Abstract
Flavones isolated from celery varied in their stability and susceptibility to deglycosylation during thermal processing at pH 3, 5, or 7. Apigenin 7-O-apiosylglucoside was converted to apigenin 7-O-glucoside when heated at pH 3 and 100 °C. Apigenin 7-O-glucoside showed little conversion or degradation at any pH after 5 h at 100 °C. Apigenin, luteolin, and chrysoeriol were most stable at pH 3 but progressively degraded at pH 5 or 7. Chamomile and celery were used to test the effects of glycosidase-rich foods and thermal processing on the stability of flavone glycosides. Apigenin 7-O-glucoside in chamomile extract was readily converted to apigenin aglycone after combination with almond, flax seed, or chickpea flour. Apigenin 7-O-apiosylglucoside in celery leaves was resistant to conversion by β-glucosidase-rich ingredients, but was converted to apigenin 7-O-glucoside at pH 2.7 when processed at 100 °C for 90 min and could then be further deglycosylated when mixed with almond or flax seed. Thus, combinations of acid hydrolysis and glycosidase enzymes in almond and flax seed were most effective for developing a flavone-rich, high aglycone food ingredient from celery.
Keywords: Apium graveolens, Flavones, Thermal processing, β-Glucosidase
1. Introduction
Flavones are a class of flavonoids found in a variety of fruits and vegetables and are most abundant in artichoke heads, kumquats, parsley, and celery (Azzini et al., 2007; Justesen, Knuthsen, & Leth, 1998; Sakakibara, Honda, Nakagawa, Ashida, & Kanazawa, 2003). The flavone apigenin exhibits toxicity to cancer cells in vitro (Engelmann et al., 2002; Gupta, Afaq, & Mukhtar, 2002; Mak, Leung, Tang, Harwood, & Ho, 2006; Manthey & Guthrie, 2002; Piantelli et al., 2006) and animal studies with flavones demonstrate the ability to attenuate the inflammatory response (Nicholas et al., 2007; Ueda, Yamazaki, & Yamazaki, 2004). However, human trials with flavone rich foods such as parsley and celery show limited bioavailability of these compounds, with maximum plasma concentrations of <1 μM (Cao, Zhang, Chen, & Zhao, 2010; Meyer, Bolarinwa, Wolfram, & Linseisen, 2006). The time of maximum plasma concentration after eating these foods was over 7 h (Cao et al., 2010; Meyer et al., 2006), indicating that the flavones are likely absorbed in the colon rather than the small intestine. Future use of flavone-rich foods for health benefits requires a better understanding of the forms that are most readily absorbed and their stability during food processing.
Both parsley and celery are rich in flavone glycosides, particularly apigenin 7-O-apiosylglucoside (apiin) (Lechtenberg, Zumdick, Gerhards, Schmidt, & Hensel, 2007; Lin, Lu, & Harnly, 2007) and malonyl apiin, which can be converted by endogenous esterases to apiin (Hostetler, Riedl, & Schwartz, 2012). Because intestinal absorption of flavonoid glycosides varies according to the aglycone core and the sugars and other functional groups attached, the glycosylation of flavones may determine their site and efficiency of absorption. Similar to other flavonoids, flavone glucosides can be hydrolysed by intestinal β-glucosidase (Németh et al., 2003) and absorbed in the small intestine. In contrast, flavonoid glycosides with disaccharide and malonyl moieties are more resistant to intestinal β-glucosidase than their simple glucoside counterparts, potentially limiting their bioavailability (Németh et al., 2003). Caco-2 models show that flavone aglycones were more readily absorbed into intestinal cells than flavone glucosides, with 10 times more apigenin or luteolin transported across the intestinal epithelium than apigenin 6-C-glucoside or luteolin 7-O-glucoside (Tian, Yang, Yang, & Wang, 2009).
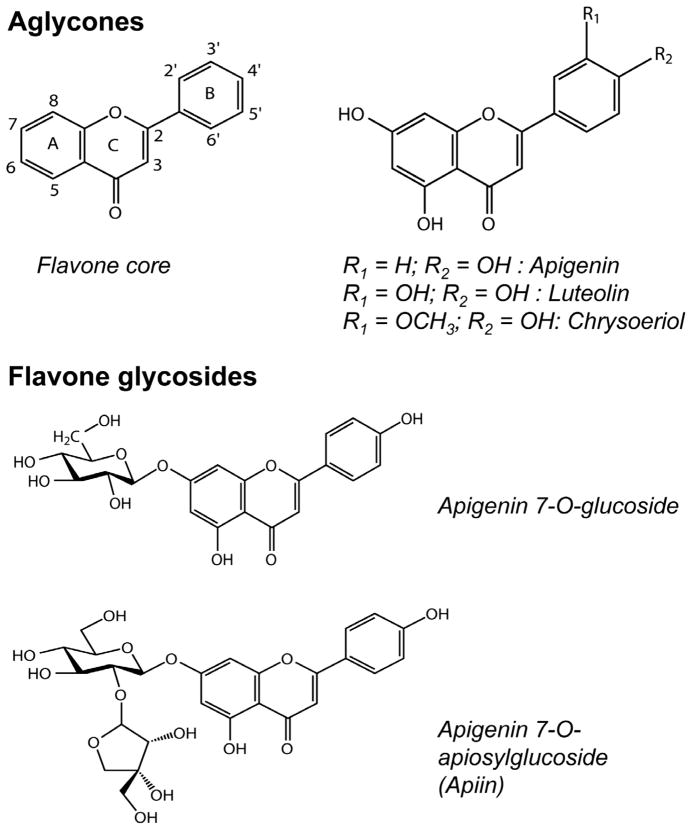
Flavone glycosides in foods may be modified slightly by endogenous enzymes such as malonyl esterases (e.g. conversion from malonylapiin to apiin) (Hostetler et al., 2012; Matern, 1983), but remain as glycosides after processing such as shredding lettuce (DuPont, Mondin, Williamson, & Price, 2000), juicing artichoke heads (Schütz, Kammerer, Carle, & Schieber, 2004), and heating orange juice (Gil-Izquierdo, Gil, & Ferreres, 2002). Rhamnosidase has been used to convert hesperetin rhamnosyl glucoside to hesperetin glucoside, thereby improving bioavailability 4-fold (Nielsen et al., 2006). The β glycoside linkage of flavonoid glycosides can also be cleaved by β-glucosidase to release flavonoid aglycones (Riedl, Zhang, Schwartz, & Vodovotz, 2005), and β-glucosidase-rich ingredients such as almond (Ducret, Trani, & Lortie, 2006), flax seeds (Fan & Conn, 1985), and chickpeas (Hösel, 1976) can potentially be used for this purpose in food processing. In an effort to develop a functional food rich in flavone aglycones and ultimately to improve the bioavailability of these compounds, several processing parameters were tested for their effects on flavone glycosylation in celery. In addition, the thermal stability of purified flavone derivatives from celery (Fig. 1) was evaluated to better understand processing effects on flavone-rich foods.
Fig. 1.
Flavone aglycones and glycosides derived from celery.
2. Experimental
2.1. Materials
Chinese celery (Apium graveolens L., Apiaceae), raw almonds (Prunus dulcis Mill., Rosaceae), flax seeds (Linum usitatissimum L., Linaceae), and chickpea flour (Cicer arietinum L., Fabaceae) were purchased from local grocery stores in Columbus, Ohio. Flavo-Natin tablets with chamomile extract were obtained from Koehler Pharma (Alsbach-Haehnlein, Germany). Formic acid, apigenin, and luteolin were from Sigma (St. Louis, MO), and apigenin 7-O-glucoside was from Chromadex (Irvine, CA). Ammonium acetate was from J. T. Baker (Phillipsburg, NJ). Phosphoric acid was obtained from Corco Chemical Corp. (Fairless Hills, PA). All other reagents were from Fisher Scientific (Fair Lawn, NJ).
2.2. Isolation of flavones by preparative HPLC
Celery leaves were crushed, lyophilised, and extracted with 2.5 mL 70% (v/v) aqueous methanol. To isolate flavone apiosylglucosides, the original extract was used. A 2 mL aliquot of the original 70% methanol extract was hydrolysed with 100 μL 12 MHCl for 1 h at 90 °C to hydrolyse flavone apiosylglucosides to flavone glucosides and aglycones. The hydrolysed extract was dried under N2 and reconstituted in 50% aqueous methanol.
Extracts were filtered through 0.45 μm nylon and separated by preparative high performance liquid chromatography (HPLC) using an HP 1050 separations module (Agilent Technologies, Santa Clara, CA), and a SunFire C18 column (150 × 19 mm id, 5 μm) and Empower software (Waters Corp., Milford, MA). The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), and the following gradient method was used: 0–1 min, 5% B; 1–16 min, 21% B; 16–35 min, 30% B; 35–40 min, 100% B; 40–45 min, 5% B. Analytical HPLC confirmed that each compound was >97% pure.
2.3. Thermal stability of purified flavones
Ammonium acetate buffer (0.1 M) was adjusted to pH 3, 5, or 7 with ammonium hydroxide or formic acid. Apigenin 7-O-apiosylglucoside (apiin), apigenin 7-O-glucoside, apigenin, luteolin, and chrysoeriol isolated from celery were dissolved in pH 3, 5 or 7 buffers to make 25 μM solutions of 4 ml each. Solutions were heated for 5 h in a boiling water bath (100 °C), taking 400 μL aliquots at 0, 60, 120, 180, 240, and 300 min. Aliquots were diluted 1:1 with methanol, sonicated, and analysed directly by HPLC.
2.4. Effects of food ingredients on flavone deglycosylation
Celery leaves were separated from stalks, macerated, and lyophilised. Almonds and flax seeds were ground in a coffee grinder. Crushed chamomile tablet (250 mg) or lyophilised celery leaves (125 mg) were combined with ground almond, ground flax seed, or chickpea flour (250 mg) in 2.5 mL sodium acetate buffer (1.4 M, pH 5.0). The mixtures were incubated at 37 °C for 20 h to allow enzymes in the ingredients to deconjugate flavone glycosides in the chamomile tablets or celery leaves. After incubation, the samples were extracted once with 2.5 mL methanol and twice with 2.5 mL 70% (v/v) aqueous methanol. The supernatants were combined and analysed by HPLC.
2.5. Thermal stability of flavones in celery
To determine the effects of heat and acidity on flavones in celery, lyophilised celery leaves (500 mg) were rehydrated in 1.5 N H3PO4 (1 mL) and left at room temperature (25 °C) for 1 h or heated in a boiling water bath (100 °C) for 90 min. After processing, the samples were extracted three times with 2.5 mL 70% (v/v) aqueous methanol and the supernatants were combined and analysed by HPLC.
2.6. Effects of food ingredients on flavone glycosides in thermally processed celery
Lyophilised celery leaves (125 mg) were rehydrated in water (1.25 mL), acidified to pH 2.7 with 1.5 N H3PO4 (940 μL), and heated in a boiling water bath (100 °C) for 90 min. Samples were then neutralised to pH 5 with 235 μL 10% NaOH and combined with almond powder, ground flax seed, or chickpea flour. Blended samples were incubated at 50 °C for 2 h and cooled on ice. After processing, the samples were extracted three times with 2.5 mL 70% (v/v) aqueous methanol and the supernatants were combined and analysed by HPLC.
2.7. HPLC analysis
Analysis of celery, chamomile tablets, and purified flavones was conducted by HPLC. Commercial apigenin, luteolin, chrysoeriol, apigenin 7-O-glucoside, and apigenin 7-O-apiosylglucoside standards were used to identify compounds by UV spectra and retention times. For quantification, the HPLC system included a Waters 2695 separations module, a 2996 photodiode array detector, a Symmetry C18 column (75 × 4.6 mm id, 3.5 μm), and Empower software (Waters Corp., Milford, MA). The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), and the column was maintained at 30 °C. For celery, chamomile tablets, apiin, apigenin 7-O-gluscoside, apigenin, and chrysoeriol, the following gradient method was used: 0–1 min, 5–19% B; 1–6.5 min, 19% B; 6.5–7 min, 19–20% B; 7–13 min, 20% B; 13–15 min, 20–55% B; 15–18 min, 55–80% B; 18–20 min, 80–100% B; 20–22 min, 100% B; 22–25 min, 5% B. Luteolin isolated from celery was quantified with the following gradient: 0–10 min, 5–30% B; 10–13 min, 30–80% B; 13–15 min, 80–100% B; 15–17 min, 100% B; 17–20 min, 5% B. Peak areas were measured at 338 nm for apigenin and 350 nm for luteolin and chrysoeriol. Because the flavone glycosides in this study shared the same wavelengths of maximum absorption and extinction coefficients as their aglycone counterparts, concentrations of all flavones were determined with aglycone standard curves.
2.8. Statistical analysis
Statistical analysis was performed with R 2.11 for Windows (R Foundation for Statistical Computing, Vienna, Austria) using one-way analysis of variance (ANOVA). Post hoc comparisons were done with Tukey’s HSD.
3. Results
3.1. Thermal stability of purified flavones
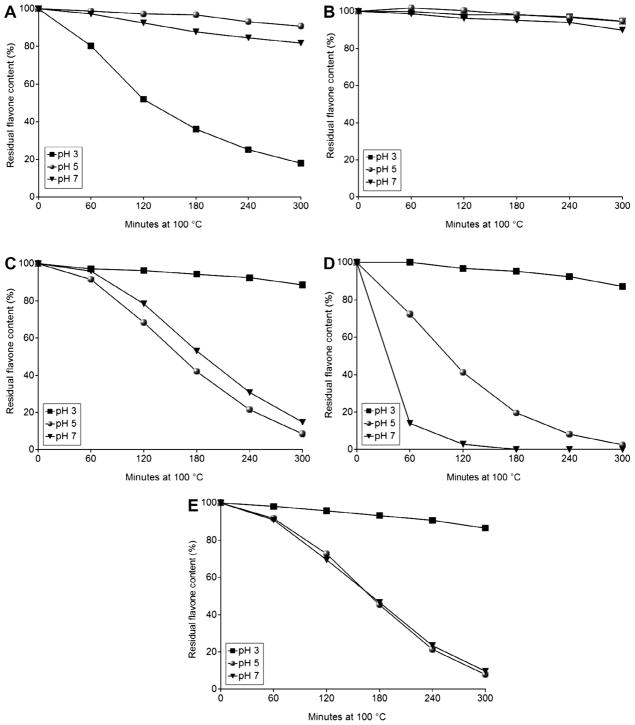
Several flavone derivatives were tested for their thermal stability. Apiin, apigenin 7-O-glucoside, apigenin, luteolin, and chrysoeriol were heated at 100 °C for up to 300 min at pH 3, 5, or 7. Apiin was relatively stable to thermal processing at pH 5 and 7, but its concentration fell rapidly after heating 1 h at pH 3 (Fig. 2A). The primary hydrolysis product of apiin at pH 3 was apigenin 7-O-glucoside (data not shown).
Fig. 2.
Deglycosylation and stability of flavone standards in aqueous solution at 100 °C for up to 300 min in pH 3, 5, or 7 ammonium acetate buffer. (A) apiin, (B) apigenin 7-O-glucoside, (C) apigenin, (D) luteolin, (E) chrysoeriol. Data are mean, n = 3. SD ≤ 10.8.
Of all flavones tested, apigenin 7-O-glucoside was the most stable to thermal processing at every pH tested, retaining 90–95% of the original flavone concentrations after 5 h of heat treatment (Fig. 2B). The flavone aglycones were all relatively stable at pH 3 but degraded rapidly under pH 5 and neutral pH conditions (Fig. 2C–E). Both apigenin and chrysoeriol showed similar thermal lability at pH 5 and 7 and degraded asymptotically (Fig. 2C and E). Luteolin degraded more rapidly at pH 5 and 7, and only 2% remained after heating 2 h at pH 7 (Fig. 2D).
3.2. Effects of food ingredients on flavone deglycosylation
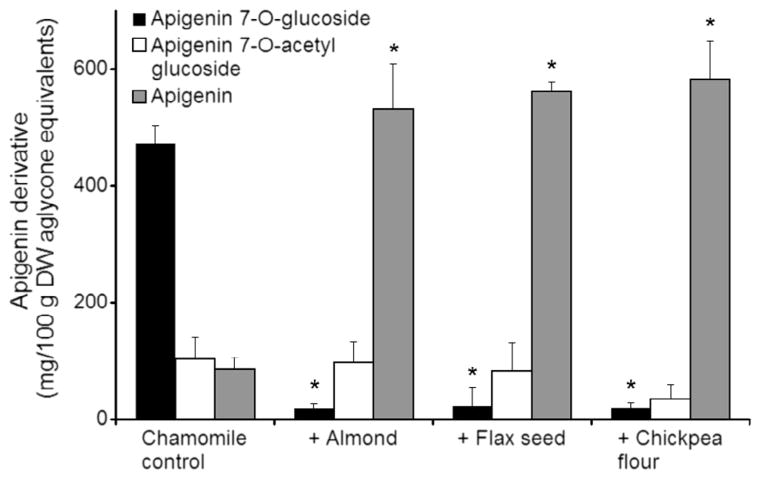
The chamomile extract contained predominantly apigenin 7-O-glucoside with smaller concentrations of apigenin 7-O-acetyl glucoside and apigenin (Fig. 3). After incubation with almond, flax seed, and chickpea flour for 20 h at 37 °C, concentrations of apigenin 7-O-glucoside decreased from over 400 mg/100 g to less than 50 mg/100 g. In the same samples, apigenin concentrations rose 4-fold from less than 100 mg/100 g to over 500 mg/100 g. The concentration of apigenin acetyl glucoside was not significantly different from the control with any of the treatments (Fig. 3). With all treatments, the concentrations of apigenin equivalents were largely conserved.
Fig. 3.
Conversion of apigenin glycosides in chamomile extract to aglycone after incubation for 20 h at 37 °C alone (control) and combined 1:1 (w/w) with other food ingredients in 1.4 M, pH 5 sodium acetate buffer. Data are mean ± SD, n = 3. P < 0.001 versus control.
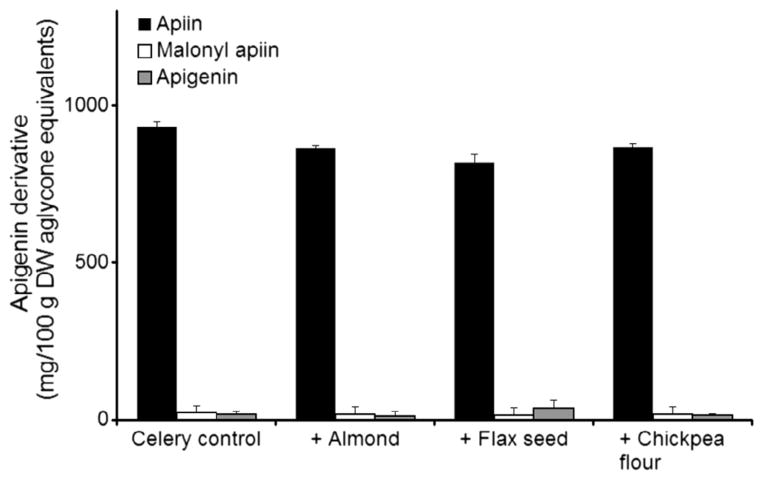
Lyophilised celery leaves contained primarily apiin, with smaller concentrations of malonyl apiin and apigenin (Fig. 4). While apigenin glycosides were the most abundant in the celery samples (75% of total flavone aglycone equivalents by weight), luteolin glycosides (14%) and chrysoeriol glycosides (11%) were also found. Almond and flax seed treatments led to significantly higher apigenin aglycone concentrations after incubation, but the concentrations only increased from 2.7% of all apigenin derivatives to 3.6% and 5.0% for almond and flax seed respectively (Fig. 4). Again, total apigenin equivalents were conserved with all treatments.
Fig. 4.
Conversion of apigenin glycosides in lyophilised celery leaves to aglycone after incubation for 20 h at 37 °C alone (control) and combined 1:2 (w/w) with other food ingredients in 1.4 M, pH 5 sodium acetate buffer. Data are mean ± SD, n = 3.
3.3. Thermal stability of flavones in celery
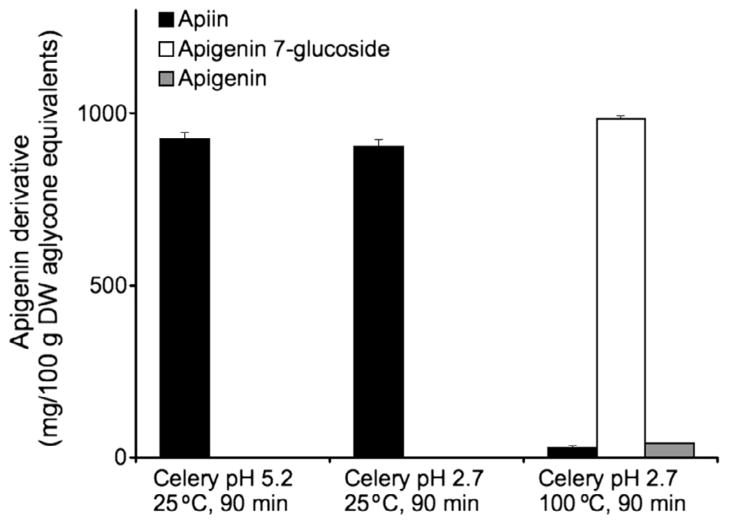
Because apigenin 7-O-glucoside was the residual product of acid hydrolysis of apiin at pH 3 and apigenin 7-O-glucoside was very resistant to thermal degradation (Fig. 2B), celery was thermally treated to maximise this conversion. When celery was acidified to pH 2.7 with phosphoric acid and left at room temperature (25 °C), the predominant apigenin derivative was apiin (Fig. 5), as was demonstrated previously with untreated celery. After acidification to pH 2.7 and thermal processing for 90 min at 100 °C, apigenin 7-O-glucoside became the most abundant derivative, with a small amount of aglycone also present (Fig. 5). The total concentration of derivatives was similar with both treatments, showing no loss of total flavones. This demonstrates that both heat and low pH are necessary for the hydrolysis of apiin to apigenin 7-O-glucoside in celery.
Fig. 5.
Conversion of apiin in celery to apigenin 7-O-glucoside and aglycone after acidification to pH 2.7 with phosphoric acid and thermal processing for 90 min at 100 °C. Data are mean ± SD, n = 3.
3.4. Effects of food ingredients on flavone glycosides in thermally processed celery
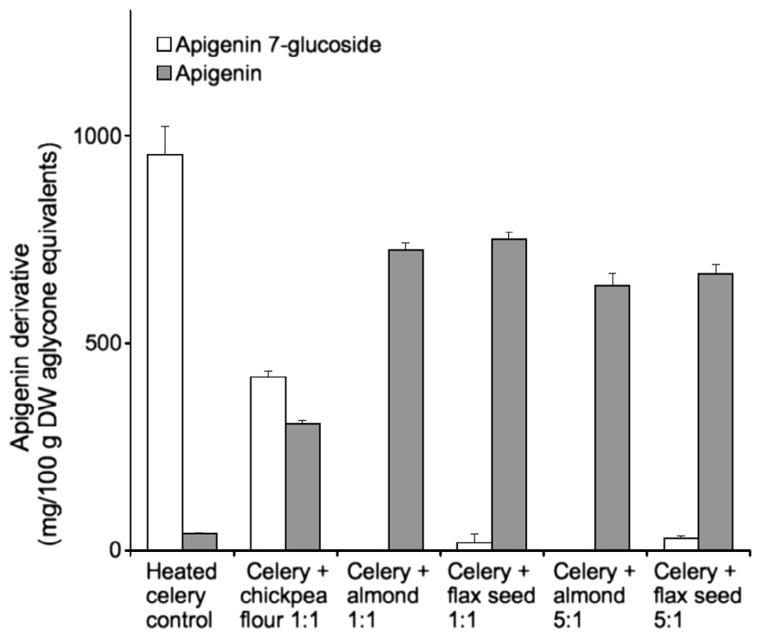
After apiin in celery was converted to apigenin 7-O-glucoside, it was neutralised to pH 5 and combined with the same enzyme-rich food ingredients used previously in order to convert apigenin 7-O-glucoside to apigenin aglycone. After blending lyophilised celery 1:1 (w/w) with almond, flax seed, or chickpea flour and incubating at 50 °C for 2 h, almond and flax seed treatments resulted in nearly complete conversion of apigenin 7-O-glucoside to aglycone, with less than 5% of apigenin glycosides remaining (Fig. 6). Although the chickpea flour treatment also produced apigenin aglycone, there was greater residual apigenin 7-O-glucoside. After mixing the celery with lower concentrations of almond or flax seed (5:1), apigenin aglycone was again the predominant flavone form present (Fig. 6). The concentration of total apigenin derivatives was 30–35% lower with the almond or flax seed treatments, indicating a substantial loss of total flavones.
Fig. 6.
Conversion of apigenin glycosides in celery to aglycone after incubation at pH 5 for 2 h at 50 °C alone (control) and combined with other food ingredients 1:1 or 5:1 (w/w). Data are mean ± SD, n = 3.
4. Discussion
Flavonoid glycosides vary in their thermal stability, and the results shown here were most similar to anthocyanins rather than flavonol glycosides or other flavone glycosides. The flavone diglycoside in the current study, apiin, hydrolysed progressively at pH 3 to afford apigenin 7-O-glucoside (Fig. 2A). Apigenin 7-O-glucoside was very stable at 100 °C regardless of pH (Fig. 2B). A similar pattern has been shown with cyanidin 3-glycosides at pH 3.5, where those with a terminal glucose moiety are more stable than those with a terminal xylose (Sadilova, Carle, & Stintzing, 2007). In contrast to the greater apiin stability at higher pH shown in the current study, the flavonol disaccharide quercetin rutinoside had greater thermal stability at pH 5 than at pH 8 (Buchner, Krumbein, Rohn, & Kroh, 2006), and apigenin malonyl glucoside and apigenin malonylacetylglucoside were more stable at pH 2 than pH 7 at 25 °C (Švehlíková et al., 2004).
The stability of flavone aglycones at pH 3 in this work is also similar to that of anthocyanins, which are known to be more stable at lower pH values (Cavalcanti, Santos, & Meireles, 2011). The flavone aglycones apigenin, luteolin, and chrysoeriol were all stable at pH 3, but degraded steadily at pH 5 or 7 (Fig. 2C–E). The most rapid degradation occurred with luteolin, which contains a catechol group on the B ring, at pH 7 (Fig. 2D). More rapid degradation has also been associated with increased hydroxylation on the B ring of anthocyanins (Sadilova et al., 2007; Woodward, Kroon, Cassidy, & Kay, 2009), and the role of oxygen in anthocyanin degradation has long been established (Daravingas & Cain, 1968). Quercetin aglycone was also more stable at pH 5 than at pH 8 (Buchner et al., 2006), in contrast to the flavone aglycones presented here, which degraded at pH 5 or 7. While all of the conditions tested in this experiment far exceed what would be used in typical food processing, the extremes in cooking times and conditions provide a picture of the relative stability of different flavone derivatives.
To test the activity of β-glucosidase-rich ingredients towards flavone glycosides, almond powder, ground flax seeds, or chickpea flour were combined with chamomile extract or lyophilised celery. Although the β-glucosidases from chickpeas have been reported to cleave isoflavone 7-O-apiosylglucosides (Hösel, 1976), this activity on apiin was not observed in our experiments with celery. Similarly, β-glucosidase from almonds can cleave isoflavone glucosides (Ismail & Hayes, 2005) but had no effect on celery flavone apiosylglucosides, apparently due to the apiose moiety, as expected. We are not aware of other studies using flax seeds to hydrolyse flavonoid glycosides. Here we found that it is effective on apigenin 7-O-glucoside but not apigenin 7-O-apiosylglucoside.
The main conversion product of apiin at pH 3 was apigenin 7-O-glucoside, and these results combined with the ability of enzyme-rich foods to hydrolyse apigenin 7-O-glucoside (Fig. 3) suggested that aglycone-rich celery could be produced by combining the two processes. By acidifying the celery homogenate to pH 2.7, heating 90 min at 100 °C was necessary for nearly complete conversion of apiin to apigenin 7-O-glucoside (Fig. 5). Experiments showed that celery can be mixed with 5:1 celery to almond and flax seeds to achieve over 95% conversion of apigenin glycosides to aglycone (Fig. 6). However, this again resulted in up to 30–35% loss of total apigenin derivatives, indicating degradation of apigenin during processing. Although pH 5 was chosen for optimal enzymatic conversion, it also favors thermal degradation of apigenin aglycone (Fig. 2C) which could explain the loss of total apigenin derivatives.
In conclusion, this study shows that although apiin from celery is resistant to β-glucosidase activity, it can be effectively hydrolysed to apigenin 7-O-glucoside with a combination of acid and heat treatments. Apigenin 7-O-glucoside can then be enzymatically converted to apigenin, resulting in an apigenin aglycone-rich food. We also show that apigenin glycosides are very resistant to degradation during thermal processing, while aglycones are labile above pH 3. Together these results provide insights into making functional foods with maximum flavone concentrations and possible improvements in bioavailability.
References
- Azzini E, Bugianesi R, Romano F, Di Venere D, Miccadei S, Durazzo A, et al. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: A pilot study. The British Journal of Nutrition. 2007;97:963–969. doi: 10.1017/S0007114507617218. [DOI] [PubMed] [Google Scholar]
- Buchner N, Krumbein A, Rohn S, Kroh LW. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Communications in Mass Spectrometry: RCM. 2006;20:3229–3235. doi: 10.1002/rcm.2720. [DOI] [PubMed] [Google Scholar]
- Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. The British Journal of Nutrition. 2010;103:249–255. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- Cavalcanti RN, Santos DT, Meireles MAA. Non-thermal stabilization mechanisms of anthocyanins in model and food systems – An overview. Food Research International. 2011;44:499–509. [Google Scholar]
- Daravingas G, Cain R. Thermal degradation of black raspberry anthocyanin pigments in model systems. Journal of Food Science. 1968;33:138–142. [Google Scholar]
- Ducret A, Trani M, Lortie R. Comparison between various commercial sources of almond β-glucosidase for the production of alkyl glucosides. Journal of Molecular Catalysis B: Enzymatic. 2006;38:91–94. [Google Scholar]
- DuPont MS, Mondin Z, Williamson G, Price KR. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce and endive. Journal of Agricultural and Food Chemistry. 2000;48:3957–3964. doi: 10.1021/jf0002387. [DOI] [PubMed] [Google Scholar]
- Engelmann C, Blot E, Panis Y, Bauer S, Trochon V, Nagy HJ, et al. Apigenin – Strong cytostatic and anti-angiogenic action in vitro contrasted by lack of efficacy in vivo. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2002;9:489–495. doi: 10.1078/09447110260573100. [DOI] [PubMed] [Google Scholar]
- Fan TW, Conn EE. Isolation and characterization of two cyanogenic beta-glucosidases from flax seeds. Archives of Biochemistry and Biophysics. 1985;243:361–373. doi: 10.1016/0003-9861(85)90513-2. [DOI] [PubMed] [Google Scholar]
- Gil-Izquierdo A, Gil MI, Ferreres F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. Journal of Agricultural and Food Chemistry. 2002;50:5107–5114. doi: 10.1021/jf020162+. [DOI] [PubMed] [Google Scholar]
- Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- Hösel W. Purification and properties of two beta-glycosidases from Cicer arietinum L. with preferential specificity for biochanin A 7-beta-apiosylglucoside (author’s transl) Hoppe-Seyler’s Zeitschrift Für Physiologische Chemie. 1976;357:1673–1681. [PubMed] [Google Scholar]
- Hostetler G, Riedl K, Schwartz S. Endogenous enzymes, heat, and pH affect flavone profiles in parsley (Petroselinum crispum var. neapolitanum) and celery (Apium graveolens) during juice processing. Journal of Agricultural and Food Chemistry. 2012;60:202–208. doi: 10.1021/jf2033736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail B, Hayes K. Beta-glycosidase activity toward different glycosidic forms of isoflavones. Journal of Agricultural and Food Chemistry. 2005;53:4918–4924. doi: 10.1021/jf0404694. [DOI] [PubMed] [Google Scholar]
- Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. Journal of Chromatography A. 1998;799:101–110. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- Lechtenberg M, Zumdick S, Gerhards C, Schmidt TJ, Hensel A. Evaluation of analytical markers characterising different drying methods of parsley leaves (Petroselinum crispum L.) Die Pharmazie. 2007;62:949–954. [PubMed] [Google Scholar]
- Lin LZ, Lu S, Harnly JM. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC–DAD–ESI/MS. Journal of Agricultural and Food Chemistry. 2007;55:1321–1326. doi: 10.1021/jf0624796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P, Leung YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through ERβ. Neoplasia. 2006;8:896–904. doi: 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. Journal of Agricultural and Food Chemistry. 2002;50:5837–5843. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- Matern U. Acylhydrolases from parsley (Petroselinum hortense). Relative distribution and properties of four esterases hydrolyzing malonic acid hemiesters of flavonoid glucosides. Archives of Biochemistry and Biophysics. 1983;224:261–271. doi: 10.1016/0003-9861(83)90209-6. [DOI] [PubMed] [Google Scholar]
- Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Annals of Nutrition & Metabolism. 2006;50:167–172. doi: 10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- Németh K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. European Journal of Nutrition. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. The Journal of Immunololgy. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- Nielsen ILF, Chee WSS, Poulsen L, Offord-Cavin E, Rasmussen SE, Frederiksen H, et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. The Journal of Nutrition. 2006;136:404–408. doi: 10.1093/jn/136.2.404. [DOI] [PubMed] [Google Scholar]
- Piantelli M, Rossi C, Iezzi M, La Sorda R, Iacobelli S, Alberti S, et al. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. Journal of Cellular Physiology. 2006;207:23–29. doi: 10.1002/jcp.20510. [DOI] [PubMed] [Google Scholar]
- Riedl KM, Zhang YC, Schwartz SJ, Vodovotz Y. Optimizing dough proofing conditions to enhance isoflavone aglycones in soy bread. Journal of Agricultural and Food Chemistry. 2005;53:8253–8258. doi: 10.1021/jf0508549. [DOI] [PubMed] [Google Scholar]
- Sadilova E, Carle R, Stintzing F. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Molecular Nutrition and Food Research. 2007;51:1461–1471. doi: 10.1002/mnfr.200700179. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. Journal of Agricultural and Food Chemistry. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- Schütz K, Kammerer D, Carle R, Schieber A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC–DAD–ESI/MSn. Journal of Agricultural and Food Chemistry. 2004;52:4090–4096. doi: 10.1021/jf049625x. [DOI] [PubMed] [Google Scholar]
- Švehlíková V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, et al. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert) Phytochemistry. 2004;65:2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Tian XJ, Yang XW, Yang X, Wang K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. International Journal of Pharmaceutics. 2009;367:58–64. doi: 10.1016/j.ijpharm.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamazaki C, Yamazaki M. A hydroxyl group of flavonoids affects oral anti-inflammatory activity and inhibition of systemic tumor necrosis factor-α production. Bioscience, Biotechnology, and Biochemistry. 2004;68:119–125. doi: 10.1271/bbb.68.119. [DOI] [PubMed] [Google Scholar]
- Woodward G, Kroon P, Cassidy A, Kay C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. Journal of Agricultural and Food Chemistry. 2009;57:5271–5278. doi: 10.1021/jf900602b. [DOI] [PubMed] [Google Scholar]