, 2 patients had stage I disease and received preoperative chemoradiation before a planned local excision, and 6 patients received chemoradiation for oligometastatic disease.
Table 1.
Patient, tumor, and treatment characteristics
| Characteristics | 3D-CRT (n = 28) | IMRT (n = 20) | P |
|---|---|---|---|
| Patient | |||
| Median age, y (range) | 61.5 (26.4–87.9) | 57.0 (39.3–86.1) | 0.594 |
| Gender, n (%) | 0.493 | ||
| Male | 14 (50.0) | 12 (60.0) | |
| Female | 14 (50.0) | 8 (40.0) | |
| Race, n (%) | 1 | ||
| White | 13 (46.4) | 10 (50.0) | |
| Black | 9 (32.1) | 7 (35%) | |
| Hispanic | 5 (17.9) | 3 (15.0) | |
| Other | 1 (3.6) | 0 (0.0) | |
| Tumor and disease | |||
| Clinical stage (AJCC 2010), n (%) | 0.675 | ||
| I | 1 (3.6) | 1 (5.0) | |
| II | 12 (42.9) | 10 (50.0) | |
| III | 10 (35.7) | 8 (40.0) | |
| Iva | 5 (17.9) | 1 (5.0) | |
| Median tumor size, cm (range)a | 4.2 (1.0–15.0) | 3.0 (1.1–10.0) | 0.059 |
| Tumor grade, n (%)a | 0.165 | ||
| G1 | 1 (3.7) | 0 (0.0) | |
| G2 | 22 (81.5) | 13 (65.0) | |
| G3 | 4 (14.8) | 7 (35.0) | |
| Treatment | |||
| Concurrent chemotherapy, n (%) | 0.679 | ||
| 5-FU | 18 (64.3) | 14 (70.0) | |
| Capecitabine | 10 (35.7) | 6 (30.0) | |
| Type of surgery, n (%) | 0.805 | ||
| None | 10 (35.7) | 6 (30.0) | |
| Lower anterior resection | 10 (35.7) | 6 (30.0) | |
| Abdominoperineal resection | 6 (21.4) | 5 (25.0) | |
| Median RT dose, Gy (range) | 50.4 (30.6–50.4) | 50.0 (45.0–50.4) | <.0001 |
| Treatment duration, d, median (range) | 39 (23–52) | 35 (32–42) | <.0001 |
| Local excision, n (%) | 2 (7.1) | 3 (15.0) | |
| Treatment break required, n (%) | 2 (7.1) | 0 (0.0) | 0.504 |
| pCR rates, n (%) | 3 (16.7) | 3 (21.4) | 1 |
| pCR rates, microscopic, n (%) | 5 (27.8) | 8 (57.1) | 0.093 |
%, percentage of the total patients in each group.
Tumor grade unavailable for 1 patient with 3D-CRT; tumor size unavailable for 1 patient with IMRT.
Overall Acute Toxicity
In comparison with the rates of overall grade 2 and higher acute toxicity (Table 2), the IMRT group was associated with significantly reduced overall morbidity compared with that in patients receiving 3D-CRT (40% vs. 75%, respectively, P = .015).
Table 2.
Acute toxicities, grade 2 or higher
| Toxicity | 3D-CRT (n = 28) | IMRT (n = 20) | P |
|---|---|---|---|
| Hematologic | 28.57 | 10.00 | 0.160 |
| Genitourinary | 7.14 | 0.00 | 0.504 |
| Dermatologic | 39.29 | 35.00 | 0.762 |
| Overall non-GI | 57.14 | 35.00 | 0.130 |
| Overall (including GI) | 75.00 | 40.00 | 0.015 |
Data are expressed as a percentage of the total patients in each group.
Forty-seven of the 48 patients completed the radiation regimen as planned. One patient treated with 3D-CRT declined further treatment at 30.6 Gy of a planned 50.4 Gy after being hospitalized for diarrhea and dehydration. Forty-six patients completed RT without interruption (3D-CRT, 93%; IMRT, 100%); 2 patients who underwent 3D-CRT had their treatment withheld: 1 for grade 3 diarrhea (treatment break of 7 days) and the other for grade 3 dehydration and grade 2 anemia (treatment break of 2 days). Chemoradiation duration favored IMRT, as the median treatment duration was 39 days with 3D-CRT and 35 days for those treated with IMRT (P < .0001; Table 1).
GI Toxicity
Acute grade 2 or higher GI toxicity experienced by patients in the 3D-CRT and IMRT groups is summarized in Table 3. Significantly less overall GI toxicity was observed among the patients receiving IMRT (30% IMRT vs. 61% 3D-CRT; P = .036). This reduction in overall GI toxicity was attributable to less diarrhea of grade 2 and higher among the patients treated with IMRT. Grade 2 or higher diarrhea was experienced by 10% of the patients in the IMRT group vs. 43% of those treated with 3D-CRT (P = .014). There were no significant differences between the 2 groups in overall and individual GI acute toxicities of grade 3 and higher, including nausea, diarrhea, vomiting, and enteritis, and only 10.71% of the 3D-CRT and 10% of the IMRT patients experienced acute grade 3 GI side effects (P = 1). No grade 4 or 5 acute GI toxicity was experienced in either the 3D-CRT or IMRT group.
Table 3.
Acute GI toxicities, grade 2 or higher
| Toxicity | 3D-CRT (n = 28) | IMRT (n = 20) | P |
|---|---|---|---|
| Nausea | 7.14 | 0.00 | 0.504 |
| Vomiting | 3.57 | 0.00 | 1 |
| Diarrhea | 42.86 | 10.00 | 0.014 |
| Enteritis | 0.00 | 0.00 | — |
| Proctitis | 3.57 | 0.00 | 1 |
| Dehydration | 7.14 | 5.00 | 1 |
| Overall GI | 60.71 | 30.00 | 0.036 |
Data are expressed as a percentage of the total patients in each group.
A univariate analysis of acute GI toxicity including all the patient, tumor, and treatment characteristics described in Table 1 showed only treatment delivery type to be significant. IMRT vs. 3D-CRT had an odds ratio of 0.286 (P = .0387), showing that the patients who received IMRT treatment were 71% less likely to experience GI toxicity than were those who had 3D-CRT.
Non-GI Toxicity
Table 2 displays grade 2 and higher non-GI acute toxicity (hematologic, genitourinary, and dermatologic). Although the rates of toxicity were lower in the patients receiving IMRT, none of the differences achieved statistical significance. No grade 4 or 5 acute non-GI toxicity was experienced in either the 3D-CRT or IMRT group.
Late Toxicity
One patient who received 3D-CRT presented with a small bowel obstruction due to presumed treatment-related fibrosis 9 months following the completion of radiation treatment and ultimately underwent a small bowel resection.
Tumor Response
Surgical resection was performed in 32 (67%) patients at a median of 10.6 weeks following the completion of chemoradiation (range, 5–26.6 weeks). Overall, there was no difference in the rate of sphincter-preserving surgery between treatment techniques (70% IMRT vs. 64.3% 3D-CRT; P = .805; Table 1). Sixteen patients did not undergo resection: 5 were given palliative treatment; 3 had rapid progression of disease, which precluded them from surgery; 1 underwent fulguration of the tumor; and 7 refused surgery. Pathologic complete response (pCR) rates were 16.7% for the 3D-CRT group and 21.4% for the IMRT group (P = 1). When those patients found to have only microscopic disease at resection were included, the rates of pCR with microscopic residual disease increased in the IMRT group (to 57%), compared to 28% of patients receiving 3D-CRT, but the difference did not reach statistical significance (P = .093).
DISCUSSION
With the use of maximal bowel displacement in our study, the acute GI toxicity profile in patients undergoing IMRT as part of a fluoropyrimidine preoperative chemoradiation regimen for rectal cancer was significantly improved, compared with that of patients undergoing 3D-CRT. Specifically, the decrease in the rate of grade 2 and higher overall GI toxicity from 61% to 30% with IMRT was most likely attributable to significantly less diarrhea. These results are consistent with recently published observations from the Mayo Clinic, which showed a similar reduction in grade 2+ GI toxicity with IMRT and are in line with our and other dosimetric analyses that showed small bowel sparing with IMRT.16,20
One of the most worrisome acute toxicities of chemoradiation to the pelvis is the development of diarrhea, an effect thought to be the direct result of radiation of the small bowel.6 The available data guiding dose limits to the small bowel to reduce the risk of acute diarrhea are variable. Robertson and colleagues8 reported that the most important dose-volume parameter predicting the development of diarrhea was 150 cm3 of small bowel receiving more than 15 Gy. Other investigators have also reported that the volume of small bowel receiving doses of 5–30 Gy strongly correlates with the development of acute diarrhea.6,8 In our previous preclinical work, with all patients positioned prone on a bowel-displacement device, the mean dose to 150 cm3 of small bowel generally exceeded 15 Gy when external iliac coverage was required, and plans were shown to be improved with IMRT.16 This result is consistent with those in a published report by Guerrero Urbano et al15 showing significant reduction in the volume of small bowel irradiated with IMRT. Our clinical results are also consistent with data reported by Engels et al,21 who found the normal tissue complication probability for diarrhea of grade 2 or higher to be reduced from 39.5% to 26.5% with IMRT (P < .01).
In this series, the rate of grade 2 or higher diarrhea with IMRT was further decreased to 10%, compared with 43% with 3D-CRT (P = .014). This agrees favorably with the 23% reported by Samuelian et al,20 who used IMRT. It should be noted, however, that in contrast to our patient cohort, the Mayo Clinic series included postoperative patients, as well as those with recurrent tumors, which may have contributed to these differences. Our low rate of diarrhea may also be due to our routine use of a bowel-displacement, prone technique. Samuelian et al20 reported that 66% of their patients were treated prone without such a device. Kim et al22 have shown that the use of IMRT with a belly-board device significantly reduces the volume of small bowel irradiated at all dose levels, when compared to IMRT without bowel displacement. It is important to note that we perform daily pretreatment kilovoltage images to assure patient setup reproducibility with prone positioning.
In addition, no patients receiving IMRT experienced grade 3 or higher diarrhea, whereas that level of diarrhea was observed in 10.71% of patients in our 3D-CRT group, consistent with the 12% rate published by the German Rectal Cancer Group, which used 3-D treatment planning.4 Of note, our univariate analysis of acute GI toxicity showed that patients who received IMRT treatment were 71% less likely to experience GI toxicity than those who underwent 3D-CRT. We are currently in the process of analyzing the amount of small bowel irradiated with each approach, in an attempt to identify significant small-bowel dose volume predictors of GI morbidity.
Our data also showed significantly shorter treatment duration with IMRT. This finding is important, as a secondary analysis of the German CAO/AIO/ARO-94 study data has demonstrated that prolongation of radiation is associated with poorer local-regional control.9 The reasons for our treatment duration findings are most likely twofold. First, the rate of GI side effects was higher with 3D-CRT; 2 of our patients treated with this modality experienced significant treatment breaks that were attributed to this toxicity. Second, we used a concurrent boost IMRT technique where the rectal tumor received 200 cGy per treatment over 25 days. It is also encouraging that our study showed similar overall pCR rates and improved downstaging with IMRT, compared to those with 3D-CRT. Such findings have yet to be reported in other series. Determining whether these results translate into improved pelvic control requires longer follow-up.
There were no significant differences in acute non-GI toxicities between the 2 treatment groups. The rates of grade 2 and higher genitourinary and hematologic toxicities observed in our cohort (Table 2) were lower than those reported by Samuelian et al,20 who noted overall hematologic toxicity of grade 2 or higher to be 26% for 3D-CRT and 42% for IMRT and genitourinary toxicity of grade 2 or higher to be 21% for 3D-CRT and 16% for IMRT. Our overall acute grade 2 or higher skin toxicity, however, exceeded that reported in their series, in which rates of 10% for IMRT and only 3% for 3D-CRT were quoted.
In an effort to further improve disease-free survival, investigations are currently examining intensified preoperative chemoradiation approaches for locally advanced rectal cancer. These aggressive combined-modality regimens have been associated with increased rates of acute GI toxicity and therefore may benefit from the implementation of IMRT. For example, in a recent phase II trial of preoperative chemoradiotherapy with 3D-CRT, capecitabine, and bevacizumab, Resch et al23 reported grade 3 diarrhea in 25% of patients.23 Similarly, in the RTOG 0247 phase II trial, diarrhea of grade 3 or higher was observed in 17% of patients who received capecitabine, oxaliplatin, and 3D-CRT. This regimen was associated with an encouraging pCR rate of 21%.24
Building off this platform and in an attempt to reduce the rates of acute GI morbidity associated with this approach, the RTOG recently completed the phase II 0822 trial examining the role of preoperative IMRT in combination with capecitabine and oxaliplatin.17 Preliminary results have suggested a small, but insignificant, benefit in GI toxicity with IMRT, when compared to that in patients treated with 3D-CRT in the RTOG 0247 trial.24 Although these results require further analysis, they may be due to the lack of maximal bowel displacement, a heterogeneous method of contouring the small bowel, and a sequential IMRT approach (IMRT delivered at 45 Gy in 25 fractions to the pelvis with a subsequent 3D-CRT boost to the mesorectum). In contrast, we used maximal bowel displacement, uniform small bowel contouring, a concurrent boost IMRT technique, and fluoropyrimidine monotherapy.
There are several points, however, that deserve consideration. First, our analysis was limited by its relatively small sample and retrospective nature. Second, the median duration of follow-up was relatively short. The dosimetric hot spots associated with IMRT may yield increased postoperative toxicity; further follow-up is therefore warranted to assess the long-term effects of chemoradiation with IMRT. In addition, although our pathologic downstaging has been quite favorable with IMRT, the durability of these responses should be confirmed.
In conclusion, IMRT, with maximal bowel displacement, has yielded favorable acute GI toxicity and downstaging profiles at our institution, as compared to 3D-CRT in patients who receive preoperative treatment with a fluoropyrimidine for rectal cancer. Long-term follow-up is necessary to assess the influence of IMRT on late postoperative effects and pelvic control. Despite the early results of RTOG 0822, we believe that the optimization and further analysis of this approach in the combined-modality management of locally advanced rectal cancer is warranted.
Acknowledgments
The authors would like to acknowledge the clinical contributions of Lawrence T. Orlina, CMD, RT, in identifying patients for this analysis. This work was presented at the 52nd Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 31–November 4, 2010, San Diego, CA.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. American Cancer Society Cancer Facts and Figures 2012. Atlanta: American Cancer Society, 2012 [Google Scholar]
- 2. Wolmark N, Wieand HS, Hyams DM, et al. : Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 92:388–396, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Roh MS, Colangelo LH, O'Connell MJ, et al. : Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27:5124–5130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauer R, Becker H, Hohenberger W, et al. : Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bosset JF, Collette L, Calais G, et al. : Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Tho LM, Glegg M, Paterson J, et al. : Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys 66:505–513, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Baglan KL, Frazier RC, Yan D, et al. : The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 52:176–183, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Robertson JM, Lockman D, Yan D, et al. : The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 70:413–418, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Fietkau R, Rodel C, Hohenberger W, et al. : Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys 67:1008–1019, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Gunderson LL, Russell AH, Llewellyn HJ, et al. : Treatment planning for colorectal cancer: radiation and surgical techniques and value of small-bowel films. Int J Radiat Oncol Biol Phys 11:1379–1393, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Ashman JB, Zelefsky MJ, Hunt MS, et al. : Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 63:765–771, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Mundt AJ, Lujan AE, Rotmensch J, et al. : Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 52:1330–1337, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Kachnic LA, Tsai HK, Coen JJ, et al. : Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 82:153–158, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Arbea L, Ramos LI, Martinez-Monge R, et al. : Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol 5:153–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerrero Urbano MT, Henrys AJ, Adams EJ, et al. : Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 65:907–916, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Martin NE, Nawaz AO, Schuller B, et al. : Dosimetric comparison of radiation techniques to the prone pelvis for rectal cancer: 3-field based on bony landmarks (2D) vs. 3-dimensonal conformal (3D) vs. intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 72:907–S548, 2008 [Google Scholar]
- 17. RTOG 0822, a phase II evaluation of preoperative chemoradiotherapy utilizing intensity modulated radiation therapy (IMRT) in combination with capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 81:S3–S4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. International Commission on Radiation Units and Measurements: Prescribing, recording and reporting photon beam therapy. Report 50 Bethesda, MD: ICRU, 1993 [Google Scholar]
- 19. Myerson RJ, Garofalo MC, El Naqa I, et al. : Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 74:824–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samuelian JM, Callister MD, Ashman JB, et al. : Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 82:1981–1987, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Engels B, De Ridder M, Tournel K, et al. : Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys 74:1476–1480, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Kim JY, Kim DY, Kim TH, et al. : Intensity-modulated radiotherapy with a belly board for rectal cancer. Int J Colorectal Dis 22:373–379, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Resch G, De Vries A, Ofner D, et al. : Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer: a 2 stage phase II clinical trial. Radiother Oncol 102:10–13, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Wong SJ, Winter K, Meropol NJ, et al. : Radiation Therapy Oncology Group 0247: a randomized phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 82:1367–1375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


