Abstract
Multiple myeloma (MM) patients exhibit consistent degrees of immune dysfunction. Regulatory T cells contribute to the establishment of an immunosuppressive status in MM patients, hence favoring disease progression.
Keywords: T regulatory cells, multiple myeloma, immune suppression, disease progression, FoxP3, suppressor cells
Multiple myeloma (MM) is a plasma cell (PC) malignancy arising from post-germinal center B cells. In MM patients, the accumulation of abnormal PCs in the bone marrow (BM) alters the homeostatic regulation of both immune and non-immune cells, causes several systemic disorders, including hypercalcemia, renal impairment, anemia, and lytic bone lesions (which are cumulatively known as “CRAB” symptoms).1 Therefore, MM patients exhibit numerical and functional defects in multiple immune cell subsets, most of which are strongly associated with poor disease outcome.
During the past decade, regulatory T cells (Tregs) have attracted great attention due to their ability to deteriorate immune responses against a wide panel of neoplasms. Tregs are generally considered as an outlet to maintain immune homeostasis in healthy individuals. Conversely, Tregs exert a major immunosuppressive activity in cancer patients. Several factors have been shown to contribute to the propagation of MM cells in the tumor microenvironment. In particular, we have recently demonstrated that CD4+ Tregs play a significant role in MM progression.2
We confirmed that the amount of CD4+ Tregs is significantly elevated in newly diagnosed and relapsed MM patients, as compared with healthy individuals.2 Functional assays revealed that CD4+ Tregs isolated from MM patients inhibit the proliferation of CD4+ T cells and the secretion of interferon γ (IFNγ) in a concentration-dependent manner.2 We then asked whether an increase in immunosuppressive CD4+ Tregs would have a clinical significance in MM patients. As we expected, patients with increased numbers of CD4+ Tregs had a significantly shorter time-to-progression than patients with low amounts of CD4+ Tregs.2 These data suggest that CD4+ Tregs accumulating in the course of MM exert immunosuppressive functions and favor disease progression.
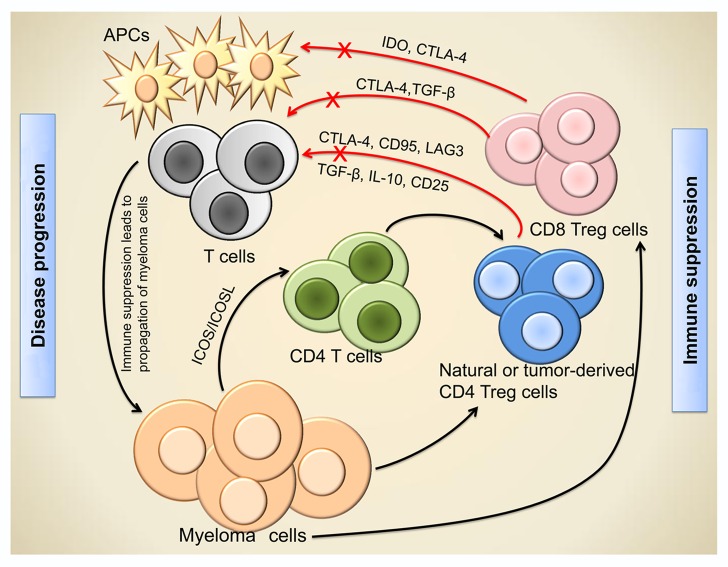
Of note, high levels of CD4+ Tregs were also associated with hypercalcemia, the IgA myeloma subtype and reduced numbers of normal PCs.2 It remains unclear whether myeloma cells stimulate the expansion of Tregs in the course of disease progression or, vice versa, disease progression is accelerated by the Treg-mediated suppression of anticancer immune responses. We believe that these processes are mutually interconnected (Fig. 1). Recently, a British study has confirmed that myeloma cells promote the differentiation of Tregs from CD4+ T cells in a cell contact-dependent manner.3 Such tumor-derived Tregs express higher levels of forkhead box P3 (FOXP3), programmed cell death 1 (PDCD1, best known as PD-1) and tumor necrosis factor receptor superfamily, member 18 (TNFRSF18, also known as glucocorticoid-induced tumor necrosis factor receptor, GITR) than natural Tregs. These findings suggest that myeloma cells harness CD4+ Tregs to suppress antitumor responses, hence sustaining disease progression (Fig. 1).
Figure 1. Regulatory T cell-mediated immunosuppression and disease progression in myeloma. Myeloma cells stimulate the differentiation of tumor-derived regulatory T cells (Tregs) from CD4+ T cells in an inducible co-stimulator (ICOS)-dependent manner, and favor the accumulation of naturally occurring CD4+ and CD8+ Tregs. Tregs suppress antitumor immune responses by inhibiting other immune cells, including T cells and antigen-presenting cells (APCs), via both cell contact-dependent and -independent mechanisms. The immunosuppression mediated by Tregs generates a path for the immune evasion and uncontrollable growth of myeloma cells, thus favoring disease progression. CTLA4, cytotoxic T-lymphocyte-associated protein 4; ICOS-L, ICOS ligand; IDO, indoleamine 2,3-dioxygenase; LAG3, lymphocyte-activation gene 3; TGFβ, transforming growth factor β.
As the molecular mechanisms underpinning the expansion and immunosuppressive functions of CD4+ Tregs are well documented, recent efforts have been dedicated to the identification and functional characterization of CD8+ Tregs in various malignant and inflammatory conditions. CD8+ Tregs exert immunosuppressive functions by several mechanisms, including the cell contact-dependent delivery of inhibitory signals, the secretion of relatively unspecific cytokines and the specific killing of effector immune cells.4 We were the first to demonstrate that the amount of CD8+ Tregs is significantly increased in MM patients as compared with healthy subjects. Similar to CD4+ Tregs, CD8+ Tregs expressed elevated levels of FOXP3, cytotoxic T lymphocyte antigen-4 (CTLA4) and CD62L, while exhibiting a CD127−/dim+ phenotype.5 RT-PCR data confirmed the deregulation of CD8+ Tregs in MM patients, clearly showing that these cells express higher levels of FOXP3 than their normal counterparts.5 Functional assays revealed that CD8+ Tregs isolated from both MM patients and healthy individuals inhibit the proliferation of CD4+ T cells as well as their ability to secrete IFNγ (but not interleukin-10, IL-10) in a concentration-dependent manner. Of note, CD4+ T cells isolated from MM patients and healthy subjects proliferated similarly in the absence of CD8+ Tregs.5 These observations suggest that although the proliferative potential of CD4+ T cells from MM patients is intact, these cells are held in check by CD8+ Tregs. Thus, not only CD4+ but also CD48+ Tregs are highly deregulated in MM patients.
The administration of immunomodulatory drugs (IMiDs) is considered as an effective therapeutic regimen for MM patients, as demonstrated by various clinical studies reporting a significant improvement in both disease-free and overall survival. We were therefore interested in assessing whether lenalidomide (one of such IMiDs) affects Tregs in MM patients. To our surprise, the administration of lenalidomide in combination with dexamethasone (a corticosteroid) significantly increased the amount of both CD4+ and CD8+ Tregs in MM patients.6,7 Such an increase in immunosuppressive Tregs upon the co-administration of lenalidomide and dexamethasone is paralleled by a decreased in the effector functions of T and NK cells.8,9 Thus, an increase in Tregs might compromise the immunotherapeutic effects of lenalidomide in MM patients.
In summary, Tregs accumulate in the course of MM, mediating immunosuppressive effects that favor not only disease progression but also infectious complications. The Treg-dependent immune evasion of MM cells and the tumor-dependent expansion of Tregs might underpin a vicious cycle that allows for disease progression. Tregs stand out as candidate targets for improving natural or therapy-elicited immune responses against a wide panel of solid tumors. Along these lines, Tregs have recently attracted clinical attention for the treatment of MM. Thus, a Phase II clinical trial is currently ongoing to test the response of MM patients to PD-1-blocking strategies (which, at least in part, counteract the immunosuppressive effects of Tregs), administered alone or in combination with a dendritic cell-based vaccine upon autologous stem cell transplantation. The results of this clinical trial, which are yet to be published, may confirm the therapeutic potential of Treg-targeting strategies for the clinical management of MM.
Acknowledgments
The authors are supported by the following grants: The Ministry of Education, Youth and Sports, MSM0021622434; Grant Agency of Czech Science Foundation, GAP304/10/1395; The Ministry of Health, Czech Republic - conceptual development of research organization, FNBr, 65269705.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25619
References
- 1.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One. 2012;7:e47077. doi: 10.1371/journal.pone.0047077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feyler S, Scott GB, Parrish C, Jarmin S, Evans P, Short M, et al. Tumour cell generation of inducible regulatory T-cells in multiple myeloma is contact-dependent and antigen-presenting cell-independent. PLoS One. 2012;7:e35981. doi: 10.1371/journal.pone.0035981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang XL, Smith TR, Kumar V. Specific control of immunity by regulatory CD8 T cells. Cell Mol Immunol. 2005;2:11–9. [PubMed] [Google Scholar]
- 5.Muthu Raja KR, Kubiczkova L, Rihova L, Piskacek M, Vsianska P, Hezova R, et al. Functionally suppressive CD8 T regulatory cells are increased in patients with multiple myeloma: a cause for immune impairment. PLoS One. 2012;7:e49446. doi: 10.1371/journal.pone.0049446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthu Raja KR, Kovarova L, Hajek R. Induction by lenalidomide and dexamethasone combination increases regulatory cells of patients with previously untreated multiple myeloma. Leuk Lymphoma. 2012;53:1406–8. doi: 10.3109/10428194.2011.652106. [DOI] [PubMed] [Google Scholar]
- 7.Muthu Raja KR, Plasil M, Rihova L, Pelcova J, Adam Z, Hájek R. Flow cytometry based enumeration and functional characterization of CD8 T regulatory cells in patients with multiple myeloma before and after lenalidomide plus dexamethasone treatment. Cytometry Part B: Clinical Cytometry. 2013 doi: 10.1002/cytob.21109. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–67. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- 9.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011;117:1605–13. doi: 10.1182/blood-2010-04-278432. [DOI] [PubMed] [Google Scholar]