Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest types of malignancy. Via a broad stimulation of the immune system, PDAC activates both antitumor immune responses and immunosuppressive mechanisms. We propose that new immunotherapeutic strategies for the management of PDAC should be designed to specifically neutralize the immunosuppressive tumor microenvironment.
Keywords: immunosuppression, immunotherapy, pancreatic cancer, regulatory T cells
Patients affected by pancreatic ductal adenocarcinoma (PDAC) have an especially poor prognosis, with 5-y survival rates of only ~1% and median survival of 4–6 mo. Upon tumor resection, 5-y survival rates increase to approximately 15%, while a 25% survival is attained in the context of adjuvant chemotherapy.1 The reasons for such a poor prognosis are multiple, including rapid tumor dissemination and the lack of early, disease-specific symptoms, which is associated with late diagnosis.2 In contrast to other neoplasms, PDAC is highly resistant to both conventional and targeted chemotherapy. Currently, regimens based on gemcitabine as a standalone intervention or 5-fluorouracil constitute the standard therapeutic approach to advanced PDAC. However, the clinical impact of these treatments is very modest. Thus, new therapeutic options are urgently needed to improve the survival of PDAC patients. In this context, immunotherapy might constitute an attractive approach.
PDAC activates tumor-specific immune responses, as demonstrated by: 1) the accumulation of cytotoxic T lymphocytes (CTLs) within neoplastic lesions,3 2) the positive association between intratumoral CTL accumulation and increased patient survival (our unpublished data), and 3) the expression of tumor-associated antigens by malignant cells4 (Fig. 1). Moreover, PDAC patients have been shown to respond to the administration of interferon α (IFNα) with a systemic activation of the immune system5 that correlates with improved survival (our unpublished data). Nonetheless, in the context of the CapRi trial, a chemoradioimmunotherapeutic approach combining 5-fluorouracil, external beam radiation therapy and systemic IFNα could not be associated with any clinical benefit for PDAC patients as compared with chemoradiotherapy alone.6 Based on these findings, we hypothesized that PDAC itself and/or its capacity to broadly stimulate the immune system may engender an immunosuppressive state that dampens antitumor immune responses (Fig. 1).
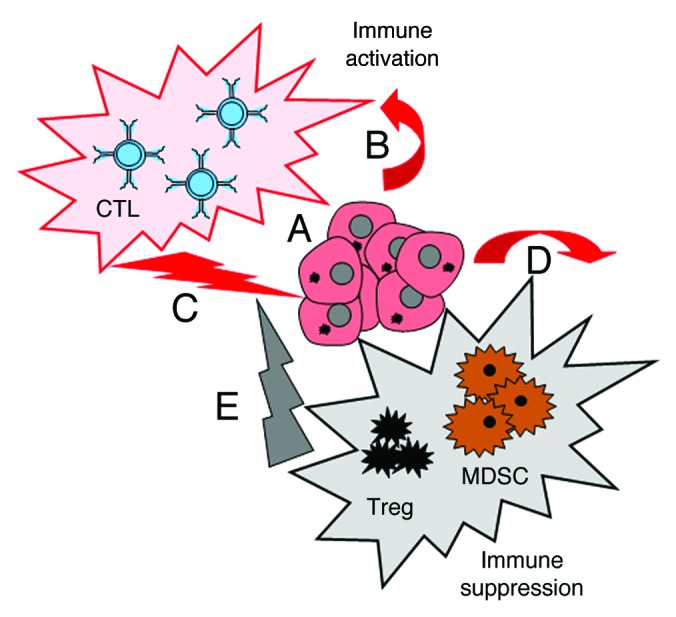
Figure 1. Double immunological consequences of pancreatic ductal adenocarcinoma. Pancreatic ductal adenocarcinoma (PDAC) cells expressing tumor-associated antigens (A) activate the immune system in PDAC patients (B). This generally results in the elicitation of PDAC-specific immune responses (C). In addition, PDAC cells can activate the immunosuppressive arm of the immune system (D), thus damping antitumor immune responses (E).
In support to this hypothesis, we observed that the PDAC patients exhibit increased numbers of circulating regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) (our unpublished data). Along similar lines, we demonstrated that orthotopic tumors generated upon the inoculation of Panc02 cells are highly infiltrated by Tregs. Remarkably, these cells exhibited an effector/memory phenotype, pointing to an enhanced immunosuppressive activity as well as to an increased proliferative potential.7 We actually demonstrated an intense replication of Tregs in the tumor microenvironment, suggesting local proliferation as the major mechanism whereby Tregs accumulate within PDACs. Increased levels of other immunosuppressive cells, namely MDSCs, were also detected within orthotopic Panc02 tumors (our unpublished data). The immunosuppressive molecule CD274 (best known as B7-H1) has been shown to be upregulated in human PDAC, strongly correlating with poor disease outcome.8 We have demonstrated that both PDAC cells and tumor-infiltrating leukocytes express B7-H1, in vivo and in vitro, in an inducible manner (our unpublished data). These results allowed us to conclude that PDAC induces not only an antitumor immune response but also a robust state of local immunosuppression driven by the accumulation of immunosuppressive cells and the upregulation of immunosuppressive molecules (Fig. 1).
Since PDAC exerts both immunostimulatory and immunosuppressive effects, and since nonspecific immunotherapeutic approaches often promote immunosuppression, too, we believe that future strategies for the clinical management of PDAC should rely on the elimination of immunosuppressive circuitries. In this context, it should be noted that the depletion of Tregs with antibodies specific for CD25 or the folate receptor can also affect activated CD4+FOXP3- and CD8+ T cells, concomitantly inhibiting the effector arm of antitumor immunity. Furthermore, an antibody-based strategy for the depletion of MDSCs is not adequate, mostly because of the elevated heterogeneity of this cell population. Conversely, low-dose chemotherapy has nowadays emerged as a promising approach for selective Treg and MDSC depletion.9
As we have recently reported, the administration of low-dose gemcitabine to mice bearing orthotopic Panc02 neoplasms results in the preferential depletion of proliferating Tregs and prolongs the survival of mice even though it does not exert any direct cytotoxicity on malignant cells.7 We have recently shown that sildenafil, an inhibitor of cyclic GMP-specific phosphodiesterase type 5 commonly employed for the treatment of erectile dysfunction, can inhibit the immunosuppressive activity of MDSCs in melanoma-bearing mice.10 We therefore administered sildenafil to mice bearing orthotopic Panc02 tumors. Such treatment could indeed decrease the amount of intratumoral MDSCs and improve the survival of PDAC-bearing mice (our unpublished data). Finally, we found that blocking B7-H1 with a specific monoclonal antibody also improves the survival of PDAC-bearing mice, mainly as it favors the activation of effector CD8+ T cells while inhibiting the immunosuppressive functions of Tregs (our unpublished data).
In summary, PDAC appears to simultaneously activate antitumor immune responses and immunosuppressive circuitries. We believe that new immunotherapeutic strategies against PDAC should be based not only on the stimulation of immune effector mechanisms, but also on the inhibition of tumor-elicited immunosuppression.
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocyte
- IFNα
interferon α
- Treg
regulatory T cell
- MDSC
myeloid-derived suppressor cell
- PDAC
pancreatic ductal adenocarcinoma
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25736
References
- 1.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006;17:403–9. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosty C, Goggins M. Early detection of pancreatic carcinoma. Hematol Oncol Clin North Am. 2002;16:37–52. doi: 10.1016/S0889-8588(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 3.Ryschich E, Nötzel T, Hinz U, Autschbach F, Ferguson J, Simon I, et al. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res. 2005;11:498–504. [PubMed] [Google Scholar]
- 4.Heller A, Zörnig I, Müller T, Giorgadze K, Frei C, Giese T, et al. Immunogenicity of SEREX-identified antigens and disease outcome in pancreatic cancer. Cancer Immunol Immunother. 2010;59:1389–400. doi: 10.1007/s00262-010-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt J, Jäger D, Hoffmann K, Büchler MW, Märten A. Impact of interferon-alpha in combined chemoradioimmunotherapy for pancreatic adenocarcinoma (CapRI): first data from the immunomonitoring. J Immunother. 2007;30:108–15. doi: 10.1097/01.cji.0000211317.15278.27. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30:4077–83. doi: 10.1200/JCO.2011.38.2960. [DOI] [PubMed] [Google Scholar]
- 7.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, et al. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133:98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 8.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 9.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33:369–83. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 10.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108:17111–6. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]


