Abstract
Thirty-five women with metabolic syndrome and high plasma low-density lipoprotein (LDL) cholesterol (≥100 mg/dl) participated in a dietary intervention consisting of a Mediterranean-style low-glycemic-load diet for 12 weeks. Participants were randomly allocated to consume diet only (n=15) or diet plus a medical food containing soy protein and plant sterols (n=20). Plasma concentrations of carotenoids, lipoprotein subfractions and oxidized LDL (OxLDL) were measured. Independent of treatment, women had a significant increase in plasma lutein (P<.0001) and β-carotene (P<.0001), while plasma lycopene was reduced (P<.05) after 12 weeks. Low-density lipoprotein cholesterol was reduced from 138±35 to 114±33 mg/dl (P<.0001). In addition, decreases were observed in the atherogenic subfractions: large very low-density lipoprotein (P<.05), small LDL (P<.00001) and medium high-density lipoprotein (P<.05). Oxidized LDL was significantly reduced by 12% in both groups (P<.01). Changes in OxLDL were inversely correlated with plasma lutein (r=−.478, P<.0001). The data indicate that women complied with the dietary regimen by increasing fruits and vegetable intake. Decreased consumption of high-glycemic foods frequently co-consumed with lycopene-rich tomato sauce such as pasta and pizza may be responsible for the lowering of this carotenoid in plasma after 12 weeks. These results also suggest that plasma lutein concentrations may protect against oxidative stress by reducing the concentrations of OxLDL.
Keywords: Mediterranean diet, Plasma carotenoids, Oxidized LDL, Metabolic syndrome
1. Introduction
Metabolic syndrome (MetS) is a cluster of metabolic alterations, including central obesity, hyperglycemia, low high-density lipoprotein cholesterol (HDL-C) concentrations, hypertension and hyper-triglyceridemia. MetS is associated with a high risk of developing cardiovascular disease (CVD), type 2 diabetes and all-cause mortality [1]. A lower prevalence of MetS has been associated with several components of the Mediterranean diet pattern, which has long been linked with greater longevity and reduced mortality and morbidity for coronary heart disease (CHD), certain cancers and other nutrition-related diseases [1–3].
An important step toward elucidating diet and CVD risk relationships in humans is the development of techniques for monitoring and characterizing dietary exposure [4]. Usually, this is accomplished by using dietary assessment instruments such as 24-h dietary recalls, food record, and food frequency questionnaires [5]. However, these tools are subjective and are prone to reporting errors [5]. A biomarker is less susceptible to the inherent errors associated with traditional diet assessment methods, may be less subject to intraindividual variation and can allow assessment of compliance in dietary intervention trials where a true placebo arm is not feasible [4].
Vegetables and fruits are key components of a Mediterranean diet [4,6–8]and represent the main sources of carotenoids in the human diet [9]. Consequently, plasma carotenoid levels have been found to be useful biomarkers of vegetable and fruit intake. Of the more than 700 naturally occurring carotenoids identified to date, six (β-carotene, β-cryptoxanthin, α-carotene, lycopene, lutein and zeaxanthin) are commonly found in blood (>95% total blood carotenoids) [10]. Carotenoids have potential antioxidant properties due to their chemical structure with abundant conjugated double bonds that interact with cellular membranes because of their fat-soluble nature [11]. The majority of circulating carotenoids are associated with lipoproteins, but predominantly with low-density lipoprotein (LDL), the major cholesterol-transporting lipoprotein [12].
Oxidative modification of LDL (OxLDL) is a key event in the oxidation hypothesis of atherogenesis and is suggested to increase the risk of CVD [13]. The ability of LDL to resist oxidation is influenced by several endogenous factors, among which the content of tocopherols and carotenoids are prominent [14]. It has been reported that particle contents of lutein/zeaxanthin, β-cryptoxanthin, β-carotene and lycopene were markedly reduced in small, dense LDL [14], a particle that is known to be more prone to oxidation.
Studies have shown inconclusive results regarding the ability of carotenoids to affect oxidation of LDL. For example, a high dietary intake of tomato products increased the resistance of LDL to oxidation in healthy normocholesterolemic adults [15]. Likewise, in diabetic patients, the increased susceptibility to LDL oxidation was normalized by β-carotene dietary supplementation [16]. In a subgroup of subjects from the Prevención con Dieta Mediterránea (PREDIMED) study, which evaluated the effects of this dietary pattern in patients on primary cardiovascular prevention, OxLDL levels decreased significantly after 3 months of consuming a Mediterranean diet [17]. However, a human study showed that supplementation with either a carotene mixture or lycopene had no effect in vitro on LDL oxidation, despite significant increases in plasma and LDL concentrations of lycopene, α-carotene and β-carotene [18].
Some studies have demonstrated that supplementation with eggs, as a source of dietary carotenoids, positively modulates plasma carotenoid and lipoprotein subclasses [19,20]. However, to the best of our knowledge, there is no information about the effect of a Mediterranean-style low-glycemic-load diet on OxLDL levels in women with MetS.
The aims of this study were (1) to measure the effects of a Mediterranean-style low-glycemic-load diet alone or in combination with a medical food containing soy protein, vitamins and plant sterols, on plasma carotenoid levels, lipoprotein subfractions and size and plasma concentrations of OxLDL; (2) to establish relationships between dietary intake of lutein, zeaxanthin, β-carotene and lycopene and their plasma concentrations in order to assess adherence to the diet; and (3) to determine associations between plasma carotenoid concentrations and OxLDL levels and lipoproteins subfractions. Our hypothesis was that the consumption of a Mediterranean-style diet would increase plasma carotenoid concentrations and that increases in circulating carotenoids would be associated with decreases in both OxLDL levels and atherogenic lipoproteins in women with MetS.
2. Experimental procedure
2.1. Materials
Lutein and zeaxanthin were purchased from Chromadex (Irvine, CA). β-Carotene was purchased from Sigma-Aldrich (St. Louis, MO). Pure lycopene was isolated and crystallized from tomato paste as previously described [21]. All solvents were high-performance liquid chromatography (HPLC) grade and were purchased from Fisher Scientific (Pittsburgh, PA). Butylated hydroxytoluene (BHT) was also purchased from Fisher Scientific. The medical food was provided by Metagenics (Gig Harbor, WA). Oxidized LDL kits were purchased from Alpco (Salem, NH).
2.2. Experimental design
We initially recruited 39 women with MetS and high plasma concentrations of LDL cholesterol (LDL-C ≥100 mg/dl). Subjects were randomly assigned to consume either a Mediterranean-style low-glycemic-load diet alone (diet-only group; n=19) or the same diet plus a medical food containing soy protein, plant sterols, rho iso-alpha acids from hops and proanthocyanidins from the acacia tree (medical food group; n=20). The composition of the medical food is presented in Table 1. Women were asked to follow their assigned diet for 12 weeks without changes in their physical activity level. All experimental protocols were approved by the University of Connecticut Institutional Review Board, and informed consents were obtained from all subjects. Four subjects from the diet-only group did not finish the study for several reasons, including difficulty in following the diet or for other personal reasons. Thus, only 15 women from the diet-only group completed the study. Plasma samples were obtained at baseline and Week 12 to measure plasma carotenoids, lipids, lipoprotein subfractions and size, and OxLDL.
Table 1.
Macronutrient, vitamins, isoflavones and plant sterol content of the medical food
| Nutrient | Amount per day |
|---|---|
| Energy (kJ) | 1394 |
| Total fat (g) | 6 |
| Protein (g) | 30 |
| Total carbohydrate (g) | 48 |
| Dietary fiber (g) | 8 |
| Retinyl palmitate (IU) | 3500 |
| Ascorbic acid (mg) | 120 |
| Cholecalciferol (IU) | 80 |
| α-Tocopheryl acetate (IU) | 22 |
| Thiamin (mg) | 1.5 |
| Riboflavin (mg) | 1.7 |
| Niacin (mg) | 20 |
| Vitamin B6 (mg) | 50 |
| Folate (μg) | 800 |
| Vitamin B12 (μg) | 60 |
| Biotin (μg) | 300 |
| Pantothenic acid (mg) | 10 |
| Isoflavones (mg) | 34 |
| Plant sterols (mg) | 4000 |
2.3. Dietary records
For the assessment of compliance, subjects provided 3-day dietary records every 2 weeks. Records included two weekdays and one weekend. Nutrient intake was evaluated using Nutritional Data Systems software Version 8.0, developed by the Nutrition Coordinating Center, University of Minnesota (Food and Nutrient Database 29, Minneapolis, MN).
2.4. Blood handling
Following a 12-h fast, whole blood was collected into tubes containing 0.10 g/100 g EDTA. Plasma was separated by centrifugation at 1500×g for 20 min at 4°C and placed into vials containing phenyl methyl sulfonyl fluoride (0.01 g/100 g), sodium azide (0.01 g/100 g) and aprotinin (0.05 g/100 g). Plasma samples were aliquoted and stored at −80°C for further analysis.
2.5. Carotenoid Extraction
Plasma (0.5 ml) was mixed with ethanol containing 0.1% BHT (0.5 ml) and 2.5 ml of HEAT [hexane/ethanol/acetone/toluene (HEAT), 10:6:7:7, v/v/v/v]. Samples were vortexed and centrifuged for 10 min at 300×g. The upper layer (containing carotenoids) was collected, and the remaining aqueous plasma mixture was extracted once more with 2.5 ml HEAT. The extracts were pooled and dried immediately under N2 gas. Dried extracts were stored at −80°C for no more than 24 h before HPLC analysis. All experiments were performed under red light.
2.6. Analysis of plasma carotenoids
The HPLC method was modified from Kopec et al. [21]. Briefly, plasma extracts were resolubilized in 150 μl of 1:1 methyl tert-butyl ether (MTBE)/methanol and filtered through nylon syringe filters (0.45 μm pore size), and 20 μl was injected onto the HPLC column. Reverse-phase separation was performed on a Waters Alliance 2695 HPLC equipped with a Waters 996 photodiode array. A YMC C30 column, 150 mm×4.96 mm ID, 5 μm particle size (Waters, Milford, MA) was used. The mobile phase consisted of Solvent A=88:5:5:2 methanol/MTBE/H2O/2% aqueous ammonium acetate solution (v/v/v/v) and Solvent B=78:20:2:2 MTBE/methanol/2% aqueous ammonium acetate solution (v/v/v/v). The following gradient, delivered at 1.7 ml/min, was used to separate the compounds: solvent B=10% at 0 min, increased linearly to 100% B over 11 min, held at 100% B for 1.5 min, rapidly returning to 10% B and holding for 4.5 min. The column temperature was 30°C. Column effluent was monitored from 210 to 600 nm. Carotenoids were identified based on retention time and ultraviolet-visible spectra coincident with authentic standards of lutein, zeaxanthin, β-carotene and lycopene. Carotenoids were quantified using external calibration curves of lutein, zeaxanthin, β-carotene and lycopene. Concentrations of each carotenoid were determined by comparing peak area to the respective calibration curve.
2.7. Plasma lipids
Total cholesterol and triglycerides were determined by enzymatic methods using Roche Diagnostics standards and kits [22]. High-density lipoprotein cholesterol was measured in the supernatant after precipitation of apolipoprotein B-containing lipoproteins [23] and LDL-C was determined using the Friedewald equation [24].
2.8. Lipoprotein subfractions and sizes
Proton nuclear magnetic resonance (H-NMR) analysis was performed on a 400-MHz NMR analyzer (BrukerBioSpin, Billerica, MA) as previously described [25]. Briefly, lipoprotein subclasses of different sizes produce a distinct lipid methyl signal of which the amplitude is directly proportional to lipoprotein particle concentration. NMR simultaneously quantifies >30 lipoprotein subclasses that are empirically grouped into nine smaller subclasses based on particle diameters: large very low-density lipoprotein (VLDL, >60 nm), medium VLDL (27–35 nm), small VLDL (23–27 nm), intermediate-density lipoprotein (IDL), large LDL (21.2–23 nm), medium LDL (19.8–21.2 nm), small LDL (18–19.8 nm), large HDL (8.8–13 nm), medium HDL (8.2–8.8 nm) and small HDL (7.3–8.2 nm). Weighted average lipoprotein particle size and diameter was calculated based on the diameter of each lipoprotein subclass multiplied by its respective relative concentration.
2.9. Oxidized LDL
Oxidized LDL was measured by ELISA kits using the monoclonal antibody 4E6 that has been utilized in numerous clinical trials [26]. The standards and samples were read at 450 nm in a spectrophotometer (Spectramax Multimode Spectrophotometer; Molecular Devices, Sunnyvale, CA). Using a polynomial curve, concentrations of OxLDL were calculated and expressed as millimoles per liter.
2.10. Statistical analysis
Repeated-measures ANOVA was used to analyze time effect, diet effect and the interaction of time and diet. The repeated measure was each subject over time and the between groups comparison was diet-only group versus diet + medical food group. Pearson correlations established associations between plasma carotenoids and OxLDL. Data are expressed as mean±S.D., and P<.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
At baseline, there were no significant differences observed in age, most characteristics associated with MetS and plasma LDL-C between the two diet groups (Table 2). However, HDL-C was significantly higher in women in the diet-only group (P<.05).
Table 2.
Age, metabolic syndrome characteristics and plasma LDL-C at baseline of women in this study by study group
| Parameter | Diet only (n=15) | Medical food (n=20) |
|---|---|---|
| Age (y) | 49.4±9.2 | 44.0±10.3 |
| Body Weight (Kg) | 89.3±14.9 | 89.2±14.0 |
| Body Mass Index (kg/m2) | 33.7±5.0 | 32.9±5.6 |
| Waist Circumference (cm) | 106.1±11.7 | 106.1±14.4 |
| Plasma Triglycerides (mg/dl) | 180.6±59 | 183.2±116.3 |
| Plasma HDL-C (mg/dl) | 59.8±13.2 | 47.0±12.4 * |
| Plasma LDL-C (mg/dl) | 148.7±46.2 | 133.5±22.9 |
| Plasma glucose (mg/dl) | 96.0±8.2 | 95.8±16.0 |
| Systolic blood pressure (mm Hg) | 125.0±14.5 | 119.6±12.5 |
| Diastolic blood pressure (mm Hg) | 89.5±15.7 | 85.0±12.5 |
Values are presented as mean±S.D. for the number of subjects indicated in parentheses.
Significantly different between groups (P<.05) as determined by Student’s t tests.
3.2. Dietary intake
Analysis of the 3-day food records showed that although women were not asked to reduce caloric intake, there was a significant decrease in energy intake for all women, which was likely associated with increased protein intake (P<.001; Table 3). Levels of the dietary carotenoids β-carotene, lutein and zeaxanthin significantly increased over 12 weeks (P<.0001), while levels of dietary lycopene decreased (P<.05). There were no significant differences between dietary groups in their intake of any of the measured or calculated nutrients.
Table 3.
Total energy and percent carbohydrate, fat and protein intake and dietary carotenoids of study participants by study group
| Nutrient | Diet only (n=15)
|
Medical food (n=20)
|
||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Energy (kJ) | 7925±3354 | 4264±2915** | 7716±2075 | 5408±2665** |
| Carbohydrate (% energy) | 42.3±11.6 | 35.6±8.4* | 43.3±6.8 | 39.8±9.7* |
| Fat (% energy) | 36.9±10.0 | 31.6±10.4* | 36.8±6.7 | 32.1±11.0* |
| Protein (% energy) | 18.6±6.3 | 25.3±6.1** | 17.7±5.0 | 24.1±4.4** |
| β-Carotene (μg/d) | 3247±2599 | 4532±2627** | 2083±1904 | 4757±3178** |
| Lutein + zeazanthin (μg/d) | 2179±2100 | 3572±2075** | 1192±751 | 3007±1886** |
| Lycopene (μg/d) | 4572±3816 | 4211±5007* | 4946±4139 | 3308±2653* |
Values are presented as mean±S.D. for the number of subjects indicated in parentheses.
Significantly different from baseline,
P<.05 and
P<.0001, as determined by repeated-measures ANOVA. There were no diet effects or interactions.
3.3. Plasma lipids, lipoprotein subfractions and size
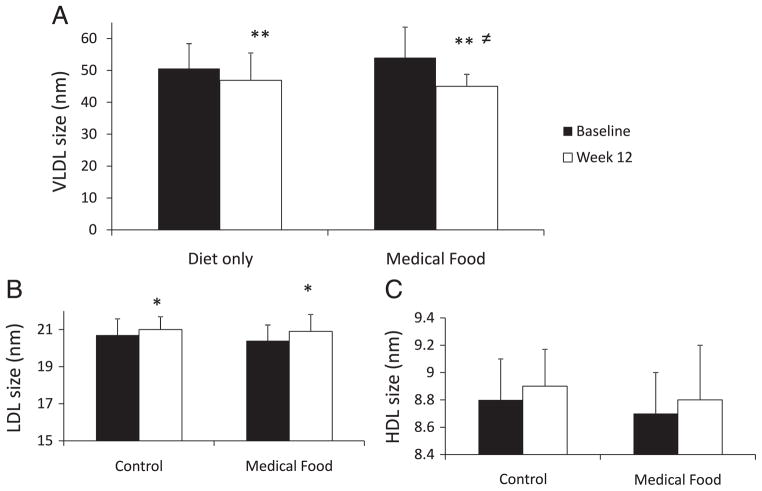
Plasma total cholesterol, LDL-C and triglyceride were reduced in both groups after 12 weeks (P<.01; Table 4). High-density lipoprotein cholesterol was significantly reduced in the diet-only group (P<.05), while no changes were observed in the medical food group. Interestingly, there were significant reductions in all lipoprotein subfractions that are considered to be atherogenic, including large VLDL (P<.001), IDL (P<.01), small LDL (P<.05) and medium HDL (P<.05). This reduction was observed in both diet groups, with no differences between groups (Table 4). Large VLDL decreased by 76% in the medical food group but only by 50% in the diet-only group. The percent difference between diet groups was significant (P<.05). Large VLDL was significantly reduced in both groups from baseline to Week 12 (P<.01; Fig. 1). However, the medical food group had a greater decrease in VLDL size after 12 weeks than the diet-only group (P<.05). Low-density lipoprotein size increased over time for both groups (P<.05), while HDL size was not affected by treatment or time.
Table 4.
Plasma lipids and atherogenic lipoprotein subfraction of participants by study group
| Parameter | Diet only (n=15)
|
Medical food (n=20)
|
||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Plasma lipids (mg/dl) | ||||
| Total cholesterol | 247.9±40.8 | 195.1±35.5** | 214.9±29.1 | 179.9±29.5** |
| LDL-C | 148.9±46.2 | 132.9±34.2** | 133.6±22.9 | 102.2±27.2** |
| HDL-C | 59.8±13.2 | 55.2±14.8* | 47.0±12.4 | 50.1±11.6≠ |
| Triglycerides | 180.6±59 | 156.8±63.4* | 183.2±116.3 | 129.5±45.8** |
| Lipoprotein concentration (mmol/l) | ||||
| Large VLDL | 4.8±3.8 | 2.4±2.1** | 8.4±8.9 | 2.0±2.4** |
| IDL | 87.3±72.6 | 56.3±56.0** | 63.6±467 | 41.2±40.9** |
| Small LDL | 999.7±627.4 | 899.4±500.1* | 1155.3±342.5 | 933.5±417.5* |
| Medium HDL | 5633±5638 | 4373±4532* | 6440±5119 | 4020±3731* |
Values are presented as mean±S.D. for the number of subjects indicated in parentheses. Significantly different from baseline,
P<.05 and
P<.0001, as determined by repeated-measures ANOVA.
There was an interactive effect in HDL-C independent of a time effect (P<.05).
Fig. 1.
VLDL (A), LDL (B) and HDL (C) size (in nanometers) at baseline (black bars) and after 12 weeks (white bar). Significantly different from baseline at **P<.01 and *P<.05; ≠ differences between groups.
3.4. Plasma carotenoids
Plasma carotenoids followed the same pattern as 3-day dietary records of carotenoid consumption (Table 5). There were significant increases in β-carotene and lutein levels in both diet groups (P<.0001) from baseline to Week 12, while plasma lycopene levels decreased over 12 weeks (P<.05). Interestingly, plasma zeaxanthin did not change over time in either group (Table 5).
Table 5.
Plasma carotenoids of participants by study group
| Carotenoid | Diet only (n=15)
|
Medical food (n=20)
|
||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| β-Carotene (nmol/l) | 191.5±95.6 | 342.9±208.5** | 206.3±137.7 | 300.8±219.1** |
| Lutein (nmol/l) | 166.7±52.7 | 209.3±79.7** | 138.7±54.2 | 159.8±65.3** |
| Zeaxanthin (nmol/l) | 33.7±12.8 | 39.9±16.8 | 27.5±11.3 | 27.5±13.2 |
| Lycopene (nmol/l) | 378.4±169.6 | 322.5±101.2* | 340.3±105.3 | 273.1±83.7* |
Values are presented as mean±S.D. for the number of subjects indicated in parenthesis. Significantly different from baseline,
P<.05 and
P<.0001, as determined by repeated-measures ANOVA. There were no diet effects or interactions.
3.5. Oxidized LDL
Oxidized LDL levels were reduced overall in both groups from baseline to Week 12 (Fig. 2A). Most of the subjects (except for two) experienced a reduction in OxLDL levels as indicated in Fig. 2B.
Fig. 2.
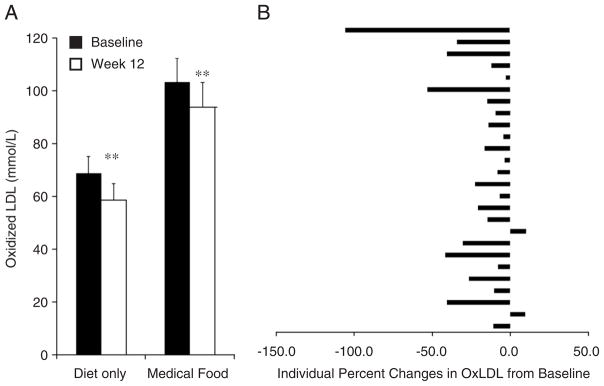
Concentrations of oxidized LDL in participants following a Mediterranean-style low-glycemic-load diet (diet-only group) or the same diet plus a medical food (medical food group) at baseline (black bars) and after 12 weeks (white bars). Significantly different at **P<.05 (A). Individual changes in oxidized LDL (B).
3.6. Correlations: plasma carotenoids, OxLDL and atherogenic lipoproteins
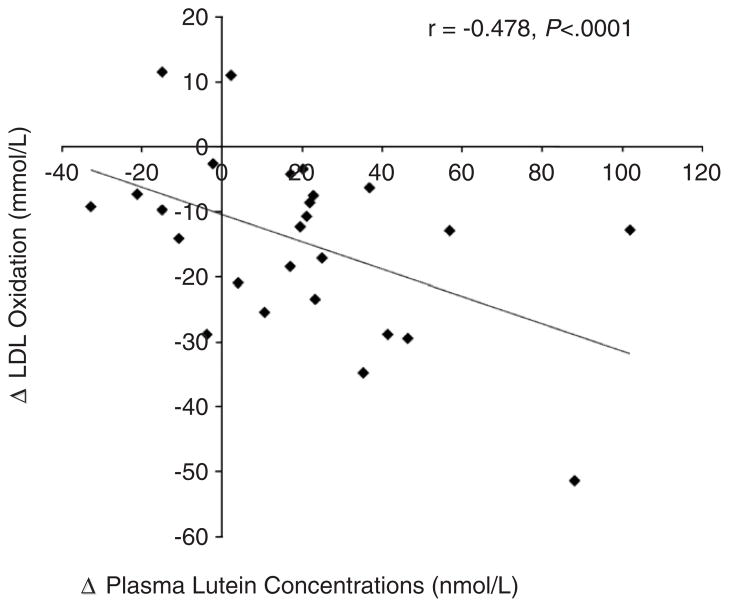
A significant inverse correlation was observed between increases in plasma lutein and reductions in OxLDL over 12 weeks (r=−.478, P<.0001; Fig. 3). Additionally, plasma lutein levels at Week 12 were inversely correlated with large VLDL (r=−.349, P<.05) and small LDL (r=−.340, P<.05) and positively correlated with LDL size (r=.337, P<.05).
Fig. 3.
Inverse correlation between changes in oxidized LDL and changes in plasma lutein level (r=−.478, P<.0001).
4. Discussion
To the best of our knowledge, this is the first randomized parallel-group human clinical trial measuring the effect of a Mediterranean-style low-glycemic-load diet on OxLDL in humans. The dietary treatment not only reduced plasma LDL-C and atherogenic lipoproteins (large VLDL and small LDL) but also plasma levels of OxLDL. The increase in plasma levels of lutein and β-carotene observed at the end of the study suggest dietary compliance. Furthermore, the change in plasma levels of lutein were inversely correlated with a change in plasma OxLDL levels.
4.1. Dietary adherence and plasma carotenoids
The term “Mediterranean diet” reflects the dietary patterns characteristics of several countries in the Mediterranean Basin during the early 1960s [2]. The common dietary food patterns, defined in 1993 at the International Conference on the Diets of the Mediterranean, include high levels of consumption of plant foods (fruits, vegetables, breads, beans, nuts and seeds), minimal consumption of processed foods, olive oil as the principal source of dietary lipids, low to moderate intake of dairy products, low frequency and level of red meat consumption, moderate to high fish and poultry intakes and a low to moderate intake of wine [2].
High-glycemic-index (GI) and high-glycemic-load (GL) diets have been positively associated with CHD and type 2 diabetes. This relationship is believed to be due to their adverse effects in increasing plasma triglycerides, reducing HDL-C and causing systemic inflammation [1,27]. Although the Mediterranean diet has been shown to produce beneficial effects on lipid metabolism, its high carbohydrate (CHO) content might hinder potentially healthy benefits in some high-CHD-risk populations, especially individuals with diabetes or insulin resistance (as seen in MetS) [28]. However, it has been shown that a modified Mediterranean diet lower in CHO (35% low GI CHO), moderately high in fat (45%, high in monounsaturated fat) and 15%–20% protein improved all the biomarkers of MetS and LDL-C after 12 months [29].
Our results indicate that women complied with the dietary regimen by making major changes from their habitual diet at baseline. Independent of treatment, women voluntarily decreased their energy intake by decreasing CHO and fat intake, as well as increasing their protein intake. In addition, all subjects increased vegetable intake. Numerous studies have shown the utility of nutritional biomarkers to assess the validity and to identify misreporting of energy and nutrient intake across different dietary assessment methods [30,31]. Plasma carotenoids have shown to be useful complementary tools for assessing adherence to the Mediterranean diet [4,7,8,32]. In this study, plasma carotenoid levels closely followed the dietary intake of carotenoids as assessed with 3-day dietary records, with increases in β-carotene and lutein levels and decreases in lycopene levels. Similar to our findings, studies have reported a range of correlations between dietary and plasma carotenoid concentrations. For example, Campbell et al. [4] evaluated 99 men and women aged 18–37 years in a cross-sectional study and found that the sum of plasma carotenoids (α-carotene, β-carotene, lutein, β-cryptoxanthin but not lycopene) was highly correlated with total vegetable and fruit intake. In addition, Djuric et al. [33] examined 69 healthy women aged 25–59 years following a modified Mediterranean diet or their own habitual diet for six months and found that increases in plasma lutein, α-carotene and β-carotene were significantly and highly correlated with the increased intake of these carotenoids compared with the control group. In a different study, Wang et al. [34] evaluated a cross-sectional sample of 2895 middle-aged and older women from the Women’s Health Study and reported significant but low correlations between plasma concentration and dietary intake of each carotenoid, except for lycopene. Our results show correlations of moderate magnitude as indicated above. Importantly, we confirm findings from these and other studies regarding decreases in dietary and plasma lycopene and positive correlations between plasma lycopene and consumption of lycopene-poor vegetables and fruits. This study encouraged the consumption of low glycemic foods; thus, significant reductions in plasma lycopene in this group of women may be related to the decreased consumption of high-glycemic foods typically served with lycopene-rich tomato sauce, such as pasta and pizza.
4.2. Plasma carotenoids, plasma lipids and lipoprotein subfractions and size
Our results indicate that women with MetS and high LDL-C levels (≥100 mg/dl) improved several MetS parameters after 12 weeks following the dietary regimen, with no differences between groups. However, the medical food group showed more pronounced decreases than the diet-only group in the mean values of LDL-C (23.5% vs. 11%) and triglycerides (29.3% vs. 13.2%) and the atherogenic lipoproteins, large VLDL (76.2% vs. 50%), small LDL (19.2% vs. 10%) and medium HDL (37.6% vs. 22.4%). The presence of plant sterols and soy protein in the medical food may be responsible for the greater reductions observed in this group, since their cholesterol-lowering effects have been previously reported [35,36].
In our study, positive changes in biomarkers of MetS were observed after only 12 weeks of intervention. In a 12-month study by Elhayany et al. [37] who evaluated 194 overweight diabetic patients following a low-CHO Mediterranean diet (35% low GI CHO, 45% fats high in MUFA and 15%–20% proteins), a traditional Mediterranean diet (50%–55% low GI CHO, 30% fats high in MUFA content and 15–20% proteins) or the American Diabetic Association diet (50%–55% CHO, 30% fats and 20% proteins) reported similar values [37]. The low-CHO diet improved all the features of the MetS and decreased LDL-C with additional reductions of 8% in LDL-C when compared to the other two diets [37].
Large VLDL particles [38] lead to the formation of a higher number of LDL particles, specifically small LDL. The observed decreases in plasma triglycerides in the current study are correlated with a decrease in the number of large VLDL. This reduction in triglycerides could be explained by the low GL in the modified Mediterranean diet, which results in less fuel available to generate and store triglycerides in hepatocytes for VLDL assembly. Also, the presence of increased fiber in the intestine (from increased fruit and vegetable consumption) might have had an effect in interrupting enterohepatic circulation of bile acids and the absorption rate of lipids [39]. Accordingly, we observed the formation of larger, less atherogenic, LDL particles and decreases in small LDL particles. We speculate that this larger LDL may have been carrying greater concentrations of carotenoids because the increases in plasma lutein at Week 12 were positively correlated with LDL size and inversely correlated with small LDL number.
4.3. Plasma carotenoids and OxLDL
The dietary regimen also substantially reduced the concentration of plasma OxLDL. The increased antioxidant content, i.e., carotenoids carried by the larger LDL, may have contributed to this finding. This hypothesis is further supported by the strong inverse correlation between plasma OxLDL and plasma lutein concentrations.
Studies have shown that supplementation with a carotene mixture had no effect on oxidative modification of LDL in vitro despite significant increases in plasma and LDL concentrations of lycopene, α-carotene and β-carotene [18]. However, results from in vitro studies do not necessarily reflect what is happening in vivo. Our results are concordant with a recent randomized controlled clinical trial assessing the effects of two traditional versions of a Mediterranean diet compared with a low-fat diet in 372 subjects on in vivo lipoprotein oxidation measured using the same antibody as in this study [40]. After 3 months of the two traditional Mediterranean diets, OxLDL levels were significantly decreased when compared with the low-fat diet. These differences remained significant even after adjusting for LDL-C: HDL-C ratio, sex, age, basal body weight and physical activity, and baseline values of OxLDL. Even though our sample size was small (n=35) compared with the above study, the significant positive changes observed in the present study suggest the effectiveness of this diet in ameliorating LDL oxidation.
One of the limitations of our study was sample size. It is difficult to find strong correlations between food data and biomarkers with a large cohort [32], and the small sample of our size could have limited the ability to find additional or stronger associations. Moreover, we did not measure the direct contribution of other plant components, such as additional antioxidants, fiber and other phytochemicals present in the Mediterranean diet, which may have also contributed to the beneficial effects observed on lipoprotein subclasses and OxLDL levels. Although the medical food included plant sterols, soy protein and other phytochemicals, we did not find significant differences between those who consumed the medical food and those who did not. The positive effects of the diet alone may have overridden our ability to observe potential benefits of individual or additional phytochemicals.
In summary, adherence to a Mediterranean-style low-glycemic-load diet is associated with a significant improvement in CVD risk factors in women with MetS and high LDL-C, as seen by significant reductions in LDL-C, atherogenic lipoprotein subfractions and OxLDL levels. The fact that plasma lutein concentrations were correlated with reduced oxidative damage to LDL and smaller, less atherogenic LDL particles, suggests that lutein may protect against atherosclerosis development and oxidative stress.
Abbreviations
- BHT
butylated hydroxytoluene
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HDL-C
HDL cholesterol
- HEAT
hexane/ethanol/acetone/toluene, 10:6:7:7
- HPLC
high-performance liquid chromatography
- LDL-C
LDL cholesterol
- MetS
metabolic syndrome
- MTBE
methyl tert-butyl ether
- NMR
nuclear magnetic resonance
- OxLDL
oxidize LDL
Footnotes
This study was supported by Metagenics, Inc. (Gig Harbor, WA).
References
- 1.Babio N, Bullo M, Salas-Salvado J. Mediterranean diet and metabolic syndrome: the evidence. Public Health Nutr. 2009;12:1607–17. doi: 10.1017/S1368980009990449. [DOI] [PubMed] [Google Scholar]
- 2.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean Diet: a systematic review. Prevention of Nutrition-Related Chronic Diseases: Scientific Foundations and Community Interventions. Nutr Rev; Fifth Nestlé Nutrition Conference; Mexico City, Mexico. October 7–8, 2004; 2006. pp. S27–47. [DOI] [PubMed] [Google Scholar]
- 3.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev. 1994;3:493–500. [PubMed] [Google Scholar]
- 5.Thompson FE, Subar AF, Loria CM, Reedy JL, Baranowski T. Need for technological innovation in dietary assessment. J Am Diet Assoc. 2010;110:48–51. doi: 10.1016/j.jada.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott KJ, Thurnham DI, Hart DJ, Bingham SA, Day K. The correlation between the intake of lutein, lycopene and beta-carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50–65 years in the UK. Br J Nutr. 1996;75:409–18. doi: 10.1079/bjn19960143. [DOI] [PubMed] [Google Scholar]
- 7.Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur J Clin Nutr. 2005;59:1387–96. doi: 10.1038/sj.ejcn.1602252. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A, Benetou V, Lagiou P, Gnardellis C, Stacewicz-Sapunzakis M, Papas A. Plasma carotenoid levels in relation to the Mediterranean diet in Greece. Int J Vitam Nutr Res. 2003;73:221–5. doi: 10.1024/0300-9831.73.3.221. [DOI] [PubMed] [Google Scholar]
- 9.Britton G, Khachick F. Carotenoids in Food. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Birkauser Verlag; Basel: 2009. pp. 45–66. [Google Scholar]
- 10.Maiani G, Caston MJ, Catasta G, Toti E, Cambrodon IG, Bysted A, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53(Suppl 2):S194–218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 11.Riccioni G. Carotenoids and cardiovascular disease. Curr Atheroscler Rep. 2009;11:434–9. doi: 10.1007/s11883-009-0065-z. [DOI] [PubMed] [Google Scholar]
- 12.Clevidence BA, Bieri JG. Association of carotenoids with human plasma lipoproteins. Methods Enzymol. 1993;214:33–46. doi: 10.1016/0076-6879(93)14051-j. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg D, Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–71. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 14.Goulinet S, Chapman MJ. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler Thromb Vasc Biol. 1997;17:786–96. doi: 10.1161/01.atv.17.4.786. [DOI] [PubMed] [Google Scholar]
- 15.Silaste ML, Alfthan G, Aro A, Kesaniemi YA, Horkko S. Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br J Nutr. 2007;98:1251–8. doi: 10.1017/S0007114507787445. [DOI] [PubMed] [Google Scholar]
- 16.Levy Y, Zaltsberg H, Ben-Amotz A, Kanter Y, Aviram M. Dietary supplementation of a natural isomer mixture of beta-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann Nutr Metab. 2000;44:54–60. doi: 10.1159/000012821. [DOI] [PubMed] [Google Scholar]
- 17.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 18.Carroll YL, Corridan BM, Morrissey PA. Lipoprotein carotenoid profiles and the susceptibility of low density lipoprotein to oxidative modification in healthy elderly volunteers. Eur J Clin Nutr. 2000;54:500–7. doi: 10.1038/sj.ejcn.1601046. [DOI] [PubMed] [Google Scholar]
- 19.Greene CM, Waters D, Clark RM, Contois JH, Fernandez ML. Plasma LDL and HDL characteristics and carotenoid content are positively influenced by egg consumption in an elderly population. Nutr Metab (Lond) 2006;3:6. doi: 10.1186/1743-7075-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutungi G, Waters D, Ratliff J, Puglisi M, Clark RM, Volek JS, et al. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J Nutr Biochem. 2010;21:261–7. doi: 10.1016/j.jnutbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, et al. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–6. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 23.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–88. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Wood RJ, Volek JS, Liu Y, Shachter NS, Contois JH, Fernandez ML. Carbohydrate restriction alters lipoprotein metabolism by modifying VLDL, LDL, and HDL subfraction distribution and size in overweight men. J Nutr. 2006;136:384–9. doi: 10.1093/jn/136.2.384. [DOI] [PubMed] [Google Scholar]
- 26.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study) Arterioscler Thromb Vasc Biol. 2002;22:1162–7. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- 27.Denova-Gutierrez E, Huitron-Bravo G, Talavera JO, Castanon S, Gallegos-Carrillo K, Flores Y, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J Nutr Metab. 2010:2010. doi: 10.1155/2010/170680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87:269S–74S. doi: 10.1093/ajcn/87.1.269S. [DOI] [PubMed] [Google Scholar]
- 29.Esposito K, Giugliano D. Mediterranean diet and the metabolic syndrome: the end of the beginning. Metab Syndr Relat Disord. 2010;8:197–200. doi: 10.1089/met.2009.0095. [DOI] [PubMed] [Google Scholar]
- 30.Bingham SA, Day NE. Using biochemical markers to assess the validity of prospective dietary assessment methods and the effect of energy adjustment. Am J Clin Nutr. 1997;65:1130S–7S. doi: 10.1093/ajcn/65.4.1130S. [DOI] [PubMed] [Google Scholar]
- 31.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158:1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 32.Bach-Faig A, Geleva D, Carrasco JL, Ribas-Barba L, Serra-Majem L. Evaluating associations between Mediterranean diet adherence indexes and biomarkers of diet and disease. Public Health Nutr. 2006;9:1110–7. doi: 10.1017/S1368980007668499. [DOI] [PubMed] [Google Scholar]
- 33.Djuric Z, Ren J, Blythe J, VanLoon G, Sen A. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutr Res. 2009;29:156–63. doi: 10.1016/j.nutres.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Gaziano JM, Norkus EP, Buring JE, Sesso HD. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am J Clin Nutr. 2008;88:747–54. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AbuMweis SS, Jones PJ. Cholesterol-lowering effect of plant sterols. Curr Atheroscler Rep. 2008;10:467–72. doi: 10.1007/s11883-008-0073-4. [DOI] [PubMed] [Google Scholar]
- 36.Weidner C, Krempf M, Bard JM, Cazaubiel M, Bell D. Cholesterol lowering effect of a soy drink enriched with plant sterols in a French population with moderate hypercholesterolemia. Lipids Health Dis. 2008;7:35. doi: 10.1186/1476-511X-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab. 2010;12:204–9. doi: 10.1111/j.1463-1326.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 38.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–65. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez ML. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol. 2001;12:35–40. doi: 10.1097/00041433-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Fito M, Guxens M, Corella D, Saez G, Estruch R, de la Torre R, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167:1195–203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]