Abstract
Attentional dysfunction in schizophrenia (SZ) is a core deficit that contributes to multiple cognitive deficits and the resulting functional disability. However, developing procognitive therapeutics for neuropsychiatric disorders have been limited by a ‘translational gap'—a lack of cognitive paradigms having cross-species translational validity and relevance. The present study was designed to perform an initial validation of the cross-species homology of the 5-choice Continuous Performance Test (5C-CPT) in healthy nonpsychiatric comparison subjects (NCS), SZ patients and mice under pharmacologic challenge. The 5C-CPT performance in SZ patients (n=20) was compared with age-matched NCS (n=23). The effects of the general muscarinic receptor antagonist scopolamine on mice (n=21) performing the 5C-CPT were also assessed. SZ subjects exhibited significantly impaired attention in the 5C-CPT, driven by reduced target detection over time and nonsignificantly increased impulsive responding. Similarly, scopolamine significantly impaired attention in mice, driven by reduced target detection and nonsignificantly increased impulsive responding. Scopolamine also negatively affected accuracy and speed of responding in mice, although these measures failed to differentiate SZ vs NCS. Thus, mice treated with scopolamine exhibited similar impairments in vigilance as seen in SZ, although the differences between the behavioral profiles warrant further study. The availability of rodent and human versions of this paradigm provides an opportunity to: (1) investigate the neuroanatomic, neurochemical and genomic architecture of abnormalities in attention observed in clinical populations such as SZ; (2) develop and refine animal models of cognitive impairments; and (3) improve cross-species translational testing for the development of treatments for these impairments.
Keywords: attention, mice, schizophrenia, scopolamine, translation, vigilance
Introduction
Current medications for patients with schizophrenia (SZ) exhibit primary efficacy at treating positive symptoms, but limited efficacy for treating negative symptoms or cognitive dysfunctions.1, 2, 3 Functional outcome correlations with cognitive performance, particularly in the domain of attention,4, 5 have galvanized research to identify procognitive treatments with the hope that amelioration of these deficits will lead to improved functional outcome.
Developing procognitive therapies for SZ will be facilitated by: (1) an understanding of the neural systems underlying the targeted cognitive processes; (2) knowledge of the neuroanatomical changes that underlie deficits in patients; (3) identifying manipulations that can recreate these deficits in animals; and (4) cognitive paradigms having cross-species translational validity to assess potential therapeutics.6 Unfortunately, the development of such procognitive therapeutics has been ‘overwhelmingly disappointing' to date,7 in part because of the use of ‘fast and dirty' cognitive paradigms with limited cross-species translational validity.8
Since,9, 10 it has been clear that patients cannot maintain attention over time. More recently, two initiatives funded by National Institute of Mental Health, the Measurement And Treatment Research to Improve Cognition in Schizophrenia (MATRICS)11, 12 and the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS),13 highlighted the need to improve attentional functioning in SZ and to develop appropriate animal models in order to quickly screen for potential therapeutic response properties.7, 14, 15, 16 Attentional deficits appear to be core aspects of cognitive dysfunction in SZ, as they are present at first episode17, 18 and persist across remitted and active phases of illness.19, 20 The detrimental impact of impaired attention in SZ is underscored by the finding that more severe deficits assessed by continuous performance tests (CPTs) are robustly associated with substantially higher costs of caregiving in these patients.21 The CPT is the ‘gold standard' test of attention in SZ, and although numerous human CPTs exist with differing demands on differing aspects of cognition, for example, perception, working memory and/or contextual processing demands,22 all CPTs use both target and nontarget stimuli.23 Thus, CPTs assess a specific aspect of attention, that is, the maintenance of vigilance over time where subjects must respond to signals (target stimuli) and inhibit from responding to noise (nontarget stimuli).24, 25 Despite the fact that CPTs are commonly used to demonstrate poorer vigilance in people with SZ throughout the session,19, 26 there are few animal CPT paradigms available that have demonstrable translational validity.
In reviews of preclinical test batteries for MATRICS16 and CNTRICS,27 the 5-choice continuous performance test (5C-CPT), 5-choice serial reaction time task (5-CSRTT) in the Cambridge Neuropsychological Test Automated Battery28 and the distractor sustained attention task (dSAT)29 were proposed as putative cross-species tests for assessing attention in rodents. Although human CPTs assess various aspects of attention, the necessity of inhibiting from responding to nontarget stimuli is considered a core feature of human CPTs and their assessing vigilance,23, 30 yet this aspect is lacking in most rodent attentional tasks. Whereas the 5-CSRTT contains only stimuli to which the rodent must respond, the dSAT requires rats to determine the presence or absence of a stimulus and respond appropriately.27 Given that a prevailing theory of the cognitive dysfunction occurring in SZ results from their inappropriate attention to irrelevant external stimuli, to model and treat such dysfunction would require the presence of irrelevant stimuli. Consistent with human CPTs, the 5C-CPT presents two stimulus types and requires both responses to relevant stimuli and the inhibition of responses to irrelevant stimuli. Hence, the 5C-CPT is a promising candidate for cross-species test for assessing the attentional construct (vigilance) measured in such tasks. In support of its cross-species relevance, a standard requirement of the validation of human CPTs is the detection of a vigilance decrement—poorer attentional performance over time—which has been demonstrated in both mice and rats in the 5C-CPT.31, 32, 33, 34
Current human CPTs commonly utilize culturally specific symbols (for example, letters or numbers), limiting their use across cultures and especially in rodent studies. To address these limitations, we developed the human 5C-CPT that incorporates a spatial array and utilizes distinct noncultural stimuli. To assess the clinical relevance of such a task, SZ patients and age-matched healthy nonpsychiatric subjects (NCS) were tested on the 5C-CPT. We hypothesized that SZ patients would exhibit significant impairments in vigilance driven primarily by poor target detection over time, consistent with other CPT studies.35, 36, 37, 38, 39, 40, 41 This impairment may relate to reduced expression of muscarinic acetylcholine receptors (mAChRs) M1 and M4 in SZ patients42 because of the importance of the cholinergic system to attention.43 Thus, we also hypothesized that administering the nonspecific mAChR antagonist scopolamine to mice would impair their 5C-CPT performance in a pattern similar to SZ patients.
Materials and methods
Human participants
Clinically stable SZ (n=20) and gender- and age-matched NCS (n=23) were recruited and tested in accordance with our established methods.44, 45 All participants were assessed for capacity to provide informed consent and, after receiving a detailed description of the study, written informed consent was obtained in accordance with University of California San Diego (UCSD) institutional review board-approved procedures (numbers 030510 and 040564). All participants received a urine toxicology screen to rule out recent drug use. Exclusion criteria also included recent substance abuse or dependence. Participants were assessed diagnostically using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,46 the Scale for the Assessment of Negative Symptoms (SANS),47 the Scale for the Assessment of Positive Symptoms (SAPS)48 and the modified Global Assessment of Functioning Scale.49 SZ patients did not have an Axis I diagnosis other than SZ, and NCS did not have any Axis I diagnosis. All participants had not experienced a neurologic insult such as significant head trauma and/or loss of consciousness. NCS were recruited through newspaper advertisements, postings on the Internet and fliers posted at UCSD Medical Center. SZ patients were recruited from community residential facilities and via physician referral. Table 1 shows demographic characteristics of the sample and clinical characteristics of the groups including the numbers receiving antipsychotic treatment. All participants were able to tolerate the testing procedures and completed the entire task, demonstrating the basic feasibility of this test in NCS and SZ.
Table 1. Healthy nonpsychiatric comparison subjects and patient demographics.
| Demographic and clinical characteristics | Nonpsychiatric comparison subjects (n=23) (±s.d., min–max) | Patients with schizophrenia (n=20) (±s.d., min–max) |
|---|---|---|
| Gender (% male) | 52 | 60 |
| Mean age (years) | 43.6 (±9.9, 20–59) | 42.8 (±8.8, 28–62) |
| Education | 13.9 (±2.5, 9–20) | 12.0a (±1.9, 9–16) |
| Smoking | 26.1 % | 42.1 % |
| Right handedness | 87.0 % | 90.0 % |
| Age of illness onset (years) | 19 (±1.1, 12–30) | |
| Duration of illness (years) | 24 (±9.2, 8–42) | |
| SAPS total score | 9.9 (±4.4, 4–17) | |
| SANS total score | 17.3 (±3.3, 7–22) | |
| GAF | 41.2 (± 0.9, 36–47) | |
| Patients receiving medication type, no. | ||
| No AP | 2 | |
| Typical AP (exclusively) | 5 | |
| Atypical AP (exclusively) | 13 | |
| Typical+atypical AP | 0 | |
Abbreviations: AP, antipsychotic; GAF, Global Assessment of Functioning Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Patient symptoms are also included.
P<0.05 when compared with healthy subjects.
Human 5C-CPT apparatus
A Dell PC with E-Prime2 software (Psychology Software Tools, Sharpsburg, PA, USA) was used for stimulus presentation and data acquisition. Subjects sat in front of a 56-cm cathode ray tube (CRT) computer screen (60 cm away). An arcade joystick was provided that subjects used with their dominant hand. The joystick was spring-mounted so that after every response the joystick would return to center.
Human 5C-CPT task
The participants were given a brief introduction to the task. The subjects were forewarned that they would see 5 white lines (3 cm) in an arc on a black background and if a white circle (∼2 cm diameter) appeared behind a line then they should move the joystick in that direction (target stimuli). Subjects were also instructed to inhibit responding if circles appeared behind every line (nontarget stimuli). See Figure 1 for an example of presentations of stimuli. Stimuli appeared for only 100 ms, although the subjects could still respond up to 1 s after the stimuli disappeared. Trials were separated by a variable intertrial interval (ITI; 0.5, 1 or 1.5 s) occurring 1 s after the stimulus of the previous trial was presented. The ITI was presented in a pseudorandom order so that no more than 3 of a specific ITI appeared consecutively. Variability of stimulus presentations was introduced in order to reduce temporal predictability of stimuli, precluding a mediating strategy that could aid performance. Moreover, this variability is consistent with the rodent 5C-CPT. Before testing in the full task, subjects were given a practice session consisting of 12 trials (10 target and 2 nontarget stimuli randomly presented). The full task consisted of 270 trials, 225 target and 45 nontarget stimuli, presented in a pseudorandom order so that no more than 3 presentations of a specific stimulus appeared consecutively. Similarly, the Conners CPT assesses attention and vigilance, utilizing a target/nontarget ratio of 9:1, and is sensitive to inattention in SZ patients.38, 50, 51 However, most CPTs present more nontarget stimuli. The high ratio of target vs nontarget stimuli is required in the rodent 5C-CPT to ensure the maintenance of responding. Hence, this ratio was maintained in the human 5C-CPT. Responses were recorded according to Table 2 and include hits and misses to target trials or false alarms (FAs) or correct rejections (CRs) to nontarget trials. Numerous calculations were also used in the analysis of performance, including hit rate (HR) and false alarm rate (FAR):
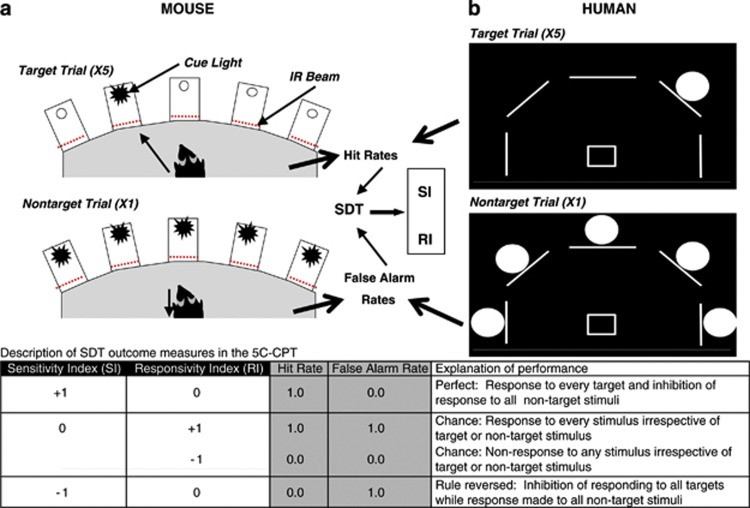
Figure 1.
Schematic of the mouse and human 5-choice continuous performance test (5C-CPT). There are five locations in which stimuli can be presented. For mice, stimuli are presented in a 5-hole operant chamber in which cue light stimuli are presented and responses of the mice are monitored using infrared (IR) beams (a). For humans, stimuli are presented in one of five locations in an arc on a computer screen, with responses made using a five-way joystick (b). In both cases, a single light stimulus represents a target trial in which subjects must respond in that location. A nontarget trial is represented by all five stimuli being presented simultaneously, to which the subject must inhibit from responding. Because there are five targets for every one nontarget trial, responding in the presence of a cue stimulus represents the prepotent response, whereas inhibiting from responding requires the control of attention. Target trials generate measures of hits and misses (target responses and omissions) resulting in a subjects' Hit Rate, whereas nontarget trials generate measures of correct rejections and false alarms, resulting in a subjects' False Alarm Rate. These two rates are utilized in signal detection theory (SDT) to generate the nonparametric measure of performance, the sensitivity index (SI). Thus, the overall combined performance can be measured (SI) and the two components of that performance can also be measured to determine whether one more than the other drives changes in SI. The table provides examples of what permutations of Hit and False Alarm Rates result in various SI and RI levels, as well as provides a description of the behavior such scores would entail.
Table 2. Description of measures produced in the 5-choice continuous performance test.
| Measure | Description |
|---|---|
| Hit | Response to target stimulus in correct location |
| Miss | Nonresponse (failure to respond) to target stimulus |
| Incorrect | Response to target stimulus but in another location |
| Correct rejection | Appropriate nonresponse to nontarget stimulus |
| False alarm | Inappropriate response to nontarget stimulus |
| Premature response | Response to no stimuli during the intertrial interval |
| Cumulative Latencies | Cumulative latencies to responses, including correct, incorrect, false alarms and premature responses |
| Hit rate | Proportion of appropriate responses to target stimuli |
| False alarm rate | Proportion of inappropriate responses to nontarget stimuli |
| Sensitivity index (attention) | Nonparametric measure examining the difference between hit and false alarm rates to determine performance |
| Responsivity index (bias) | Nonparametric measure examining the combination of hit and false alarm rates to determine responsivity to stimuli |
| Accuracy | Proportion of correct compared with incorrect responses |
| % Omissions | Percentage of misses |
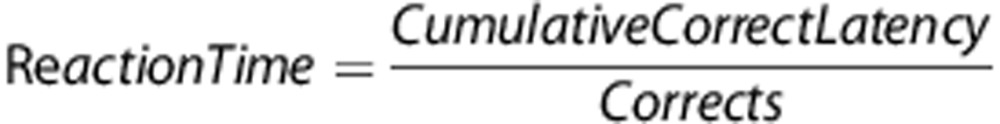 |
 |
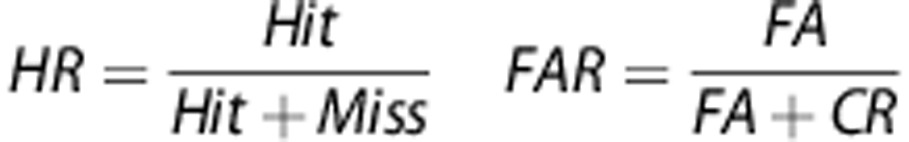 |
Based upon these basic parameters, signal detection indices52, 53 were then calculated to assess both sensitivity index and responsivity index bias. The sensitivity and responsivity indices were calculated using the following formulas:
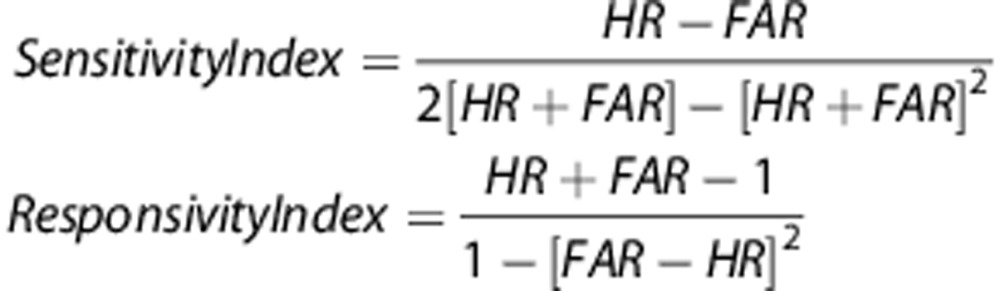 |
Sensitivity index provides a nonparametric assessment of sensitivity to appropriate responding.54 Values for sensitivity index vary from −1 to +1 (see Figure 1), with +1 indicating that all signal events were responded to while all nonsignal events were inhibited from responding to, whereas 0 indicates chance levels of distinguishing between signal and nonsignal events. To mirror the use of sensitivity index, the nonparametric response bias measure responsivity index54 was chosen to provide a measure of the ‘tendency to respond', with lower numbers indicating a conservative response strategy, whereas higher numbers equating to liberal responding.54, 55, 56 Both sensitivity index and responsivity index are based on the same geometric logic and are appropriate for use with single choice procedures (that is, responding or not).55
Animal subjects
Male C57BL/6N mice (n=16) were obtained from Jackson Laboratories (Bar Harbor, ME, USA) at ∼3 months of age (20–30 g). Mice were housed separately in groups of maximum 4 per cage and maintained at 85% of free-feeding weight, with water available ad libitum, and housed in a vivarium on a reversed day/night cycle (lights off at 0700 and on at 1900 h). Mice were brought to the laboratory 30 min before testing between 0900 and 1200 h. Procedures were approved by the UCSD institutional animal care and use committee and conformed to the National Institutes of Health (NIH) Guidelines.
Animal 5C-CPT apparatus
Training and testing took place in four 5-hole operant chambers (25 × 25 × 25 cm, Med Associates, St Albans, VT, USA). Each chamber consisted of an array of five square holes (2.5 × 2.5 × 2.5 cm) arranged horizontally on a curved wall 2.5 cm above the grid floor opposite a food delivery magazine (Lafayette Instruments, Lafayette, IN, USA) at floor level and a house light near the ceiling. The chamber was located in a sound-attenuating box, ventilated by a fan that also provided a low level of background noise. An infrared camera installed in each chamber enabled the monitoring of performance during training and testing. Mice were trained to respond with a nose-poke to an illuminated LED recessed into the holes. Responses were detected by infrared beams mounted vertically located 3 mm from the opening of the hole. Liquid reinforcement in the form of strawberry milkshake (Nesquik (Vevey, Switzerland) plus non-fat milk, 30 μl) was delivered by peristaltic pump (Lafayette Instruments) to a well located in the magazine opposite the 5-hole wall. Magazine entries were monitored using an infrared beam mounted horizontally, 5 mm from the floor and recessed 6 mm into the magazine. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates) using custom programming.33
Animal 5C-CPT training and testing
In order to learn the basic response patterns required for the 5C-CPT, mice were first trained in the 5-CSRTT daily, 5 days per week as described previously.57 Each session lasted 30 min or 120 trials, whichever was completed first. Each trial was initiated by the mouse nose-poking, then removing its nose from the magazine. After a 5-s ITI, a light stimulus appeared in one of the five apertures located opposite the magazine. A nose-poke in the lit aperture during the stimulus duration (SD) plus a 2-s limited hold period resulted in a correct (Hit) response being registered and a reward being delivered in the magazine. A nose-poke in any other aperture over this period was registered as an incorrect response and resulted in a 4-s timeout (TO). Failure to respond in any aperture during the SD+limited hold was registered as an omission (omission+incorrect=Miss) and also resulted in a TO. Response in any aperture during the ITI registered a premature response and triggered a TO. The next trial began when the mouse entered, then exited the magazine. The SD started at 20 s and was reduced to 10, 8 and 4 s after the attainment of each criterion (a mean correct latency less than half the current SD for two consecutive days) across sessions. At this point, mice were transferred to a variable ITI (3–7 s). Once performance stabilized (∼3 days), the mice were then transferred to the 5C-CPT (Figure 1). For the 5C-CPT, 100 trials were target trials, identical to trials described in the 5CSRTT where a cue stimulus appeared in any 1 of the 5 apertures, and 20 trials were nontarget trials, unique to the 5C-CPT, in which all 5 apertures were illuminated and the mouse was required to inhibit from responding. Consistent with human CPTs,30 successful inhibition of a response to a nontarget stimulus resulted in a correct rejection being recorded and reward delivered. Responding to a nontarget stimulus however, resulted in a false alarm (FA) being registered and a TO occurring. These nontarget stimuli were interspersed pseudorandomly within the 100 target trials (maximum of 3 sequential nontarget trials). False alarm latency was also recorded. Training took ∼45 training sessions from 5-CSRTT to criterion in the 5C-CPT.
Assessing the effects of scopolamine on animal 5C-CPT
All C57BL/6N mice had been exposed previously to nicotine in a single study.58 After a 2-week washout period, scopolamine was administered (vehicle, 0.1, 0.3 and 1 mg kg−1, intraperitoneal, 15 min preinjection) in a crossover design. Vehicle (saline) was administered on Thursday, Friday and Monday to acclimate the mice to receiving injections. Allocated doses of scopolamine or vehicle were administered on Tuesday and Friday for 2 weeks, with vehicle administration occurring on the day before scopolamine administration. On each drug-challenge day, the 5C-CPT performance of mice was challenged in the extended session task challenge of 240 trials with a variable SD (0.75, 1.25 and 2.00 s).
Drug preparation
Scopolamine hydrobromide was purchased from Sigma-Aldrich (St Louis, MO, USA), and dissolved in saline. Scopolamine was administered at 5 ml kg−1 intraperitoneally in a 15-min preinjection time. Doses were chosen based on scopolamine-induced disruption of preference in mice59 and impairment of mouse 5-CSRTT performance.60, 61, 62
Statistical analysis
Human 5C-CPT performance was analyzed using a one-way analysis of covariance with groups as a between-subject factor, trial block as a within-subject factor and years of education entered as a covariate because groups differed on this measure. Performance was also analyzed over three-trial blocks and compared between groups using a two-way repeated measures analysis of covariance with trial block as a within-subject factor, group as a between-subject factor and years of education as a covariate. Tukey's post hoc analyses were performed on main effects or interactions with trial block. Mouse 5C-CPT performance was analyzed using a two-factor repeated measures analysis of variance with drug and trial block as within-subject factors. Data were analyzed using SPSS 19.0 (Chicago, IL, USA). The α was set to 0.05.
Results
Human 5C-CPT assessment
Overall performance
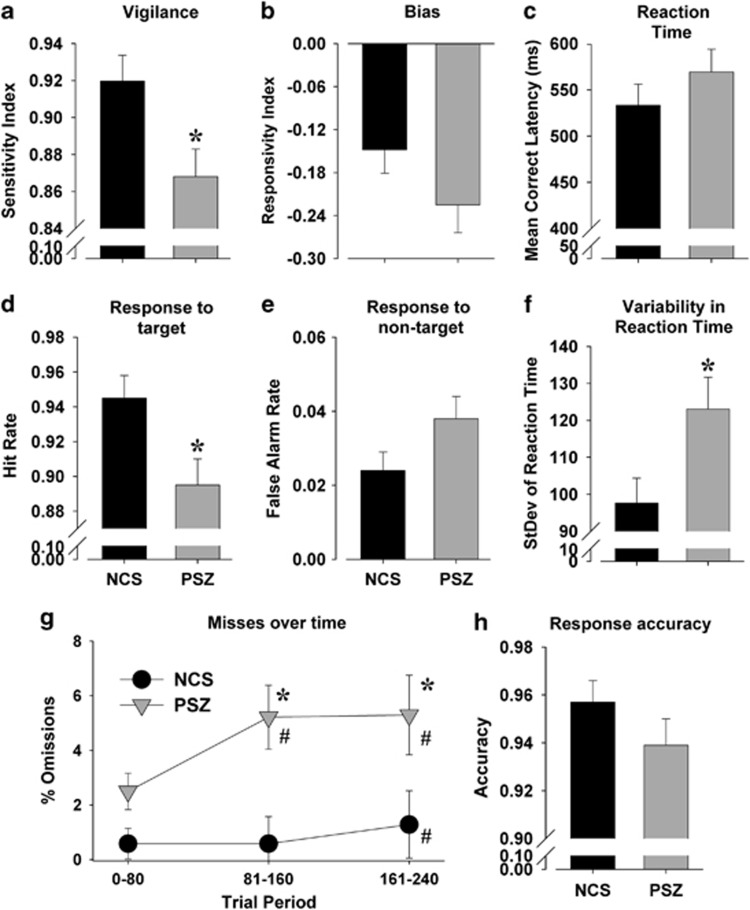
People with SZ exhibited significantly poorer performance relative to NCS as measured by their sensitivity index (F(2, 40)=5.5, P<0.01; Figure 2a). There were no significant differences between groups in response strategy (bias), as measured by responsivity index (F(2, 40)=1.9, nonsignificant (NS); Figure 2b) or reaction time (F(2, 40)=2.2, P=0.12; Figure 2c). As hypothesized, the poorer performance of SZ patients was driven by fewer responses to target stimuli (F(2, 40)=5.1, P<0.05; Figure 2d), as no differences in nontarget responding were observed (F(2, 40)=1.5, NS; Figure 2e). SZ patients also exhibited greater reaction time variability (F(2, 40)=4.8, P<0.01; Figure 2f). Not a single premature response was recorded for either group and accuracy to responding to the target location also did not differ (F<1, NS; Figure 2h).
Figure 2.
The performance of people with schizophrenia (SZ) and healthy nonpsychiatric comparison subjects (NCS) in the 5-choice continuous performance test (5C-CPT). People with SZ (n=20) exhibited impaired attention compared with NCS (n=23) as measured by a reduced sensitivity index (a). This attentional deficit was unlikely because of changes in bias of responding given the lack of difference in responsivity index between the two groups (b). Reaction times were slower in SZ but not significantly so (c). Poorer attention was driven by a significant reduction in hit rate (d), with only a modest increase in false alarm responses (e). Variability in reaction time was significantly larger in patients with SZ (f). The poorer overall hit rate of SZ subjects was driven by increased misses over time with a decrement in performance occurring earlier and to a greater extent than NCS (g). Although the accuracy of performance was reduced in people with SZ compared with NCS, this difference was not significant (h). Data presented as mean+s.e.m., *P<0.05 when compared with NCS, #P<0.05 when compared with trial period 0–80.
Within-session performance
No effects of trial period or trial period × group interaction were observed for overall measures of sensitivity or responsivity indices, reaction time or accuracy (F<1.8, NS). A group × trial block interaction was observed for percent omissions (F(2, 80)=4.2, P<0.05; Figure 2g), however, with post hoc analysis of covariances revealing a significant block effect, indicative of performance decrements over time that was evident in both NCS (F(2, 42)=4.5, P<0.05) and SZ (F(2, 36)=4.3, P<0.05) groups. Tukey's post hoc analyses revealed that NCS exhibited a higher proportion of omissions in trial period 3 compared with trial period 1 (P<0.05), whereas SZ subjects exhibited higher proportion of omissions in trial periods 2 and 3 compared with trial period 1 (P<0.05). Finally, when analyzed within trial periods, patients exhibited increased omissions compared with NCS in trial periods 2 (F(2, 40)=3.7, P<0.05) and 3 (F(2, 40)=4.2, P<0.05), but not trial period 1 (F(2, 40)=2.0, NS) that, taken together, demonstrate that SZ patients exhibited a greater decrement in performance over time in a manner that is indicative of a sustained attention impairment.
The effects of scopolamine on mouse 5C-CPT performance
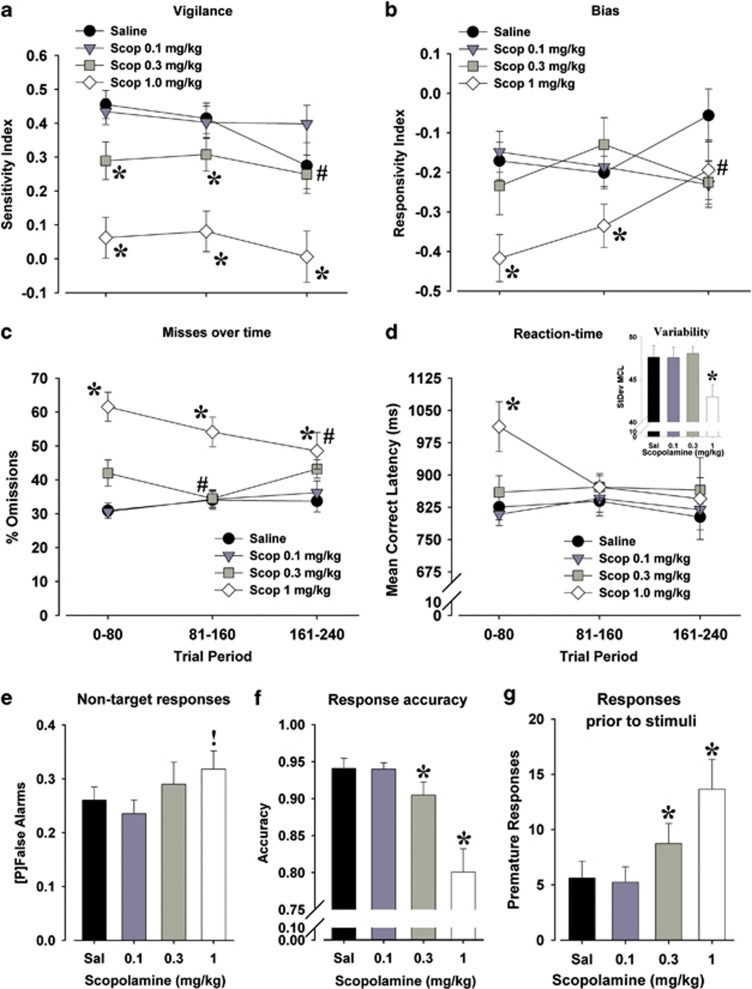
Consistent with findings in SZ, scopolamine impaired mouse 5C-CPT performance as demonstrated by reduced sensitivity index (F(3, 48)=9.2, P<0.0001; Figure 3a) at the highest dose when compared with vehicle (P<0.05). Importantly, a vigilance decrement was observed in vehicle-treated mice (F(2, 34)=3.9, P<0.05; Figure 3a), where their sensitivity index in trial period 3 was worse than trial period 1 (P<0.05), and trending worse than trial period 2 (P<0.1). However, the sensitivity index did not differ over trial periods in mice treated with scopolamine at any dose (F<1, NS). Scopolamine deleteriously affected the sensitivity index in each trial period (F(3, 48)>7, P<0.001), where 0.3 and 1 mg kg−1 lowered performance in trial periods 1 and 2, whereas 1 mg kg−1 also lowered performance in trial period 3 when compared with vehicle (P<0.05).
Figure 3.
The effects of scopolamine (Scop; 0.1, 0.3 and 1.0 mg kg−1) on mouse performance of the 5-choice continuous performance test (5C-CPT). Scopolamine at 0.3 and 1.0 mg kg−1 impaired the attention of mice as measured by the sensitivity index, which occurred in all three trial periods at 1.0 mg kg−1, but only in the first and second trial periods at 0.3 mg kg−1 because of performance dropping in saline-treated mice in the third trial period (a). Bias was largely unaffected by trial period except in the mice treated with 1.0 mg kg−1 scopolamine who exhibited an initially more conservative response profile (reduced responsivity index) that normalized with saline by trial period 3 (b). Scopolamine impaired attention in mice primarily by increasing the number of targets missed, occurring at every trial period for mice treated with 1.0 mg kg−1, although performance improved over time (c). Slowed reaction time of mice treated with scopolamine (1.0 mg kg−1) was primarily observed in the first trial period only (d), with the highest dose reducing the variability of reaction time (inset). Scopolamine treatment tended to increase the probability [P] of make a false alarm response to nontargets (e). Scopolamine treatment reduced accuracy of responding in mice to lit vs unlut holes (f). Finally, scopolamine increased the propensity of mice to respond before a stimulus appearing (premature responses; g). Data presented as mean±s.e.m., *P<0.05; !P<0.1 when compared with vehicle-treated mice; #P<0.05 compared with trial period 1.
Overall, scopolamine treatment did not affect bias of responding in mice (F(3, 48)=1.5, NS; Figure 3b) although there was an interaction with trial period (F(6, 102)=2.8, P<0.05). Only 1 mg kg−1 significantly affected bias over time (F(2, 34)=5.8, P<0.01), with a higher response rate in trial period 3 compared with 1 and 2 (P<0.05). This effect was consistent with a lower bias in 1 mg kg−1 scopolamine-treated mice in trial period 1 (P<0.05), a trend to lower in trial period 2 (P<0.1) and equivalent to vehicle in trial period 3 (P>0.1).
Scopolamine-treatment tended to slow target responding in mice (F(3, 48)=2.3, P<0.1; Figure 3c), which interacted with time (F(6, 102)=2.2, P=0.055). No dose individually differed from vehicle (P>0.1), nor did any dose change across trial periods (F<2, NS). Significant post hoc analyses were limited to 1 mg kg−1 scopolamine-treated mice being slower than vehicle-treated mice in trial period 1 only (P<0.05). Latencies to incorrect, false alarm or premature responses were not affected by scopolamine (F<1.4, NS), nor did they interact with trial period. Scopolamine treatment decreased the variability in the latency of target responding (F(3, 48)=4.5, P<0.01; see inset in Figure 3c), an effect that did not interact with trial period (F<1, NS). Scopolamine treatment at 1 mg kg−1 reduced such variability compared with vehicle (P<0.05).
Consistent with SZ, the scopolamine-induced impairment of attention was driven primarily by increased percent omissions (F(3, 48)=6.0, P<0.005; Figure 3d), with 0.3 and 1 mg kg−1 doses differing from vehicle (P<0.1 and 0.05, respectively). Scopolamine treatment and trial period interacted on the effect of target responses (F(6, 102)=2.6, P<0.05). No trial period effect was observed for saline-treated mice (F<1.2, NS), whereas scopolamine doses affected target responses over time with a trend at 0.1 mg kg−1 (F(2, 34)=3.1, P<0.1), a significant effect at 0.3 mg kg−1 (F(2, 34)=4.6, P<0.01) but no effect at 1 mg kg−1 (F<1.8, NS). Both 0.1 and 0.3 mg kg−1 scopolamine-treated mice exhibited lower percent omissions in trial period 3 compared with 1, whereas 0.4 mg kg−1 also differed between trial periods 2 and 1 (P<0.05).
Again, similar to SZ, scopolamine also modestly increased nontarget responses (F(3, 48)=3.1, P<0.05; Figure 3e), as 1 mg kg−1 produced a trend-level increase in the false alarm rate (P<0.1). No interaction of scopolamine treatment and trial period was observed (F<1, NS). Unlike SZ, however, scopolamine treatment reduced the accuracy of responding in mice (F(3, 48)=3.1, P<0.05; Figure 3f) at 0.3 and 1.0 mg kg−1 doses (P<0.05 compared with vehicle). Scopolamine treatment also increased premature responding in mice (F(3, 48)=3.6, P<0.05; Figure 3g) at both 0.3 and 1 mg kg−1 when compared with vehicle (P<0.05). Scopolamine effects did not interact with trial period in either accuracy or premature responses (F<1, NS).
Discussion
The ability of people to maintain vigilance to relevant stimuli over a period of time is a necessity for optimal functioning in daily activities. The results of this study indicate that the human 5C-CPT is sensitive to detecting specific and global impairments of vigilance in SZ. These deficits are driven by misses to target stimuli that become exaggerated over time (that is, a vigilance decrement). Importantly, mice can also perform the 5C-CPT and exhibit performance indices that are comparable to those observed in humans. Treatment with the general mAChR antagonist scopolamine impaired mouse 5C-CPT performance in a pattern that shared some parallels with, but did not exactly recreate, the deficit profile exhibited by SZ patients. Thus, the present results support the emerging view of the 5C-CPT as a reverse-translational cross-species test that can characterize specific attentional deficits in people with SZ. The 5C-CPT provides a methodology for cross-species studies to develop animal models and screen putative proattentive therapeutics.
Impaired vigilance in patients with SZ
As noted above, the present data indicate that the 5C-CPT is sensitive to impaired vigilance in SZ. These deficits are manifested primarily via reduced target responses with a concomitant increased variability in reaction time, consistent with many other CPT tasks that have been widely used in human studies,35, 36 including the Conner's CPT37, 38 and the degraded stimulus-CPT.39, 40, 41 Indeed, people with SZ exhibit similar impaired CPT performance irrespective of the use of auditory, visual or combined auditory/visual stimuli.63 An apparent increase in nontarget responses, although not significant, also suggested that SZ subjects exhibited difficulties in inhibiting responses to nontarget stimuli. Thus, despite a reduced rate of detecting targets, the bias (rate of responding) was unaltered in people with SZ, which is consistent with other CPT studies.64, 65 The lack of altered response bias supports the hypothesis that the impaired sensitivity index of patients with SZ reflects impaired attentional performance.38, 41, 66
The significant increases in missed targets in SZ across testing blocks in the 5C-CPT is consistent with the long-standing view since Kraepelin and Bleuler that patients cannot maintain their vigilance over time.67 Although SZ subjects in the Raindrops Attentional Task designed by Hahn et al.67 demonstrated increased missed targets after 10 min, modest deficits were detectable in the 5C-CPT after only 6 min. When the 5C-CPT task length is extended to 24 min, even greater vigilance decrements are observed (Young JW and Light GA, unpublished observations). That the Raindrops Task and the current 5C-CPT revealed such impaired attention over time contrasts with other studies in SZ (see, for example, refs, 19 and 26) and could reflect the use of multiple stimuli that the subject must attend to requiring selective attention, as opposed to one stimulus in the center of the visual field as is typical for most CPTs.25, 30, 68
The 5C-CPT measures other aspects of attention that are also measured in the 5-CSRTT. For example, accuracy of responding to stimuli (lit) vs blank locations and responding before a stimulus presentation (premature response) can be measured in both tasks. In the present 5C-CPT studies, SZ patients exhibited comparable accuracy to NCS. In addition, no premature responses were registered in either group, supporting the hypothesis that this variable does not reflect impaired attention and/or impulsivity associated with SZ. Moreover, these data support a dissociation in the mechanisms subserving false alarms and premature responses, as has been demonstrated in mice.34 Patients with SZ and NCS responded to nontarget stimuli (false alarms), but did not respond when no stimuli were present (premature responses). Premature responses have been observed in children with attention deficit hyperactivity disorder performing a stop signal reaction time task69 or a CPT variant,70 although the latter is not always observed in children with attention deficit hyperactivity disorder71 or children at risk for SZ72 in this CPT variant. Premature response errors may therefore be more prominent in children than adults, in some clinical populations more than others, or require specialized equipment to detect.73 Future studies are needed to investigate whether premature responses can be observed in patients with attention deficit hyperactivity disorder and/or in NCS with manipulations to the current task. When SZ patients were tested in the human 5-CSRTT, which requires only responses to targets, premature responses have not been reported, nor have differences in accuracy, but patients exhibited increased variability of reaction time.74 This deficit has been described as evidence of poor attention and/or visual motor skills in patients.75, 76 The present data therefore support the importance of including nontarget stimuli for disentangling the inattention associated with SZ, as measured by increased nonresponses to relevant (target) stimuli over time.
Although some studies suggest an association of attentional dysfunction as measured by CPTs and severity of positive77, 78 or negative79 symptoms in people with SZ, no significant associations of performance in the 5C-CPT and clinical symptoms (SAPS/SANS) were observed in this cohort. A robust negative correlation (r=−0.66) was observed, however, between responsivity bias and clinician ratings of psychosocial functioning, with more conservative responding being associated with better daily functioning.
Scopolamine-induced disruption of attention in mice
Before the human and rodent 5C-CPT paradigms are deployed in larger-scale clinical trials or in more extensive preclinical assays, some critical questions must be answered. For example, how well do the rodent 5C-CPT studies model the similarly observed deficits in people with SZ? In this initial proof-of-concept study, mice undergoing a scopolamine challenge were assessed in order to compare human and rodent behavioral patterns of responding. As predicted, scopolamine impaired overall mouse performance of the 5C-CPT with many similarities to people with SZ. A scopolamine-induced deficit in vigilance was observed, with no evidence of an effect on biased responding or reaction time. As in SZ, the poorer performance of scopolamine-treated mice was driven by a reduction in target responses, with a modest increase in nontarget responses. Unlike SZ, however, scopolamine-treated mice exhibited other effects including impaired accuracy with reduced rather than increased reaction time variability. These mice also exhibited increased premature responses whereas none were recorded in humans. Scopolamine may therefore exert a wider range of effects that do not parallel those seen in patients with SZ. The present data are consistent with the literature whereby scopolamine-induced effects on accuracy and premature responses were also reported in mice performing the 5-CSRTT.60, 61, 62
In contrast to mouse 5-CSRTT studies, the present study demonstrated a vigilance decrement in task performance of mice treated with saline, which is consistent with other reports in mice and rats.31, 32, 33, 34 This contrast between studies is likely because this decrement was observed as a reduced sensitivity index (not available in the 5-CSRTT) but not accuracy (the primary 5-CSRTT performance variable). However, scopolamine-treated mice did not exhibit a vigilance decrement, with vigilance being worse in every trial period. On examination of the percentage of target omissions, the measure affected over time in SZ subjects, mice treated with scopolamine actually improved over time. This improved performance over time after scopolamine treatment is likely a result of its pharmacokinetic properties with a half-life of 40 min.80 Scopolamine-induced deficits of human CPT performance are observed for up to 7 h, which likely reflects the longer half-life of scopolamine in humans.81, 82
Thus, with the primary attentional deficits of people with SZ being impaired vigilance that worsens over time, scopolamine-induced disruption in performance is an imperfect model. Of course, in the present study we examined the performance in patients currently stable on a variety of antipsychotic medications, and hence a proper comparison would be to coadminister to mice scopolamine with chronic dopamine D2 blockade.83 Although future studies could explore the effects of such coadministration, the effects of scopolamine alone may better model the impaired attention observed in patients with Alzheimer's disease,84, 85 where an exacerbation of deficits over time only occurs with degraded86 but not distinct86, 87 stimuli. Given that distinct mAChRs differentially affect pre- and post-synaptic activity in the cortex,88 producing more distinct behavioral effects,89, 90 future studies could also compare the effects of more selective mAChR ligands on mouse 5C-CPT performance.
Cross-species 5C-CPT performance comparison
It is clear that mice,33, 34, 91 rats31, 32 and humans (present studies) can perform the 5C-CPT. The current study enables the comparison of performance of mice and humans on the task. Because verbal instructions are available for humans, extended training is not required, although a practice session was provided to acclimatize the subject to the joystick and stimulus presentation. Mice, on the other hand, took ∼4 months to train to a stable level of performance in the 5C-CPT. The human task lasted only 8 min, whereas the mouse challenge lasted 60 min in the present studies. The difference in length may mediate the clear vigilance decrement described here in mice and in previous studies,33 whereas only an increase in omissions was observed in humans. This possibility is supported by observations of the emergence of a significant vigilance decrement in a longer (that is, 24 min) human 5C-CPT (Young JW and Serences J, unpublished observations). In terms of absolute performance on the task, mice exhibit poorer performance across all measures (for example, sensitivity index scores of 0.4 compared with human scores of 0.9; 1.0 being perfect, see Table 1). Although the proportion of target responses is lower in mice (0.62) compared with humans (0.94), this difference is not driven by a reduced response rate as the proportion of nontarget responses is higher in mice (0.26) as compared with humans (0.02). Hence, humans (−0.13) and mice (−0.13) exhibit a comparable response bias, indicating that they are less likely to respond than at chance levels. The quantitative performance levels differed between the two species with human vigilance levels almost perfect (0.92), whereas mice were roughly between human levels and chance (0.45). Such differences between rats and humans were also observed in the sustained attention task. In contrast, qualitatively similar effects of distraction were observed between the two species.29 Similarly, in the present studies, although quantitative differences in attentional performance were observed between mice and humans, the similar drop in performance over time provides a qualitative similarity, supporting that the 5C-CPT assesses similar attentional processes across species. When attempting to match human and rodent performance levels in another paradigm,92 were required to use longer SDs and larger stimuli. By maintaining the mouse task parameters for consistency with previous reports,33, 34, 91 meaningful comparisons could be made to these reports. Interestingly, the level of bias shown by mice and humans is similar to that reported in rats,31, 32 suggesting similar strategies of responding at least. Investigating the differential effects of distractors on task performance across species, as has been done in the dSAT,29 may provide further information on possible qualitative differences between the species. Interestingly, genetic abnormalities such as 5-HT3 and catechol-O-methyltransferase allelic variations can affect CPT performance.93, 94, 95 Transgenic mice with these humanized allelic variants could enable further cross-species comparisons mediating attentional performance. Given that the 5C-CPT was: (1) originally developed for use in mice;33, 34 (2) has also been used to study rat models of behavior relevant to SZ;31, 32 (3) is amenable to functional magnetic resonance image testing;96, 97 and (4) is sensitive to impaired attention in patients with SZ (present studies), the 5C-CPT may provide a viable translational tool with which to examine vigilance deficits in neuropsychiatric patients,27 test putative models98, 99 and discover treatments for such disorders.58 Although future studies will incorporate each of these points, they will also determine the consistency of human 5C-CPT performance with more established attentional measures.
In summary, the human 5C-CPT is sensitive to impaired attention in patients with SZ. Specifically, people with SZ appear more sensitive than NCS to a decrement in hit rate over time. Although there was significant overlap in the pattern of human/mouse results in the four conditions presented (NCS and SZ, placebo- and scopolamine-treated mice), the scopolamine-induced 5C-CPT deficits appear to provide an imperfect model of attentional dysfunction in SZ because of the lack of vigilance decrement sensitivity. Nonetheless, the present studies demonstrate substantial translational homology using this attentional task. Finally, although the cross-species availability of the 5C-CPT may benefit SZ research, impaired CPT performance is also present in other neuropsychiatric populations such as bipolar disorder, attention deficit hyperactivity disorder and Alzheimer's disease. It will therefore be useful to assess these clinical populations to determine the pattern of the responding they exhibit and compare this pattern with the abnormalities seen in people with SZ.
Acknowledgments
We thank Ms Mahalah Buell, Joyce Sprock and Marlena Pela for their assistance. These studies were supported by NIH Grants R01-MH071916, R01-MH079777 and R01-MH042228, as well as by the Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center and NARSAD Awards.The funding support did not provide any role in the design, collection or analysis of studies.
The authors declare no conflict of interest.
References
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Mintz J, Kopelowicz A.CUtLASS confirms CATIE Arch Gen Psychiatry 200764978author reply 9-80. [DOI] [PubMed] [Google Scholar]
- Parks JJ, Radke AQ, Tandon R. Impact of the CATIE findings on state mental health policy. Psychiatr Serv. 2008;59:534–536. doi: 10.1176/ps.2008.59.5.534. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia. Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Kern RS, Baade LE, Fenton WS, Gold JM, et al. Functional co-primary measures for clinical trials in schizophrenia: results from the MATRICS Psychometric and Standardization Study. Am J Psychiatry. 2008;165:221–228. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Olivier B, Joels M, Kahn RS. From antipsychotic to anti-schizophrenia drugs: role of animal models. Trends Pharmacol Sci. 2012;33:515–521. doi: 10.1016/j.tips.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Geyer MA, Gold LH, Grace AA. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull. 2005;31:888–894. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- Sarter M. Animal cognition: defining the issues. Neurosci Biobehav Rev. 2004;28:645–650. doi: 10.1016/j.neubiorev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenology. E&S Livingston: Edinburgh, UK; 1919. [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. International Universities Press: New York, NY; 1950. [Google Scholar]
- Marder SR. The NIMH-MATRICS project for developing cognition-enhancing agents for schizophrenia. Dialogues Clin Neurosci. 2006;8:109–113. doi: 10.31887/DCNS.2006.8.1/smarder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Arnsten A, Buchanan RW, Cohen JD, Geyer M, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schizophr Bull. 2009;35:109–114. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA. New opportunities in the treatment of cognitive impairments associated with schizophrenia. Curr Dir Psychol Sci. 2010;19:264–269. [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pineda P, Baeza I, Perez-Gomez M, Vendrell P, Junque C, Bargallo N, et al. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19 (2 Pt 1:365–375. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- Kleinlogel H, Strik W, Begre S. Increased NoGo-anteriorisation in first-episode schizophrenia patients during Continuous Performance Test. Clin Neurophysiol. 2007;118:2683–2691. doi: 10.1016/j.clinph.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophr Bull. 2009;35:182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SY, Chen PS, Yang YK, Liao YC, Lee YD, Yeh TL, et al. Correlation between performance on the Continuous Performance Test and economic costs in patients with schizophrenia. Psychiatry Clin Neurosci. 2003;57:373–377. doi: 10.1046/j.1440-1819.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Light G, Palumbo DR. The sustained attention test: a measure of attentional disturbance. Computers in Human Behavior. 1998;14:463. [Google Scholar]
- Borgaro S, Pogge DL, DeLuca VA, Bilginer L, Stokes J, Harvey PD. Convergence of different versions of the continuous performance test: clinical and scientific implications. J Clin Exp Neuropsychol. 2003;25:283–292. doi: 10.1076/jcen.25.2.283.13646. [DOI] [PubMed] [Google Scholar]
- Mackworth NH. Researches on the Measurement of Human Performance. His Majesty's Stationery Office: London; 1950. [Google Scholar]
- Parasuraman R. The Attentive Brain. MIT Press: Cambridge; 1998. [Google Scholar]
- Nuechterlein KH.Vigilance in schizoprhenia and related disordersIn: Steinhauer SR, Gruzelier JH, Zubin J (eds).Neuropsychology, Pscyhophysiology, and Information Processing Handbook of Schizophrenia Elsevier Science: New York; 1991397–433. [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW.CNTRICS final animal model task selection: Control of attention Neurosci Biobehav Rev 2012(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Morris R, Evenden J, Sahakian B, Robbins T.Computer aided assessment of dementia: comparative studies of neuropsychological deficits in Alzheimer's type dementia and Parkinson's diseaseIn: Stahl S, Iversen S, Goodman E (eds).Cognitive Neurochemistry Oxford University Press: Oxford; 198721–36. [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention. Arch Clin Neuropsychol. 2002;17:235–272. [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. D(1) receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology (Berl) 2011;220:129–141. doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology. 2011;62:1432–1441. doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund BR, Orbeck AL, Landro NI. Vigilance deficits in schizophrenics and affectively disturbed patients. Acta Psychiatr Scand. 1992;86:207–212. doi: 10.1111/j.1600-0447.1992.tb03253.x. [DOI] [PubMed] [Google Scholar]
- Bedwell JS, Kamath V, Baksh E. Comparison of three computer-administered cognitive tasks as putative endophenotypes of schizophrenia. Schizophr Res. 2006;88:36–46. doi: 10.1016/j.schres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Kelley M, Egan G, Green A, Wilcox L, Boshoven W, et al. Lack of relationship between acoustic startle and cognitive variables in schizophrenia and control subjects. Psychiatry Res. 2011;187:324–328. doi: 10.1016/j.psychres.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker PH, Tsai J, Henninger LL, Vohs JL, Viverito K. Decrements in sustained attention across trials in a continuous performance test: associations with social functioning in schizophrenia. J Nerv Ment Dis. 2010;198:154–158. doi: 10.1097/NMD.0b013e3181cc5215. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Faux SF, McCarley RW, Shenton ME, Sands SF. Measurement of visual sustained attention in schizophrenia using signal detection analysis and a newly developed computerized CPT task. Schizophr Res. -Dec. 1990;3:329–332. doi: 10.1016/0920-9964(90)90018-3. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Faux SF, McCarley RW, Sands SF, Horvath TB, Peterson A. Neuroleptics improve sustained attention in schizophrenia. A study using signal detection theory. Neuropsychopharmacology. 1991;4:145–149. [PubMed] [Google Scholar]
- Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. J Abnorm Psychol. 1983;92:4–28. doi: 10.1037//0021-843x.92.1.4. [DOI] [PubMed] [Google Scholar]
- Raedler TJ. Comparison of the in-vivo muscarinic cholinergic receptor availability in patients treated with clozapine and olanzapine. Int J Neuropsychopharmacol. 2007;10:275–280. doi: 10.1017/S1461145706006584. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2012;62:1544–1553. doi: 10.1016/j.neuropharm.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Rissling AJ, Pascual-Marqui R, Kirihara K, Pela M, Sprock J, et al. Neural substrates of normal and impaired preattentive sensory discrimination in large cohorts of nonpsychiatric subjects and schizophrenia patients as indexed by MMN and P3a change detection responses. Neuroimage. 2012;66C:594–603. doi: 10.1016/j.neuroimage.2012.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Braff DL, Swerdlow NR, Hellemann G, Rassovsky Y, Sprock J, et al. Disentangling early sensory information processing deficits in schizophrenia. Clin Neurophysiol. 2012;123:1942–1949. doi: 10.1016/j.clinph.2012.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSMIV Axis 1. Disorders - Patient Edition. New York State Psychiatric Institute: New York; 1995. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa: Iowa City; 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa: Iowa City; 1984. [Google Scholar]
- Hall RC. Global assessment of funcitoning; a modified scale. Pscyhosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Hunter MA, Huang M, Smith AK, Chen Y, Sadek J, et al. Termporal and frontal cortical thickness associations with M100 auditory gating and attention in healthy controls and individuals with schizophrenia. Schiz Res. 2012;140:250–257. doi: 10.1016/j.schres.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakvik B, Stiles T, Hugdhal K. Experiencing malevolent voices is associated with attentional dysfunction in psychotic patients. Scand J Psychol. 2013;54:72–77. doi: 10.1111/sjop.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley & Sons: New York; 1966. [Google Scholar]
- McNicol D. A Primer of Signal Detection Theory. George Allen & Unwin: London; 1972. [Google Scholar]
- Frey PW, Colliver JA. Sensitivity and responsibility measures for discrimination learning. Learn Motiv. 1973;4:327–342. [Google Scholar]
- Marston HM. Analysis of cognitive function in animals, the value of SDT. Brain Res Cogn Brain Res. 1996;3:269–277. doi: 10.1016/0926-6410(96)00012-2. [DOI] [PubMed] [Google Scholar]
- Sahgal A. Some limitations of indices derived from signal detection theory: evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology (Berl) 1987;91:517–520. doi: 10.1007/BF00216022. [DOI] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013;240:119–133. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe JP, Nielsen EO, Christensen JK, Peters D, Timmermann DB, Olsen GM. Alpha7 nicotinic acetylcholine receptor activation ameliorates scopolamine-induced behavioural changes in a modified continuous Y-maze task in mice. Eur J Pharmacol. 2009;602:58–65. doi: 10.1016/j.ejphar.2008.09.035. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Fransen F, Duytschaever H, Grantham C, Megens AA. Attentional performance of (C57BL/6Jx129Sv)F2 mice in the five-choice serial reaction time task. Physiol Behav. 2006;89:692–703. doi: 10.1016/j.physbeh.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–587. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Mussgay L, Hertwig R. Signal detection indices in schizophrenics on a visual, auditory, and bimodal Continuous Performance Test. Schizophr Res. 1990;3:303–310. doi: 10.1016/0920-9964(90)90014-x. [DOI] [PubMed] [Google Scholar]
- Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Report on a continuous performance test. Arch Gen Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, James GA, Boshoven W, Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res. 2011;125:169–173. doi: 10.1016/j.schres.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Matveeva TM, Harvey AN, Luck SJ, et al. Kraepelin and Bleuler had it right: people with schizophrenia have deficits sustaining attention over time. J Abnorm Psychol. 2012;121:641–648. doi: 10.1037/a0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci. 2001;13:326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol. 2003;31:315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Wada N, Yamashita Y, Matsuishi T, Ohtani Y, Kato H. The test of variables of attention (TOVA) is useful in the diagnosis of Japanese male children with attention deficit hyperactivity disorder. Brain Dev. 2000;22:378–382. doi: 10.1016/s0387-7604(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Huang YS, Chao CC, Wu YY, Chen YY, Chen CK. Acute effects of methylphenidate on performance during the Test of Variables of Attention in children with attention deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2007;61:219–225. doi: 10.1111/j.1440-1819.2007.01653.x. [DOI] [PubMed] [Google Scholar]
- Ozan E, Deveci E, Oral M, Karahan U, Oral E, Aydin N, et al. Neurocognitive functioning in a group of offspring genetically at high-risk for schizophrenia in Eastern Turkey. Brain Res Bull. 2010;82:218–223. doi: 10.1016/j.brainresbull.2010.04.013. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. Ocular-motor delayed-response task performance among schizophrenia patients. Neuropsychobiology. 1996;34:67–71. doi: 10.1159/000119294. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34:1161–1177. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Stip E, Poulin J, Melun JP, Godbout R, Guillem F, et al. Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin. 2007;23:575–583. doi: 10.1185/030079906X167372. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Soholm B, Fink-Jensen A, Lublin H, Glenthoj BY. Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2007;30:3–12. doi: 10.1097/01.WNF.0000240940.67241.F6. [DOI] [PubMed] [Google Scholar]
- Nelson EB, Sax KW, Strakowski SM. Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. Am J Psychiatry. 1998;155:137–139. doi: 10.1176/ajp.155.1.137. [DOI] [PubMed] [Google Scholar]
- Pandurangi AK, Sax KW, Pelonero AL, Goldberg SC. Sustained attention and positive formal thought disorder in schizophrenia. Schizophr Res. 1994;13:109–116. doi: 10.1016/0920-9964(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Liu SK, Hwu HG, Chen WJ. Clinical symptom dimensions and deficits on the Continuous Performance Test in schizophrenia. Schizophr Res. 1997;25:211–219. doi: 10.1016/s0920-9964(97)00026-1. [DOI] [PubMed] [Google Scholar]
- Marks MJ, O'Connor MF, Artman LD, Burch JB, Collins AC. Chronic scopolamine treatment and brain cholinergic function. Pharmacol Biochem Behav. 1984;20:771–777. doi: 10.1016/0091-3057(84)90198-9. [DOI] [PubMed] [Google Scholar]
- Lenz RA, Baker JD, Locke C, Rueter LE, Mohler EG, Wesnes K, et al. The scopolamine model as a pharmacodynamic marker in early drug development. Psychopharmacology (Berl) 2012;220:97–107. doi: 10.1007/s00213-011-2456-4. [DOI] [PubMed] [Google Scholar]
- Ebert U, Siepmann M, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. J Clin Pharmacol. 1998;38:720–726. doi: 10.1002/j.1552-4604.1998.tb04812.x. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2012;62:1381–1390. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Tariot PN. Pharmacologic models of Alzheimer's disease. Psychiatr Clin North Am. 1991;14:287–308. [PubMed] [Google Scholar]
- Ebert U, Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- Berardi AM, Parasuraman R, Haxby JV. Sustained attention in mild Alzheimer's disease. Dev Neuropsychol. 2005;28:507–537. doi: 10.1207/s15326942dn2801_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Cocchini G, Della Sala S, Logie RH, Spinnler H. Working memory and vigilance: evidence from normal aging and Alzheimer's disease. Brain Cogn. 1999;41:87–108. doi: 10.1006/brcg.1999.1097. [DOI] [PubMed] [Google Scholar]
- Gigout S, Jones GA, Wierschke S, Davies CH, Watson JM, Deisz RA. Distinct muscarinic acetylcholine receptor subtypes mediate pre- and postsynaptic effects in rat neocortex. BMC Neurosci. 2012;13:42. doi: 10.1186/1471-2202-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS One. 2012;7:e35896. doi: 10.1371/journal.pone.0035896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Benignus VA, Case MW. Signal detection behavior in humans and rats: a comparison with matched tasks. Behavioral processes. 2003;64:121–129. doi: 10.1016/s0376-6357(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Lennertz L, Wagner M, Frommann I, Schulze-Rauschenbach S, Schuhmacher A, Kuhn KU, et al. A coding variant of the novel serotonin receptor subunit 5-HT3E influences sustained attention in schizophrenia patients. Eur Neuropsychopharmacol. 2010;20:414–420. doi: 10.1016/j.euroneuro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Markov V, Krug A, Krach S, Jansen A, Eggermann T, Zerres K, et al. Impact of schizophrenia-risk gene dysbindin 1 on brain activation in bilateral middle frontal gyrus during a working memory task in healthy individuals. Hum Brain Mapp. 2010;31:266–275. doi: 10.1002/hbm.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AH, Opgen-Rhein C, Urbanek C, Hahn E, Ta TM, Seidelsohn M, et al. COMT Val 158 Met polymorphism is associated with cognitive flexibility in a signal discrimination task in schizophrenia. Pharmacopsychiatry. 2009;42:141–144. doi: 10.1055/s-0028-1112132. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Dawes SE, Asgaard GL, Young JW. Abnormalities of brain responses during vigilance and inhibition in bipolar disorder. Bipolar Disord. 2011;13 (Suppl 1:42. [Google Scholar]
- McKenna B, Young JW, Dawes SE, Asgaard GL, Eyler LT. Bridging the bench to bedside gap: validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res. 2013;212:183–191. doi: 10.1016/j.pscychresns.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011;25:934–943. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]