Abstract
Lycopene is a non-provitamin A carotenoid that is responsible for the red to pink colors seen in tomatoes, pink grapefruit, and other foods. Processed tomato products are the primary dietary lycopene source in the United States. Unlike many other natural compounds, lycopene is generally stable to processing when present in the plant tissue matrix. Recently, lycopene has also been studied in relation to its potential health effects. Although promising data from epidemiological, as well as cell culture and animal, studies suggest that lycopene and the consumption of lycopene containing foods may affect cancer or cardiovascular disease risk, more clinical trial data is needed to support this hypothesis. In addition, future studies are required to understand the mechanism(s) whereby lycopene or its metabolites are proven to possess biological activity in humans.
Keywords: lycopene, carotenoids, lycopenoids, bioavailability, cancer, cardiovascular disease
INTRODUCTION
Epidemiological research indicates that diets rich in fruits and vegetables are associated with a decreased risk of chronic diseases such as cardiovascular disease (CVD) (Ignarro et al. 2007) and cancer (Block et al. 1992). It is estimated that approximately 50% of cancer cases and 35% of cancer deaths in the United States can be attributed to poor diet (Williams et al. 1999). Epidemiological studies have associated tomato consumption with a decreased risk of prostate cancer ( Jain et al. 1999, Giovannucci et al. 2002) and CVD (Arab & Steck 2000). Despite this evidence, it is not clear which individual compounds present in tomato, such as lycopene, impart these potential benefits or whether other constituents of tomatoes and tomato products produce beneficial effects.
Lycopene, a naturally occurring red carotenoid pigment found in tomatoes, pink grapefruit, watermelon, papaya, guava, and other fruits, has been extensively studied for more than 70 years, with more than 2000 articles published in peer-reviewed journals and 4000 other publications (scientific and otherwise) written on the subject. Most of these articles have focused on lycopene derived from tomatoes. Given the vast amount of information already published about lycopene, we focus on the recent literature (1999–2009) related to the health effects of tomato lycopene in humans. Although in vitro and animal studies are vitally important to understanding the mechanisms behind potential health effects, human studies, specifically clinical trials, are ultimately used to determine the effect of dietary constituents on health, as well as to set nutrition and food labeling policy. For more information on in vitro and animal studies related to the potential health effects of lycopene, please see the reviews by Rao et al. (2006) and Cohen (2002). Over the past decade, lycopene-containing foods (primarily tomato products) and lycopene supplements have been reported to affect diseases ranging from cancer to heart disease to asthma (Dahan et al. 2008, Riccioni et al. 2008, Wood et al. 2008). Most recently, researchers have begun to investigate lycopenoids, oxidative metabolites of lycopene, based on the possibility that these lycopenoids may produce biological effects (Erdman et al. 2009).
Given the scope of literature published on the potential health benefits of this carotenoid in the diet, herein we review publications related to lycopene chemistry, sources, intake, and bioavailability. In addition, we summarize the literature on the presence and formation of lycopenoids, and discuss the most promising directions for future lycopene research.
LYCOPENE CHEMISTRY
Lycopene is one pigment in a large family of plant pigments known as carotenoids. Carotenoids produce colors ranging from the yellow color of squash, to the orange color of pumpkins, to the red color of tomatoes. Carotenoids also contribute to some plant food aromas (Rodriguez- Bustamante & Sanchez 2007). Some carotenoids also possess provitamin A activity and have shown potent antioxidant activity. To date, more than 700 carotenoids have been identified (Britton et al. 2004). There are two primary types of carotenoids: hydrocarbon carotenoids and xanthophylls. Hydrocarbon carotenoids, such as lycopene, are composed entirely of hydrogen and carbon. In contrast, xanthophylls, such as lutein, contain oxygen in addition to carbon and hydrogen (Furr & Clark 1997). Some hydrocarbon carotenoids, such as β-carotene and α-carotene, can be enzymatically cleaved to form vitamin A. Lycopene lacks provitamin A activity because of the absence of a terminal beta ionone ring (Rao & Rao 2007). Carotenoids typically contain 40 carbons. Apo-carotenoids contain less than 40 carbons. The prefix apo is used to identify carotenoids that have been shortened in length by one or more carbons (Britton et al. 2004). Regardless of the number of carbons, all carotenoids possess an isoprenoid backbone (Britton et al. 2004).
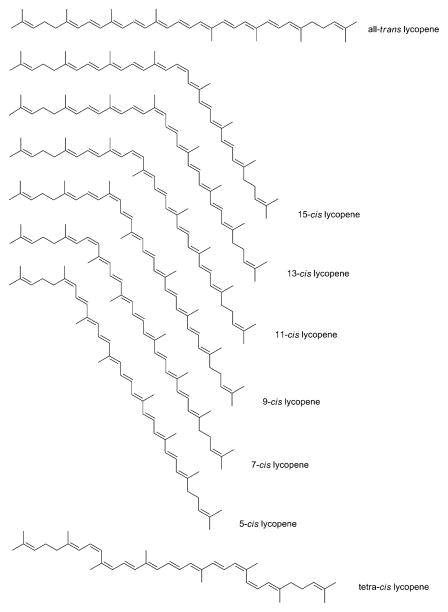
The chemical formula for lycopene is C40H56. The 11 conjugated and 2 unconjugated double bonds present in lycopene allow for extensive isomerization, resulting in 1056 theoretical cis-trans configurations (Zechmeister 1944). Only a few isomers are actually found in nature, however, (Figure 1) with the all-trans configuration of lycopene being the most common isomer found in foods (Nguyen & Schwartz 2000). The thermodynamic stabilities of the common lycopene isomers have been determined relative to the all-trans isomer. The 5-cis isomer was found to be the most stable followed by all-trans, 9-cis, 13-cis, 15-cis, 7-cis, and 11-cis (Chasse et al. 2001). The lycopene isomers found in human blood plasma, breastmilk, and human tissues are mainly of the cis-isomer type (Hadley et al. 2003, Allen et al. 2002, Ferruzzi et al. 2001, Boileau et al. 2002). The color of lycopene is directly related to its isomeric form. The all-trans isomer and most other isomers of lycopene are red, whereas tetra-cis-lycopene possesses an orange hue (Nguyen & Schwartz 2000, Zechmeister 1944).
Figure 1.
Structure of lycopene and several isomers.
Reactive oxygen species (ROS) are oxygen-containing molecules that either are or have the potential to generate free radicals. Overproduction of ROS results in a condition known as oxidative stress, which has been linked to both cancer and cardiovascular disease (Halliwell 1994). Carotenoids, including lycopene, can be potent antioxidant molecules and are especially effective at scavenging the ROS singlet oxygen. Of the carotenoids, lycopene is the most effective singlet oxygen scavenger in vitro (Sies & Stahl 1995). This antioxidant activity has been proposed as a mechanism for the potential health benefits of carotenoids (Sies & Stahl 1995, Agarwal & Rao 2000). Recently, this antioxidant mechanism has been called into question given the low concentration of lycopene in the body relative to other antioxidant molecules, such as vitamin E (Erdman et al. 2009). This has led to speculation that observed health benefits may be due to the effect of lycopene or oxidative metabolites on gene expression (Erdman et al. 2009).
LYCOPENE SOURCES, INTAKE, AND BIOAVAILABILITY
More than 80% of dietary lycopene intake in the U.S. is derived from processed tomato products such as ketchup, tomato juice, spaghetti sauce, and pizza sauce (Clinton 1998). The amount of lycopene present in processed foods is often much higher than that found in fresh foods given that processing often involves concentration via water loss. For example, ketchup contains 9.9–13.44 mg lycopene/100 g, whereas fresh tomatoes contain anywhere from 0.88–7.74 mg lycopene/100 g wet weight (Rao et al. 1998, Nguyen & Schwartz 1998).
Dietary intake of lycopene varies greatly depending upon the studied population. The average Italian consumes 14.3 mg/day of total carotenoids (Lucarini et al. 2006). Lycopene makes up the largest proportion of carotenoids in the Italian diet, with an average intake of 7.4 mg/day (Lucarini et al. 2006). The average daily intake of lycopene in the United States ranges from 6.6–10.5 mg/day for men and from 5.7–10.4 mg/day for women (Porrini & Riso 2005). The average reported daily lycopene intake is 1.1 mg/day in the United Kingdom, 1.6 mg/day in Spain, 3.8 mg/day in Australia, 4.8 mg/day in France, and 4.9 mg/day in the Netherlands (Porrini & Riso 2005).
Lycopene bioavailability can be affected by a number of factors, including food processing and dietary composition. Lycopene can occur in several forms in fresh plant foods, including carotenoid-protein complexes in chloroplasts or in crystalline form inside chromoplasts (Parada & Aguilera 2007). The effects of processing and storage on lycopene structure and stability are of interest for a number of reasons. Improper processing and storage (i.e., exposure to light and oxygen) may alter the ratio of lycopene isomers or degrade lycopene entirely, making these food products less desirable to the consumer (Xianquan et al. 2005). Traditional commercial processing methods do not have a significant effect on lycopene levels or on cis/trans isomerization (Nguyen & Schwartz 1998). In fact, thermal processing generally improves lycopene bioavailability by disrupting cellular membranes, which allows lycopene to be released from the tissue matrix (Nguyen et al. 2001). Multiple studies have shown that lycopene from thermally processed tomato products is more bioavailable than lycopene from fresh tomatoes (Gärnter et al. 1997, Stahl & Sies 1992, Allen et al. 2002).
The absolute amount of lycopene absorbed does not appear to vary greatly with dose. A study performed in men observed lycopene absorption after consumption of one serving of tomato juice (Diwadkar-Navsariwala et al. 2003). Different volumes of tomato juice, with a constant percent fat, were administered to deliver 10 mg to 120 mg of lycopene. The range of lycopene absorbed, independent of dose, was between 1.8 mg and 14.3 mg, with an average of 4.7 mg. The amount of lycopene absorbed by the men consuming juice containing 120 mg lycopene was not significantly different from that absorbed by the men consuming juice containing 10 mg lycopene. These authors suggested that inter-individual differences, not dose, has the greatest impact on the amount of lycopene absorbed (Diwadkar-Navsariwala et al. 2003).
Lycopene bioavailability is greatly affected by dietary composition. Given that lycopene is a lipid-soluble compound, consuming it with fat increases its bioavailability. For example, consuming salads with full-fat dressing results in higher blood carotenoid levels than eating salads with reduced fat dressing. When salads were consumed without fat in the same study, no measurable lycopene uptake occurred (Brown et al. 2004). A study by Unlu et al. (2005) showed a similar result, whereby the consumption of tomato salsa with avocado (as lipid source) led to a 4.4-fold increase in lycopene absorption as compared with salsa without avocado.
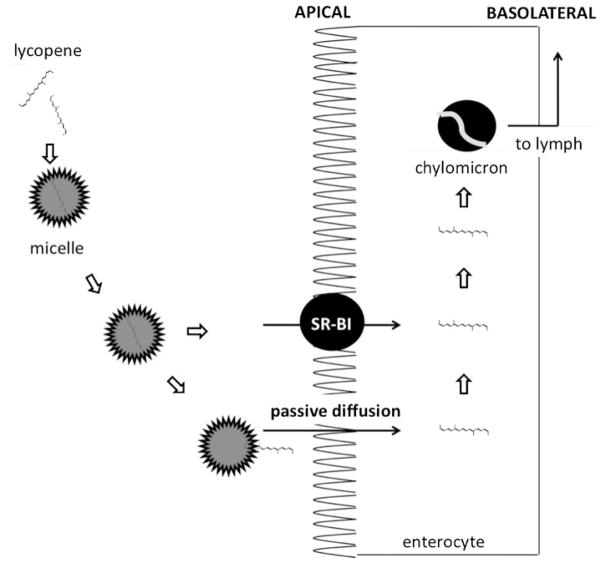
A schematic of lycopene digestion and absorption is shown in Figure 2. Once ingested, lycopene must first be released from the food matrix before it is incorporated into mixed micelles. Micelles contain bile salts, cholesterol, and fatty acids from the meal, and the amphiphilic nature of the micelle structure helps to keep the lipophilic nutrients soluble in the aqueous digesta (During & Harrison 2004). The micelles approach the unstirred water layer of the apical side of the intestinal cells (enterocytes), and lycopene passively diffuses across the apical membrane (During & Harrison 2004). Historically, researchers believed that lycopene was absorbed by the same route as dietary lipids, i.e., passive diffusion (Furr & Clark 1997), and this is still believed to be at least partially true. However, in the past five years, investigators have discovered that lycopene absorption can be facilitated by a cholesterol membrane transporter known as scavenger receptor class B type I (SR-BI) (During et al. 2005, Moussa et al. 2008). Research has also suggested that lycopene absorption may be facilitated by other transporters, but this has not yet been confirmed (During et al. 2005, Moussa et al. 2008). Once inside the enterocyte, lycopene is packaged with other dietary lipids into chylomicrons (During & Harrison 2004). Chylomicrons are then transported across the basolateral membrane and make their way into the lymphatic system, which eventually releases chylomicrons into the blood.
Figure 2.
Digestion and absorption of lycopene in the small intestine. Data adapted from During & Harrison (2004) and During et al. (2005).
Competition by other carotenoids or cholesterol may also influence lycopene absorption. One post-prandial human study demonstrated that co-consumption of tomato puree (30 mg lycopene) + spinach lutein (30 mg) or encapsulated lutein (30 mg) reduced chylomicron lycopene levels by 70% and 61% respectively, as compared with lycopene levels observed after consumption of tomato puree alone (Tyssandier et al. 2002). However, when subjects consumed these foods daily for three weeks at half of the previous dose (15 mg of lycopene + 15 mg lutein), no difference was observed in steady-state plasma levels of lycopene (Tyssandier et al. 2002). A human study by Johnson et al. (1997) observed that co-administration of β-carotene crystals in oil (60 mg) with lycopene crystals in oil (60 mg) increased the 24-hour postprandial serum area under the curve (AUC) of lycopene by four-fold as compared with the administration of lycopene alone. It is unclear whether absorption of lycopene as part of a food product would increase if co-consumed with a β-carotene–containing food product. Considering the recent research demonstrating the transport of lycopene by cholesterol transporter SR-BI, it is likely that genetic differences in SR-BI expression may also affect the absorption of lycopene. However, no studies have yet been published on this topic.
In addition, lycopene absorption may be affected by other factors such as probiotics and single nucleotide polymorphisms (SNPs). Consumption of a probiotic-containing yogurt versus a regular yogurt lowered blood lycopene levels in a group of 17 women who consumed probiotics for a total of 4 weeks (Fabian & Emaldfa 2007). This study suggests that probiotics may affect lycopene bioavailability or metabolism (Fabian & Emaldfa 2007). Borel et al. (2007) reported that human blood carotenoid levels are influenced by SNPs in apolipoproteins A-IV and B, which are associated with lipid transport.
Greater than 90% of the lycopene found in processed tomato products is in the all-trans conformation (Nguyen & Schwartz 1998, Nguyen et al. 2001, Boileau et al. 2002). In vivo studies demonstrate that the cis-isomers of lycopene appear to be more bioavailable than the all-trans isomer (Stahl & Sies 1992, Unlu et al. 2007). In vitro experiments support the conclusion that increased bioavailability of lycopene cis-isomers is at least partially due to increased micellarization and increased uptake by the enterocyte relative to all-trans lycopene (Failla et al. 2008).
Human organs store lycopene to varying degrees. Lycopene is found in the highest concentrations in the liver, testes, adrenal glands, and adipose tissues (Kun et al. 2006). It is found in lower concentrations in the kidney, ovary, lung, and prostate (Kun et al. 2006).
POTENTIAL HEALTH BENEFITS OF LYCOPENE
Over the past decade, the effects of lycopene have been studied with respect to a wide range of diseases (various cancers, CVD) (Dahan et al. 2008, Riccioni et al. 2008). Here we summarize data from recent human studies examining lycopene’s biological effects on these disease processes.
Cancer
Cancer is the second leading cause of death in the United States, with approximately 1.5 million new cases of cancer diagnosed in 2008 (American Cancer Society 2008). The consumption of tomatoes and tomato products has been associated with a reduced incidence of a number of different types of cancers, most notably prostate, lung, and stomach (Giovannucci 1999).
Of the diseases studied in relation to lycopene, prostate cancer is one of the most well-researched. In addition to prostate cancer, benign prostatic hyperplasia (BPH), the age-related non-cancerous overgrowth of the prostate gland, also negatively affects men’s health. Some of the strongest epidemiological evidence to support an association between tomato product consumption and a reduced incidence of prostate cancer has come from the Health Professionals Follow-Up Study (HFPS). Most recently, a prospective observational study by Giovannucci et al. (2002) collected food frequency questionnaire (FFQ) data from the HPFS group of 47,365 men in 1986, 1990, and 1994. Intake of ≥2 servings of tomato sauce per week was associated with a reduced risk of prostate cancer [relative risk (RR) = 0.77 relative to <1 serving of tomato sauce per month, Ptrend < 0.001]. Lycopene intake was also associated with a reduced risk of prostate cancer, but the association was weaker (Giovannucci et al. 2002). A study by Lu et al. (2001) quantified lycopene in blood plasma of 65 prostate cancer patients and 132 cancer-free controls. A significant inverse association between prostate cancer and plasma lycopene concentration [odds ratio (OR) = 0.17, Ptrend = 0.005] was observed between the highest and lowest quintiles of intake (Lu et al. 2001). Another group of researchers observed men (n = 4770) from the placebo arm of the Prostate Cancer Prevention Trial from 1994–2003 who were free of BPH at baseline (Kristal et al. 2008). Over the course of the study, 876 men developed BPH (Kristal et al. 2008). FFQ data revealed that there was an 18% reduction in risk of BPH in men who consumed the greatest amount of lycopene as a food or a supplement (Ptrend = 0.056). A recent meta-analysis demonstrated a modest inverse relationship between intake of raw tomatoes (RR = 0.89 for highest versus lowest quartile of intake) and tomato products (RR = 0.81 for highest versus lowest quartile of intake) and prostate cancer (Etminan et al. 2004). Collectively, these studies suggest that the consumption of lycopene or lycopene-containing foods reduces the risk for developing prostate cancer.
In contrast, other observational studies have found weak (Chang et al. 2005) or inconclusive (Peters et al. 2007) evidence supporting a link between prostate cancer or BPH and lycopene intake.
Since 1999, at least 12 clinical trials have examined the relationship between tomato products or lycopene containing supplements and prostate cancer. Most of these studies measured prostate specific antigen (PSA). PSA levels are routinely measured to determine prostate cancer risk and to monitor prostate cancer treatment (Chen et al. 2001). In one study by Chen et al. (2001), 32 prostate cancer patients consumed tomato sauce daily for three weeks (30 mg lycopene/day) before a radical prostatectomy. Serum PSA levels decreased after the dietary intervention by 20% (p < 0.001). In addition, analysis of the prostate tissue revealed a decrease in the ratio of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a DNA adduct indicative of oxidative stress and associated with cancer, to 2′-deoxyguanosine (a marker of oxidative DNA damage) in the treated patients as compared with random controls (ratio = 0.76 versus 1.06, respectively; p = 0.03) (Chen et al. 2001). A separate clinical study examined the effects of tomato and tomato product consumption with and without soy protein isolate in men (n = 41) with recurrent, asymptomatic prostate cancer (Grainger et al. 2008). Consumption of tomatoes and tomato products daily (target intake level 25 mg/day lycopene) for eight weeks reduced serum PSA levels in 34% of the subjects (Grainger et al. 2008). A phase II clinical trial was performed in men (n = 71) diagnosed with prostate cancer investigating the effects on PSA levels of a tomato extract capsule (Lyc-o-Mato®, 15mg/day lycopene) consumed twice daily for up to 6 months with or without soy isoflavones (Vaishampayan et al. 2007). Lyc-o-Mato® is a tomato extract oleoresin containing 15 mg lycopene along with other tomato phytochemicals including phytoene (1.5 mg), phytofluene (1.4 mg), β-carotene (0.4 mg), and tocopherols (5 mg) (Voskuil et al. 2008). These researchers observed that subjects in both treatment groups had a decline in the rate of rise of PSA levels (Vaishampayan et al. 2007). A placebo-controlled clinical study, in 37 men with confirmed BPH, had men consume either a LycoVit® synthetic lycopene supplement (15 mg/day) or a placebo pill daily for six months (Schwarz et al. 2008). The consumption of the LycoVit® tablet reduced serum PSA levels by 10% (p < 0.05) over the course of six months, whereas there was no change in serum PSA in the placebo group (Schwarz et al. 2008). In contrast to the clinical trials showing a reduction in PSA levels as a surrogate marker for prostate cancer status, some studies observed a weak effect (Barber et al. 2006) or no effect (Clark et al. 2006, Bunker et al. 2007, Jatoi et al. 2007) of tomato consumption or lycopene supplementation on prostate cancer risk.
Epidemiological evidence has suggested that consumption of lycopene containing foods may decrease risk for breast cancer. A prospective cohort study by Cui et al. (2008) found that lycopene consumption, as estimated by an FFQ, was inversely associated with estrogen and progesterone receptor positive breast cancer risk in postmenopausal women (n = 84,805) followed for an average of 7.6 years (RR = 0.85 for highest quartile of intake as compared with lowest quartile of intake, Ptrend = 0.064). Two case-control studies comparing the dietary habits of women with and without breast cancer also observed a significant decrease in the odds ratio of those who consumed the highest amount versus the lowest amount of dietary lycopene (Ronco et al. 1999, Levi et al. 2001).
Effects on biomarkers of breast cancer risk have also been investigated. A cross-sectional study of 207 breast cancer survivors by Thomson et al. (2007) demonstrated that increased plasma lycopene concentrations were modestly correlated with reduced levels of 8-OHdG. A recent randomized, placebo-controlled, double-blind, crossover trial conducted by Voskuil et al. (2008) determined that tomato-extract supplementation (Lyc-o-Mato®, 30 mg/day lycopene) for two months in premenopausal women with a high breast cancer risk (n = 36) reduced free insulin-like growth factor-I (IGF-I) by 7.0% (p < 0.05). IGF-I is a biomarker associated with increased breast cancer risk in premenopausal women (Hankinson et al. 1998).
Lung cancer is the leading cause of cancer death in the U.S. for both men and women. Most lung cancers are related to tobacco use. Lung cancer is of particular interest with regards to carotenoid research given the increased risk of lung cancer development observed in smokers consuming a β-carotene supplement (Omenn et al. 1996, Albanes et al. 1996). Most recently, the VITamins And Lifestyle (VITAL) cohort study observed how previous β-carotene supplement usage was correlated with incidence of lung cancer development in 77,126 free-living Washington State residents. This study reported that β-carotene supplementation was associated with a threefold increase in lung cancer incidence (Satia et al. 2009). Epidemiological studies suggest that higher intake of lycopene is associated with either a reduced risk of lung cancer (Yuan et al. 2001, Holick et al. 2002, Michaud et al. 2000, Ito et al. 2005b), or no change in lung cancer risk, as compared with lower intake levels (Yuan et al. 2003, Rohan et al. 2002, Voorrips et al. 2000, Knekt et al. 1999, Steinmetz et al. 1993, Satia et al. 2009). A recent meta-analysis by Gallicchio et al. (2008) summarizes the available data on lycopene and lung cancer.
There have also been mixed results in the epidemiological studies examining the association between lycopene and colorectal cancer risk. In a prospective cohort study of 3182 free-living subjects in rural Japan, higher serum levels of lycopene were significantly associated with a reduced risk of colorectal cancer mortality (Ito et al. 2005a). However, a case-control study by Kune & Watson (2006), a meta-analysis of 11 cohort studies by Männisto et al. (2007), and a study by Leung et al. (2008) found that lycopene intake and plasma lycopene levels were not associated with colorectal cancer risk or survival in patients already diagnosed with cancer.
Several clinical trials have reported beneficial effects of lycopene-containing supplements on colorectal cancer biomarkers. A randomized, placebo-controlled, double-blind crossover study conducted by Vrieling et al. (2007) demonstrated that tomato derived lycopene supplementation (Lyc-o-Mato®, 30 mg/day lycopene) for eight weeks in 40 men and 31 postmenopausal women at high risk for colorectal cancer increased serum insulin-like growth factor binding protein-1 (IGFBP-1) concentrations in women by 22%. Serum IGFPB-2 concentrations in men and women were also increased by 8.2% and 7.8%, respectively (Vrieling et al. 2007). IGFBPs have been shown to bind and inactivate IGF (higher concentrations of IGF are associated with a higher risk of developing cancer) (Vrieling et al. 2007). Another double-blind, randomized, placebo-controlled study by Walfisch et al. (2007) observed the effects of lycopene supplementation on IGF levels in colon cancer patients. This study found that supplementation with tomato extract (Lyc-o-Mato®, 30 mg/day lycopene) in 30 patients waiting for colectomy surgery led to a 25% decrease in plasma IGF-I concentration (p = 0.02). A 24% decrease in the ratio of IGF-I/IGFBP-3 was also observed (p = 0.03) (Walfisch et al. 2007). This was considered to be a positive result as a study by Ma et al. (1999) reported that men in the highest quintile of plasma IGF-I concentrations had an increased risk of colorectal cancer compared with men in the lowest quintile (p = 0.02). In another study, 20 healthy subjects participated in a double-blind crossover dietary intervention and consumed a tomato juice beverage (250 ml of Lyc-o-Mato® drink, 5.7 mg lycopene, 3.7 mg phytoene, 2.7 mg phytofluene, 1 mg β-carotene, and 1.8 mg α-tocopherol), and a placebo beverage for 26 days each, with a 26-day washout in between (Riso et al. 2006). Blood plasma levels of IGF-I were inversely correlated with lycopene consumption (Riso et al. 2006). In contrast, a study by Graydon et al. (2007) did not find evidence to suggest that synthetic lycopene reduces IGF-I and IGFBP-3 in healthy men.
Gastric cancer is not common in the United States, but is a commondisease in Korea, Japan, and other Asian countries. The majority of human studies examining the effects of lycopene on gastric cancer have been observational rather than clinical. A 12-year case-control study of 191 cases and 570 controls within a cohort of 18,244 middle-aged men in Shanghai, China observed higher serum levels of lycopene in individuals who developed gastric cancer as compared with controls (Yuan et al. 2004). Similarly, a 2 year case-control study in Uruguay observed a strong inverse relationship between dietary lycopene intake (as determined by FFQ) and gastric cancer when comparing 120 cases of stomach cancer with 360 controls (De Stefani et al. 2000). A prospective cohort study observed a subset of 243 subjects from the α-tocopherol, β-carotene (ATBC) Cancer Prevention trial who were diagnosed with gastric adenocarcinomas and compared them with the ~29,000 male smokers in the original trial (Nouraie et al. 2005). Data from this study demonstrated that higher lycopene intake, as determined by an FFQ, was correlated with a reduced risk of gastric noncardia cancer (hazard ratio = 0.67). Gastric noncardia cancer is a type of gastric cancer strongly associated with Helicobacter pylori (Nouraie et al. 2005). In contrast, a number of observational studies from the Netherlands, Spain, Sweden, and Japan found no association between lycopene intake or plasma lycopene levels and gastric cancer risk (Botterweck et al. 2000, Garcia-Closas et al. 1999, Larsson et al. 2007, Persson et al. 2008).
Few studies have investigated the effect of lycopene on pancreatic cancer. Early evidence has suggested that increased levels of serum lycopene were associated with a reduced risk of pancreatic cancer (Burney et al. 1989, Comstock et al. 1991). More recently, a case-control study of Kuwaitis (11 cases with matched controls) by Abiaka et al. (2001) found that low plasma lycopene levels (0.12 μM for cases versus 0.72 μM for controls) were associated with pancreatic cancer (p<0.0001). Another case-control study (462 cases and 4721 population-based controls) in Canada by Nkondjock et al. (2005) reported that increased dietary lycopene intake (assessed by FFQ) was associated with a reduction in pancreatic cancer risk in men when comparing the highest versus lowest quintiles of intake (OR = 0.69, Ptrend = 0.026).
There are a limited number of epidemiological studies that have been done regarding lycopene and ovarian cancer. A case-control study (549 cases, 516 controls) by Cramer et al. (2001) in pre- and postmenopausal women found that dietary lycopene intake (determined by FFQ) was significantly and inversely associated with ovarian cancer risk (OR = 0.53 for the highest quintile as compared with the lowest quintile of intake, Ptrend = 0.003). A recent case-control study (45 cases, 135 controls) by Jeong et al. (2009) of Korean women also found that increased plasma lycopene was associated with reduced risk of ovarian cancer (OR = 0.09 between highest and lowest tertiles of intake, Ptrend = <0.0001). In contrast to these studies, others have found no relationship between serum lycopene and ovarian cancer risk (Helzlsouer et al. 1996, Zhang et al. 2007).
Cardiovascular Disease
Cardiovascular disease (CVD) is the leading cause of death in the United States and was responsible for more than 652,000 deaths in 2005 (U.S. Mortality Data 2008). Increased plasma lycopene levels have been associated with reductions in CVD risk and have also been reported to improve biomarkers associated with CVD. For example, a study by Sesso et al. (2003) of 38,445 women found that higher levels of tomato-based product intake were associated with a reduced risk of cardiovascular disease (RR = 0.71, Ptrend = 0.029) and myocardial infarction (RR = 0.43, Ptrend = 0.033) between the highest and lowest quintiles of intake. A separate study in Finnish men (n = 1028) found an inverse association between serum lycopene concentrations and common carotid artery intima-media thickness, a measure of early atherosclerosis (Rissanen et al. 2003). In addition, a study using a subset of data (n = 4557) from the third National Health and Nutrition Survey (NHANES III) observed decreased serum levels of carotenoids (including lycopene) in individuals with higher levels of C-reactive protein, a marker of inflammation (Kritchevsky et al. 2000).
Some clinical trials have also supported a relationship between cardiovascular disease and lycopene intake. Some studies have shown that lycopene may reduce cholesterol synthesis and increase low-density lipoprotein (LDL) degradation (Arab & Steck 2000). A randomized crossover study by Agarwal & Rao (1998) used four different treatments: placebo (0 mg lycopene), tomato juice (50.4 mg lycopene), spaghetti sauce (39.2 mg lycopene), and tomato oleoresin (75 mg lycopene). Nineteen healthy subjects consumed each treatment daily for one week and went through a one-week washout period between each treatment week. Serum lycopene concentration doubled in subjects on the lycopene-containing treatments. In addition, a significant decrease in serum lipid peroxidation and LDL oxidation was observed after subjects consumed any one of the three lycopene-containing treatments (Agarwal & Rao 1998). In a study by Hadley et al. (2003), healthy individuals received one of three tomato treatments for 15 days (condensed tomato soup, ready-to- serve tomato soup, or V8® vegetable juice). Blood samples were taken at baseline and after treatment, and the LDL + VLDL (very-low-density lipoprotein) fraction was exposed ex vivo to oxidative stress (Hadley et al. 2003). The lipoprotein oxidation lag period, a measure of protection against oxidative stress, was significantly increased in all three treatment groups (Hadley et al. 2003). A separate clinical trial by Shen et al. (2007) treated 24 subjects with either fresh tomato, tomato juice, or a lycopene drink (all delivering 40 mg lycopene/day) for six weeks. This study found that triglyceride levels and LDL cholesterol were decreased, and high-density lipoprotein (HDL) cholesterol increased in subjects who consumed fresh tomato and tomato juice. In a more recent study, 18 healthy men and women consumed a soy-tomato beverage daily (21 mg lycopene/day) for eight weeks (Bohn et al. 2009). Again, consumption of the beverage significantly reduced the susceptibility of the LDL + VLDL blood plasma fraction to oxidative damage. In addition, HDL cholesterol levels significantly increased, and the ratio of total cholesterol/HDL cholesterol significantly decreased over the course of the study (Bohn et al. 2009).
In contrast, a four-week dietary intervention study by Paterson et al. (2006) observed that consumption of a carotenoid-rich diet did not have an effect on plasma antioxidant status or markers of oxidative stress.
Other Diseases
Although studies on the ability of lycopene to modify cancer and CVD risk are most prevalent, there have been numerous other diseases that have also been investigated in relation to lycopene consumption. These conditions include ultra violet (UV)-induced sunburn, gingivitis, osteoporosis, mental disorders, and asthma.
The ability of lycopene to affect UV-induced sunburn was investigated in nine healthy individuals consuming 40 g tomato paste (~16 mg lycopene) with olive oil daily for 10 weeks. The control group (n = 10) consumed olive oil alone. At week = 0 and week = 10, subjects were irradiated with a solar simulator, and the a-value (i.e., redness) of skin tone was measured by chromatometry. Individuals in the treatment group had a 32% reduction in a-values between week = 0 and week = 10, and 40% lower a-values as compared with controls. This study indicates that the tomato paste treatment was protective against UV-induced sunburn (Stahl et al. 2001). A separate clinical study was performed in 36 healthy adults, whereby subjects consumed synthetic lycopene alone, a soft-gel encapsulated tomato extract, or a tomato drink for 12 weeks. Dorsal skin was irradiated at weeks 0, 4, and 12 with artificial UV light at levels high enough to cause mild sunburn. The subjects consuming the tomato extract and tomato drink had a 38% and 48% decrease, respectively, in solar simulator–induced sunburn at week 12, compared with only a 25% decrease in the group treated with synthetic lycopene (Aust et al. 2005).
A randomized, placebo-controlled, split-mouth study of gingivitis was performed by Chandra et al. (2007) in 20 healthy subjects with clinical signs of gingivitis. The treatment group (n = 10) was supplemented with 8 mg/day lycopene (LycoRed®), whereas the control group (n = 10) received a placebo daily for two weeks. In this study, patients receiving the lycopene treatment showed statistically significant reductions in gingivitis and bleeding index (Chandra et al. 2007).
Low serum levels of lycopene have also been associated with increased risk of psychiatric disorders (Li & Zhang 2007). A cross-sectional study using NHANES III data (n = 6680) observed an inverse association between serum levels of certain vitamins and carotenoids and whether subjects had attempted suicide (Li & Zhang 2007). Suicide attempters had lower levels of antioxidant vitamins and carotenoids in their serum than did nonattempters. The difference between mean serum lycopene levels in suicide attempters versus nonattempters = 0.142 μmol/L (Li & Zhang 2007).
Research has also suggested that lycopene may have a therapeutic effect on asthma. A randomized, crossover study of 17 asthmatic adults treated with placebo, tomato extract (Lyc-o-Mato®, 45 mg/day lycopene), and tomato juice (45 mg/day of lycopene) for seven days showed a reduced airway neutrophil influx and a reduced sputum neutrophil elastase activity after the tomato extract and tomato juice treatment (Wood et al. 2008). During placebo treatment, plasma lycopene concentration decreased, percent neutrophils increased, and neutrophil elastase levels increased (Wood et al. 2008). This study suggests that intake of lycopene may improve lung function in asthmatics.
Finally, a recent report also indicated that dietary lycopene intake, as measured by FFQ, was inversely related to risk of fracture in a 17-year follow-up study of elderly adults (Sahni et al. 2009). Although all of these studies are preliminary, they have presented positive results and indicate potential directions for future lycopene research and dietary interventions with tomato products.
LYCOPENOIDS: POTENTIAL OXIDATIVE METABOLITES OF LYCOPENE
Researchers have recently used the term lycopenoids to refer to oxidative products of lycopene that contain at least one oxygen atom (Lindshield et al. 2007). Historically, lycopenoids have been reported in tomatoes and tomato products, but only recently have they garnered attention from individuals interested in the potential health benefits of lycopene or its metabolites. This newfound interest is largely stimulated by the recent discovery of a second carotenoid cleavage enzyme in mammals (β-carotene oxygenase 2, or BCO2) and the possibility that it may cleave lycopene to produce metabolites (Kiefer et al. 2001). Previous to this report, only one carotenoid cleavage enzyme, the enzyme responsible for converting provitamin A carotenoids into vitamin A, was known to exist in mammals.
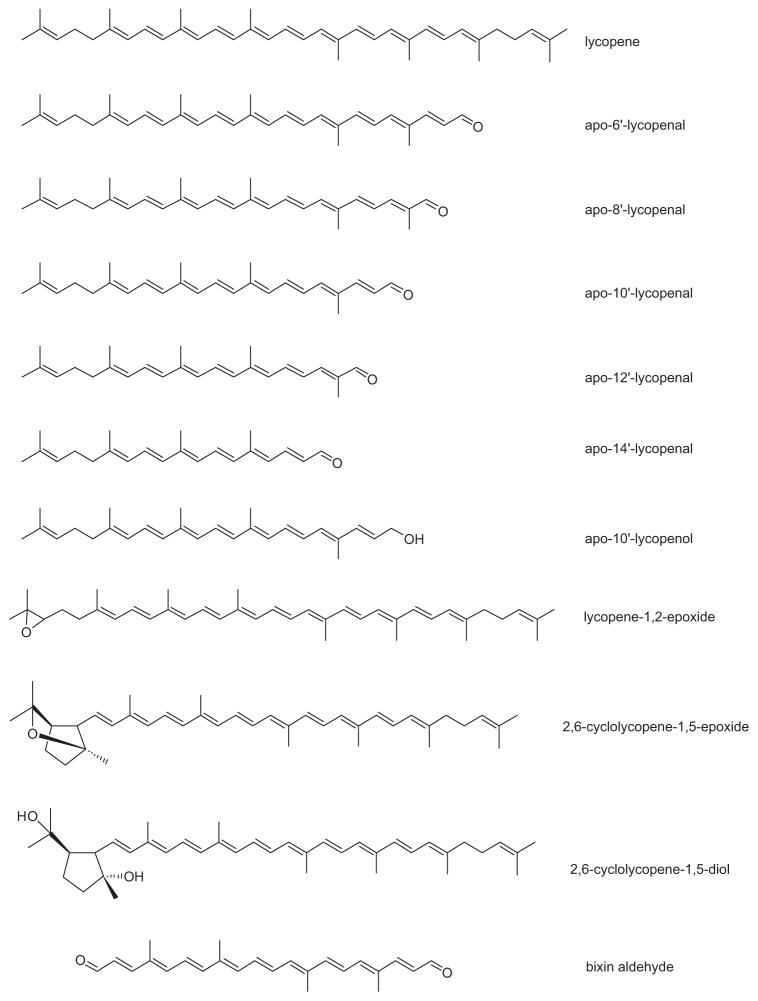
A handful of lycopenoids have been identified in food products. Apo-6′-lycopenal and apo-8′-lycopenal were reported in tomato paste (Winterstein et al. 1960). Later in 1973, Ben-Aziz et al. confirmed the presence of apo-6′-lycopenal and apo-8′-lycopenal in raw tomatoes, indicating that these products are present in the unprocessed fruit as well. Recently, we observed apo-10′-, -12′-, and -14′-lycopenal (in addition to the previously reported apo-6′- and apo-8′-lycopenals) in raw tomatoes, watermelon, grapefruit, and tomato products (Kopec et al. 2008, 2009). Epoxide derivatives have also been reported in tomatoes. Lycopene-1,2-epoxide and lycopene-5,6-epoxide were identified in tomatoes and tomato products by multiple groups (Britton & Goodwin 1969, 1975; Ben-Aziz et al. 1973; Kamber & Pfander 1984; Khachik et al. 1992). Later, nuclear magnetic resonance (NMR) research revealed that lycopene-5,6-epoxide had been misidentified and was actually 2,6-cyclolycopene-1,5-epoxide (Khachik et al. 1998b). An alcohol derivative, 2,6-cyclolycopene- 1,5-diol, was also identified in tomato products and later found to be present in human blood plasma and breast milk (Khachik et al. 1992, 1997, 1998a). These researchers hypothesized that 2,6-cyclolycopene-1,5-epoxide was converted to 2,6-cyclolycopene-1,5-diol in humans, presumably during digestion, given that none of the epoxide was identified in human samples, and the alcohol is present in small quantities in the food (Khachik et al. 1998b).
The presence of apo-lycopenoids in tomato tissues is not surprising. Analysis of the Arabidopsis genome has revealed nine potential carotenoid cleavage enzymes (Schwartz et al. 2001). Carotenoid cleavage dioxygenase 1 (CCD1) in the annatto plant has been shown to cleave lycopene to produce bixin aldehyde, a precursor of the carotenoids bixin and norbixin (Bouvier et al. 2003). CCD1 from tomato has also been shown to cleave lycopene at the 5′-6′ and 9′-10′ double bonds when transfected into an Escherichia coli model that accumulates lycopene (Vogel et al. 2008). The nonvolatile fragments of these reactions would be apo-6′-lycopenal and apo-10′-lycopenal, respectively (see Figure 3), although they were not investigated in this study (Vogel et al. 2008). Carotenoid cleavage dioxygenase 7 (CCD7) has been shown to cleave the 9′-10′ bond of lycopene (Schwartz et al. 2004). However, it is not known if the other identified CCDs cleave lycopene. Alternatively, lycopene could be broken down nonspecifically by reaction with reactive oxygen species or co-oxidation with other lipids in the plant tissue.
Figure 3.
Structure of various lycopenoids.
More recently, lycopenoids have been identified in the tissues of animals consuming lycopene. In one study, ferrets consumed a lycopene supplement (LycoVit® powder, 10% lycopene) daily for nine weeks (Hu et al. 2006). Following sacrifice, apo-10′-lycopenol was found in the lung tissue of these animals (Hu et al. 2006). In a separate study by Gajic et al. (2006), rats were fed lycopene as part of their daily diet for 50 days, followed by a radioactive dose of lycopene on day 51. Rat livers were then extracted and apo-8′-lycopenal and putative apo-12′-lycopenal were identified (Gajic et al. 2006). In addition, we identified apo-6′, -8′-, -10′-, -12′-, and -14′-lycopenal in the blood plasma of humans who had consumed 300 mL of a tomato juice based beverage daily for eight weeks (Kopec et al. 2008, 2009).
The presence of lycopenoids in animals has been hypothesized to be the result of enzymatic cleavage. In fact, lycopene has been shown to be cleaved enzymatically by BCO2 in vitro (Keifer et al. 2001, Hu et al. 2006). Other researchers have incubated lycopene with various tissue homogenates ex vivo and produced oxidative products, but it is unclear whether these are products of a BCO reaction or whether they could be produced in vivo (dos Anjos Ferreira et al. 2004).
There are very limited data on the biological effects of lycopenoids. Apo-10′-lycopenoic acid has been shown to inhibit the growth of BEAS-2B human bronchial epithelial cells in vitro (Lian & Wang 2008). The feeding of apo-10′-lycopenoic acid has also been shown to reduce lung tumor multiplicity in an A/J mouse model, an effect that was dose dependent (Lian et al. 2007).
CONCLUSIONS
In this paper, we summarized lycopene chemistry, sources, intake, bioavailability, and recent data regarding the potential health effects of lycopene. Many questions remain, however. First, and most importantly, does lycopene produce health effects in humans? A review of the epidemiological literature by Giovannucci (2005) suggests that it is still too early to determine whether tomatoes or lycopene have health benefits.
Further, it is unclear whether apparent reductions in disease risk observed in epidemiological and short-term prospective studies result from the whole tomato or from lycopene alone (Giovannucci 2005, Clinton 2005). Lycopene studies have been performed with tomato products, tomato-based supplements, and synthetic lycopene. These treatments are not interchangeable and should not be considered equivalent. In fact, the distinction between lycopene and tomatoes was made by the Food and Drug Administration (FDA) in their 2005 review of the literature to evaluate a proposed health claim on tomatoes, lycopene, and cancer (Kavanaugh et al. 2007). The FDA concluded that there is “no credible evidence to support an association between lycopene intake and a reduced risk of prostate, lung, colorectal, gastric, breast, ovarian, endometrial or pancreatic cancer,” but the FDA found “very limited evidence to support an association between tomato consumption and reduced risks of prostate, ovarian, gastric, and pancreatic cancers” (Kavanaugh et al. 2007). This conclusion was based on the limited high-quality human clinical trial data necessary to meet FDA standards and could change if more rigorously designed studies with positive results are reported.
As with other natural products with potential health benefits, lycopene continues to be researched because of promising epidemiological data as well as encouraging results in cell culture and animal studies. Discrepancies between animal data, which are generally positive, and human data, which are less clear, may be due to differences in carotenoid absorption and metabolism in humans relative to other species as well as inter-individual differences in humans. Animal studies are typically conducted using inbred animals, reducing genetic variability and producing clearer results. Lycopene’s effects may vary from person to person based on dietary lycopene and fat intake, probiotics, genetic differences in metabolism, and other factors. One further limitation of human studies is the lack of easily accessible, specific disease biomarkers to estimate the effects of a treatment on a disease process. Although PSA is frequently used to monitor prostate-cancer risk, and LDL is widely accepted as a marker for cardiovascular disease, many other diseases lack specific biomarkers. For this reason, more general markers, such as IGF-I or 8-OHdG, are often used to assess the effects of lycopene or tomato products on biochemical disease processes.
When considering the effects of a dietary component on health, it is difficult to separate the effect of a single compound from that of multiple compounds found in whole foods and whole diets. If lycopene in tomatoes does affect health, is it the major active component, or does it act synergistically with other bioactive compounds in tomatoes (provitamin A, flavonoids, vitamin C, fiber, etc.)? In fact, tomato flavonoids, including rutin, quercetin, naringenin, have been reported to have potential health effects. Quercetin and rutin have been shown to reduce IGF-1-induced prostate cell proliferation in vitro (Wang et al. 2003). Quercetin has been shown to reduce neutrophil-induced LDL oxidation (Loke et al. 2008). Naringenin chalcone from tomato skin has also been shown to produce anti-inflammatory effects in mice (Yamamoto et al. 2004). These studies provide evidence that flavonoids may play a role in the health effects of tomatoes and tomato products. Other studies have demonstrated that glycoalkaloids present in tomato also produce multiple biological effects (see review by Friedman 2002). In addition, recent research has suggested that water soluble components of tomatoes may reduce platelet aggregation, a risk factor for cardiovascular disease (O’Kennedy et al. 2006), although the components present in this fraction have not been clearly identified. Likewise, some authors have suggested that blood levels of lycopene and other carotenoids are simply indicative of a diet that includes fruits and vegetables. A growing body of evidence indicates that whole foods may be more effective than individual compounds for lowering disease risk (Clinton 2005, Gómez-Romero et al. 2007).
If lycopene does produce positive health effects, what are the mechanisms involved? Does lycopene act by itself, or is it metabolized into a biologically active compound in humans? Are the oxidative products of lycopene found in foods (lycopenoids) absorbed? Lycopene can be a potent antioxidant under the appropriate chemical conditions, but it is present at such low levels in the human body some researchers have speculated that any health effects observed may be due to the effects of lycopene or its metabolites on gene regulation (Erdman et al. 2009). However, the accumulation of lycopene in some organs may allow for multiple mechanisms of action, including antioxidant activity. Evidence from animal studies suggests that lycopene could be metabolized in the human body (Hu et al. 2006, Gajic et al. 2006), whereas data from human studies suggest that oxidative products of lycopene can be absorbed and further metabolized in humans (Khachik et al. 1992, 1997, 1998a, 1998b). It is possible that there are other bioactive lycopene metabolites that are yet to be identified. Further work must be done to understand how lycopene is metabolized in fruits and vegetables and whether these products are absorbed into the bloodstream and distributed and stored in tissues. In addition, more research is needed to understand how lycopene is metabolized in humans and whether these metabolites have biological effects.
Acknowledgments
We would like to thank Ruth Watkins for her help with the technical editing of this manuscript.
Glossary
- CVD
cardiovascular disease
- Isoprenoid
refers to an unsaturated, branched 5-carbon structure
- Reactive oxygen species (ROS)
oxygen-containing molecules that either are or have the potential to generate free radicals
- Oxidative stress
an imbalance between the oxidative and reductive reactions in living systems favoring oxidation to the extent that damage may result
- Single nucleotide polymorphisms (SNPs)
variations in a single DNA base pair in a gene that can affect the function of proteins produced from the gene
- BPH
benign prostatic hyperplasia
- FFQ
food frequency questionnaire
- RR
relative risk
- OR
odds ratio
- Prostate specific antigen (PSA)
a biomarker commonly used to monitor the risk or progression of prostate cancer
- 8-hydroxy-2′-deoxyguanosine (8-OHdG)
a DNA adduct indicative of oxidative stress, which could lead to cancer or other diseases
- Randomized placebo-controlled, double-blind, crossover trial
clinical trial in which treatments are randomly assigned, neither researchers nor participants are aware of treatment groupings, and each participant receives each participant received each treatment over the course of the study
- Insulin-like growth factor-1 (IGF-I)
a biomarker of growth that is sometimes used to monitor the development and progression of several cancers, including prostate, lung, and colon
Footnotes
The Annual Review of Food Science and Technology is online at food.annualreviews.org
RELATED RESOURCES
http://www.foodsafety.gov/~dms/qhclyco2.html
http://www.cancer.org/docroot/ETO/content/ETO_5_3X_Lycopene.asp
http://www.dietandcancerreport.org/
http://whqlibdoc.who.int/publications/2004/9241546123_chap8.pdf
Errata
An online log of corrections to Annual Review of Food Science and Technology articles may be found at http://food.annualreviews.org
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Erica N. Story, Email: estory@ncsu.edu.
Rachel E. Kopec, Email: kopec.4@osu.edu.
Steven J. Schwartz, Email: schwartz.177@osu.edu.
G. Keith Harris, Email: gkharris@ncsu.edu.
LITERATURE CITED
- Abiaka CD, Al-Awadi FM, Al-Sayer H, Gulshan S, Behbehani A, Farghaly M. Plasma micronutrient antioxidant in cancer patients. Cancer Detect Prev. 2001;25(3):245–53. [PubMed] [Google Scholar]
- Agarwal S, Rao AV. Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids. 1998;33(10):981–84. doi: 10.1007/s11745-998-0295-6. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000;163(6):739–44. [PMC free article] [PubMed] [Google Scholar]
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Can Inst. 1996;88(21):1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- Allen CM, Smith AM, Clinton SK, Schwartz SJ. Tomato consumption increases lycopene isomer concentrations in breast milk and plasma of lactating women. J Am Diet Assoc. 2002;102(9):1257–62. doi: 10.1016/s0002-8223(02)90278-6. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71(6):1691S–95S. doi: 10.1093/ajcn/71.6.1691S. [DOI] [PubMed] [Google Scholar]
- Aust O, Stahl W, Sies H, Tronnier H, Heinrich U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int J Vitam Nutr Res. 2005;75(1):54–60. doi: 10.1024/0300-9831.75.1.54. [DOI] [PubMed] [Google Scholar]
- Barber NJ, Zhang X, Zhu G, Pramanik R, Barber JA, et al. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006;9(4):407–13. doi: 10.1038/sj.pcan.4500895. [DOI] [PubMed] [Google Scholar]
- Ben-Aziz A, Britton G, Goodwin TW. The carotene epoxides of tomatoes. Phytochemistry. 1973;12(11):2759–64. [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18(1):1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Bohn T, Blackwood M, Francis D, Tian Q, Schwartz SJ, Clinton SK. Bioavailability of phytochemical constituents from a novel soy fortified lycopene rich tomato juice developed for targeted cancer prevention trials. 2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau TWM, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med. 2002;227(10):914–19. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- Borel P, Moussa M, Reboul E, Lyan B, Defoort C, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137(12):2653–59. doi: 10.1093/jn/137.12.2653. [DOI] [PubMed] [Google Scholar]
- Botterweck AAM, Van Den Brandt PA, Goldbohm RA. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma. Cancer. 2000;88(4):737–48. [PubMed] [Google Scholar]
- Bouvier F, Dogbo O, Camara B. Biosynthesis of the food and cosmetic plant pigment bixin (annatto) Science. 2003;300(5628):2089–91. doi: 10.1126/science.1085162. [DOI] [PubMed] [Google Scholar]
- Britton G, Goodwin TW. The occurrence of phytoene 1,2-oxide and related carotenoids in tomatoes. Phytochemistry. 1969;8(11):2257–58. [Google Scholar]
- Britton G, Goodwin TW. Carotene epoxides from the delta tomato mutant. Phytochemistry. 1975;14(11):2530–32. [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H. Carotenoids Handbook. Basel/Boston/Berlin: Birkhäuser Verlag; 2004. [Google Scholar]
- Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressing as measured with electrochemical detection. Am J Clin Nutr. 2004;80(2):396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- Bunker CH, McDonald AC, Evans RW, de la Rosa N, Boumosleh JM, Patrick AL. A randomized trial of lycopene supplementation in Tobago men with high prostate cancer risk. Nutr Cancer. 2007;57(2):130–37. doi: 10.1080/01635580701274046. [DOI] [PubMed] [Google Scholar]
- Burney PGJ, Comstock GW, Morris JS. Serological precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989;49(5):895–900. doi: 10.1093/ajcn/49.5.895. [DOI] [PubMed] [Google Scholar]
- Chandra RV, Prabhuji ML, Roopa DA, Ravirajan S, Kishore HC. Efficacy of lycopene in the treatment of gingivitis: a randomized, placebo-controlled clinical trial. Oral Health Prev Dent. 2007;5(4):327–36. [PubMed] [Google Scholar]
- Chang S, Erdman JW, Jr, Clinton SK, Vadiveloo M, Strom SS, et al. Relationship between plasma carotenoids and prostate cancer. Nutr Cancer. 2005;53(2):127–34. doi: 10.1207/s15327914nc5302_1. [DOI] [PubMed] [Google Scholar]
- Chasse GA, Mak ML, Deretey E, Farkas I, Torday LL, et al. An ab initio computational study on selected lycopene isomers. J Mol Struc. 2001;571:27–37. [Google Scholar]
- Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, et al. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93(24):1872–79. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- Clark PE, Hall MC, Borden LS, Jr, Miller AA, Hu JJ, et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67(6):1257–61. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56(2):35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Clinton SK. Tomatoes or lycopene: a role in prostate carcinogenesis? J Nutr. 2005;135(8):2057S–59S. doi: 10.1093/jn/135.8.2057S. [DOI] [PubMed] [Google Scholar]
- Cohen L. A review of animal model studies of tomato carotenoids, lycopene, and cancer chemoprevention. Exp Biol Med. 2002;227(10):864–68. doi: 10.1177/153537020222701005. [DOI] [PubMed] [Google Scholar]
- Comstock GW, Helzlsouer KJ, Bush TL. Prediagnostic serum levels of carotenoids and vitamin E as related to subsequent cancer in Washington Country, Maryland. Am J Clin Nutr. 1991;53(1):260S–64S. doi: 10.1093/ajcn/53.1.260S. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Kuper H, Harlow BL, Titus-Ernstoff L. Carotenoids, antioxidants and ovarian cancer risk in pre- and postmenopausal women. Int J Cancer. 2001;94(1):128–34. doi: 10.1002/ijc.1435. [DOI] [PubMed] [Google Scholar]
- Cui Y, Shikany JM, Liu S, Shagufta Y, Rohan TE. Selected antioxidants and risk of hormone receptor-defined invasive breast cancers among postmenopausal women in the Women’s Health Observational Study. Am J Clin Nutr. 2008;87(4):1009–18. doi: 10.1093/ajcn/87.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan K, Fennal M, Kumar NB. Lycopene in the prevention of prostate cancer. J Soc Integr Oncol. 2008;6(1):29–36. [PubMed] [Google Scholar]
- De Stefani E, Boffetta P, Brennan P, Deneo-Pellegrini H, Carzoglio JC, et al. Dietary carotenoids and risk of gastric cancer: a case-control study in Uruguay. Eur J Cancer Prev. 2000;9(5):329–34. doi: 10.1097/00008469-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Diwadkar-Navsariwala V, Novotny J, Gustin DM, Sosman JA, Rodvold KA, et al. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res. 2003;44(10):1927–39. doi: 10.1194/jlr.M300130-JLR200. [DOI] [PubMed] [Google Scholar]
- dos Anjos Ferreira AL, Yeum K-J, Russell RM, Krinsky NI, Tang G. Enzymatic and oxidative metabolites of lycopene. J Nutr Biochem. 2004;15(8):493–502. doi: 10.1016/j.jnutbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in caco-2 cells treated with ezetimibe. J Nutr. 2005;135(10):2305–12. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- During A, Harrison EH. Intestinal absorption and metabolism of carotenoids: insights from cell culture. Arch Biochem Biophys. 2004;430(1):77–88. doi: 10.1016/j.abb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys. 2009;483(2):229–35. doi: 10.1016/j.abb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13(3):340–45. [PubMed] [Google Scholar]
- Fabian E, Elmadfa I. The effect of daily consumption of probiotic and conventional yoghurt on oxidant and anti-oxidant parameters in plasma of young healthy women. Int J Vitam Nutr Res. 2007;77(2):79–88. doi: 10.1024/0300-9831.77.2.79. [DOI] [PubMed] [Google Scholar]
- Failla ML, Chitchumroonchokchai C, Ishida BK. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J Nutr. 2008;138(3):482–86. doi: 10.1093/jn/138.3.482. [DOI] [PubMed] [Google Scholar]
- Ferruzzi M, Nguyen ML, Sander LC, Rock CL, Schwartz SJ. Analysis of lycopene geometrical isomers in biological microsamples by liquid chromatography with coulometric array detection. J Chromatogr B. 2001;760(2):289–99. doi: 10.1016/s0378-4347(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Friedman M. Tomato glycoalkaloids: role in the plant and in the diet. J Agric Food Chem. 2002;50(21):5751–80. doi: 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- Furr HC, Clark RM. Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem. 1997;8(7):364–77. [Google Scholar]
- Gajic M, Zaripheh S, Sun FR, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136(6):1552–57. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Boyd K, Matanoshi G, Tao XG, Chen L, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88(2):372–83. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- Garcia-Closas R, Gonzalez CA, Agudo A, Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999;10(1):71–75. doi: 10.1023/a:1008867108960. [DOI] [PubMed] [Google Scholar]
- Gärtner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr. 1997;66(1):116–22. doi: 10.1093/ajcn/66.1.116. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiological literature. J Natl Cancer Inst. 1999;91(4):317–31. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94(5):391–98. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135(8):2030S–31S. doi: 10.1093/jn/135.8.2030S. [DOI] [PubMed] [Google Scholar]
- Gómez-Romero M, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A. Analytical determination of antioxidants in tomato: typical components of the Mediterranean diet. J Sep Sci. 2007;30(4):452–61. doi: 10.1002/jssc.200600400. [DOI] [PubMed] [Google Scholar]
- Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TWM, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145–54. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- Graydon R, Gilchrist SECM, Young IS, Obermüller-Jevic U, Hasselwander O, Woodside JV. Effect of lycopene supplementation on insulin-like growth factor-1 and insulin-like growth factor binding protein- 3: a double-blind, placebo-controlled trial. Eur J Clin Nutr. 2007;61(10):1196–200. doi: 10.1038/sj.ejcn.1602632. [DOI] [PubMed] [Google Scholar]
- Hadley CW, Clinton SK, Schwartz SJ. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J Nutr. 2003;133(3):727–32. doi: 10.1093/jn/133.3.727. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human diseases: curiosity, cause, or consequence? Lancet. 1994;334(8924):1–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, et al. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet. 1998;351(9113):1393–96. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ, Alberg AJ, Norkus EP, Morris JS, Hoffman SC, Comstock GW. Prospective study of serum micronutrients and ovarian cancer. J Natl Cancer Inst. 1996;88(1):32–37. doi: 10.1093/jnci/88.1.32. [DOI] [PubMed] [Google Scholar]
- Holick CN, Michaud DS, Stolzenberg-Solomon R, Mayne ST, Pietinen P, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol. 2002;156(6):536–47. doi: 10.1093/aje/kwf072. [DOI] [PubMed] [Google Scholar]
- Hu K, Liu C, Ernst H, Krinsky N, Russell R, Wang X. The biochemical characterization of ferret carotene-9,10-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281(28):19327–38. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. 2007;73(2):326–40. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kurata M, Hioki R, Suzuki K, Ochiai J, Aoki K. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: a population-based follow-up study of inhabitants of a rural area of Japan. Asian Pac J Cancer Prev. 2005a;6(1):10–15. [PubMed] [Google Scholar]
- Ito Y, Wakai K, Suzuki K, Ozasa K, Watanabe Y, et al. Lung cancer mortality and serum levels of carotenoids, retinol, tocopherols, and folic acid in men and women: a case-control study nested in the JACC Study. J Epidemiol Suppl. 2005b;2:S140–49. doi: 10.2188/jea.15.S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34(2):173–84. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- Jatoi A, Burch P, Hillman D, Vanyo JM, Dakhil S, et al. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: results of a phase II study from the north central cancer treatment group. Urol. 2007;69(2):289–94. doi: 10.1016/j.urology.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Jeong NH, Song ES, Lee JM, Lee KB, Kim MK, et al. Plasma carotenoids, retinol and tocopherol levels and the risk of ovarian cancer. Acta Obstet Gynecol Scand. 2009;88(4):457–62. doi: 10.1080/00016340902807215. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Qin J, Krinsky NI, Russell RM. Ingestion by men of a combined dose of beta-carotene and lycopene does not affect the absorption of beta-carotene but improves that of lycopene. J Nutr. 1997;127(9):1833–37. doi: 10.1093/jn/127.9.1833. [DOI] [PubMed] [Google Scholar]
- Kamber M, Pfander H. Separation of carotenoids by high-performance liquid chromatography III 1,2-epoxycarotenoids. J Chromatogr. 1984;295(1):295–98. [Google Scholar]
- Kavanaugh CJ, Trumbo PR, Ellwood KC. The US Food and Drug Administration’s evidence based review for qualified health claims: tomatoes, lycopene and cancer. J Natl Cancer Inst. 2007;99(14):1074–85. doi: 10.1093/jnci/djm037. [DOI] [PubMed] [Google Scholar]
- Khachik F, Goli MB, Beecher GR, Holden J, Lusby WR, et al. Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetables. J Agric Food Chem. 1992;40(3):390–98. [Google Scholar]
- Khachik F, Pfander H, Traber B. Proposed mechanisms for the formation of synthetic and naturally occurring metabolites of lycopene in tomato products and human serum. J Agric Food Chem. 1998a;46(12):4885–90. [Google Scholar]
- Khachik F, Spangler CJ, Smith JC, Canfield LM, Pfander H, Steck A. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69(10):1873–81. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- Khachik F, Steck A, Niggli UA, Pfander H. Partial synthesis and structural elucidation of the oxidative metabolites of lycopene identified in tomato paste, tomato juice, and human serum. J Agric Food Chem. 1998b;46(12):4874–84. [Google Scholar]
- Kiefer C, Hessel S, Lampert JM, Vogt K, Leferer MO, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276(17):14110–16. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- Knekt P, Järvinen R, Teppo L, Aromaa A, Seppänen R. Role of various carotenoids in lung cancer prevention. J Natl Cancer Inst. 1999;91(2):182–84. doi: 10.1093/jnci/91.2.182. [DOI] [PubMed] [Google Scholar]
- Kopec RE, Riedl KM, Curley RW, Jr, Harrison EH, Schwartz SJ. Presence of apo-lycopenals in food products and human blood plasma. FASEB J. 2008;22:1105.8. (Abstr.) [Google Scholar]
- Kopec RE, Riedl KM, Harrison EH, Curley RW Jr, Hruszkewycz DP, et al. Presence of apo-lycopenals in food products and human blood plasma. 2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, et al. Dietary patterns, supplemental use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167(8):925–34. doi: 10.1093/aje/kwm389. [DOI] [PubMed] [Google Scholar]
- Kritchevsky SB, Bush AJ, Pahor M, Gross MD. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152(11):1065–71. doi: 10.1093/aje/152.11.1065. [DOI] [PubMed] [Google Scholar]
- Kune G, Watson L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56(1):11–21. doi: 10.1207/s15327914nc5601_3. [DOI] [PubMed] [Google Scholar]
- Kun Y, Lule US, Xiao-Lin D. Lycopene: its properties and relationship to human health. Food Rev Intr. 2006;22(4):309–33. [Google Scholar]
- Larsson SC, Bergkvist L, Näslund I, Rutegård J, Wolk A. Vitamin A, retinol, and carotenoids and the risk of gastric cancer: a prospective cohort study. Am J Clin Nutr. 2007;85(2):497–503. doi: 10.1093/ajcn/85.2.497. [DOI] [PubMed] [Google Scholar]
- Leung YL, Crozier JEM, Talwar D, O’Reilly DSJ, McKee RF, et al. Vitamin antioxidants, lipid peroxidation, tumor state, the systemic inflammatory response and survival in patients with colorectal cancer. Int J Cancer. 2008;123(10):2460–64. doi: 10.1002/ijc.23811. [DOI] [PubMed] [Google Scholar]
- Levi F, Pasche C, Lucchini F, La Vecchia C. Dietary intake of selected micronutrients and breast-cancer risk. Int J Cancer. 2001;91(2):260–63. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1041>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang J. Serum concentrations of antioxidant vitamins and carotenoids are low in individuals with a history of attempted suicide. Nutr Neurosci. 2007;10(1–2):51–58. doi: 10.1080/10284150701250747. [DOI] [PubMed] [Google Scholar]
- Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28(7):1567–74. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123(6):1262–68. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: Are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458(2):136–40. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Loke WM, Proudfoot JM, McKinley AJ, Needs PW, Kroon PA, et al. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J Agric Food Chem. 2008;56(10):3609–15. doi: 10.1021/jf8003042. [DOI] [PubMed] [Google Scholar]
- Lu QY, Hung JC, Heber D, Go VLW, Reuter VE, et al. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(7):749–56. [PubMed] [Google Scholar]
- Lucarini M, Lanzi S, D’Evoli L, Aguizzi A, Lombardi-Boccia G. Intake of vitamin A and carotenoids from the Italian population–results of an Italian total diet study. Int J Vitam Nutr Res. 2006;76(3):103–9. doi: 10.1024/0300-9831.76.3.103. [DOI] [PubMed] [Google Scholar]
- Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-1 and IGF-binding protein-3. J Natl Cancer Inst. 1999;91(7):620–25. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- Männisto S, Yaun S, Hunter D, Spiegelman D, Adami H, et al. Dietary carotenoids and risk of colorectal cancer in a pooled analysis of 11 cohort studies. Am J Epidemiol. 2007;165(3):246–55. doi: 10.1093/aje/kwk009. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Feskanich D, Rimm EB, Golditz GA, Speizer FE, et al. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr. 2000;72(4):990–97. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- Moussa M, Landrier J, Reboul E, Ghiringhelli O, Comera C, et al. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J Nutr. 2008;138(8):1432–36. doi: 10.1093/jn/138.8.1432. [DOI] [PubMed] [Google Scholar]
- Nguyen ML, Francis D, Schwartz SJ. Thermal isomerisation susceptibility of carotenoids in different tomato varieties. J Sci Food Agric. 2001;81(9):910–17. [Google Scholar]
- Nguyen ML, Schwartz SJ. Lycopene stability during food processing. Proc Soc Exp Biol Med. 1998;218(2):101–5. doi: 10.3181/00379727-218-44274. [DOI] [PubMed] [Google Scholar]
- Nguyen ML, Schwartz SJ. Lycopene. In: Lauro GL, Francis FJ, editors. Natural Food Colorants: Science and Technology. New York: Marcel Dekker, Inc; 2000. pp. 153–92. [Google Scholar]
- Nkondjock A, Ghadirian P, Johnson KC, Krewski D Canadian Cancer Registries Epidemiology Research Group . Dietary intake of lycopene is associated with reduced pancreatic cancer risk. J Nutr. 2005;135(3):592–97. doi: 10.1093/jn/135.3.592. [DOI] [PubMed] [Google Scholar]
- Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, et al. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2087–92. doi: 10.1158/1055-9965.EPI-05-0038. [DOI] [PubMed] [Google Scholar]
- O’Kennedy N, Crosbie L, Whelan S, Luther V, Horgan G, et al. Effects of tomato extract on platelet function: a double-blinded crossover study in healthy humans. Am J Clin Nutr. 2006;84(3):561–69. doi: 10.1093/ajcn/84.3.561. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, et al. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J Nat Can Inst. 1996;88(21):1550–59. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- Parada J, Aguilera JM. Food microstructure affects the bioavailability of several nutrients. J Food Sci. 2007;72(2):R21–32. doi: 10.1111/j.1750-3841.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Paterson E, Gordon MH, Niwat C, George TW, Parr L, et al. Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter markers of oxidative stress or cardiovascular risk factors. J Nutr. 2006;136(11):2849–55. doi: 10.1093/jn/136.11.2849. [DOI] [PubMed] [Google Scholar]
- Persson C, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, et al. Plasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: a nested case-control study. Carcinogenesis. 2008;29(5):1042–48. doi: 10.1093/carcin/bgn072. [DOI] [PubMed] [Google Scholar]
- Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, et al. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2007;16(5):962–68. doi: 10.1158/1055-9965.EPI-06-0861. [DOI] [PubMed] [Google Scholar]
- Porrini M, Riso P. What are typical lycopene intakes? J Nutr. 2005;135(8):2042S–45S. doi: 10.1093/jn/135.8.2042S. [DOI] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Rao AV, Ray MR, Rao LG. Lycopene. Adv Food Nutr Res. 2006;51:99–164. doi: 10.1016/S1043-4526(06)51002-2. [DOI] [PubMed] [Google Scholar]
- Rao AV, Waseem Z, Agarwal S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res Intern. 1998;31(10):737–41. [Google Scholar]
- Riccioni G, Mancini B, di Ilio E, Bucciarelli T, D’Orazio N. Protective effect of lycopene in cardiovascular disease. Eur Rev Med Pharacol Sci. 2008;12(3):183–90. [PubMed] [Google Scholar]
- Riso P, Brusamolino A, Martinetti A, Porrini M. Effect of a tomato drink intervention on insulin-like growth factor (IGF)-1 serum levels in healthy subjects. Nutr Cancer. 2006;55(2):157–62. doi: 10.1207/s15327914nc5502_6. [DOI] [PubMed] [Google Scholar]
- Rissanen TH, Voutilainen S, Nyyssonen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2003;77(1):133–38. doi: 10.1093/ajcn/77.1.133. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bustamante E, Sanchez S. Microbial production of C-13 norisoprenoids and other aroma compounds via carotenoid cleavage. Crit Rev Micro. 2007;33(3):211–30. doi: 10.1080/10408410701473306. [DOI] [PubMed] [Google Scholar]
- Rohan TE, Jain M, Howe GR, Miller AB. A cohort study of dietary carotenoids and lung cancer risk in women (Canada) Cancer Causes Control. 2002;13(3):231–37. doi: 10.1023/a:1015048619413. [DOI] [PubMed] [Google Scholar]
- Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer. 1999;35(2):111–19. doi: 10.1207/S15327914NC352_3. [DOI] [PubMed] [Google Scholar]
- Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham osteoporosis study. J Bone Miner Res. 2009;24(6):1086–94. doi: 10.1359/JBMR.090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satia JA, Littman A, Slatore CG, Galanko JA, White E. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the Vitamins And Lifestyle (VITAL) study. Am J Epidemiol. 2009;69(7):815–28. doi: 10.1093/aje/kwn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Loewen MC. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem. 2004;279(45):46940–45. doi: 10.1074/jbc.M409004200. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JAD. Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem. 2001;276(27):25208–11. doi: 10.1074/jbc.M102146200. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Obermüller-Jevic UC, Hellmis E, Koch W, Jacobi G, Biesalski HK. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008;138(1):49–53. doi: 10.1093/jn/138.1.49. [DOI] [PubMed] [Google Scholar]
- Sesso H, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr. 2003;133(7):2336–41. doi: 10.1093/jn/133.7.2336. [DOI] [PubMed] [Google Scholar]
- Shen YC, Chen SL, Wang CK. Contribution of tomato phenolics to antioxidation and down-regulation of blood lipids. J Agric Food Chem. 2007;55(16):6475–81. doi: 10.1021/jf070799z. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W. Vitamin E and Vitamin C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62(6):1315S–21S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- Stahl W, Heinrich U, Wiseman S, Eichler O, Seis H, Tronnier H. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131(5):1449–51. doi: 10.1093/jn/131.5.1449. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122(11):2161–66. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD, Folsom AR. Vegetables, fruit, and lung cancer in the Iowa Women’s Health Study. Cancer Res. 1993;53(3):536–43. [PubMed] [Google Scholar]
- Thomson CA, Stendell-Hollis NR, Rock CL, Cussler EC, Flatt SW, Pierce JP. Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2008–15. doi: 10.1158/1055-9965.EPI-07-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyssandier V, Cardinault N, Caris-Veyrat C, Amiot MJ, Grolier P, et al. Vegetable-borne lutein, lycopene, and beta-carotene compete for incorporation into chylomicrons, with no adverse effect on the medium-term (3-wk) plasma status of carotenoids in humans. Am J Clin Nutr. 2002;75(3):526–34. doi: 10.1093/ajcn/75.3.526. [DOI] [PubMed] [Google Scholar]
- Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135(3):431–36. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- Unlu NZ, Bohn T, Francis D, Clinton SK, Schwartz SJ. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-β-carotene varieties of tomatoes. J Agric Food Chem. 2007;55(4):1597–1603. doi: 10.1021/jf062337b. [DOI] [PubMed] [Google Scholar]
- US Mortality Data 2005. National Center for Health Statistics, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- Vogel JT, Tan B, McCarty DR, Klee HJ. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem. 2008;283(17):11364–73. doi: 10.1074/jbc.M710106200. [DOI] [PubMed] [Google Scholar]
- Voorrips LE, Goldbohm RA, Brants HA, van Poppel GA, Strumans F, et al. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(4):357–65. [PubMed] [Google Scholar]
- Voskuil DW, Vrieling A, Korse CM, Beijnen JH, Bonfrer JMC, et al. Effects of lycopene on the insulin-like growth factor (IGF) system in premenopausal breast cancer survivors and women at high familial breast cancer risk. Nutr Cancer. 2008;60(3):342–53. doi: 10.1080/01635580701861777. [DOI] [PubMed] [Google Scholar]
- Vrieling A, Voskuil DW, Bonfrer JM, Korse CM, van Doorn J, et al. Lycopene supplementation elevates circulating insulin-like growth factor-binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. Am J Clin Nutr. 2007;86(5):1456–62. doi: 10.1093/ajcn/86.5.1456. [DOI] [PubMed] [Google Scholar]
- Walfisch S, Walfisch Y, Kirilov E, Linde N, Mnitentag H, et al. Tomato lycopene extract supplementation decreases insulin-growth factor-I levels in colon cancer patients. Eur J Cancer Prev. 2007;16(4):298–303. doi: 10.1097/01.cej.0000236251.09232.7b. [DOI] [PubMed] [Google Scholar]
- Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133(7):2367–76. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- Williams GM, Williams CL, Weisburger JH. Diet and cancer prevention: the fiber first diet. Toxicol Sci. 1999;52(2 Supplement):72–86. [PubMed] [Google Scholar]
- Winterstein A, Studer A, Ruegg R. Neuere Ergebnisse der Carotinoidforschung. Chemische Berichte- Recueil. 1960;93(12):2951–65. [Google Scholar]
- Wood LG, Garg ML, Powell H, Gibson PG. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008;42(1):94–102. doi: 10.1080/10715760701767307. [DOI] [PubMed] [Google Scholar]
- Xianquan S, Shi J, Kakuda Y, Yueming J. Stability of lycopene during food processing and storage. J Med Food. 2005;8(4):413–22. doi: 10.1089/jmf.2005.8.413. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yoshimura M, Yamaguchi F, Kouchi T, Tsuji R, et al. Anti-allergic activity of naringenin chalcone from a tomato skin extract. Biosci Biotechnol Biochem. 2004;68(8):1706–11. doi: 10.1271/bbb.68.1706. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Ross RK, Chu XD, Gao YT, Yu MC. Prediagnostic levels of serum beta-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2001;10(7):767–73. [PubMed] [Google Scholar]
- Yuan JM, Ross RK, Gao YT, Qu YH, Chu XD, Yu MC. Prediagnostic levels of serum micronutrients in relation to risk of gastric cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2004;13(11):1772–80. [PubMed] [Google Scholar]
- Yuan JM, Stram DO, Arakawa K, Lee H, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2003;12(9):890–98. [PubMed] [Google Scholar]
- Zechmeister L. Cis-trans isomerization and stereochemistry of carotenoids and diphenyl-polyenes. Chem Rev. 1944;34(2):267–344. [Google Scholar]
- Zhang M, D’Arcy C, Holman J, Binns CW. Intake of specific carotenoids and risk of epithelial ovarian cancer. Br J Nutr. 2007;98(1):187–93. doi: 10.1017/S0007114507690011. [DOI] [PubMed] [Google Scholar]