Abstract
Introduction: In this work, the use of β-cyclodextrine (β-CD)-modified dendrimers as a nanocapsule with a biocompatible shell have studied. β-CD-modifieddendrimers have designed and synthesized to enhance the loading capacity of the final dendrimers with encapsulation properties. Methods: To achieve β-CD-modified dendrimers, first citric acid dendrimers were synthesized and then the end functional groups of dendrimers were grafted to β-CD through ester linkages. The molecular structures of resulted dendrimers were verified using common spectroscopic methods such as 1H NMR, FT-IR and the diameters of obtained nanocarriers were evaluated with using dynamic light scattering (DLS) experiments. The isolated dendrimers were utilized as the drug delivery agents and the encapsulation and the controlled release of guest drug molecule Naltrexone (NLX) was investigated in different pH’s using UV spectroscopy method. Results: It was established that the loading capacity of dendrimers depend on several factors such as their generation and the structure and number of conjugated modifier end groups. Conclusion: Increasing in the number of branches and the size of interior voids and number of conjugated β-CDs cause to enhance the loading capacity.
Keywords: Dendrimer, Nanocarrier, Citric acid, β-Cyclodextrin, Encapsulation, Naltrexone
Introduction
Polymeric drug delivery systems (DDS) can develop bioavailability, therapeutic efficacy and decrease of the side effects of clinically recognized drugs.1 Among these polymeric DDS, dendrimers are known as potential drug delivery systems in biomedical and pharmaceutical sciences in recent years.2,3Dendrimers, a new category of highly branched polymers with featured properties, such as a structurally well-defined, shape and numerous functional groups on the peripheral 4-8, monodispersity, nanoscopic architecture9,10 and hydrophobic or hydrophilic cavities in the interior region with host-guest entrapment properties.11,12 Considering these features, dendrimers prove perfect candidates for their applications in numerous fields such as drug delivery devices.13-16Several efforts have been made to design dendrimers as drug carriers. It has been shown that dendrimers can be covalently attached to the drug molecules 17,18 or encapsulates the drug molecules as a container. 19 In contrast, physically entrapped inside the dendritic structures are attentions to be useful because the drugs stay undamaged within the dendrimers. 9,20,21 Compared with free drug molecules, these non-covalent inclusions or complexes offer several featured advantages, such as enhancement of drug stability, solubility and controlled release of drugs from the matrixes. These properties together express that encapsulation with dendrimers is a general approach for drug release systems.
On the other hand, cyclodextrins (CDs) are cyclic oligosaccharides created with 6, 7 or 8 glucopyranoseunits respectively named as α-, β- or γ-cyclodextrin. Their hydrophobic interior (cavity) allows to form inclusion complexes with organic compounds through formation of non-covalent inclusion complexes. 22 β-CD have a polar exterior and apolar interior cavity 23 and is well known for its capability to form host–guest inclusion complexes with hydrophobic drugs.24,25 Encapsulation ability of CDs has found numerous applications in fields such as drug delivery systems. β-CD is the most positive to be solubilized of molecules but the solubilization capability of β-CD is restricted by its poor solubility in water (1.85 g/100 ml at 20 °C) 26 and almost unsolvable complexes are often achieved with greatly a polar molecules.22Diverse chemical modifications have been so far projected for improvement of solubility of β-CD and its complexes in aqueous medium, such as preparation of β-CD conjugates with biocompatible polar artificial polymers. 27,28 In recent times, it has been publicized that the merging of CDs into polymeric nanoparticles can enhance the drug loading and modifies drug release. 29,30
We have previously reported synthesis of some of linear–dendritic macromolecules from dendritic citric acid–poly (ethylene glycol)–dendritic citric acid and their application for encapsulation and release of some of guest molecules. 6,7,31 Hear the preparation of a citric acid-based dendrimers bearing β-CD as conjugated side-chains by using dicyclohexylcarbodiimide (DCC) as a coupling agent and the evaluation of its ability for the encapsulation of NLX and as biocompatible surface modified is reported. The use of the desired dendrimers as pH sensitive carriers for NLX has also been studied. Dendrimers were loaded with NLX and the release pattern of the drug in biological conditions was investigated. Influences of the dendrimer generation and number of grafted β-CD with dendrimers on their encapsulation ability have been described.
Materials and Methods
Materials
Poly (ethylene glycol) 600 diacid (acid number 175, 96–98%, from Fluka) was dried over Na2SO4 (Merck Chemical Co., Germany). β-cyclodextrin (from Fluka) dried in oven at 90 ˚C for 8 h. Citric acid and pyridine (purified with refluxing over NaOH for 2 h and subsequent distillation) were obtained from Merck Chemical co. (Germany). ρ-Toluenesulfonic acid (TSA) were purified by conventional methods before use. Thionyl chloride (Merck Chemical Co., Germany) was refluxed on linseed oil for 2 h. Sodium azide was purchased from Fluka. DCC was purchased from Merck Chemical Co. (Germany). N,N-dimethylformamide (DMF) (from Fluka) was dried and distilled under reduced pressure. Dialysis membrane D7884 was purchased from Sigma-Aldrich Co. (Steinhein, Germany) (retains molecular weights greater than 2000 and releases smaller than 1200). Naltrexone hydrochloride was obtained from Sigma (St. Louis, MO, USA) and neutralized with NaOH 0.25M. Figure 1 shows the structure of NLX. Other reagents and solvents purchased from Merck Chemical Co. (Germany).
Figure 1.
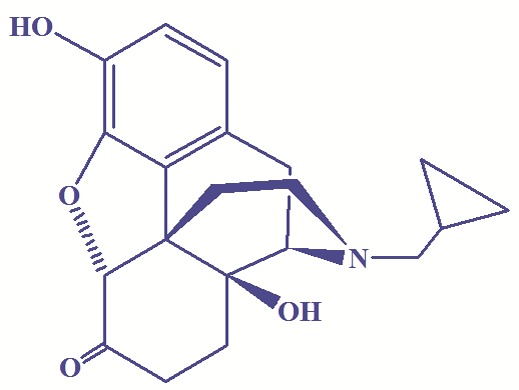
Chemical structure of naltrexone.
Instrumental measurements
FT–IR spectra were recorded on a Bruker Model Tensor-27 spectrometer (Kyoto, Japan). 1H NMR spectra were recorded with (Bruker spectra spin 400 MHz, Leipzig, Germany) using DMSO-d6, acetone deuterium and D2O as a solvent. The UV absorption spectra were recorded using 1700 Shimadzu spectrophotometer. Particle sizes were determined with dynamic light scattering by commercially available equipment Zetasizer Nano ZS from Malvern using a 4-mW He–Ne laser (633 nm wavelength) with a fixed detector angle of 173°.
The synthesis of Gn (n=1-2)-β-CD
Compounds G1and G2 with slightly modification were synthesized through divergent method as has been previously reported with carboxyl end-capped groups.6Functionalized dendrimers of G1 and G2 were reacted with β-CD in the presence of pyridine after activation of acidic groups with DCC and the compounds Gn (n=1-2)-β-CD were obtained, respectively. Compounds Gn (n=1-2)-β-CD were synthesized with conjugation of Gn (n=1-2) to β-CD through the formation of an ester band. For this purpose, A solution of G1 and or G2 (0.25 g) in 20 ml of dry DMF was added to a round-bottom flask equipped with reflux condenser, argon inlet, dropping funnel and magnetic stirrer. Dry pyridine (0.2 ml) was added to this solution by dropping funnel within 15 min. The mixture was stirred vigorously for 20 min. To this solution, DCC (0.65 g) in 15 ml dry DMF was added as a coupling agent at 0 °C by dropping funnel and was stirred for 30 min. After a drop wise addition of a solution of β-CD in 15 ml of DMF, the mixture was stirred at 0 °C for 2 h, then under argon at room temperature for 48 h and for 24 h at 50-65 °C. The solution was filtered off and was placed at 4°C for 24 h and again it was filtered off and the solvent was removed under vacuum. The product was precipitated in diethyl ether and was washed by acetone. Then the product was dissolved in the methanol, and filtered off then were precipitated in diethyl ether several times for removal of unreacted β-CD. The solution was filtered and the solvent was removed under vacuum. The product was dissolved in 5 ml water and stirred for 24 h at room temperature. The solution was filtered off and poured in 5 ml of water at 25 °C. The mixture was conducted into cellophane membrane dialysis bag. The bag was closed and transferred into a flask containing 100 ml of water maintained at 25 °C. The external water was continuously stirred for two days. The external water was removed after 24 h and added 100 ml fresh water. The product was removed from dialysis bag and dried under vacuum at 50 °C and the purified compound was obtained as a brown solid.
Preparation of Gn-β-CD (n=1–2)/NLX complexes
For the preparation of Gn-β-CD (n=1–2)/NLX complexes, first the dendrimers were dissolved in 20 ml DMF and these solutions were added to a round-bottom flask equipped with reflux condenser and magnetic stirrer containing a solution of drug (excess of NLX) in 20 ml DMF. The solutions were stirred for 2 h at 35-45 ˚C. Complexes were precipitated in n-hexane and then dissolved in water, filtered and precipitated in diethylether. The obtained sample was dried at 35 ˚C for 3 h in a vacuum oven.
In vitro release studies
In vitro release of drug–dendrimer complexes was carried out by the dialysis method. A quantity of dried dendrimer/drug complexes (66 mg) were added to a 5 ml of aqueous buffered solution (pH 10, 7.4, and 1) at 37 °C. The resulting solution was loaded into a cellophane membrane dialysis bag. The cellophane bag was closed and transferred into glass beakers containing 25 ml of the equal buffer solution maintained at 37 °C. The outer phase was stirred continuously with a magnetic stirrer at 800 rev/min and a sampling (3 ml) was withdrawn at regular selected intervals and replaced with 3 ml fresh buffer. The NLX released in to the medium was analyzed through a UV spectrophotometer to characterize the release of NLX and determined from the calibration curve achieved previously under the same conditions. The absorbance of the outer phase was monitored at 282 nm (pH 1 and 7.4) and 291 nm (pH 10) by a 1700 Shimadzu UV spectrophotometer to characterize the concentration of NLX using a 1 cm quartz cell. The results were presented in the terms of cumulative release as a function of time.
Particle size analysis
Particle size of complexes were measured through dynamic light scattering (DLS) using a Malvern Zeta-Sizer Nano ZSequipped with a 4-mW He–Ne laser (633 nm wavelength) with a fixed detector angle of 173°. Samples were dissolved in distilled water, and Light scattering experiments were carried out at 25 °C and were started 10 min after the cuvette was placed in the DLS apparatus to allow the temperature to equilibrate. About 1 ml of the sample was transferred to a special dust free light-scattering cell. The temperature was controlled to within ±0.02 °C. All samples were quietly inverted for homogenization before measurement. The particle sizes were measured based on their average diameters.
Results and discussion
Synthesis
Compound G1 and G2 (Figure 2) were synthesized through divergent method as has been previously reported.6 Subsequent synthesis of these compounds, we have covalently conjugated the dendrimerGn (n=1-2) to β-CD by the formation of an ester band and the compound G1-β-CD and G2-β-CD were synthesized respectively. The 1H NMR spectrum of the Gn (n=1-2)-β-CD conjugate shows signals originating from both Gn (n=1-2) and β-CD. 1H NMR data of these compounds shows a quartet at 2.72–3.06 ppm as AB system for the CH2 protons of citric acid, the anomeric protons of β-CD at 4.81-4.83 and CH2OCO-dendrimer of β-CD at 4.2-4.4 can be recognized. All these chemical shifts were in agreement with the projected structure of these compounds.
Figure 2.
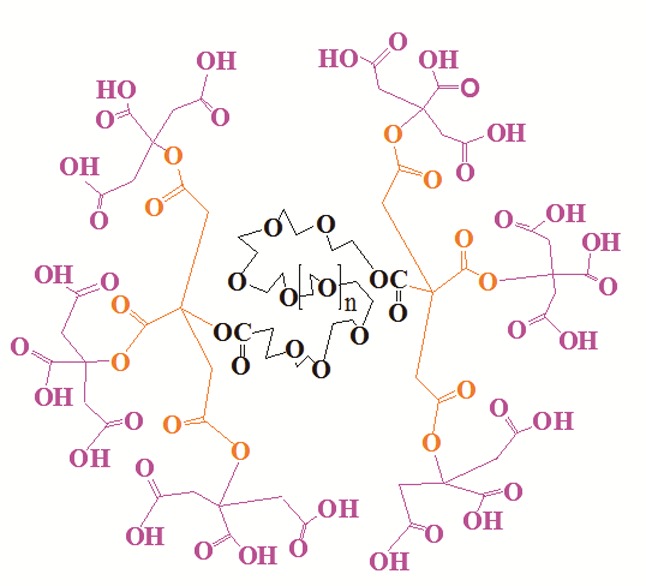
Schematic representation of second generation if dendrimer (G2).
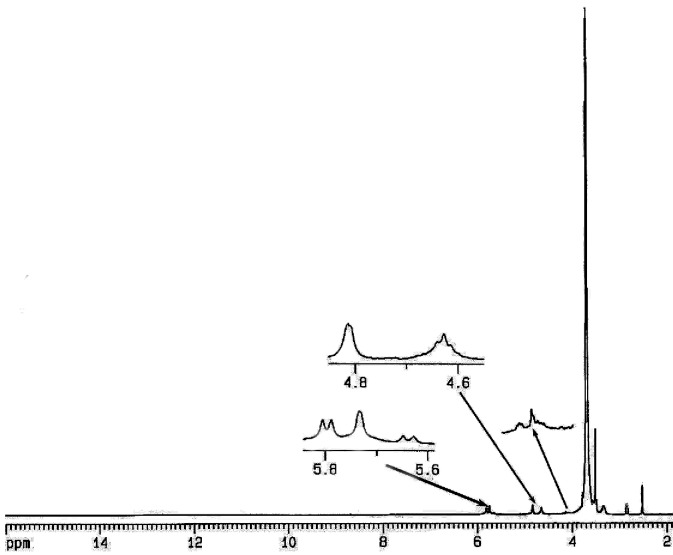
The average number of β-CD in the Gn (n=1-2)-β-CD dendrimers prepared was evaluated from their 1H NMR spectra. 1H-NMR spectrum of the resulting Gn (n=1-2)-β-CD was measured and the molar ratio of dendrimer and β-CD was calculated from the peak areas of anomeric protons of β-CD and citric acid protons of the dendrimer. From the integration ratio of the signal at 4.81-4.83 ppm (corresponding to the anomeric protons of β-CD) to the signal at 2.72-3.06 ppm corresponding to the four protons of citric acid, approximately 2:3 molecule of β-CD were attached to each molecule of G1-β-CD and G2-β-CD, respectively. For example Figure 3 displays the 1H NMR spectrum of G1-β-CD.
Figure 3.
1H NMR spectrum of G2-β-CD in DMSO-d6.
FT-IR spectra of these compounds showed a band around 1031-1033 cm-1 that could be assigned as characteristic stretching vibration of C–OH of β-CD.A strong and wide band at 3416–3423 cm-1 was the absorption of hydroxyl groups from both β-CD and dendrimer components.
In the FT-IR spectra of Gn (n=1-2)-β-CD (for example FT-IR spectrum of G2-β-CD s shown in Figure 4), the strong peak at 1739-1741 cm-1 could be assigned to the stretching vibration of carbonyl group in which was not observed in β-CD spectrum. The results indicated that the carboxymethylation occurs at C6position. The FT-IR spectrum of Gn (n=1-2)-β-CD indicateda C–H asymmetrical stretching vibration of the dendrimer segment at 2858-2860 cm-1, which is not appeared in β-CD. The absorption at 1151-1154 cm-1 (C–O stretch) confirmed the presence of ether group of PEG.
Figure 4.
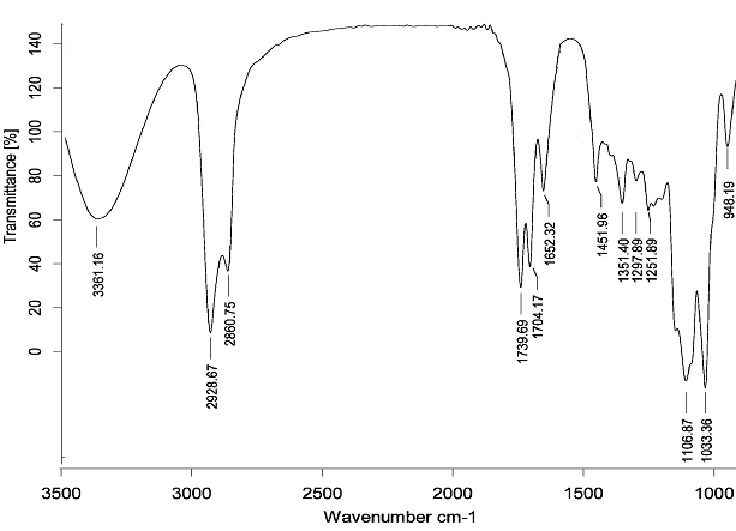
FT-IR spectrum of G2-β-CD.
1H NMR (400 MHz, DMSO-d6): δ=2.72-3.06 (q, citric acid CH2), 3.16-3.83 (br, β-CD H2, H4, H5, H3, H6a, H6b and PEG -OCH2CH2O-), 4-4.1 (br, PEG -COCH2O-), 4.2-4.4 (br, β-CD -CH2OCO-dendrimer), 4.6 (br, β-CD OH6), 4.81-4.83 (br, β-CD H1), 5.6 (br, β-CD OH3) and 5.73 (br, β-CD OH2). IR (KBr, cm-1): 3416-3423 (ν, OH β-CD and COOH dendrimer), 2931 and 2858-2860 (ν, C–H), 1739-1741 and 1705 (ν, ester and acid C=O), 1151-1154 (ν, C-O-C), 1100 and 1031 (ν, C-O).
Drug encapsulation and in vitro release
The resultant dendrimer-cyclodextrin conjugates revealed enormously high water solubility. Generally, dendrimers serve as carriers of biologically active agents by encapsulating them in the interior or, joining them on the exterior edging of the dendrimers. dendrimer-cyclodextrin conjugates have been synthesized, and the encapsulation of NLX drug, using these structures has been attempted (Figure 5). The measurements of UV from complexes confirmed the presence of drug in the resulted complexes. For determining the amount of drug encapsulated in the complex, 66 mg of dried drug/dendrimer complexes was dissolved in 100 ml of water. After complete dissolution of the drug/dendrimer complexes, the amount of drug in drug/dendrimer complexes was measured by UV spectrometer and the results are shown in Table 1. The UV detection for determining the quantity ofdrug inside the complexeswas measured at 282 nm.
Figure 5.
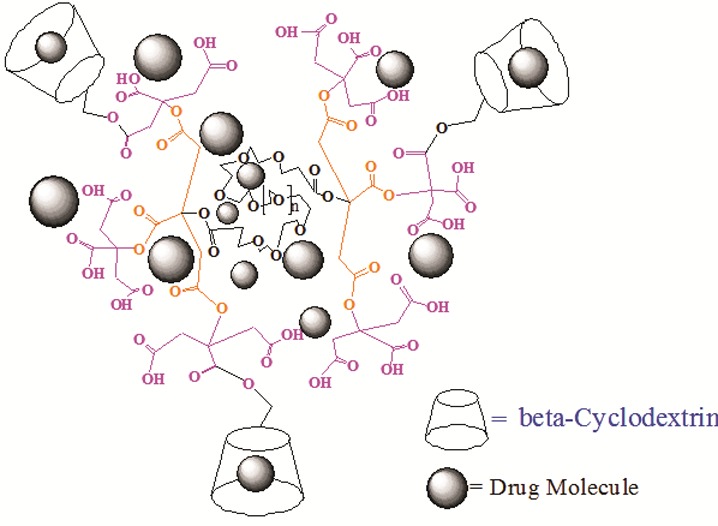
Schematic representation of drug encapsulation with G2-β-CD conjugates.
Table 1. Drug loading efficiency of Gn(n=1-2) /NLX and Gn(n=1-2)-β-CD/NLX complexes.
| Compound |
Naltrexone Loading (%) |
| G1/NLX | 8 |
| G2/NLX | 22 |
| G1-ß-CD/NLX | 17 |
| G2-ß-CD/NLX | 32 |
NLX loading efficiency of the complexes was determined as fallowing:
Amount of trapped drug in the complexes) g) = (A/B)
Loading efficiency (%) = (C/A) × 100
where A, B and C are the primary feeding amount of drug (g), determined free drug content (g) and amount of trapped drug in the complexes (g) respectively.
As shown in Table 1 the loading efficiency% of carried drug by dendrimers was enhanced with the rising of dendrimer generations. The drug content and percent of encapsulation efficiency was varying between 8 and 32%. The maximum encapsulation of NLX was observed with G2-β-CD in distilled water of about 32%. The increased entrapment was possibly due to increase the number of acidic groups (for electrostatic interactions), cavities (for trap of drugs) in higher generations and the number of grafted β-CD’s cavities. Coating with β-CD increased drug entrapment further because of the hydrophobic cavity of CDs is capable of including a variety of hydrophobic compounds using host-guest complexation. Whenever, it seems the ionic interaction between dendrimers and guest molecule and host–guest interactions including hydrophobic interactions, hydrogen-bonding and Vander Waals interactions influence the ability of dendrimers as drug carriers.
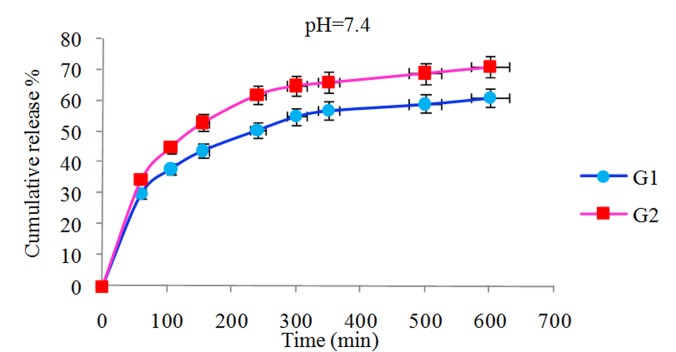
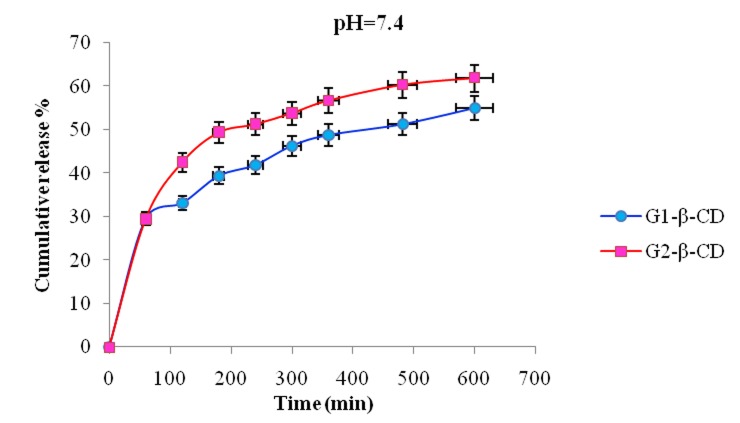
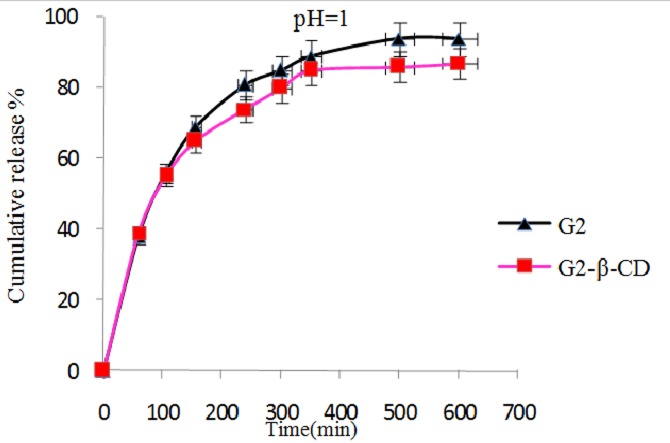
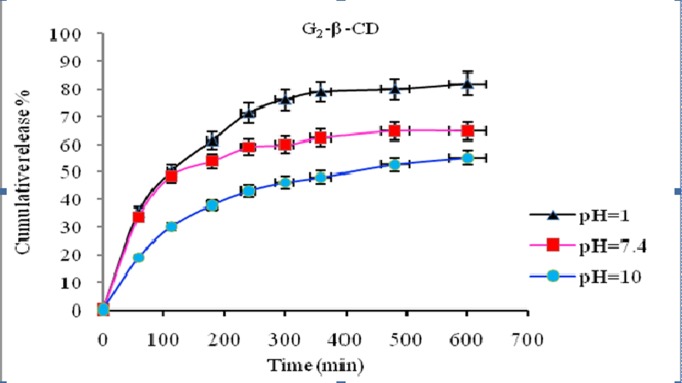
In vitro release behavior of NLX from the NLX/dendrimer complexes were examined in buffered solution at different pH values (pH 1 and pH 7.4 λmax=282; pH 10 λmax=291 and 37 °C). UV absorbance measurements were carried out for the characterization of NLX concentration in the complex solution. In vitro release rate of NLX from dendrimers having β-CD and without β-CD in end groups of dendrimer is compared and subsequent results were obtained. The comparison of the data showed that in different pH’s the NLX released in different rates. In pH 10 the rate of release of NLX was slower than pH 1 and 7.4. Interestingly, in all cases after approximately 600 min, the release of NLX from the complexes is approximately completed and was changed to a very slow rate. Figures 6 and 7 display the controlled release of NLX from Gn (n=1-2) and Gn (n=1-2)-β-CD at pH 7.4, respectively. As shown, the rate of release and the amount of released drugs in complexes (pH 7.4) were increased with the increase of generations but the rate of release in Gn (n=1-2)-β-CD was found to be less compared to their corresponding dendrimers (Gn (n=1-2)) and these results is related to the hydrophobic interactions between β-CD cavity and NLX that leading to stable complexes and steric hindrance preventing the drug release from open structure. Also, Figure 8 shows the release profiles of NLX from G2 and G2-β-CD at 37°C at pH 1. As shown, the rate of release for G2 is faster than to G2-β-CD. Control released in this case is related to the stable complexes between drug and β-CD cavity. The Figure 9 shows the rate of release of NLX from G2-β-CD at pH 1, 7.4 and 10. As shown, the rate of release in pH 1 is faster, because the complexes in this pH are not stable and the esteric bonds are hydrolyzed. Also, the results showed that the drug release rate from all kinds of the complexes was faster in the initial period of time. This rapid release was due to the free drug remaining at the surface that was not entrapped competently inside the dendrimer matrix. After this initial burst, slow and constant release rate is observed. The slow release after the initial burst release was as a result of the low dispersion of NLX from complexes. Again as shown the rate of released drug at pH 7.4 is higher than pH 10 and lower than the pH 1. This result could be related to the relative stability of the complexes in the above different pH’s.
Figure 6.
Release of NLX from Gn (n=1-2)/NLX complexes as a function of time and pH (pH 7.4, 37 ˚C).
Figure 7.
Release of NLX from Gn (n=1-2)-β-CD/complexes as a function of time and pH (pH 7.4, 37 ˚C).
Figure 8.
Release of NLX from G2 and G2-β-CD/complexes as a function of time and pH (pH 1, 37 ˚C).
Figure 9.
Release of NLX from G2-β-CD/complexes as a function of time and pH (pH 1, 7.4 and 10 and 37 ˚C).
Particle size analysis
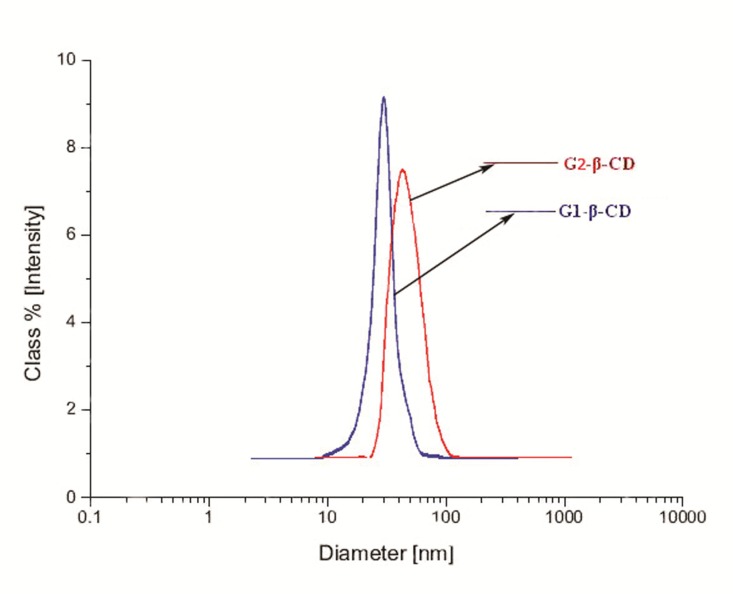
Particle sizes were determined with DLS experiments in distilled water and laser diffraction particle size analyzer. DLS results present that the average diameters of G1-β-CD and G2-β-CD/NLX complexes were 50 and 70 nm, respectively (Figure 10). These dendrimers exhibited a narrow size distribution.
Figure 10.
The size distribution profiles of G1-β-CD and G2-β-CD dendrimers estimated by DLS.
Conclusion
As the novel drug nanocarrier systems having the interior sites with encapsulation sites for drugs and also with biocompatible surface, some new dendrimers of citric acid and poly (ethylene glycol) having conjugated β-CD was designed and synthesized through the DCC coupling reagent. Complexes of the synthesized dendrimers with NLX have been developed. Rate of release of guest molecules showed that in each case the release profile depend on several types of interactions between host and guest molecule and it is influenced with generation of linear–dendritic macromolecules and pH. Also, finding data showed that the β-CD-coated dendrimers can be utilized for sustained release delivery of NLX. Therefore, all the obtained results exhibited that the β-CD-conjugated biodegradable citric acid dendrimers have potential as candidates for an efficient drug carrier system due to their relative stability in aqueous solution and their ability in drug encapsulation and release properties.
Ethical issues
None to be declared.
Conflict of interests
The authors declare no conflict of interests.
Acknowledgment
Authors are grateful to acknowledge the University of Tabriz and Research Center for Pharmaceutical Nanotechnology for their financial supports of this work.
References
- 1.Vicent MJ, Duncan R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol. 2006;24:39–47. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Haririan I, Shafieealavidjeh M, Khorramizadeh MR, Shafieeardestani M, Zareighane Z, Namazi H. Anionic linear-globular dendrimer-cis-platinum (II) conjugates promote cytotoxicity in vitro against different cancer cell lines. Int J Nanomedicine. 2010;5:63–75. doi: 10.2147/ijn.s8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alavidjeh MS, Haririan I, Khorramizadeh MR, Ghane ZZ, Ardestani MS, Namazi H. Anionic linear-globular dendrimers: biocompatible hybrid materials with potential uses in nanomedicine. J Mater Sci-Mater. 2010;21:1121–1133. doi: 10.1007/s10856-009-3978-8. [DOI] [PubMed] [Google Scholar]
- 4.Tomalia DA, Baker H, Dewald JR. et al. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym J. 1985;17:117–132. [Google Scholar]
- 5.Didehban K, Namazi H, Entezami AA. Triazine-based Dendrimers as Liquid Crystals: Synthesis and Characterization. Iranian Polymer Journal. 2009;18:731–741. [Google Scholar]
- 6.Namazi H, Adeli M. Novel linear-globular thermoreversible hydrogel aba type copolymers from dendritic citric acid as the a blocks and poly (ethyleneglycol) as the b block. European Polymer Journal. 2003;39:1491–1500. [Google Scholar]
- 7.Namazi H, Adeli M. Dendrimers of citric acid and poly (ethylene glycol) as the new drug-delivery agents. Biomaterials. 2005;26:1175–1183. doi: 10.1016/j.biomaterials.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Namazi H, Jafarirad S. Hybrid Organic/Inorganic Dendritic Triblock Copolymers: Synthesis, Nanostructure Characterization, and Micellar Behavior. J Appl Polym Sci. 2010;117:1085–1094. [Google Scholar]
- 9.Lee CC, Mackay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 10.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Bio chem Soc Trans. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 11.Tomalia DA. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Progress in Polymer Science. 2005;30:294–324. [Google Scholar]
- 12.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 13.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Gingras M, Raimundo JM, Chabre YM. Cleavable dendrimers. Angew Chem Int Ed Engl. 2007;46:1010–1017. doi: 10.1002/anie.200601962. [DOI] [PubMed] [Google Scholar]
- 15.Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug DiscovToday. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 16.Frampton MJ, Anderson HL. Insulated molecular wires. Angew Chem Int Ed Engl. 2007;46:1028–1064. doi: 10.1002/anie.200601780. [DOI] [PubMed] [Google Scholar]
- 17.Lai PS, Lou PJ, Peng CL. et al. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. Control Release. 2007;122:39–46. doi: 10.1016/j.jconrel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Gupta U, Agashe HB, Asthana A, Jain NK. Dendrimers: Novel polymeric nanoarchitectures for solubility enhancement. Biomacromolecules. 2006;7:649–658. doi: 10.1021/bm050802s. [DOI] [PubMed] [Google Scholar]
- 19.Namazi H, Jafarirad S. Controlled release of linear-dendritic hybrids of carbosiloxane dendrimer: the effect of hybrid's amphiphilicity on drug-incorporation; hybrid-drug interactions and hydrolytic behavior of nanocarriers. Int jpharm. 2011;407:167–173. doi: 10.1016/j.ijpharm.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Newkome GR, Moorefield CN, Baker GR, Johnson AL, Behera RK. Alkane Cascade Polymers Possessing Micellar Topology:Micellanoic AcidDerivatives. Angewandte ChemieInternational Edition in English. 1991;30:1176–1178. [Google Scholar]
- 21.Hawker CJ, Wooley KL, Frechet JMJ. Unimolecular Micelles and Globular Amphiphiles - Dendritic Macromolecules as Novel Recyclable Solubilization Agents. Journal of the Chemical Society-Perkin Transactions 1 1993; (12):1287-1297. [Google Scholar]
- 22.Rekharsky MV, Inoue Y. Complexation Thermodynamics of Cyclodextrins. Chem Rev. 1998;98:1875–1918. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 23.Szejtli J. Cyclodextrins and their inclusion complexes. Budapest: Akadémiaikiadó; 1982. [Google Scholar]
- 24.Connors KA. The Stability of Cyclodextrin Complexes in Solution. Chem Rev. 1997;97:1325–1358. doi: 10.1021/cr960371r. [DOI] [PubMed] [Google Scholar]
- 25.Namazi H, Kanani A. Synthesis of new prodrugs based on beta-CD as the natural compounds containing beta-lactam antibiotics. Journal of Bioactive and Compatible Polymers. 2007;22:77–88. [Google Scholar]
- 26.Szejtli J. Introduction and General Overview of Cyclodextrin Chemistry. Chem Rev. 1998;98:1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 27.Salmaso S, Semenzato A, Caliceti P. et al. Specific antitumor targetable beta-cyclodextrin-poly(ethylene glycol)-folic acid drug delivery bioconjugate. Bioconjug Chem. 2004;15:997–1004. doi: 10.1021/bc034186d. [DOI] [PubMed] [Google Scholar]
- 28.Giammona G, Cavallaro G, Maniscalco L, Craparo EF, Pitarresi G. Synthesis and characterisation of novel chemical conjugates based on alpha,beta-polyaspartylhydrazide and beta-cyclodextrins. European Polymer Journal. 2006;42:2715–2729. [Google Scholar]
- 29.Temtem M, Pompeu D, Jaraquemada G, Cabrita EJ, Casimiro T, Aguiar-ricardo A. Development of PMMA membranes functionalized with hydroxypropyl-beta-cyclodextrins for controlled drug delivery using a supercritical CO(2)-assisted technology. Int J Pharm. 2009;376:110–115. doi: 10.1016/j.ijpharm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 30.Namazi H, Kanani A. Investigation diffusion mechanism of beta-lactam conjugated telechelic polymers of PEG and beta-cyclodextrin as the new nanosized drug carrier devices. Carbohydrate Polymers. 2009;76:46–50. [Google Scholar]
- 31.Namazi H, Adeli M, Zarnegar Z, Jafari S, Dadkhah A, Shukla A. Encapsulation of nanoparticles using linear-dendritic macromolecules. Colloid and Polymer Science. 2007;285:1527–1533. [Google Scholar]