Abstract
To elucidate the pathomechanism leading to obstructive vascular disease in patients with elastin deficiency, we compared both elastogenesis and proliferation rate of cultured aortic smooth-muscle cells (SMCs) and skin fibroblasts from five healthy control subjects, four patients with isolated supravalvular aortic stenosis (SVAS), and five patients with Williams-Beuren syndrome (WBS). Mutations were determined in each patient with SVAS and in each patient with WBS. Three mutations found in patients with SVAS were shown to result in null alleles. RNA blot hybridization, immunostaining, and metabolic labeling experiments demonstrated that SVAS cells and WBS cells have reduced elastin mRNA levels and that they consequently deposit low amounts of insoluble elastin. Although SVAS cells laid down ∼50% of the elastin made by normal cells, WBS cells deposited only 15% of the elastin made by normal cells. The observed difference in elastin-gene expression was not caused by a difference in the stability of elastin mRNA in SVAS cells compared with WBS cells, but it did indicate that gene-interaction effects may contribute to the complex phenotype observed in patients with WBS. Abnormally low levels of elastin deposition in SVAS cells and in WBS cells were found to coincide with an increase in proliferation rate, which could be reversed by addition of exogenous insoluble elastin. We conclude that insoluble elastin is an important regulator of cellular proliferation. Thus, the reduced net deposition of insoluble elastin in arterial walls of patients with either SVAS or WBS leads to the increased proliferation of arterial SMCs. This results in the formation of multilayer thickening of the tunica media of large arteries and, consequently, in the development of hyperplastic intimal lesions leading to segmental arterial occlusion.
Introduction
The resilience of vascular walls, skin, ligaments, and lungs are maintained by elastic fibers in the extracellular matrix (ECM). These fibers are organized into distinct structures in different tissues: ropelike fibers in lung, skin, and ligaments; honeycomblike networks in elastic cartilage; and concentric lamellae in blood vessels (for reviews, see Rosenbloom et al. 1993; Pasquali-Ronchetti and Baccarani-Contri 1997). Ultrastructurally, elastic fibers are complex structures with two major components: an amorphous core of extensively cross-linked elastin that makes up the bulk (>90%) of the fiber, and 10–12-nm microfibrils composed of several distinct glycoproteins (Vrhovski and Weiss 1998; Debelle and Tamburro 1999). Elastin is a polymer of precursor protein (tropoelastin) molecules, which have to be secreted from fibroblasts, endothelial cells, chondroblasts, or vascular smooth-muscle cells (SMCs) and then properly assembled on a microfibrillar scaffold in the extracellullar space and covalently cross-linked by lysyl oxidase (LO) (Kagan and Trackman 1991). Impaired elastogenesis coincides with increased SMC migration and proliferation, causing physiological occlusion of the fetal ductus arteriosus (Hinek et al. 1991; Hinek and Rabinovitch 1993). A similar association of deficient deposition of elastin and increased cell proliferation occurs during the formation of hyperplastic atherosclerotic plaques, as well as during intimal thickening in posttransplantation arteriopathy and during angioplasty-induced arterial restenosis (MacLeod et al. 1994; Ju and Dixon 1996).
We have previously reported that proper elastogenesis depends on the presence of 67-kD elastin-binding protein (EBP) encoded by alternatively spliced mRNA from the β-galactosidase gene (GLB1) (Hinek et al. 1988, 1993; Privitera et al. 1998). We have established that the EBP acts as a recycling molecular “chaperone” protecting the highly hydrophobic tropoelastin molecules from intracellular self-aggregation and premature degradation and facilitates their orderly assembly (Hinek and Rabinovitch 1994; Hinek et al. 1995). We also found that the cell-surface–residing EBP mediates cellular attachment to elastic fibers and acts as a “true receptor,” which, on contact with soluble elastin-derived peptides, transduces intracellular signals facilitating cellular motility and proliferation (Hinek 1994, 1996; Jung et al. 1998, 1999). Recent studies in our laboratories, based on natural models of inherited human connective-tissue diseases, have shed more light on the pathophysiological association between impaired elastogenesis and heightened cellular proliferation.
We have shown that patients with GM-1 gangliosidosis who have nonsense mutations in GLB1 (also encoding the EBP) manifest a primary EBP deficiency and, consequently, an impaired elastogenesis (Hinek et al. 2000b). We also have demonstrated that intracellular and pericellular accumulation of dermatan sulfate in Hurler disease (Hinek and Wilson 2000), as well as accumulation of chondroitin sulfate in Costello syndrome (Hinek et al. 2000a), cause functional inactivation of the EBP, which results in inadequate deposition of insoluble elastin, despite normal synthesis of tropoelastin and adequate production of components of the microfibrillar scaffold (microfibril-associated proteins and fibrillin). Our studies also have indicated that, in the latter two diseases, impaired deposition of insoluble elastin and accumulation of small elastin-derived peptides coincide with increased cell proliferation. Impaired elastogenesis and increased cell growth more than likely contribute to the development of connective-tissue abnormalities, including occlusive arterial lesions, found to be associated with these disorders.
These results of our previous studies were consistent with studies of mice with a targeted inactivation of the elastin gene (Eln). Homozygous null mutants for the elastin gene, ELN−/−, develop a progressive arterial disorder characterized by heightened proliferation of arterial SMCs, leading to thickening of the arterial wall and narrowing of the lumen and resulting in neonatal death (Li et al. 1998a, 1998b).
To investigate further whether reduced elastin deposition is directly linked to increased cellular proliferation, the present study compared elastogenesis and the proliferation rates of cultured aortic SMCs and skin fibroblasts from healthy control subjects and from patients with two diseases—supravalvular aortic stenosis (SVAS [MIM 185500]) and Williams-Beuren syndrome (WBS [MIM 194050])—characterized by primary genetic disorders of ELN.
SVAS, an inherited obstructive arterial disease, may occur either as an isolated condition (Eisenberg et al. 1964) or as part of a complex developmental syndrome, WBS (Williams et al. 1961; Beuren et al. 1962). Isolated SVAS is caused by point mutations (Li et al. 1997; Tassabehji et al. 1997; Boeckel et al. 1999; Urbán et al. 1999, 2000a, 2001; Metcalfe et al. 2000; Dedic et al. 2001), translocations (Curran et al. 1993; von Dadelszen et al. 2000), and deletions (Ewart et al. 1993, 1994; Olson et al. 1995; Fryssira et al. 1997) in ELN. Most ELN mutations in SVAS result in premature termination codons, some of which have also been shown to cause nonsense-mediated decay (NMD) of mutant mRNA products (Urbán et al. 2000a, 2001). In WBS, patients are characterized by a complex developmental phenotype that, in addition to aortic stenosis, includes specific facial features, growth deficiency, developmental delay, mental retardation, a specific neurobehavioral profile, and connective-tissue abnormalities, all caused by a genomic deletion of 1.6 Mb in chromosome 7. This deletion encompasses at least 16 (reviewed by Francke [1999]) and potentially as many as 24 (DeSilva et al. 2002) genes, including ELN. Histologic evaluation of the vascular system in patients with either SVAS or WBS indicates that these syndromes should be considered generalized diseases of the arterial wall, causing segmental narrowing of the aorta and of the pulmonary, carotid, cerebral, renal, and coronary arteries (O'Connor et al. 1985; Rein et al. 1993; Kaplan et al. 1995). In children with SVAS, narrowing of the coronary arteries may lead to myocardial infarcts and sudden death (Conway et al. 1990), and cerebral artery stenoses may lead to cerebral infarcts and stroke (Kaplan et al. 1995). In both the syndromic and the isolated forms, stenotic regions of the aorta are characterized by disorganized lamellar architecture of the tunica media and thickening of the intima, which contains numerous SMCs and is surrounded by an elastin-poor ECM (O’Connor et al. 1985). Nonstenotic regions of the aorta in patients with either SVAS or WBS have a normal lumen diameter but an abnormally high aortic-wall diameter, resulting from a two- to threefold increase in SMC layers surrounded by thinner than normal elastic lamellae. Thus, we hypothesized that inadequate deposition of insoluble elastin may be pathophysiologically connected to SMC hyperplasia during development of vascular thickenings in SVAS and in WBS.
The results presented here indicate a strong correlation between abnormally low levels of elastin deposition and increased cell-proliferation rate of WBS and SVAS cells. We also show that the increased proliferation rate of ELN+/− cells can be reversed by addition of exogenous insoluble elastin.
Patients, Material, and Methods
Patients and Samples
The genotypes and characteristics of the study subjects are summarized in table 1. Detailed clinical and molecular descriptions of families SVAS-9 (175902) and SVAS-8 (UF125) and of patient 11719 have been published elsewhere (Kumar et al. 1993; Urbán et al. 2000a, 2001; von Dadelszen et al. 2000). An additional patient with SVAS, patient 2250, underwent mutational analysis as part of the present study. Clinical diagnosis of all patients with SVAS was established by echocardiography. All patients with WBS who were enrolled in this study also had SVAS, as determined by echocardiography or cardiac catheterization. After clinical diagnosis, molecular diagnosis of patients with WBS was established by FISH with a probe containing loci ELN, LIMK1, and D7S613 (Vysis), with peripheral-blood lymphocytes used as samples.
Table 1.
Characteristics of Subjects
| Subject (Age [Sex]) | Diagnosis | Mutation | Source(s) of Samples |
| 9001 (31 years [M]) | Normal | … | Fibroblasts |
| UF130 (21 years [M]) | Normal | … | Fibroblasts |
| 4184 (3 years [M]) | Normal | … | Fibroblasts |
| 4212 (7 mo [M]) | Normal | … | Fibroblasts |
| 9006 (2 mo [M]) | Normal | … | Aortic SMCs |
| UF125 (49 years [F]) | SVAS | 1829G→A | Fibroblasts |
| 175902 (3 years [M]) | SVAS | 1195delG | Fibroblasts |
| 2250 (3 years [M]) | SVAS | 1238delG | Fibroblasts, aortic SMCs |
| 11719 (3 years [F]) | SVAS | t (6;7) | Fibroblasts |
| 207092 (6 years [M]) | WBS | WSCR deletiona | Fibroblasts |
| 6367 (2 years [M]) | WBS | WSCR deletiona | Fibroblasts |
| 14073 (6 years [M]) | WBS | WSCR deletiona | Fibroblasts |
| 14966 (3 mo [M]) | WBS | WSCR deletiona | Fibroblasts |
| 1001 (teenage [F]) | WBS | WSCR deletiona | Aortic SMCs |
Determined on the basis of FISH analysis.
Skin biopsies were taken as 4-mm punch biopsies from healthy donors or from tissue excised as part of either cardiac surgery or catheterization from a sun-protected region (e.g., upper inner arm, chest, or inguinal region). Biopsy samples were diced and explanted in α-MEM (modified Eagle’s medium) supplemented with 10% fetal bovine serum (FBS), 25 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), l-glutamine, and antibiotics. Aortic tissue samples were excised from a patient with SVAS (patient 2250) and from a patient with WBS (patient 1001), during surgical correction of aortic stenosis. Tissue samples were diced, and cells were isolated by digestion with collagenase and elastase, as described elsewhere (Oakes et al. 1982). Passage two of aortic SMCs from a healthy control subject (subject 9006) was purchased from Clonetics.
Material
All chemical-grade reagents were obtained from Sigma. α-MEM, cell-culture media, FBS, and other cell-culture products were obtained from GIBCO Life Technologies. Powdered bovine ligamentum nuchae insoluble elastin and a polyclonal antibody to tropoelastin were purchased from Elastin Products. Monoclonal antibody against smooth-muscle α-actin, polyclonal antibody to human von Willebrand factor and secondary antibodies—fluorescein-conjugated goat anti-rabbit (GAR-FITC) and fluorescein-conjugated goat anti-mouse (GAM-FITC)—were purchased from Sigma. Monoclonal antibody to fibronectin (mAB1940) was from Chemicon, and polyclonal antibody to LO was a generous gift from Dr. Katalin Ciszar (University of Hawaii). The DNeasy Tissue System for DNA assay was from Qiagen. The BigDye cycle sequencing kit was from Perkin-Elmer. The superscript preamplification system was from Life Technologies; the TRI-reagent was from the Molecular Research Center (Cincinnati); and the bromodeoxyuridine (BrdU) labeling and immunostaining kit was from Zymed Laboratories. Radiolabeled reagents, [3H]-valine, and [3H]-thymidine were purchased from Amersham Canada.
Histopathology
We examined 4-μm-thick histological sections of aorta, pulmonary artery, and extramural coronary arteries, which were obtained during autopsy of two male children (1 and 2 years old) with SVAS, two patients (1 and 2 years old) with WBS, and two 2-year-old male children who died of non–vascular-related diseases. All histological sections were stained with modified Movat's pentachrome method (Garvey et al. 1986), which shows elastin as black, glycosaminoglycans as green, collagen as yellow, smooth muscle as red, and nuclei as dark blue. The distribution of black-stained material in Movat's method has been shown to overlap completely with immunodetectable elastin (de Chalain et al. 1999).
Cell Cultures
Cells were maintained in α-MEM supplemented with 20 mM HEPES, 1% l-glutamate, 1% sodium pyruvate, nonessential amino acids, 1% antibiotics, and 10% FBS and were routinely passaged by trypsinization. All the experiments described below were performed by using SMCs at passages 2–5 and fibroblasts at passages 4–8. Primary SMC cultures were found to be positive for indirect immunofluorescent staining with a monoclonal antibody against smooth-muscle α-actin (A2547; Sigma) and were found to be negative for a polyclonal antibody against human von Willebrand factor (F3520; Sigma).
Mutational Analysis
Mutational analysis of patient 2250 was conducted as described elsewhere (Urbán et al. 2000a). In brief, a combination of single-strand conformation analysis (SSCA) and conformation-sensitive gel electrophoresis (CSGE [Ganguly et al. 1993]) was used to maximize the efficiency of mutation detection. Each individual exon of ELN was amplified with flanking intronic sequences, in a radioactive PCR. Aliquots of reaction products were subsequently taken and analyzed, in a parallel fashion, by SSCA and CSGE. Exons with mobility shifts were further analyzed, by direct sequence analysis. The nucleotide numbering used in the present study reflects the coding region of ELN, as described elsewhere (Tassabehji et al. 1997). This region contains all exons, including exons 22 and 26A (Bashir et al. 1989) but not the alternatively spliced exon 24A (exon 12A in the original nomenclature) (Fazio et al. 1991).
RNA Expression Studies
Fibroblasts were cultured to confluence at passages 4–8, in Dulbecco’s α-MEM supplemented with 10% FBS, 25 mM HEPES, l-glutamine, and antibiotics. For NMD studies, parallel confluent cultures were incubated for 4 h, with or without 100 μg of cycloheximide (CHX)/ml, before RNA extraction. RNA was extracted by use of TRI-reagent. For analysis of allelic expression, 0.5 μg of total RNA was used to generate first-strand cDNA, by the superscript preamplification system and random primers. First-strand cDNA was amplified either by oligonucleotide HEE18.1S (5′-GGG ATC CCA GGT GCT GCG GTT-3′) (which is complementary to exon 18) or by oligonucleotides MH1 (5′-CGG AGT TGG AGG CAT TC-3′) and MH2 (5′-ACC GTA CTT GGC AGC CTT-3′) (which are complementary to exons 20 and 21, respectively), resulting in a product of 287 bp or 193 bp, respectively. RT-PCR products were analyzed by direct sequencing using the BigDye cycle sequencing kit. Alternatively, radioactive PCR products were generated by end-labeled oligonucleotide MH2 and then were analyzed by SSCA. Steady-state levels of elastin mRNA were analyzed by RNA blot hybridization using a human elastin cDNA recombinant H-11 (Olson et al. 1995) as a probe, as described elsewhere (Urbán et al. 2000a). For mRNA stability studies, parallel triplicate cultures were grown to confluence in six-well plates. Total mRNA was isolated from untreated cultures and from cultures treated, for 6, 12, or 24 h, with 60 μM of 5,6-dichlorobenzimidazole riboside (Zhang et al. 1995), and samples were analyzed by RNA blot hybridization using probe H-11.
Immunostaining
Subconfluent 2-d-old cultures of aortic SMCs and fibroblasts, from healthy control subjects and from patients with either SVAS or WBS, which do not deposit elastic fibers, as well as 10-d-old confluent cultures of these cells, which produce abundant ECM, were used. All cultures were fixed in cold 100% methanol at −20°C for 30 min, were blocked with 1% normal goat serum, and then were incubated for 1 h, either with 20 μg of anti-S-GAL antibody/ml, which recognizes the elastin-binding domain of the elastin receptor (Hinek et al. 1993), or with 10 μg of antibody to LO/ml, an enzyme responsible for cross-linking of the secreted tropoelastin. The 10-d-old confluent cultures of normal fibroblasts, SVAS fibroblasts, and WBS fibroblasts were immunostained for 1 h, with either 20 μg of polyclonal antibody to tropoelastin/ml, which also detects insoluble extracellular elastin (Prosser et al. 1991), or with 1 μg of monoclonal antibody to fibronectin/ml, as described elsewhere (Hinek and Wilson 2000). All cultures were then incubated for an additional 1 h, with the appropriate GAR-FITC or GAM-FITC secondary antibodies. Nuclei were counterstained with propidium iodide. As a control for all the immunostaining, either preimmune rabbit IgG or normal mouse ascites fluid was substituted for the primary antibodies. Secondary antibody alone was used as an additional control. Quantitative morphometric analysis of 10-d-old cultures immunostained with antibodies against ECM components was performed by use of an Olympus AH-3 microscope attached to a CCD camera (Optronics) and a computer-generated video analysis system (Image-Pro Plus software; Media Cybernetics). Fifty low-power (×20) fields from three separate cultures derived from three different patients in each analyzed group (i.e., normal, SVAS, and WBS ) were analyzed, and the area occupied by the particular immunodetectable component was quantified. The abundance of each component was then expressed as a percentage area of the entire analyzed field.
Assay of Insoluble Elastin
In each experiment, either SMCs from a healthy control subject (subject 9006), from a patient with SVAS (patient 2250), and from a patient with WBS (patient 1001) (table 1) or fibroblasts derived from four patients belonging to each experimental group (table 1) were plated in 60-mm culture dishes (100,000 cells/dish) and were grown to confluence for 48 h. Then, 2 μCi of [3H]-valine was added to each dish, along with fresh media. Cultures then were either incubated for the next 2 d or maintained in a time course as long as 10 d in the presence of radioactive valine (fresh media were added at days 3 and 6). At each time point of the course, quadruplicate cultures belonging to each experimental group were terminated, at days 3, 6, and 10, and insoluble elastin was assessed. After the removal of the media, cell layers containing insoluble elastin deposited in ECM were washed in 0.1 M acetic acid and then were scraped in 0.1 N NaOH, were sedimented by centrifugation, and, to dissolve all ECM components except elastin, were boiled, for 45 min, in 0.5 ml of 0.1 N NaOH. The resulting pellets containing the insoluble elastin were then dissolved by being boiled in 200 μl of 5.7 N HCl for 1 h, and the aliquots were mixed with scintillation fluid and were counted (Hinek et al. 1993; Hinek and Rabinovitch 1994). Aliquots taken from each culture were also used for DNA determination by the DNeasy Tissue System from Qiagen. Final results reflecting amounts of metabolically labeled insoluble elastin were expressed as counts per minute per microgram of DNA.
Assessment of Cell Proliferation
In each experiment, SMCs or fibroblasts were seeded in six-well dishes, at a density of 50,000 cells/well, in α-MEM containing 10% FBS. The medium was changed 24 h later, and parallel cultures were maintained for periods of 1–10 d, as specified in the figure legends. At the end of each incubation period, the cell proliferation was studied in each culture, by use of the BrdU labeling and immunostaining kit, according to the manufacturer's instructions. Cells from individual cultures were also trypsinized and counted in a hemocytometer. Additionally, assay of total DNA was performed at each end point, by the DNeasy Tissue System (Rodems et al. 1998). Parallel sextuplicate cultures incubated as described above were also exposed to [3H]-thymidine (2 μCi/well) for the last 24 h. These cultures were then washed in PBS and were treated with cold 5% trichloroacetic acid twice for 10 min at 4°C. Then, 0.5 ml of 0.3 N NaOH was added to all dishes, for 30 min. Subsequently, 200-μl aliquots of each culture were mixed with scintillation fluid and were counted.
In a separate series of experiments, the incorporation of [3H]-thymidine by SMCs or fibroblasts derived from all listed healthy control subjects and from patients with either SVAS or WBS was also measured in 3-d-old quadruplicate cultures. All cells were plated at the same initial density of 50,000 cells/dish and were maintained either in medium (containing 10% FBS) in the presence or absence of 1 mg of insoluble elastin/ ml or in medium that was exposed only to insoluble elastin. This medium, also containing 10% of FBS, was preincubated, for 1 h, with 1 mg of insoluble elastin/ml. Elastin was then removed by centrifugation, and the supernatant was added to the cell cultures. Results from two different experiments were combined and were expressed as the percentage of change, compared with the results for untreated controls within the same cell type.
Data Analysis
In all biochemical studies, means and SDs were calculated, and statistical analyses were performed by analysis of variance.
Results
Medial Thickening and Formation of Neointima in Arteries of Patients with either SVAS or WBS
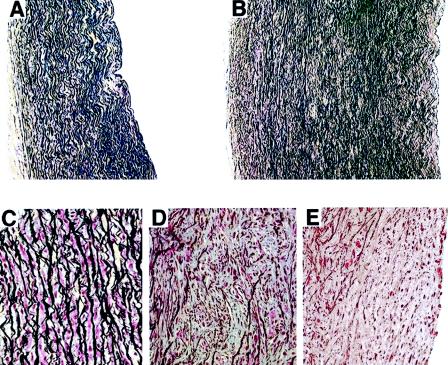
To illustrate structural changes in SVAS arteries and in WBS arteries, we compared histologic sections of the ascending aorta of infants with either isolated SVAS or isolated WBS versus controls taken from infants who died of nonvascular causes. Our assessment confirmed that nonstenotic regions of aorta from patients with either SVAS or WBS had thickened tunica media characterized by an increased number of layers of SMCs surrounded by thinner-than-normal elastic lamellae (fig. 1A and B). Assessment of medial layers of the stenotic regions in these cases indicated that, in contrast to normal aortic tunica media composed of parallel elastic lamellae separated by single layers of SMCs (fig. 1C), the lamellar architecture of the tunica media was disorganized and contained multiple clusters of SMCs separated by thin, fragmented elastic fibers (fig. 1D). The obstructive intimal thickening detected in the SVAS aorta and the WBS aorta (fig. 1E) was formed by numerous SMCs surrounded by an elastin-poor ECM. Our patients with either SVAS or WBS who were evaluated also showed concentric intimal hyperplasia, with a reduction in the lumen of the main trunk of the pulmonary artery and in the coronary arteries, characterized by the presence of scarce, fragmented elastic fibers (data not shown).
Figure 1.
A and B, Representative photomicrographs comparing a transverse section of the ascending aorta of a healthy 2-year-old child (A) and the nonstenotic region of the ascending aorta of 2-year-old patient with WBS (B) (staining is with Movat's pentachrome; original magnification ×100). Nonstenotic regions of the WBS aorta demonstrate a larger-than-normal aortic-wall diameter, resulting from an increase in the number of SMC layers surrounded by abnormally thin elastic lamellae. C–E, Higher-power images of sections stained with Movat's pentachrome (magnification ×400), comparing the tunica media of the aorta of a healthy 2-year-old child (C) and the medial (D) and neointimal (E) layers of the stenotic region of the aorta of a 2-year-old patient with WBS. In contrast to the normal aortic tunica media composed of parallel elastic lamellae separated by single layers of SMC, in the stenotic region the lamellar architecture of the tunica media is disorganized and contains multiple clusters of SMC separated by thin, fragmented elastic fibers (D). The intimal thickening of the WBS aorta is composed of numerous SMC surrounded by elastin-poor ECM (E).
Mutational Analysis of SVAS Samples and WBS Samples—and Functional Haploinsufficiency in SVAS
To be able to work with fibroblasts and SMC samples of defined genotypes, we analyzed mutations in all patients included in the present study (table 1). A characteristic microdeletion in chromosomal region 7q11.2 was found by FISH analysis of peripheral blood samples of all patients with WBS. We used cells from patients with SVAS and previously analyzed mutations, including patients 175902 (SVAS-9; Urbán et al. 2000a), UF125 (Urbán et al. 2001), and 11719 (von Dadelszen et al. 2000). For two of these patients (patients 175902 and UF125), we had shown, in previous studies (Urbán et al. 2000a, 2001), that the mutations represent functionally inactive (null) alleles.
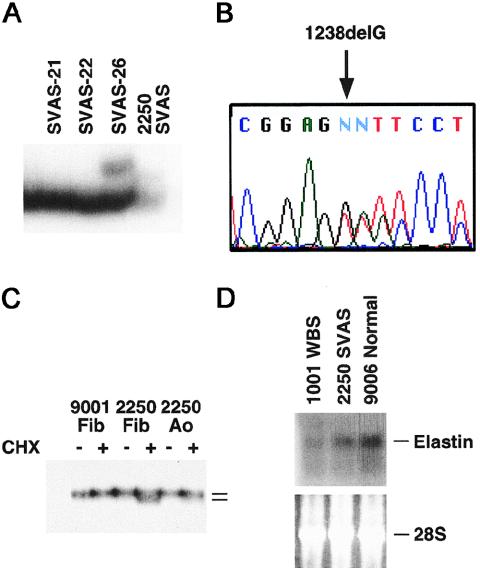
One additional patient with SVAS, who served as a donor for both skin fibroblasts and aortic SMCs, underwent a mutational analysis, which identified mutation 1238delG (fig. 2A and B). RT-PCR analysis of the expression of the mutation both in skin fibroblasts and in aortic SMCs from this patient indicated that the mutant allele was not expressed (fig. 2C). The expression of the mutant mRNA was restored by CHX treatment, indicating that this frameshift mutation results in a premature termination codon that activates the NMD mechanism. Similar to mutations 1195delG and 1829G→A, therefore, mutation 1238delG is a null allele. Consistent with this finding, we observed reduced elastin mRNA levels both in aortic SMCs and in fibroblasts from this patient and from other patients with either SVAS or WBS, when RNA blot hybridization was used (figs. 2D and 3).
Figure 2.
Identification of a new elastin-gene mutation in patient 2250 from family SVAS-31, and reduced elastin mRNA expression in SVAS SMCs and WBS SMCs. A, CSGE of ELN exon 20, which detects a mobility shift in patient 2250 (arrow). Samples from families SVAS-21 and SVAS-22 are included as negative controls, and a sample from family SVAS-26 is included as a positive control. B, DNA sequence analysis of exon 20, which identifies a guanosine deletion at position 1238 (1238delG), which is predicted to result in premature termination of translation. C, RT-PCR analysis of exon 20 in skin fibroblasts (Fib) and aortic SMCs (Ao) in patient 2250, which indicates that elastin mRNA containing mutation 1238delG is degraded by the NMD mechanism. Inhibition of NMD by cycloheximide (CHX) restores the expression of the mutant transcript. Samples from a normal skin-fibroblast line (9001) are included as controls. D, RNA blot analysis of elastin mRNA in aortic SMC from a patient with WBS (1001), a patient with nonsyndromic SVAS (2250), and a healthy control subject (9006), which show reduced total elastin mRNA in WBS and SVAS samples. Note that elastin mRNA abundance is less in WBS than in SVAS. Ethidium bromide staining of 28S rRNA is included as a control.
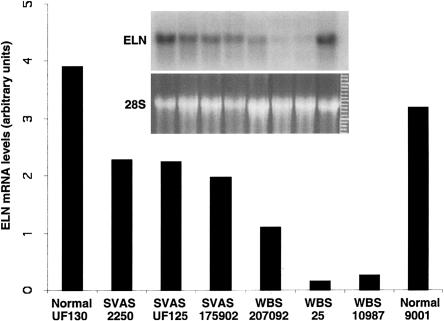
Figure 3.
Phosphorimager analysis of elastin mRNA abundance in normal, SVAS, and WBS fibroblasts. RNA samples were analyzed by blot hybridization using elastin cDNA probe H-11. The intensity of the hybridization signal (gels “ELN” [inset]) was normalized to the abundance of 28S rRNA (gels “28S” [inset]) in the corresponding gel stained with ethidium bromide, and the resulting values are shown, in the bar graph, in arbitrary units. The order of the samples in the inset is the same as in that in the bar graph.
Interestingly, WBS cells showed significantly (P<.01; t test) lower elastin mRNA levels than did SVAS cells (figs. 2D and 3). In fibroblasts the mean mRNA levels were 61% ± 4% of normal levels, whereas in the WBS cells elastin mRNA levels were 14% ± 14% of normal levels. To investigate why elastin mRNA levels were reduced below the expected 50% of normal levels, we studied the stability of elastin mRNA by treating a healthy control subject (subject 9001), a patient with SVAS (patient 175902), and a patient with WBS (patient 207092) with the transcription inhibitor DRB (5,6-dichlorobenzimidazole riboside), for 0, 6, 12, and 24 h. The half-life of elastin mRNA was 13.5 ± 1.25 h in SVAS cells and 13.25 ± 0.9 h in WBS cells. Thus, there was no significant difference, in elastin mRNA stability, between SVAS cells and WBS cells.
To exclude the possibility of somatic mosaicism for ELN deletions, we conducted FISH analysis on aortic-cell isolates. Disomy was confirmed in patient 2250 with SVAS, and monosomy was confirmed in patient 1001 with WBS, with 30 mitoses analyzed in each sample (data not shown).
Inverse Relationship between Deposition of Insoluble Elastin and Cellular Proliferation
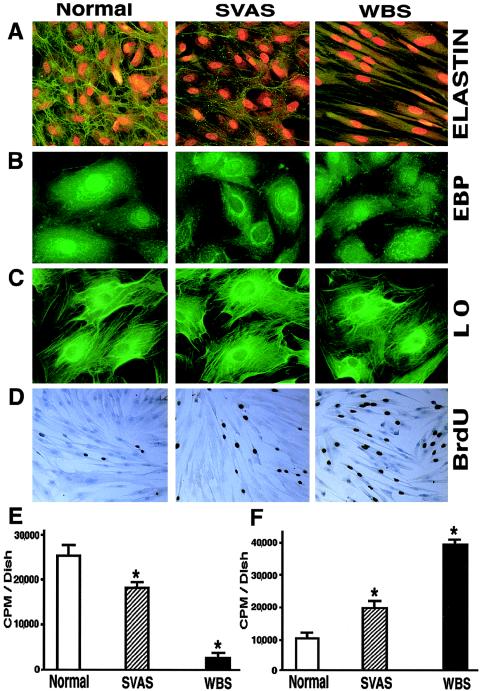
To analyze elastin deposition in cells with null mutations of ELN, we used morphometric analysis of indirect immunofluorescent staining and metabolic labeling followed by biochemical isolation of insoluble (extracellular) elastin. These studies indicated that cultured aortic SMCs obtained from a patient with SVAS and from a patient with WBS produced ∼30% less and ∼90% less insoluble elastin, respectively, than did SMCs derived from healthy subjects (fig. 4A and E). Concomitantly, we showed a two- to fourfold increase in the proliferation of SVAS SMCs and WBS SMCs, respectively, compared with those in normal controls, by measuring the incorporation of [3H]-thymidine (fig. 4F). A corresponding increase of cell proliferation was observed by following the incorporation of BrdU, by use of immunohistochemical staining (fig. 4D).
Figure 4.
A, Representative photomicrographs of 10-d-old cultures of human aortic SMC immunostained with specific antibodies to elastin (magnification ×100). Nuclei were counterstained with propidium iodide. B and C, Representative higher-power images (magnification ×400) comparing expression of the EBP and LO in 3-d-old cultures of aortic SMC. D, Representative photomicrographs of 10-d-old cultures of human aortic SMC metabolically labeled with BrdU and immunostained with anti-BrdU antibodies (brown nuclei), demonstrating higher-than-normal proliferating rates of SVAS cells and of WBS cells (magnification ×40). E and F, Results (mean ± SD) of quantitative analysis of a typical experiment using 3-d-long metabolic labeling of quadruplicate cultures with radioactive valine, followed by biochemical isolation of insoluble elastin, which demonstrates that aortic SMC from patients with SVAS and patients with WBS deposit, respectively, low and very low amounts of insoluble elastin (E), and of measurements of [3H]-thymidine incorporation into the parallel quadruplicate cultures, which indicate that abnormally low levels of insoluble elastin are associated with an inversely proportional increase in the proliferation rate of SMC in patients with SVAS and patients with WBS (F) (* [P<.001]).
Immunofluorescent microscopic studies revealed that, in cultured SMCs and fibroblasts derived from patients with either SVAS or WBS, immunodetectable expression of EBP and LO was similar to that in their counterparts derived from healthy control individuals (fig. 4B and C). Morphometric analysis of 10-d-old cultures immunostained with anti-elastin and anti-fibronectin antibodies revealed that SVAS fibroblasts and WBS fibroblasts produced 48% and 87% less insoluble elastin, respectively, than did fibroblasts derived from healthy control subjects. Interestingly, this low deposition of extracellular elastin by SVAS fibroblasts and WBS fibroblasts coincided with, respectively, 36% and 84% increases in production of fibronectin, compared with levels observed in cultures of normal fibroblasts. Similar levels of increase in deposition of fibronectin, compared with levels observed in cultures of normal SMCs, were visible also in SVAS aortic SMCs and in WBS aortic SMCs, which showed 42% and 88% increases, respectively.
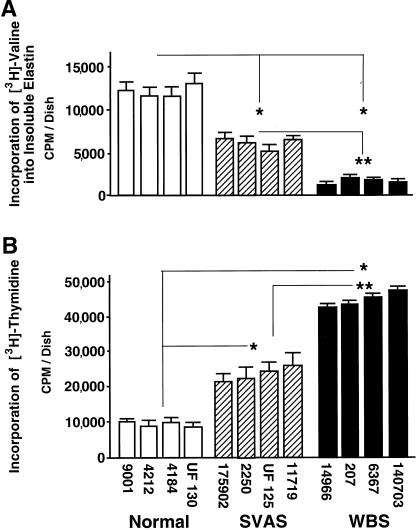
To further establish that the observed inverse relationship between elastin deposition and cell proliferation is not due merely to random interindividual variations, we studied groups of normal fibroblasts, SVAS fibroblasts, and WBS fibroblasts, from four individuals in each group. Elastin deposition as assessed by metabolic labeling and by biochemical isolation of elastin was reduced in SVAS fibroblast cultures and in WBS fibroblast cultures, to ∼50% and ∼15% of normal values, respectively (fig. 5A). By measuring [3H]-thymidine incorporation in the same group of SVAS fibroblasts and in the same group of WBS fibroblasts, we demonstrated respective twofold and fourfold increases in proliferation rate, compared with the levels in normal cells (fig. 5B).
Figure 5.
Results (mean ± SD) of quantitative assay of insoluble elastin (metabolically labeled with [3H]-valine) deposited in 3-d-old cultures of human fibroblasts (A) and assessment of cellular proliferation rate in parallel cultures, measured by incorporation of [3H]-thymidine for the last 24 h (B). Fibroblasts from all four patients with SVAS deposited consistently lower amounts of insoluble elastin than did those from four healthy control subjects. Fibroblasts from all four patients with WBS deposited even lower amounts of insoluble elastin than did those from patients with isolated SVAS. These abnormally low levels are associated with an inversely proportional increase in proliferation rates. Combined results from three separate experiments, with quadruplicate cultures of fibroblasts derived from each patient (initially plated 100,000 cells /dish), indicate that differences between experimental groups, normal versus SVAS, normal versus WBS (* [P<.002]), and SVAS versus WBS (** [P<.05]) are statistically significant. Differences between cultures derived from different patients within each experimental groups are not statistically significant.
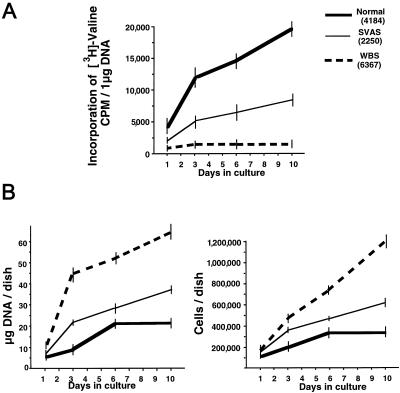
To investigate the kinetics of elastin deposition and cell proliferation, we studied these variables in 1–10-d-old cultures of selected fibroblast cell lines, which were densely plated (100,000 cells/dish) to reach an immediate confluence. Throughout the entire study period, normal cells consistently deposited the most elastin, SVAS cells deposited intermediate amounts, and WBS cells deposited the least (fig. 6A). Assessment of cell proliferation, by measurement of DNA content and by cell counting, revealed that both WBS fibroblasts and SVAS fibroblasts showed a consistent increase in proliferation rates, compared with that in normal controls (fig. 6B). Moreover, proliferation rates of these confluent fibroblasts, which quickly started production of their ECM, were always inversely proportional to amounts of insoluble elastin deposited during the entire 10-d-long time course. Interestingly, results of an additional experiment showed that cells (initially plated 10,000 cells /dish) that remained subconfluent in 2-d-old cultures and that did not deposit abundant ECM did not demonstrate any significant differences, in proliferation rates, between normal fibroblasts, SVAS fibroblasts, and WBS fibroblasts (data not shown).
Figure 6.
Results of a typical experiment when quantitative assays of metabolically labeled insoluble elastin (A) and assessments of cellular proliferation (B), measured by total DNA assay and cell counting, were performed in the time course. Results consistently showed that the abnormally high proliferation rate of cultured SVAS fibroblasts and of WBS fibroblasts is inversely proportional to their deposition of insoluble elastin during the entire 10-d culture period. At each time point, quadruplicate cultures of fibroblasts derived from each patient (initially plated 100,000 cells/dish) were assessed. Statistical evaluation showed that at 3, 6, and 10 d, the time-point differences between normal versus SVAS, normal versus WBS, and SVAS versus WBS were statistically significant.
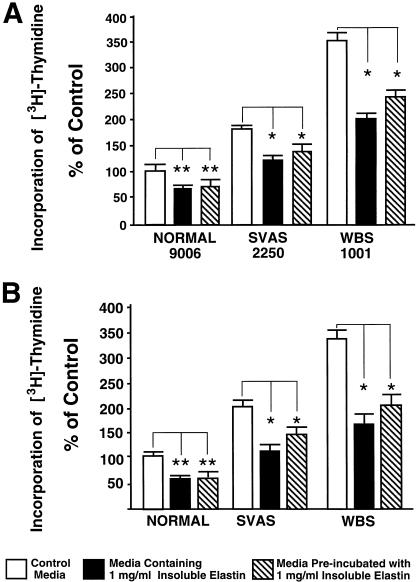
Since our experiments clearly demonstrate that both SVAS cells with a reduced rate of elastin synthesis and WBS cells with a reduced rate of elastin synthesis show increased proliferation rates (figs. 4–7), we attempted to reverse this hyperproliferative phenotype, by adding exogenous insoluble elastin to cultures of SMCs and fibroblasts. Our results indicate that supplementation with insoluble elastin significantly lowers the proliferation rates of all tested cell types (fig. 7). Meaningfully, a similar decrease in proliferation rates was observed also in cultures of cells maintained in media that were preincubated with insoluble elastin (fig. 7).
Figure 7.
Results of typical experiment comparing [3H]-thymidine incorporation into quadruplicate cultures of aortic SMC (A) and skin fibroblasts (B), derived from healthy control subjects, patients with SVAS, and patients with WBS, which were maintained for 3 d in the presence or absence of either 1 mg of exogenous insoluble elastin/ml or media that were preincubated for only 1 h with the 1 mg of exogenous insoluble elastin/ml. Statistical evaluations indicate that higher-than-normal proliferation of SMC and fibroblasts derived from patients with SVAS and patients with WBS was significantly reduced either in cultures treated with exogenous insoluble elastin or when maintained in media preincubated with insoluble elastin (* [P<.002]). Both treatments also decreased the proliferation rate of normal fibroblasts (** [P<.05]).
Discussion
Results of our present study, which uses an experimental model involving cells from patients with either SVAS or WBS who exhibit the primary genetic defects of ELN, show for the first time a direct causative relationship between reduced elastin deposition and increased proliferation of human cells. Results of our in vitro studies with aortic SMCs and skin fibroblasts clearly show that an inverse relationship between deposition of insoluble elastin and heightened cellular proliferation is an important feature of both the SVAS phenotype and the WBS phenotype.
Functional Haploinsufficiency in SVAS
ELN mutations have been shown to cause SVAS (Curran et al. 1993; Ewart et al. 1994; Olson et al. 1995; Fryssira et al. 1997; Li et al. 1997; Tassabehji et al. 1997; Boeckel et al. 1999; Urbán et al. 1999, 2000a, 2001; Metcalfe et al. 2000; von Dadelszen et al. 2000; Dedic et al. 2001). However, few studies have investigated the mechanism by which these mutations cause disease. We have recently shown that premature termination mutations in ELN activate an NMD mechanism that degrades mutant mRNA and renders the mutations as functionally inactive, null alleles (Urbán et al. 2000a, 2001). We have further shown that these null mutations result in reduced synthesis and secretion of tropoelastin. The studies presented here extend our previous results in several ways. We have shown nonsense mediated degradation (i.e., NMD) of the mutant mRNA in a new SVAS allele and have used it to demonstrate for the first time that NMD of mutant elastin mRNA is active not only in skin fibroblasts but also in aortic SMCs, the most relevant cell type for the pathogenesis of SVAS. Furthermore, our current data demonstrate that SVAS alleles not only cause reduced ELN expression at the mRNA and protein levels but also result in reduced deposition of elastic fibers. The latter is not a trivial finding, since elastic-fiber deposition requires the interaction and assembly of multiple cellular and extracellular proteins, including EBP (Hinek and Rabinovitch 1994; Hinek et al. 1995), fibrillin-1 and fibrillin-2 (Trask et al. 2000), microfibril-associated glycoproteins (Brown-Augsburger et al. 1996), and fibulin -5 (Nakamura et al. 2002). Thus, our findings indicate that tropoelastin synthesis and secretion is a rate-limiting step in elastic-fiber assembly.
Elastin Biosynthesis in WBS
WBS is caused by segmental monosomy of a 1.6-Mb region in 7q11.2. Since the WBS critical region (WSCR) includes ELN, the pathomechanism of vascular disease in WBS is clearly elastin haploinsufficiency. One concern with analysis of the effects of elastin haploinsufficiency in WBS is the potential influence that the deletion of other WSCR genes has on the phenotypic expression. Clinical studies to compare the natural history of syndromic and nonsyndromic SVAS are currently unavailable. However, early studies on the pathology and histology of vascular lesions suggested that syndromic and nonsyndromic elastin arteriopathy have similar characteristics (O’Connor et al. 1985). Our studies, too, suggest that vascular lesions in nonsyndromic and syndromic forms of SVAS are very similar.
In contrast, our in vitro studies demonstrate a reproducible difference, in steady-state levels of elastin mRNA and in elastin deposition, between isolated SVAS SMCs and isolated WBS SMCs. SMCs and dermal fibroblasts from all patients with WBS whom we studied had significantly reduced elastin mRNA levels and deposited less elastin than did the same types of cells from patients with nonsyndromic SVAS. We initially speculated that such a significant difference in net deposition was related to the possible loss of the remaining copy of ELN by WBS cells. FISH analysis of SMCs isolated from the stenotic region of the aorta of one patient with WBS, patient 1001, did not confirm such a two-hit hypothesis.
In addition to gene copy number, other determinants of mRNA steady-state levels are the rates of transcription, the transfer of mRNA from the nucleus to the cytoplasm, and the rate of mRNA degradation. We have demonstrated that elastin mRNA stability is the same in SVAS cells and in WBS cells and thus we assume that, as a result of haploinsufficiency of one or more deleted genes within the WSCR, WBS cells have either reduced transcription of ELN or reduced nuclear transport of elastin mRNA. Indeed, among the 16 genes deleted in WBS, there are 4—GTF2IRD1, WBSCR9, WBSCR14, and TBL2—that encode putative transcription factors. We speculate that, if one or more of these putative nuclear factors up-regulate ELN transcription, haploinsufficiency of these genes may result in the markedly reduced expression of ELN in WBS cells that we have observed. Independent of the exact mechanism of reduced ELN expression in WBS, our results indicate the existence of a significant gene interaction between ELN and other genes within the WSCR. This and other gene-interaction effects may contribute to difficulties associated with elucidation of the contribution that individual deleted genes make to the WBS phenotype.
Quantitative and qualitative studies on elastic-fiber morphology in WBS skin (Dridi et al. 1999, Urbán et al. 2000b) have indicated that reduced dermal elastin deposition is associated with a soft, smooth, “velvety” skin phenotype. Similar studies on the skin of patients with isolated SVAS have indicated normal skin histology (Dridi et al. 1999; Z.U., unpublished results). Our in vitro data demonstrating reduced elastin deposition by WBS cells, compared with that in isolated SVAS cells, support these earlier data. Thus, elastin-biosynthesis differences between syndromic and nonsyndromic forms of SVAS may exist in vivo. However, currently available data are insufficient to test whether such phenotypic differences exist between the vascular characteristics of patients with isolated SVAS and those of patients WBS.
Elastin and Cell Proliferation
The chemotactic, chemokinetic, and proliferative effects of proteolytic degradation products of elastin have been well established (Senior et al. 1980; Kamoun et al. 1995; Wachi et al. 1995; Jung et al. 1998, 1999); however, until recently, the function of insoluble elastin was thought to be limited to the conferring of resilience to tissues. The vascular phenotype of elastin deficiency, however, is not suggestive of mechanical failure. Previous studies (O’Connor et al. 1985; Li et al. 1998b) and the results of the present study indicate that elastin arteriopathy in SVAS is characterized by a uniform wall thickening with preserved lamellar structure but an increased number of lamellae. In addition, the segmental obstructive disease found in patients with SVAS is characterized by disrupted lamellae, clumping of SMCs, and an elastin-poor ECM. Segmental stenoses of arteries in SVAS are preferentially localized to areas subject to the highest hemodynamic stress, such as the sinotubular junction and the ascending region of the aorta and the bifurcation of major arteries.
Our results from in vitro experiments provide strong evidence that a primary deficiency in deposition of insoluble elastin in patients with either SVAS or WBS may be responsible for increased cell proliferation in vivo. The proportional inverse relationship between elastin deposition and cell-proliferation rate became apparent in all confluent cultures of SMCs and fibroblasts derived from different patients as soon as cells started to produce ECM. Interestingly, we did not detect any significant differences in proliferation rate in 48-h-old subconfluent cultures of normal cells, SVAS cells, and WBS cells, which do not contact each other and which do not deposit elastin. These results suggest that reduced elastin deposition may contribute substantially to the heightened proliferation of arterial SMCs in vivo, independent of hemodynamic stress–related mitogenic signals (Iwasaki et al. 2000). Moreover, our finding that the addition of exogenous insoluble elastin was able to normalize the proliferation rate of cultured SVAS cells and of cultured WBS cells suggests that the proper accumulation of extracellular elastin may be a negative regulator of cellular proliferation. The results that we have presented are consistent with both (1) the findings by Li et al. (1998a, 1998b), who reported development of severe obstructive vascular disease leading to perinatal lethality of homozygous Eln−/− mutant mice and (2) our previous report (Hinek and Wilson 2000) on impaired elastogenesis coinciding with the heightened proliferation of cells derived from patients with Hurler disease, who often develop occlusive arterial lesions similar to those in SVAS.
Potential Mechanisms for the Antiproliferative Effect of Elastin
The present study did not directly address the molecular mechanism by which insoluble elastin regulates cell proliferation. However, on the basis of the results of our experiments testing the effects of exogenous insoluble elastin, one can speculate that interactions between cells and elastin may either initiate antimitogenic signals or block the mitogenic signals transduced by the common growth factors present in the FBS-containing medium. The cell-proliferation decrease caused by insoluble elastin likely results from several overlapping mechanisms. First, physical contact between large particles of insoluble elastin and the cell surface may cause both aggregation of EBP molecules and masking of adjacent growth-factor receptors that normally transduce mitogenic signals induced by serum-derived growth factors. This notion is consistent with our previous findings that cell-surface EBP can mask the adjacent interleukin type I receptors interacting with IL-1B and, thereby, can decrease the cellular response to this cytokine (Hinek et al. 1996). Second, large hydrophobic particles of exogenous insoluble elastin may attract soluble tropoelastin-derived peptides present in the conditioned media and cause their precipitation (coacervation). Such a depletion of soluble fragments of tropoelastin, a factor known to generate signals facilitating proliferation in many cell types, including vascular SMCs, skin fibroblasts, and astrocytomas (Kamoun et al. 1995; Wachi et al. 1995; Jung et al. 1998, 1999), may indirectly down-regulate cellular proliferation. Moreover, insoluble elastin deposited in arterial tunica media may sequestrate the EBP molecules from the surface of adjacent cells (Hinek and Rabinovitch 1994), thereby reducing the likelihood of mitogenic response to soluble elastin-derived peptides. Results of experiments showing that all tested cell types reduced their proliferation rates when maintained in media (containing FBS) that were preincubated with insoluble elastin strongly suggest that this exogenous compound sequestrated certain growth factors from the FBS-containing media.
Regardless of the exact molecular mechanism of the antiproliferative effect of elastin, our results provide new insight into the pathomechanism of arterial occlusive disease in patients with either SVAS or WBS, linking increased cell proliferation to impaired elastogenesis. We suggest that initial impaired deposition of insoluble elastin, caused by haploinsufficiency of ELN, contributes to increased proliferation of fetal SMCs. This, in turn, results in thick aortic tunica media marked by an increased number of cell layers. The inability of the nonresilient ECM to absorb strong physical forces created by the increase in blood pressure in the ascending aorta and by a turbulent blood flow in bifurcations of the major arteries may lead to further transduction of stress-related mitogenic signals that contribute to persistent proliferation and migration of arterial SMCs, causing, during the postnatal period, arterial stenoses in patients with either SVAS or WBS. The data presented in the present study also encourage further studies aimed at the development of therapeutic strategies that seek to identify enhanced elastin deposition that might be beneficial not only for patients with either SVAS or WBS but also for those with both atherosclerosis and angioplasty-induced arterial stenoses.
Acknowledgments
This study was supported by National Institutes of Health grants AR 467379 and RR16453 (both to Z.U.), American Heart Association grant 0150587N (to Z.U.), Canadian Institute of Health Research grants PG 13920 and MT 13719 (both to A.H.), and Heart and Stroke Foundation of Canada grants NA 4381 and CI 4198 (both to A.H.). A.H. is a Career Investigator of the Heart and Stroke Foundation of Ontario. We also thank Dr. Margaret R. Wallace and Dr. Koen Devriendt, for contributing skin fibroblast samples, and Dr. Gregory Wilson, for providing the autopsy material from patients with either SVAS or WBS.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/omim (for SVAS [MIM 185500] and WBS [MIM 194050])
References
- Bashir MM, Indik Z, Yeh,H., Ornstein-Goldstein N, Rosenbloom JC, Abrams W, Fazio M, Uitto J, Rosenbloom J (1989) Characterization of the complete human elastin gene. J Biol Chem 264:8887–8891 [PubMed] [Google Scholar]
- Beuren AJ, Apitz J, Harmjanz D (1962) Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation 26:1235–1240 [DOI] [PubMed] [Google Scholar]
- Boeckel T, Dierks A, Vergopoulos A, Bähring S, Knoblauch H, Müller-Myhsok B, Baron H, Aydin A, Bein G, Luft FC, Schuster H (1999) A new mutation in the elastin gene causing supravalvular aortic stenosis. Am J Cardiol 83:1141–1143 [DOI] [PubMed] [Google Scholar]
- Brown-Augsburger P, Broekelmann T, Rosenbloom J, Mecham RP (1996) Functional domains on elastin and microfibril-associated glycoprotein involved in elastic fibre assembly. Biochem J 318:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EE Jr, Noonan J, Marion RW, Steeg CN (1990) Myocardial infarction leading to sudden death in the Williams syndrome: report of three cases. J Pediatr 117:593–595 [DOI] [PubMed] [Google Scholar]
- Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT (1993) The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell 73:159–168 [DOI] [PubMed] [Google Scholar]
- Debelle L, Tamburro AM (1999) Elastin: molecular description and function. Int J Biochem Cell Biol 31:261–272 [DOI] [PubMed] [Google Scholar]
- de Chalain T, Phillips JH, Hinek A (1999) Bioengineering of elastic cartilage with aggregated porcine and human auricular chondrocytes and hydrogels containing alginate, collagen, and kappa-elastin. J Biomed Mater Res 44:280–288 [DOI] [PubMed] [Google Scholar]
- Dedic J, Weiss AS, Katahira J, Yu B, Trent RJ, Urbán Z (2001) A novel elastin gene mutation (1281delC) in a family with supravalvular aortic stenosis: a mutation cluster within exon 20. Hum Mutat 17:81 [DOI] [PubMed] [Google Scholar]
- DeSilva U, Elnitski L, Idol JR, Doyle JL, Gan W, Thomas JW, Schwartz S, Dietrich NL, Beckstrom-Sternberg SM, McDowell JC, Blakesley RW, Bouffard GG, Thomas PJ, Touchman JW, Miller W, Green ED (2002) Generation and comparative analysis of approximately 3.3 Mb of mouse genomic sequence orthologous to the region of human chromosome 7q11.23 implicated in Williams syndrome. Genome Res 12:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi SM, Ghomrasseni S, Bonnet D, Aggoun Y, Vabres P, Bodemer C, Lyonnet S, de Prost Y, Fraitag S, Pellat B, Sidi D, Godeau G (1999) Skin elastic fibers in Williams syndrome. Am J Med Genet 87:134–138 [PubMed] [Google Scholar]
- Eisenberg R, Young D, Jacobson B, Boito A (1964) Familial supravalvular aortic stenosis. Am J Dis Child 108:341–347 [DOI] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Ensing GJ, Loker J, Moore C, Leppert M, Keating MT (1993) A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proc Natl Acad Sci USA 90:3226–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart AK, Weishan J, Atkinson D, Morris CA, Keating MT (1994) Supravalvular aortic stenosis associated with a deletion disrupting the elastin gene. J Clin Invest 93:1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio MJ., Mattei M, Passage E, Chu M, Black D, Soloman E, Davidson JM, Uitto J (1991) Human elastin gene: new evidence for localization to the long arm of chromosome 7. Am J Hum Genet 48:696–703 [PMC free article] [PubMed] [Google Scholar]
- Francke U (1999) Williams-Beuren syndrome: genes and mechanisms. Hum Mol Genet 8:1947–1954 [DOI] [PubMed] [Google Scholar]
- Fryssira H, Palmer R, Hallidie-Smith KA, Taylor J, Donnai D, Reardon W (1997) Fluorescent in situ hybridisation (FISH) for hemizygous deletion at the elastin locus in patients with isolated supravalvular aortic stenosis. J Med Genet 34:306–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Rock MJ, Prockop DJ (1993) Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA 90:10325–10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W, Fathi A, Bigelow F, Carpenter B, Jimenez C (1986) Improved Movat pentachrome stain. Stain Technol 61:60–62 [DOI] [PubMed] [Google Scholar]
- Hinek A (1994) Nature and the multiple functions of the 67-kD elastin/laminin binding protein. Cell Adhes Commun 2:185–193 [DOI] [PubMed] [Google Scholar]
- ——— (1996) Biological roles of the non-integrin elastin/laminin receptor. Biol Chem 377:471–480 [PubMed] [Google Scholar]
- Hinek A, Keeley FW, Callahan J (1995) Recycling of the 67-kD elastin binding protein in arterial myocytes is imperative for secretion of tropoelastin. Exp Cell Res 220:312–324 [DOI] [PubMed] [Google Scholar]
- Hinek A, Mecham RP, Keeley F, Rabinovitch M (1991) Impaired elastin fiber assembly is related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest 88:2083–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Molossi S, Rabinovitch M (1996) Functional interplay between interleukin-1 receptor and elastin binding protein regulates fibronectin production in coronary artery smooth muscle cells. Exp Cell Res 225:122–131 [DOI] [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M (1993) The ductus arteriosus migratory smooth muscle cell phenotype processes tropoelastin to a 52 kD product associated with impaired assembly of elastic laminae. J Biol Chem 268:1405–1413 [PubMed] [Google Scholar]
- ——— (1994) 67-kD elastin-binding protein is a protective “companion” of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol 126:563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J (1993) The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest 91:1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R (2000a) Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet 66:859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Wilson SE (2000) Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol 156:925–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Wrenn DS, Mecham RP, Barondes SH (1988) The elastin receptor: a galactoside-binding protein. Science 239:1539–1541 [DOI] [PubMed] [Google Scholar]
- Hinek A, Zhang S, Smith AC, Callahan JW (2000b) Impaired elastic-fiber assembly by fibroblasts from patients with either Morquio B disease or infantile GM1-gangliosidosis is linked to deficiency in the 67-kD spliced variant of β-galactosidase. Am J Hum Genet 67:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y (2000) Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol 278:H521–H529 [DOI] [PubMed] [Google Scholar]
- Ju H, Dixon IMC (1996) Extracellular matrix and cardiovascular diseases. Can J Cardiol 12:1259–1267 [PubMed] [Google Scholar]
- Jung S, Hinek A, Tsugu A, Hubbard SL, Ackerley C, Becker LE, Rutka JT (1999) Astrocytoma cell interaction with elastin substrates: implications for astrocytoma invasive potential. Glia 25:179–189 [DOI] [PubMed] [Google Scholar]
- Jung S, Rutka JT, Hinek A (1998) Tropoelastin and elastin degradation products promote proliferation of human astrocytoma cell lines. J Neuropathol Exp Neurol 57:439–448 [DOI] [PubMed] [Google Scholar]
- Kagan HM, Trackman PC (1991) Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol 5:206–210 [DOI] [PubMed] [Google Scholar]
- Kamoun A, Landeau JM, Godeau G, Wallach J, Duchesnay A, Pellat B, Hornebeck W (1995) Growth stimulation of human skin fibroblasts by elastin-derived peptides. Cell Adhes Commun 3:273–278 [DOI] [PubMed] [Google Scholar]
- Kaplan P, Levinson M, Kaplan BS (1995) Cerebral artery stenoses in Williams syndrome cause strokes in childhood. J Pediatr 126:943–945 [DOI] [PubMed] [Google Scholar]
- Kumar A, Stalker HJ, Williams CA (1993) Concurrence of supravalvular aortic stenosis and peripheral pulmonary stenosis in three generations of a family: a form of arterial dysplasia. Am J Med Genet 45:739–742 [DOI] [PubMed] [Google Scholar]
- Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT (1998a) Elastin is an essential determinant of arterial morphogenesis. Nature 393:276–280 [DOI] [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT (1998b) Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102:1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT (1997) Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet 6:1021–1028 [DOI] [PubMed] [Google Scholar]
- MacLeod DC, Strauss BH, de Jong M, Escaned J, Umans VA, van Suylen RJ, Verkerk A, de Feyter PJ, Serruys PW (1994) Proliferation and extracellular matrix synthesis of smooth muscle cells cultured from human coronary atherosclerotic and restenotic lesions. J Am Coll Cardiol 23:59–65 [DOI] [PubMed] [Google Scholar]
- Metcalfe K, Rucka AK, Smoot L, Hofstadler G, Tuzler G, McKeown P, Siu V, Rauch A, Dean J, Dennis N, Ellis I, Reardon W, Cytrynbaum C, Osborne L, Yates JR, Read AP, Donnai D, Tassabehji M (2000) Elastin: mutational spectrum in supravalvular aortic stenosis. Eur J Hum Genet 8:955–963 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J Jr, Honjo T, Chien KR (2002) Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415:171–175 [DOI] [PubMed] [Google Scholar]
- Oakes BW, Batty AC, Handley CJ, Sandberg LB (1982) The synthesis of elastin, collagen, and glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle: an ultrastructural and biochemical study. Eur J Cell Biol 27:34–46 [PubMed] [Google Scholar]
- O’Connor WN, Davis JB Jr, Geissler R, Cottrill CM, Noonan JA, Todd EP (1985) Supravalvular aortic stenosis: clinical and pathological observations in six patients. Arch Pathol Lab Med 109:179–185 [PubMed] [Google Scholar]
- Olson TM, Michels VV, Urban Z, Csiszar K, Christiano AM, Driscoll DJ, Feldt RH, Boyd CD, Thibodeau SN (1995) A 30 kb deletion within the elastin gene results in familial supravalvular aortic stenosis. Hum Mol Genet 4:1677–1679 [DOI] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I, Baccarani-Contri M (1997) Elastic fiber during development and aging. Microsc Res Tech 38:428–435 [DOI] [PubMed] [Google Scholar]
- Privitera S, Prody CA, Callahan JW, Hinek A (1998) The 67-kDa enzymatically inactive alternatively spliced variant of β-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem 273:6319–6326 [DOI] [PubMed] [Google Scholar]
- Prosser J, Whitehouse LA, Parks WC, Hinek A, Park PW, Mecham RP (1991) Polyclonal antibodies to tropoelastin and specific detection and measurement of tropoelastin in vitro. Connect Tissue Res 25:265-279 [DOI] [PubMed] [Google Scholar]
- Rein AJ, Preminger TJ, Perry SB, Lock JE, Sanders SP (1993) Generalized arteriopathy in Williams syndrome: an intravascular ultrasound study. J Am Coll Cardiol 21:1727–1730 [DOI] [PubMed] [Google Scholar]
- Rodems SM, Clark CL, Spector DH (1998) Separate DNA elements containing ATF/CREB and IE86 binding sites differentially regulate the human cytomegalovirus UL112–113 promoter at early and late times in the infection. J Virol 72:2697–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, Mecham R (1993) Extracellular matrix 4: the elastic fiber. FASEB J 7:1208–1218 [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Mecham RP (1980) Chemotactic activity of elastin-derived peptides. J Clin Invest 66:859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Donnai D, Hurst J, Reardon W, Burch M, Read AP (1997) Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet 6:1029–1036 [DOI] [PubMed] [Google Scholar]
- Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP (2000) Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J Biol Chem 275:24400–24406 [DOI] [PubMed] [Google Scholar]
- Urbán Z, Michels VV, Thibodeau SN, Davis EC, Bonnefont JP, Munnich A, Eyskens B, Gewillig M, Devriendt K, Boyd CD (2000a) Isolated supravalvular aortic stenosis: functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum Genet 106:577–588 [DOI] [PubMed] [Google Scholar]
- Urbán Z, Michels VV, Thibodeau SN, Donis-Keller H, Csiszár K, Boyd CD (1999) Supravalvular aortic stenosis: a splice site mutation within the elastin gene results in reduced expression of two aberrantly spliced transcripts. Hum Genet 104:135–142 [DOI] [PubMed] [Google Scholar]
- Urbán Z, Peyrol S, Plauchu H, Zabot MT, Lebwohl M, Schilling K, Green M, Boyd CD, Csiszar K (2000b) Elastin gene deletions in Williams syndrome patients result in altered deposition of elastic fibers in skin and a subclinical dermal phenotype. Pediatr Dermatol 17:12–20 [DOI] [PubMed] [Google Scholar]
- Urbán Z, Zhang Y, Davis EC, Maeda G, Kumar A, Stalker H, Belmont J, Boyd CD, Wallace MR (2001) Supravalvular aortic stenosis: genetic and molecular dissection of a complex mutation in the elastin gene. Hum Genet 109:512–520 [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Chitayat D, Winsor EJ, Cohen H, MacDonald C, Taylor G, Rose T, Hornberger LK (2000) De novo 46,XX,t(6;7)(q27;q11;23) associated with severe cardiovascular manifestations characteristic of supravalvular aortic stenosis and Williams syndrome. Am J Med Genet 90:270–275 [DOI] [PubMed] [Google Scholar]
- Vrhovski B, Weiss AS (1998) Biochemistry of tropoelastin. Eur J Biochem 258:1–18 [DOI] [PubMed] [Google Scholar]
- Wachi H, Seyama Y, Yamashita S, Tajima S (1995) Cell cycle-dependent regulation of elastin gene in cultured chick vascular smooth-muscle cells. Biochem J 309:575–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JCP, Berratt-Boyes BG, Lowe JB (1961) Supravalvular aortic stenosis. Circulation 24:1311–1318 [DOI] [PubMed] [Google Scholar]
- Zhang MC, Giro M, Quaglino D Jr, Davidson JM (1995) Transforming growth factor-beta reverses a posttranscriptional defect in elastin synthesis in a cutis laxa skin fibroblast strain. J Clin Invest 95:986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]